1. Introduction
Since the identification of acquired immunodeficiency syndrome (AIDS) in 1981, HIV has infected approximately 85.6 million people, leading to 40.4 million deaths. As of 2022, 53% of the 39 million people living with HIV are women and girls. Despite improvements in survival rates due to antiretroviral therapy (ART), cervical cancer remains prevalent among HIV-infected individuals. By 2030, it is projected that cervical cancer will result in 727,500 new cases and 432,000 deaths globally. Human papillomavirus (HPV) is a well-established cause of cervical cancer, with HIV infection increasing the prevalence of high-risk HPV (HR-HPV) and cervical intraepithelial neoplasia (CIN). HIV and HPV share transmission routes, and HIV exacerbates cervical carcinogenesis through mechanisms such as inhibition of tumour suppressor genes and alteration of cell cycle regulation. Chronic inflammation and cytokine imbalances, notably increased IL-10 levels, are linked to cervical cancer progression. Despite ART improving overall health, cervical cancer remains a significant issue, highlighting the need for better screening and prevention [
1,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18].
The introduction of HPV vaccines has improved prospects for cervical cancer prevention in sub-Saharan Africa. With many countries eligible for GAVI support, understanding factors affecting vaccine implementation is crucial. Studies indicate that HPV vaccination followed by regular screenings is cost-effective, emphasising the need for evidence-based policies to improve cervical cancer outcomes. Despite challenges, HPV vaccination offers hope for cancer control, highlighting the importance of scientific approaches to overcome barriers and achieve vaccination targets.
2. HPV and Cervical Dysplasia in HIV-Positive Women
A cross-sectional study in Brazil aimed to evaluate cytokine levels in cervicovaginal lavage and their association with HPV infection, HIV viral suppression, and other factors among HIV-positive women. This study revealed a high prevalence of specific HPV types, including HPV52, HPV51, HPV16, HPV18, HPV35, and HPV66. Notably, HR HPV prevalence was higher in HIV-positive men (25.7%) compared to HIV-negative men (15.8%). HPV16 was the most common subtype, with age and lower CD4 counts increasing the risk of cervical dysplasia. Most patients were asymptomatic, with peak HPV incidence observed in the 20-40 age group. These findings underscore the importance of routine Pap smear screening for early detection and management of dysplasia in HIV-infected women [
2,
19,
20,
21,
22].
3. ART and HPV-Related Cervical Lesions
Despite ART improving life expectancy, its impact on cervical cancer is unclear. A systematic review of 22 studies in sub-Saharan Africa examined ART’s effects on HPV prevalence and cervical abnormalities. Women on HAART showed a reduced likelihood of developing squamous intraepithelial lesions (SILs). Evidence suggests that HAART, particularly when combined with higher CD4 counts, reduces HR-HPV prevalence. Severe immunosuppression without HAART increases HR-HPV risk. While ART reduces the prevalence of CIN2 and CIN3, its impact on invasive cervical cancer remains inconclusive. Enhanced surveillance and further research on ART’s impact on cervical cancer are needed [
3,
23]. Women living with HIV (WLWH) face a high risk of cervical cancer due to high-risk Human papillomavirus (hrHPV). This study examined HPV prevalence, genotypes, and cervical cancer risk factors in 250 sexually active WLWH in Accra. Cervical swabs tested for HPV showed a 60% overall prevalence, with 44.4% for hrHPV. Employment and HAART were protective against hrHPV, while longer HIV diagnosis durations increased risk. Genotype analysis revealed 25% had hrHPV group 1 (including types 16 and 18) and 46.8% had multiple HPV types. The study highlights the need for regular HPV screening in high-risk groups to prevent cervical cancer [
24]. Cervical cancer screening methods are consistent across women, regardless of HIV status, and depend on resource availability. These methods include visual inspections (VIA and VILI), cytology-based tests (Pap smear and LBC), and HPV DNA testing. VIA allows training of lower cadre staff and offers the benefit of concurrent screening and treatment, although it has a high false positive rate. The choice of method depends on available resources [
25,
26,
27,
28,
29].
4. Systematic Review and Meta-Analysis on ART and HPV
A comprehensive review and meta-analysis of 31 studies assessed ART’s association with HR HPV prevalence and cervical abnormalities. The analysis included 6,537 women for HR HPV prevalence and 9,288 for high-grade cervical lesions (HSIL-CIN2+). ART was associated with a lower prevalence of HR HPV (adjusted odds ratio [aOR] 0.83) and a reduced prevalence of HSIL-CIN2+ (0.65). Longitudinal studies indicated that ART decreased HSIL-CIN2+ incidence (0.59), reduced progression of SIL (adjusted hazard ratio [aHR] 0.64), and increased regression of SIL or CIN (1.54). Among 15,846 women, ART was linked to a reduced incidence of invasive cervical cancer (crude HR 0.40). These findings suggest that early ART initiation and adherence may reduce cervical lesion incidence and progression, highlighting the need for further cohort studies to confirm these effects [
4]. The interactions between antiretroviral therapy (ART) and the progression of high-risk HPV and cervical lesions in women living with HIV are not well understood. Observational studies vary in design and outcomes, complicating the assessment of ART’s true impact. Although systematic reviews have examined the association between ART and high-risk HPV and cervical lesions, no meta-analysis has quantified the risk for ART users versus ART-naive women. With the growing number of women on ART, better understanding the effects of ART, immune recovery, and virological control on HPV and cervical lesion progression is crucial for effective screening programmes [
30,
31,
32].
The effect of antiretroviral therapy (ART) on anal high-risk HPV and lesion progression in people living with HIV is not well understood. This systematic review and meta-analysis examined the association of ART, HIV-RNA plasma viral load (PVL), and CD4 cell counts with anal HPV infection, anal intraepithelial neoplasia (AIN), and anal cancer. From 6777 studies, 122 matched the inclusion criteria, covering 417,006 individuals. ART users had a 35% lower prevalence of high-risk HPV than ART-naive individuals, with prolonged ART reducing high-risk HPV prevalence by 10% per year. Undetectable PVL was associated with lower HSIL-AIN2+ prevalence and reduced anal cancer risk by 44% when sustained for over a year. Each 100-cell/μL increase in nadir CD4 counts decreased anal cancer incidence by 40%. Although most studies were cross-sectional and few adjusted for confounders, the analysis suggests that effective, early ART initiation at high nadir CD4 counts may reduce anal HPV infection and anal cancer risk in people living with HIV [
33].
Recent epidemiologic studies have shown a significant increase in anal cancer among specific male sub-populations, particularly men who have sex with men and HIV-positive individuals. Fewer studies have examined the changing epidemiology of anal cancer in women. However, it is evident that HIV-positive women face a significantly increased risk, with anal cancer incidence rising from 0 per 100,000 (1980-1989) to approximately 11 per 100,000 (1996-2004). This highlights that squamous cell carcinoma of the anus (SCCA) is an escalating issue for women in the United States, especially those who are HIV-positive [
34,
35,
36].
This study systematically reviewed publications on the epidemiology of anal HPV infection, anal intraepithelial neoplasia (AIN), and anal cancer in women, focusing on research from January 1997 to September 2013, during the combined antiretroviral therapy era. The review included 37 publications on anal HPV and cytology and 23 on anal cancer. Among HIV-positive women, anal high-risk HPV (HR-HPV) prevalence ranged from 16-85%, compared to 4-86% in HIV-negative women. HIV-negative women with HPV-related pathology of the vulva, vagina, or cervix had anal HR-HPV prevalence of 23-86%, versus 5-22% in those without known pathology. Histological high-grade squamous intraepithelial lesions (AIN2 or greater) were found in 3-26% of HIV-positive women, 0-9% in women with lower genital tract pathology, and 0-3% in HIV-negative women without such pathology. Anal cancer incidence ranged from 3.9 to 30 per 100,000 in HIV-infected women, 0.8 to 63.8 per 100,000 in women with a history of cervical cancer or CIN3, and 0.55 to 2.4 per 100,000 in the general population. The review indicates a high prevalence of anal HPV infection and dysplasia, particularly in HIV-positive women and those with HPV-related lower genital tract pathology, and an increasing incidence of anal cancer despite widespread antiretroviral therapy use [
37].
5. HPV Prevalence among Men and Anal Cancer
HPV is a significant cause of sexually transmitted diseases, with high-risk types such as HPV16 and HPV18 linked to various cancers, including cervical, penile, anal, and head-and-neck cancers. The prevalence of HPV and related cancers is notably high in sub-Saharan Africa, where both HPV and HIV rates are elevated, and effective screening is limited. A systematic review and meta-analysis of 11 studies involving 9,342 men from sub-Saharan Africa revealed a high HPV prevalence, with overall rates ranging from 19.1% to 100%. The pooled prevalence was 78.2% among HIV-positive men and 49.4% among HIV-negative men. HPV16 and HPV52 were the most common HR types, while HPV6 was the predominant low-risk type. No clear age-related trend in HPV prevalence was observed. These findings indicate a substantial HPV burden among men in the region and underscore the potential benefits of HPV vaccination programs [
7].
This study assessed anal HPV and HSIL prevalence in men aged 50+ in San Francisco. Among 129 men who have sex with men living with HIV (MSMLWH) and 109 not living with HIV (MSM-not-LWH), 47% and 37% had anal HSIL, respectively. HPV-16 was found in 19% of MSMLWH and 22% of MSM-not-LWH. Although increasing age was not linked to higher HSIL or HPV prevalence, HPV-16 and other oncogenic HPV types were strongly associated with anal HSIL. Given that treating HSIL can prevent anal cancer, screening for anal cancer in older MSM is recommended [
38].
Anogenital HPV infection is the most common STI globally, affecting skin and mucosal cells, and is linked to various lesions and cancers, including anal carcinoma. Immunosuppression, such as from HIV, increases the risk of HPV acquisition and anal dysplasia. Anal cancer is a prevalent non-AIDS-defining disease in people living with HIV, especially women and MSM. High-risk HPV genotypes are associated with a higher likelihood of precursor lesions for anogenital and oropharyngeal cancers. HIV-positive MSM without oncogenic HPV genotypes and normal anal cytology are not at increased risk of HSIL or anal cancer [
39,
40,
41,
42,
43,
44,
45,
46,
47].
Men who have sex with men (MSM) face a high risk of HPV-associated anal cancer. This study reviewed data on anal HPV prevalence, high-grade anal intraepithelial neoplasia (AIN), and anal cancer in MSM. Among HIV-positive MSM, the prevalence of anal HPV-16 was 35.4%, with high-grade AIN at 29.1%, and anal cancer incidence at 45.9 per 100,000. In HIV-negative MSM, the prevalence of HPV-16 was 12.5%, with high-grade AIN at 21.5%, and anal cancer incidence at 5.1 per 100,000. Data on incident HPV and high-grade AIN were limited. Despite the high prevalence of HPV and precursors, progression to cancer appears lower than for cervical lesions, highlighting the need for large, prospective studies to develop screening guidelines [
48].
Anal cancer, caused by HPV, is a significant risk for HIV-infected men. This cross-sectional study from the CARH·MEN cohort at Hospital Germans Trias i Pujol (Spain) examined HPV prevalence and genotype distribution in HIV-infected men, including MSM and men who have sex with women (MSW). Cytological abnormalities were found in 40% of MSM and 20% of MSW. High-resolution anoscopy revealed lesions in 67% of MSM and 54% of MSW, with HPV16 being the most prevalent high-risk genotype. All HSIL cases were HR-HPV positive. The study concludes that anal cancer screening should be offered to all HIV-infected men, regardless of sexual orientation [
49].
This study of people living with HIV and a history of malignancy found high prevalence rates of HR-HPV types 16 and 18, with 89% infected. Anal HSIL was observed in 38% of patients. The results underscore the need for regular anal cancer screening in this population [
50].
This study investigated anal high-grade squamous intraepithelial lesions (HSIL) among men who have sex with men (MSM) and transgender women, who started antiretroviral therapy during acute HIV acquisition in Bangkok. Of 93 participants with a median age of 26, 11.8% had baseline histologic anal HSIL. The incidence of new HSIL was 19.7 per 100 person-years. Factors linked to incident anal HSIL included anal HPV types 16, 18/45, other high-risk HPV types, syphilis infection, and a CD4 count <350 cells/mm³. Despite antiretroviral therapy, anal HSIL prevalence and incidence remained higher compared to those without HIV. Regular screening and management of anal HSIL are vital in long-term HIV care for MSM [
51].
In recent decades, cervical cancer incidence has decreased due to cervical cytology screening, which identifies precursors such as high-grade cervical intraepithelial neoplasia (CIN) 2-3. Ablation of these lesions has notably reduced cervical cancer rates. The highest incidence of cervical cancer is now seen in regions without routine screening. Anal cancer, biologically similar to cervical cancer, is also linked to human papillomavirus (HPV). High-grade anal intraepithelial neoplasia (HGAIN), the anal equivalent of CIN, can progress to anal cancer. Screening for HGAIN involves anal cytology and high-resolution anoscopy (HRA)-directed biopsy, mirroring cervical cancer detection techniques. Given the similarities between cervical and anal cancer, it is hypothesised that removing HGAIN could potentially reduce anal cancer risk. HPV is a common sexually transmitted infection, with higher prevalence in individuals with HIV compared to HIV-negative counterparts. HIV-positive individuals exhibit increased rates of HPV-associated CIN and AIN, as well as higher incidences of anogenital cancers, including anal cancer. Despite the effectiveness of highly active antiretroviral therapy (HAART) in extending life, it does not significantly reduce the incidence of anogenital cancer precursor lesions, regression of existing high-grade lesions, or HPV clearance. This has led to a growing concern that, with increased survival times, HIV-positive individuals may face a higher risk of anal cancer due to persistent HPV infections and HGAIN. Recent studies have highlighted the high incidence of anal cancer among HIV-positive men who have sex with men (MSM). For example, Piketty et al. reported an incidence of 75 cases per 100,000 person-years in France, while D’Souza et al. found 137 cases per 100,000 person-years in the US. Patel et al. reported 78 cases per 100,000 person-years, all exceeding the highest reported incidence of cervical cancer globally. Screening and treating HGAIN could potentially reduce anal cancer incidence if treatment effectively prevents cancer progression. High-risk groups, including HIV-positive MSM, HIV-positive women, and others with compromised immune systems, might benefit from such interventions. Despite successful treatment of early-stage anal cancer with chemoradiation, this approach is associated with significant morbidity. Preventive screening and treatment of HGAIN are thus preferable. Arguments against routine anal screening include a lack of evidence showing that treating HGAIN reduces anal cancer rates, insufficient cost-effectiveness data, and limited knowledge on effective treatment modalities for HGAIN. Additionally, a high proportion of HIV-positive individuals have HGAIN, suggesting that large numbers would need treatment. Risks associated with unnecessary treatments and the need for further research on biomarkers to predict cancer progression are also concerns. Moreover, there are practical issues such as the limited number of trained clinicians and the costs and potential adverse effects of HGAIN treatments [
52,
53,
54,
55,
56,
57,
58,
59].
6. HPV and HIV in Tanzania
A study in Tanzania assessed HPV prevalence and type distribution among 1,813 men, revealing an overall HPV prevalence of 20.5%. The most common HR HPV types were HPV52, HPV51, HPV16, HPV18, HPV35, and HPV66. HIV-positive men had a significantly higher prevalence of HR HPV (25.7%) compared to HIV-negative men (15.8%, P = 0.0027). Although HPV16, HPV18, and multiple HR HPV types were somewhat more common in HIV-positive individuals, these differences were not statistically significant for other HPV types. This study highlights the high prevalence of specific HPV types and suggests that HIV status has a limited impact on HPV type distribution. Consequently, HPV vaccines are likely effective in preventing HPV infection irrespective of HIV status [
8].
A study conducted in Dar es Salaam, Tanzania, aimed to investigate the prevalence and association of human papillomavirus (HPV) infections with HIV among cervical cancer patients and non-cancer patients. Southern blot hybridisation and PCR were used to analyse tumour biopsies from 53 women with cervical or vaginal cancer at Ocean Road Hospital and cervical swabs from 359 non-cancer patients at the Muhimbili University College of Health Sciences. The findings revealed that HPV types 16 and 18 were detected in 38% and 32% of the cervical cancer biopsies, respectively. In contrast, 59% of the non-cancerous women had HPV-DNA. Among the HPV infections in non-cancer patients, types 16 and 18 were present in 13.2% and 17.5% of cases, respectively. This indicates a notably high prevalence of HPV type 18 in Tanzania compared to other regions. Key risk factors for HPV-DNA presence in non-cancer patients included young age and HIV infection. The study showed that HPV types 16 and 18 were significantly associated with trichomonas vaginalis infection, single status, early age at first intercourse, and young age at menarche. Notably, young age at menarche was found to have the most substantial impact on HPV infection risk. Although 12.8% of non-cancer patients were HIV-positive, there was no observed increase in cervical cytological abnormalities or cervical cancer rates among these women. The overall rate of cervical cytological abnormalities was 2.8%. HIV-positive patients had a higher likelihood of having HPV types 16 and 18 compared to other HPV types, but this did not correlate with a higher rate of cervical abnormalities or cancer. Furthermore, the HIV prevalence in cervical cancer patients was relatively low at 3%. The study concluded that, in this cross-sectional analysis, there was no clear association between HIV infection and cervical cytological abnormalities or cervical cancer [
60].
A cross-sectional study at Bugando Medical Centre, Mwanza, Tanzania, investigated HPV genotypes associated with cervical squamous intraepithelial lesions (SIL) among HIV-infected women between August and October 2014. Exfoliated cervical cells from 255 HIV-positive women (mean age 39.2 years) were analysed using PCR and sequencing. HPV DNA was found in 54.1% of the women, with 26 different genotypes identified, including 17 high-risk (HR) types. The most common HR genotypes were HPV-52, HPV-58, HPV-35, and HPV-16. Women with CD4 counts below 100 and those with SIL had significantly higher risks of HPV infection. Specifically, the risk ratios for HPV positivity were 1.20 for low CD4 counts and 1.37 for SIL. The study highlights the prevalence of uncommon HR HPV genotypes in HIV-positive women with low CD4 counts and underscores the need to assess HPV vaccine effectiveness in settings where HIV is widespread [
61].
A study using a deterministic transmission-dynamic compartment model assessed the impact of HIV interventions on cervical cancer in Tanzania from 1995 to 2070. Tanzania faces a high burden of both HIV and cervical cancer, with a 5.5% HIV prevalence in women in 2018 and a cervical cancer incidence rate of 59.1 per 100,000 women annually. The model projected that voluntary medical male circumcision (VMMC) has prevented 2,843 cervical cancer cases and 1,039 deaths from 1995 to 2020. By 2070, VMMC is expected to reduce cervical cancer incidence and mortality rates by 28% and 26%, respectively. Anti-retroviral therapy (ART) is anticipated to temporarily increase cervical cancer cases and deaths due to extended life expectancy but will ultimately lower these rates by 7% and 5%, compared to VMMC alone. The combined use of ART and targeted pre-exposure prophylaxis (PrEP) is predicted to further decrease incidence and mortality rates to 35.82 and 25.35 per 100,000 women, respectively, by 2070. These findings underscore that while ART may initially increase cervical cancer rates, ongoing HIV prevention efforts will significantly reduce cervical cancer incidence and mortality in the long term [
62].
Women living with HIV (WLWH) face a significantly increased risk of acquiring HPV infection and developing cervical cancerous lesions, with their risk being six times higher than that of HIV-negative women. This elevated risk persists even among younger women. Despite the availability of effective prevention, early diagnosis, and treatment methods, WLWH remain particularly vulnerable. To address the global burden of cervical cancer (CC), the World Health Organization (WHO) has proposed the “90–70–90” targets for 2030. These targets aim for 90% of girls to be fully vaccinated against HPV by 15 years of age, 70% of women to be screened with a high-performance test by 35 and 45 years of age, and 90% of women with cervical cancer to receive treatment and care. Tanzania, where this study is focused, has the highest incidence of cervical cancer in East Africa. In 2020, the country reported 10,241 new cases, with a cumulative risk of 25.3% for new cases and 24.2% for cancer-related deaths annually, according to the IARC/WHO Report. Among WLWH in Tanzania, high-risk HPV (HR-HPV) positivity rates are alarmingly high, reaching 46.7%. Current research in Dar es Salaam, Moshi, and Mwanza is investigating the distribution of various HPV genotypes in these populations. CC is preventable through HPV vaccination. Following a successful pilot project in 2014 in the Kilimanjaro region for girls aged 9–14 years, Tanzania’s Ministry of Health (MoH) introduced a nationwide vaccination programme in 2018 for 14-year-old girls. By the end of 2019, vaccine coverage was 78% for the first dose and 49% for the second dose. However, challenges remain, including insufficient coverage of all high-risk HPV types and a substantial older population that remains unvaccinated. This underscores the need for ongoing secondary prevention. In addition to vaccination, Tanzania’s MoH established a nationwide screen-and-treat programme in 2011, utilising speculum-aided visual inspection with acetic acid (VIA) and cryotherapy for treating precancerous lesions. For WLWH, screening is recommended upon HIV diagnosis and annually thereafter, ideally integrated into routine HIV care. This should also include screening and treatment for sexually transmitted infections (STIs) and female genital schistosomiasis (GS), which are associated with higher cervical cancer risk [
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76].
7. HPV and Cervical Cancer in Sub-Saharan Africa
Cervical cancer rates in sub-Saharan Africa are alarmingly high, with over 75,000 new cases and 50,000 deaths annually. The region hosts diverse HPV genotypes, with high-risk types such as HPV-16, -18, -35, and -52 prevalent. A systematic review and meta-analysis of 27 studies involving 16,506 participants found a pooled HR HPV prevalence of 34% (95% CI: 29-39%). Among 3,075 women, HPV-16 was the most common genotype (13.8%), followed by HPV-52 (9.9%) and HPV-18 (9%). HPV-16 and -52 were predominant in Eastern and Southern Africa, while HPV-16 and -35 were more common in Western Africa. The varied prevalence of HR HPV highlights the need for targeted vaccination and prevention strategies tailored to regional differences [
6].
Developing countries aim to use HPV testing as a primary cervical cancer screening method, requiring triage tests for HPV-positive women. This study compared visual inspection with acetic acid (VIA) and cytology for detecting cervical intraepithelial neoplasia Grade 2 or higher (CIN2+) in 846 women from Yaoundé and Edea, Cameroon. Of the 259 HPV-positive women, 198 were randomly assigned to VIA or cytology. VIA had a sensitivity of 25.0% and specificity of 74.2%, while cytology showed a sensitivity of 90.0% and specificity of 85.2%. The ROC area for cytology was 0.910, significantly higher than VIA’s 0.496. VIA was inferior to cytology, necessitating further research for optimal triage methods [
77].
This systematic review and meta-analysis assessed the accuracy of visual inspection with acetic acid (VIA), visual inspection with Lugol’s iodine (VILI), and HPV testing as standalone methods for cervical cancer screening in sub-Saharan Africa. Fifteen moderate-quality studies were included, with sample sizes of 61,381 for VIA, 46,435 for VILI, and 11,322 for HPV testing. CIN2+ prevalence ranged from 2.3% in VILI studies to 4.9% in HPV testing studies. Positivity rates were 16.5% for VILI, 16.8% for VIA, and 25.8% for HPV testing. VILI had higher pooled sensitivity (95.1%) compared to VIA (82.4%) and similar specificity (87.2% versus 87.4%). HPV testing had comparable accuracy to VIA and VILI. VILI, being simple and affordable, demonstrated higher sensitivity than VIA, making it a viable alternative to cytology for primary screening. Implementation studies are recommended to evaluate the impact of these screening methods on cervical cancer incidence and outcomes in the region [
78].
The advent of highly effective HPV vaccines has revitalised efforts to eliminate cervical cancer, a leading cause of cancer mortality among women in low-resource countries. Approximately 90% of the 260,000 annual cervical cancer deaths occur in less-developed regions. Unvaccinated women can be safeguarded through screening and treating precancerous lesions. The WHO recommends HPV testing for women aged 30 to 49 where resources permit, as these tests are more sensitive than VIA or Pap smears, enable longer screening intervals of at least five years, and can be performed with self-collected samples. The American Society of Clinical Oncology also endorses HPV testing as the preferred screening method in low-resource settings. Given sub-Saharan Africa’s high cervical cancer rates, assessing the feasibility of implementing HPV testing as the primary screening method is crucial. This review examined policy documents and literature from Kenya, Tanzania, and Uganda and involved consultations with key government and NGO personnel in Tanzania and Uganda. The consultations, involving 25 to 30 individuals in each country, focused on current policies and practices, the evidence required by governments to support policy changes, and the decision-making processes for updating screening policies. The findings highlight the opportunities and challenges of introducing HPV testing in these regions, emphasising the need for evidence-based policies to improve cervical cancer prevention and outcomes [
79,
80,
81].
The availability of HPV vaccination and alternative screening tests has significantly improved the prospects for cervical cancer prevention in sub-Saharan Africa (SSA). The inclusion of the HPV vaccine in GAVI’s portfolio has enhanced the potential for its introduction in GAVI-eligible countries. Investments in vaccine storage, distribution, delivery infrastructure, and human resources will accelerate HPV vaccination through school- and campaign-based approaches. Over the past 15 years, alternative screening methods to cytology, such as visual inspection with acetic acid (VIA) and HPV testing, have been extensively evaluated in Africa and other low-resource settings. VIA is particularly suitable for low-resource environments, though its efficacy is likely lower than that of HPV testing. Introducing VIA screening programmes can help develop the necessary infrastructure for future affordable HPV testing. Integrating these programmes with existing HIV/AIDS control initiatives is another strategy to enhance infrastructure and screening services in SSA. This manuscript outlines the infrastructural requirements for an integrated approach that aims to vaccinate single-year cohorts of girls aged 9-13 and screen women over 30 using VIA or rapid HPV tests [
82].
Using population and epidemiologic data for 48 sub-Saharan African countries, a model-based approach estimated cervical cancer cases, deaths averted, disability-adjusted life years (DALYs) averted, and cost-effectiveness ratios for HPV vaccination of pre-adolescent girls. Data from Uganda and South Africa informed estimates for cancer risk reduction and cost-effectiveness. With 70% vaccination coverage, over 670,000 cervical cancer cases could be prevented among five birth cohorts, with over 90% of cases averted in GAVI-eligible countries. Variations in health benefits were due to differences in cancer rates, population size, and age structure. More than half of DALYs averted were in Nigeria, Tanzania, Uganda, the Democratic Republic of the Congo, Ethiopia, and Mozambique. At I
$5 per vaccinated girl, HPV vaccination was cost-saving in 38 countries and cost I
$300 per DALY averted or less in the others. Pre-adolescent HPV vaccination followed by three lifetime screenings cost I
$300 per year of life saved in Uganda and I
$1,000 in South Africa. HPV vaccination could be very cost-effective at less than I
$25-I
$50 per vaccinated girl. In-country decision-makers must consider affordability, acceptability, feasibility, and competing health priorities for cervical cancer prevention decisions [
83].
Cervical cancer is a major public health issue in sub-Saharan Africa (SSA), being the second leading cause of cancer among women and the primary cause of female cancer deaths. Incidence and mortality rates are much higher than in high-income countries due to challenges in implementing screening programmes. HPV vaccination offers a promising strategy for primary prevention. With many SSA countries now eligible for GAVI support, it is crucial to understand the factors affecting vaccine implementation. This article examines the epidemiology of cervical cancer in SSA and the status of HPV vaccination programmes, highlighting Rwanda’s success. Key factors in Rwanda’s effective programme included government support, school-based delivery, social mobilisation, and outreach to out-of-school girls, providing valuable insights for other SSA countries.
Table 1 shows key epidemiological data for cervical cancer and HPV for SSA [
84].
Cervical cancer is the leading cause of cancer deaths among women in sub-Saharan Africa (SSA), accounting for 24.55% of global cervical cancer mortality. HPV vaccination offers hope for cancer control, and by 2020, 18 SSA countries had national programmes. However, high-population countries have lower coverage, and national rollouts may not match demonstration project success. Prioritising West African countries and learning from early adopters is essential for sustaining high coverage [
85]. This study assessed knowledge, awareness, and acceptability of cervical cancer, HPV, and HPV vaccines in sub-Saharan Africa (SSA). While willingness to vaccinate was high, knowledge was low. Only six countries met the GAVI Alliance criteria for HPV vaccine support, with 70% DTP3 coverage being crucial. Most SSA countries are unprepared for national HPV vaccination rollouts. Education and pilot programs are needed to leverage acceptability and qualify for GAVI support. GAVI, the Vaccine Alliance, is a global health partnership established in 2000 to improve access to vaccines for children in low-income countries. It aims to increase immunisation coverage and reduce vaccine-preventable diseases by providing funding and support for the introduction of new and underused vaccines [
86].
8. HPV and ART Interaction
In 2013, 35 million people were living with HIV globally, with 71% in sub-Saharan Africa. Cervical cancer remains the most common female cancer in the region, with HR-HPV DNA present in 99.7% of cervical cancers. HPV types 16 and 18 are responsible for about 70% of cases. Despite the introduction of prophylactic HPV vaccines, HPV 16’s persistence and resistance to clearance remain challenges. WHO recommends HPV vaccination for adolescent girls and women up to 26 years in resource-limited settings. Access to ART has improved in sub-Saharan Africa, yet its impact on HPV 16 prevalence and cervical cancer remains inconclusive. A meta-analysis indicated that women on HAART had a lower HR-HPV prevalence, suggesting a primary prevention opportunity for unvaccinated women. However, HPV 16’s ability to evade immune surveillance compared to other genotypes poses challenges. Enhanced surveillance is needed to understand HAART’s impact on HPV 16 and cervical cancer. The review highlights the need for further research to clarify HAART’s role in managing HPV 16 and cervical cancer in HIV-positive women in Africa [
5].
In Kampala, Uganda, a study from 2017 to 2020 found that women living with HIV (WLWH) on antiretroviral therapy (ART) had a higher prevalence of high-risk HPV (hrHPV) and cervical intraepithelial neoplasia (CIN) compared to HIV-negative women. Despite ART, WLWH were at increased risk for multiple hrHPV types and high-grade cervical abnormalities, highlighting the need for targeted cervical cancer prevention and research [
87]. Vulvar cancer (VC), though rare, is increasingly prevalent, particularly among younger women due to HPV. Early-stage VC is typically managed with surgery, while advanced cases require multimodal treatments including chemotherapy and radiotherapy. Due to its rarity, randomised controlled trials are limited, and prognosis remains poor. Recent advances in immunoncology highlight the potential of immune checkpoint inhibitors (ICIs) in improving outcomes [
88]. Cervical cancer development is linked to the integration of human papillomavirus (HPV) genomes into host chromosomes, leading to epigenetic changes and dysregulation of host gene expression. Using ‘HPV Integrated Site Capture’ (HISC) and ‘HPV16-Specific Region Capture Hi-C’ techniques, we discovered that HPV integration typically occurs through microhomology-mediated repair (MHMR), which can result in host sequence deletion or amplification. Chromatin interactions between the integrated HPV genome and host chromosomes disrupt host gene regulation within topologically associating domains (TADs). These findings suggest that HPV integration influences host gene expression through changes in genome interactions rather than proximity to cancer-causing genes [
89].
HIV and HPV co-infection is prevalent, especially among men who have sex with men (MSM), and is associated with impaired HPV clearance due to HIV-induced immune dysfunction. Despite effective antiretroviral therapy (ART), HPV-related cancer rates remain high among PLWH. Public health strategies, including universal HPV vaccination and targeted screening programs, aim to reduce HPV transmission and related disease burden [
90].
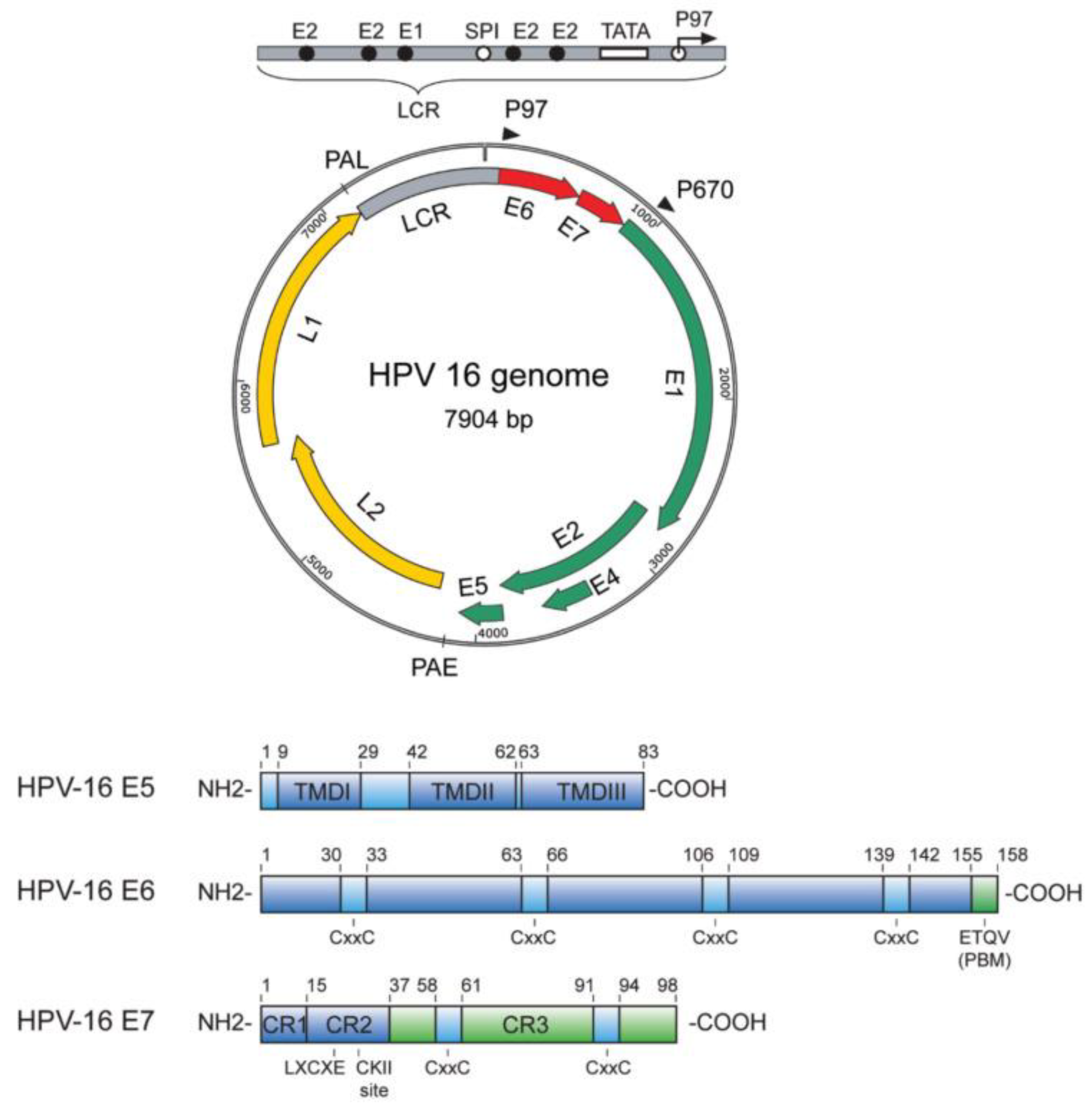
Figure 1.
Representation of the HPV genome. Taken from [
91].
Figure 1.
Representation of the HPV genome. Taken from [
91].
HIV-infected individuals have a higher incidence of HPV-associated anogenital and oropharyngeal cancers compared to those without HIV. HIV may also increase the risk of other cancers, including those of the head, neck, liver, lung, and kidney. This study reveals that prolonged exposure to cell-free HIV-1 virions or HIV-1 proteins gp120 and tat induces epithelial-mesenchymal transition (EMT) and enhances invasiveness in HPV-16-immortalised anal and cervical epithelial cells, as well as in HPV-16-infected and HPV-16-negative oral cancer cells. EMT, driven by gp120 and tat, leads to cell detachment and reattachment with intermediate EMT markers, including stem cell markers CD133 and CD44. This suggests that HIV-1 promotes de-differentiation of neoplastic cells into cancer stem cells, which may be resistant to treatment. Interventions targeting TGF-β1, MAPK signalling, vimentin, and restoring E-cadherin expression, alongside ART, could mitigate HIV-1’s role in advancing both HPV-associated and HPV-independent epithelial cancers [
92].
The study explores the interaction between Aurora kinase A (AurA) and the E6 oncoprotein of human papillomavirus (HPV), which is crucial for HPV-induced carcinogenesis. AurA, a mitotic regulator often dysregulated in cancers, binds preferentially to the E6 protein, and this association is vital for the proliferation and survival of HPV-positive cells. The research identified that the C-terminus of E6, upstream of its PDZ binding motif, is essential for forming the AurA-E6 complex in the nucleus. The level of E6 expression correlates positively with AurA expression. Functionally, the AurA-E6 interaction regulates the expression of cyclin E and phosphorylated histone H3, impacting G1/S and mitotic phases of the cell cycle. Depleting AurA also reduced the invasiveness of HPV-positive cells, though inhibiting AurA alone may not fully diminish the oncogenic potential of E6. This study reveals how HPV E6 exploits AurA to disrupt cell cycle checkpoints and drive cancer progression. These insights suggest that targeting the AurA-E6 complex could be a promising therapeutic strategy for HPV-associated cancers [
93].
The study addresses the increasing incidence of oropharyngeal squamous cell carcinoma (OPSCC) and the need to understand its etiology for effective treatment. Using an integrative genomics approach, researchers analysed RNA-Sequencing (RNA-Seq) data from 46 HPV-positive head and neck squamous cell carcinoma cases and 25 normal controls. Differential marker selection, based on a log2FoldChange (FC) score of 2 and adjusted p-value < 0.01, identified 714 genes, which were then refined to 73 using the Particle Swarm Optimization (PSO) algorithm. Machine learning models revealed seven key genes—ECT2, LAMC2, DSG2, FAT1, PLOD2, COL1A1, and PLAU—that significantly contribute to model performance. These genes were linked to OPSCC through gene set enrichment analysis, protein-protein interactions, and disease ontology mining. Survival analysis highlighted strong over-expression of three key genes in OPSCC samples from “The Cancer Genome Atlas.” These findings provide crucial insights into OPSCC pathogenesis and potential targets for therapy [
94].
9. Conclusions
The ongoing global challenge of cervical cancer, particularly among women living with HIV, underscores the critical need for comprehensive prevention and screening strategies. Since the identification of AIDS, the prevalence of HIV has led to a significant burden of cervical cancer, exacerbated by the presence of high-risk HPV types. Although antiretroviral therapy (ART) has substantially improved survival rates, its effect on cervical cancer remains ambiguous, especially concerning invasive cases.
Studies reveal that ART reduces the prevalence of high-risk HPV and cervical lesions, yet the full impact on cervical cancer incidence requires further investigation. Regular screening methods, including HPV testing, visual inspection with acetic acid (VIA), and cytology, are essential tools in managing and preventing cervical cancer. HPV vaccination programmes, notably in sub-Saharan Africa, represent a promising advancement in reducing the incidence of cervical cancer. The high prevalence of HPV among both HIV-positive and HIV-negative individuals highlights the efficacy of HPV vaccines in mitigating infection rates and preventing cancer.
Given the high cervical cancer rates in sub-Saharan Africa and the substantial benefits of HPV vaccination, there is a pressing need to address barriers to vaccine implementation. With GAVI’s support, integrating HPV vaccination into existing health infrastructures and ensuring accessible screening services are crucial steps. Evidence suggests that vaccination combined with regular screenings is both cost-effective and essential for reducing cervical cancer mortality. Continued research, policy development, and resource allocation are necessary to overcome challenges and advance global efforts in cervical cancer prevention and control.
The global challenge of cervical cancer, particularly among women living with HIV (WLWH), highlights the urgent need for effective prevention and screening strategies. Despite improvements in survival rates due to antiretroviral therapy (ART), its impact on cervical cancer remains unclear, especially concerning invasive cases. High-risk HPV types, prevalent in both HIV-positive and HIV-negative populations, contribute significantly to cervical cancer incidence. ART has shown to lower the prevalence of high-risk HPV and cervical lesions, but its overall effect on cervical cancer incidence requires further research. Regular screening methods, including HPV testing, visual inspection with acetic acid (VIA), and cytology, are crucial for managing and preventing cervical cancer. HPV vaccination programmes, especially in sub-Saharan Africa, offer a promising advancement in reducing cervical cancer rates. Addressing barriers to vaccine implementation, integrating HPV vaccination into health systems, and ensuring accessible screening services are essential. Evidence suggests that combining vaccination with regular screenings is cost-effective and vital for reducing cervical cancer mortality. Ongoing research, policy development, and resource allocation are critical to overcoming challenges and advancing global cervical cancer prevention and control efforts [
95].
Author Contributions
Investigation, A.J.-V., D.G., R.T., R.A.-F. Conceptualization, A.J.-V.; Software, D.G., Methodology, A.J.-V., and O.A.-M.; Supervision, D.G. Writing—original draft preparation, A.J.-V., D.G., & R.A.-F. All authors participated in the review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data availability in this study is available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schindler, S., Netto, E., Deminco, F., Figueiredo, C. A., de Andrade, C. M., Alves, A. R., & Brites, C. Detection of cytokines in cervicovaginal lavage in HIV-infected women and its association with high-risk human papillomavirus. Frontiers in immunology. 2024, 15, 1416204. [CrossRef]
- Jain, B. B., Adhikary, T., Sadhukhan, P. C., & Nandi, A. Human papilloma virus infection of uterine cervix and spectrum of cervical pathology in human immunodeficiency virus/AIDS. Journal of cancer research and therapeutics. 2021, 17(6), 1462–1467. [CrossRef]
- Menon, S., Rossi, R., Zdraveska, N., Kariisa, M., Acharya, S. D., Vanden Broeck, D., & Callens, S. Associations between highly active antiretroviral therapy and the presence of HPV, premalignant and malignant cervical lesions in sub-Saharan Africa, a systematic review: current evidence and directions for future research. BMJ open. 2017, 7(8), e015123. [CrossRef]
- Kelly, H., Weiss, H. A., Benavente, Y., de Sanjose, S., Mayaud, P., & ART and HPV Review Group Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. The lancet. HIV. 2018, 5(1), e45–e58. [CrossRef]
- Menon, S., Rossi, R., Kariisa, M., Acharya, S. D., Zdraveska, N., Mahmood, S., Callens, S., & Ndizeye, Z. Relationship between Highly Active Antiretroviral Therapy (HAART) and human papillomavirus type 16 (HPV 16) infection among women in Sub-Saharan Africa and public health implications: A systematic review. PloS one. 2019, 14(3), e0213086. [CrossRef]
- Bogale, A. L., Belay, N. B., Medhin, G., & Ali, J. H. Molecular epidemiology of human papillomavirus among HIV infected women in developing countries: systematic review and meta-analysis. Virology journal. 2020, 17(1), 179. [CrossRef]
- Olesen, T. B., Munk, C., Christensen, J., Andersen, K. K., & Kjaer, S. K. Human papillomavirus prevalence among men in sub-Saharan Africa: a systematic review and meta-analysis. Sexually transmitted infections. 2014, 90(6), 455–462. [CrossRef]
- Olesen, T. B., Iftner, T., Mwaiselage, J., Kahesa, C., Rasch, V., Ngoma, T., Munk, C., & Kjaer, S. K. Prevalence and type distribution of human papillomavirus among 1813 men in Tanzania and the relationship to HIV status. Sexually transmitted diseases. 2013, 40(7), 592–598. [CrossRef]
- Zheng, J. J., Miao, J. R., Wu, Q., Yu, C. X., Mu, L., & Song, J. H. Correlation between HPV-negative cervical lesions and cervical microenvironment. Taiwanese journal of obstetrics & gynecology. 2020, 59(6), 855–861. [CrossRef]
- Fernandes JV, Medeiros Fernandes TA DE, Azevedo JC DE, Cobucci RN DE, Carvalho MG, Andrade VS, et al. Link between chronic inflammation and human papillomavirus- induced carcinogenesis (Review). Oncol Lett. 2015, 9, 1015–26.
- Lin W, Niu Z, Zhang H, Kong Y, Wang Z, Yang X, et al.. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int J Clin Exp Pathol. 2019, 12, 3604–12.
- UNAIDS . Global HIV & AIDS statistics – Fact sheet. 2023. Available online at: https://www.unaids.org/en/resources/fact-sheet (Accessed July 16, 2024).
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated Malignancies. Curr Opin HIV AIDS. 2017, 12, 6–11.
- Centers for Disease Control . Pneumocystis pneumonia Vol. 30. Los Angeles: MMWR Morb Mortal Wkly Rep. 1981, p. 250–2.
- World Health Organization . HIV (2024). Available online at: https://www.who.int/data/gho/data/themes/hiv-aids (Accessed July 06, 2024).
- International Agency for Research on Cancer . Cancer Tomorrow (2023). Available online at: https://gco.iarc.fr/tomorrow/en/dataviz/bars?sexes=2&single_unit=50000&cancers=23&years=2030 (Accessed July 16, 2024).
- Lekoane KMB, Kuupiel D, Mashamba-Thompson TP, Ginindza TG. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa: scoping review. Syst Rev. 2020, 9, 88.
- Guha D, Chatterjee R. Cytokine levels in HIV infected and uninfected Indian women: correlation 326 with other STAs. Exp Mol Pathol. 2009, 86, 65–8.
- Joshi, S. N., Gopalkrishna, V., Kumar, B. K., Dutta, S., Nyaynirgune, P., Thakar, M., Tripathy, S., Mehendale, S., & Paranjape, R. Cervical squamous intra-epithelial changes and human papillomavirus infection in women infected with human immunodeficiency virus in Pune, India. Journal of medical virology. 2005, 76(4), 470–475. [CrossRef]
- Pantanowitz, L., & Michelow, P. (2011). Review of human immunodeficiency virus (HIV) and squamous lesions of the uterine cervix. Diagnostic cytopathology 2011, 39(1), 65–72. [CrossRef]
- Stewart, K. A., Allen, S. M., Chesnokova, A. E., Syed, F., & Levison, J. E. Incidence of abnormal cervical and vaginal cytology among women over age 65 years living with human immunodeficiency virus. American journal of obstetrics and gynecology. 2020, 222(5), 486.e1–486e10. [CrossRef]
- Gaete, S., Auguste, A., Bhakkan, B., Peruvien, J., Herrmann-Storck, C., Socrier, Y., Diedhiou, A., & Deloumeaux, J. Frequent high-risk HPV co-infections excluding types 16 or 18 in cervical neoplasia in Guadeloupe. BMC cancer. 2021, 21(1), 281. [CrossRef]
- Menon, S., Rossi, R., Kariisa, M., & Callens, S. Determining the HPV vaccine schedule for a HIV-infected population in sub Saharan Africa, a commentary. Virology journal. 2018, 15(1), 129. [CrossRef]
- Agyare Gyane, F., Modey, E., Maya, E., Bonney, E. Y., Abaidoo-Myles, A., Paintsil, E., & Torpey, K. Prevalence and risk factors associated with high-risk human papillomavirus infection among women living with HIV (WLWH) at a tertiary health facility in Accra, Ghana. PloS one. 2024, 19(5), e0303535. PloS one. 2024, 19. [CrossRef]
- Viviano M, De Beaudrap P, Tebeu PM, Fouogue JT, Vassilakos P, Petignat P. A review of screening strategies for cervical cancer in human immunodeficiency virus-positive women in sub-Saharan Africa. International Journal of Women’s Health. 2017, 9, 69–79. [CrossRef]
- Mungo C, Barker E, Randa M, Ambaka J, Ogollah Osongo C. Integration of cervical cancer screening into HIV/AIDS care in low income countries: a moral imperative. Ecancermedicalscience. 2021, 15. [CrossRef]
- Fokom-Domgue J, Combescure C, Fokom-Defo V, Tebeu PM, Vassilakos P, Kengne AP, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. 2015, 351, h3084. [CrossRef]
- Lee JM. Screening of uterine cervical cancer in low-resource settings. J Gynecol Oncol. 2012, 23, 137–138. [CrossRef]
- Mishra G, Pimple S, Gupta S. Cervical cancer screening in low resource settings: Cytology versus HPV triage for VIA positive women. Int J Prev Med, 2019; 10, 138. [CrossRef]
- Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: current evidence and directions for future research. Infect Agent Cancer. 2010, 5, 8. [CrossRef]
- Kelly H, Mayaud P, de Sanjose S. Concomitant infection of HIV and HPV: what are the consequences? Curr Obstet Gynecol Rep. 2015, 4.
- Denslow SA, Rositch AF, Firnhaber C, Ting J, Smith JS. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS. 2014, 25, 163–177. [CrossRef] [PubMed]
- Kelly, H., Chikandiwa, A., Alemany Vilches, L., Palefsky, J. M., de Sanjose, S., & Mayaud, P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. The lancet. HIV. 2020, 7(4), e262–e278. [CrossRef]
- Patel P, Hanson DL, Sullivan PS, et al. Incidence of Types of Cancer among HIV-Infected Persons Compared with the General Population in the United States, 1992–2003. Annals of Internal Medicine. 2008, 148(10), 728–736. [CrossRef] [PubMed]
- Howlader N, N A, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) 2012.
- Chaturvedi, AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010, 46(4 Suppl), S20–26. [Google Scholar] [CrossRef]
- Stier, E. A., Sebring, M. C., Mendez, A. E., Ba, F. S., Trimble, D. D., & Chiao, E. Y. (2015). Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. American journal of obstetrics and gynecology. 2015, 213(3), 278–309. [CrossRef]
- Hernandez, A. L., Hilton, J. F., Weatherly, C. S., Berry-Lawhorn, J. M., Jay, N., Brickman, C., Wang, C. J., Kauffman, J., Calderon, J., Farhat, S., Da Costa, M., Akha, A. S., Darragh, T., & Palefsky, J. M. (2024). Prevalence of Anal Human Papillomavirus Infection and Anal High-Grade Squamous Intraepithelial Lesions Among Men Who Have Sex With Men 50 Years and Older Living With or Without HIV. Journal of acquired immune deficiency syndromes. 1999, 96(5), 439–446. [CrossRef]
- Sendagorta-Cudós E., Burgos-Cibrián J., Rodríguez-Iglesias M. Infecciones genitales por el virus del papiloma humano. Microbiol. Clínica. 2019, 37, 324–334. [CrossRef]
- Burd E.M., Dean C.L. Human Papillomavirus. Microbiol. Spectr. 2016, 4, DMIH2-0001-2015. [CrossRef]
- Hidalgo-Tenorio C., García-Martínez C.M., Pasquau J., Omar-Mohamed-Balgahata M., López-Ruz M., López-Hidalgo J., Gil-Anguita C. Risk factors for ≥high-grade anal intraepithelial lesions in MSM living with HIV and the response to topical and surgical treatments. PLoS ONE. 2021, 16:e0245870. [CrossRef]
- Wang C.C.J., Palefsky J.M. HPV-Associated Anal Cancer in the HIV/AIDS Patient. Cancer Treat Res. 2019, 2019, 183–209.
- Darwich L., Videla S., Cañadas M.P., Piñol M., García-Cuyàs F., Vela S., Molina-López R.A., Coll J., Sirera G., Clotet B., et al. Distribution of human papillomavirus genotypes in anal cytological and histological specimens from HIV-infected men who have sex with men and men who have sex with women. Dis. Colon Rectum. 2013, 56, 1043–1052. [CrossRef]
- Cameron J.E., Hagensee M. HPV-Associated Oropharyngeal Cancer in the HIV/AIDS Patient. Cancer Treat Res. 2019, 2019, 131–181.
- Hidalgo-Tenorio C., de Jesus S.E., Esquivias J., Pasquau J. Alta prevalencia e incidencia de lesiones precursoras de cáncer anal asociada a la infección por VPH en mujeres VIH positivas en la era tardía del TAR. Microbiol. Clínica. 2018, 36, 555–562. [CrossRef]
- Clarke M.A., Cheung L.C., Lorey T., Hare B., Landy R., Tokugawa D., Gage J.C., Darragh T.M., Castle P.E., Wentzensen N. 5-Year Prospective Evaluation of Cytology, Han Papillomavirus Testing, and Biomarkers for Detection of Anal Precancer in Human Immunodeficiency Virus–Positive Men Who Have Sex with Men. Clin. Infect. Dis. 2019, 69, 631–638. [CrossRef]
- Sendagorta E., Herranz P., Guadalajara H., Bernardino J.I., Viguer J.M., Beato M.J., García-Olmo D., Peña J.M. Prevalence of abnormal anal cytology and high-grade squamous intraepithelial lesions among a cohort of HIV-infected men who have sex with men. Dis. Colon Rectum. 2014, 57, 475–481. [CrossRef]
- Machalek, D. A., Poynten, M., Jin, F., Fairley, C. K., Farnsworth, A., Garland, S. M., Hillman, R. J., Petoumenos, K., Roberts, J., Tabrizi, S. N., Templeton, D. J., & Grulich, A. E. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. The Lancet. Oncology. 2012, 13(5), 487–500. [CrossRef]
- Darwich, L., Videla, S., Cañadas, M. P., Piñol, M., García-Cuyàs, F., Vela, S., Molina-López, R. A., Coll, J., Sirera, G., Clotet, B., & Can Ruti HIV-HPV Team (2013). Distribution of human papillomavirus genotypes in anal cytological and histological specimens from HIV-infected men who have sex with men and men who have sex with women. Diseases of the colon and rectum. 2013, 56(9), 1043–1052. [CrossRef]
- Barquet-Muñoz, S. A., López-Morales, R. A., Stier, E. A., Mejorada-Pulido, E., Solís-Ramírez, D., Jay, N., Moctezuma, P., Morales-Aguirre, M., García-Carrancá, A., Méndez-Martínez, R., Martin-Onraët, A., Pérez-Montiel, D., Mendoza-Palacios, M. J., & Volkow, P. Prevalence of anal high-risk human papillomavirus (HR-HPV) types in people living with HIV and a history of cancer. HIV medicine. 2024, 10.1111/hiv.13684. Advance online publication. [CrossRef]
- Thitipatarakorn, S., Teeratakulpisarn, N., Nonenoy, S., Klinsukontakul, A., Suriwong, S., Makphol, J., Hongchookiat, P., Chaya-Ananchot, T., Chinlaertworasiri, N., Mingkwanrungruang, P., Sacdalan, C., Poltavee, K., Pankam, T., Kerr, S. J., Ramautarsing, R., Colby, D., & Phanuphak, N. (2024). Prevalence and incidence of anal high-grade squamous intraepithelial lesions in a cohort of cisgender men and transgender women who have sex with men diagnosed and treated during acute HIV acquisition in Bangkok, Thailand. Journal of the International AIDS Society. 2024, 27(5), e26242. [CrossRef]
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009, 124(10), 2375–83. [CrossRef] [PubMed]
- Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ journal of surgery. 2006, 76(8), 715–7. [CrossRef]
- Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum. 2009, 52(2), 239–47. [CrossRef] [PubMed]
- Chin-Hong PV, Berry JM, Cheng SC, Catania JA, Da Costa M, Darragh D, et al. A population-based study of human papillomavirus-associated anal neoplasia in HIV-positive and HIV-negative men using self-collected specimens: the TPOP study. Annals Int Med. 2008, 149(5), 300–6.
- Saiag P, Bauhofer A, Bouscarat F, Aquilina C, Ortonne JP, Dupin N, et al. Imiquimod 5% cream for external genital or perianal warts in human immunodeficiency virus-positive patients treated with highly active antiretroviral therapy: an open-label, noncomparative study. The British Journal of Dermatology. 2009 May 15; e-pub.
- Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. Aids. 2008, 22(10), 1203–11.
- D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and Epidemiology of Anal Cancer in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008, 48(4), 491–9.
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008, 148(10), 728–36. [CrossRef]
- ter Meulen, J., Eberhardt, H. C., Luande, J., Mgaya, H. N., Chang-Claude, J., Mtiro, H., Mhina, M., Kashaija, P., Ockert, S., & Yu, X. Human papillomavirus (HPV) infection, HIV infection and cervical cancer in Tanzania, east Africa. International journal of cancer. 1992, 51(4), 515–521. [CrossRef]
- Mujuni, F., Mirambo, M. M., Rambau, P., Klaus, K., Andreas, M., Matovelo, D., Majigo, M., Kasang, C., & Mshana, S. E. Variability of high risk HPV genotypes among HIV infected women in Mwanza, Tanzania- the need for evaluation of current vaccine effectiveness in developing countries. Infectious agents and cancer. 2016, 11, 49. Infectious agents and cancer. 2016, 11, 49. [CrossRef]
- Hall, M. T., Smith, M. A., Simms, K. T., Barnabas, R. V., Canfell, K., & Murray, J. M. The past, present and future impact of HIV prevention and control on HPV and cervical disease in Tanzania: A modelling study. PloS one. 2020, 15(5), e0231388. [CrossRef]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68(6), 394–424.
- Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018, 32(6), 795–808.
- Looker KJ, Rönn MM, Brock PM, Brisson M, Drolet M, Mayaud P, et al. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc. 2018, 21(6), e25110.
- Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017, 389(10071), 847–60.
- Mapanga W, Girdler-Brown B, Feresu SA, Chipato T, Singh E. Prevention of cervical cancer in HIV-seropositive women from developing countries through cervical cancer screening: a systematic review. Syst Rev. 2018, 7(1), 1–27.
- Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ 1999, 318(7188), 904.
- Abraham AG, D’Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013, 62(4), 405–13. [CrossRef] [PubMed]
- Mohammed DY, Shukla P, Babayants Y, Sison R, Slim J. Increased proportions of HIV-infected women met cervical cancer screening guideline in 2016. Int J Womens Health. 2018, 16(10), 83–7.
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. 2020.
- Chambuso RS, Shadrack S, Lidenge SJ, Mwakibete N, Medeiros RM. Influence of HIV/AIDS on cervical cancer: a retrospective study from Tanzania. J Glob Oncol. 2016, 3(1), 72–8.
- McHome B, Swai P, Wu C, Katanga J, Kahesa C, Manongi R, et al. Comprehensive Cervical Cancer Prevention in Tanzania (CONCEPT) study: Cohort profile. BMJ Open. 2020, 10(9), e038531.
- Runge AS, Bernstein ME, Lucas AN, Tewari KS. Cervical cancer in Tanzania: a systematic review of current challenges in six domains. Gynecol Oncol Rep. 2019, 1, 29.
- Mphuru A, Li AJ, Kyesi F, Mwengee W, Mazige F, Nshunju R, et al. National introduction of human papillomavirus (HPV) vaccine in Tanzania: Programmatic decision-making and implementation. Vaccine. 2022, 40, A2-9.
- Linde DS, Rasch V, Mwaiselage JD, Gammeltoft TM. Competing needs: a qualitative study of cervical cancer screening attendance among HPV-positive women in Tanzania. BMJ Open. 2019, 9(2), e024011.
- Bigoni, J., Gundar, M., Tebeu, P. M., Bongoe, A., Schäfer, S., Fokom-Domgue, J., Catarino, R., Tincho, E. F., Bougel, S., Vassilakos, P., & Petignat, P. Cervical cancer screening in sub-Saharan Africa: a randomized trial of VIA versus cytology for triage of HPV-positive women. International journal of cancer. 2015, 137(1), 127–134. [CrossRef]
- Fokom-Domgue, J., Combescure, C., Fokom-Defo, V., Tebeu, P. M., Vassilakos, P., Kengne, A. P., & Petignat, P. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ (Clinical research ed.) 2015, 351, h3084. [CrossRef]
- M. Drolet, É. Bénard, M.C. Boily, H. Ali, L. Baandrup, H. Bauer, , M. Brisson.
- Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15 (5), 565-580.
- Ferlay, I. Soerjomataram, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, F.Bray. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015, 136, E359-E386.
- J. Jeronimo, P.E. Castle, S. Temin, L. Denny, G.V. Vandana, J.J. Kim, S.Shastri. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J. Glob. Oncol. 2016, 3 (5), 635-657.
- Sankaranarayanan, R., Anorlu, R., Sangwa-Lugoma, G., & Denny, L. A. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine. 2013, 31, F47–F52. [CrossRef]
- Kim, J. J., Campos, N. G., O’Shea, M., Diaz, M., & Mutyaba, I. (2013). Model-based impact and cost-effectiveness of cervical cancer prevention in sub-Saharan Africa. Vaccine. 2013, 31, F60–F72. [CrossRef]
- Black, E., & Richmond, R. Prevention of Cervical Cancer in Sub-Saharan Africa: The Advantages and Challenges of HPV Vaccination. Vaccines. 2018, 6. [CrossRef]
- Ngcobo, N., Jaca, A., Iwu-Jaja, C. J., & Mavundza, E. (2021). Reflection: burden of cervical cancer in Sub-Saharan Africa and progress with HPV vaccination. Current opinion in immunology. 2021, 71, 21–26. [CrossRef]
- Perlman, S., Wamai, R. G., Bain, P. A., Welty, T., Welty, E., & Ogembo, J. G. (2014). Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: a systematic review. PloS one. 2014, 9(3), e90912. [CrossRef]
- Nakisige, C., Adams, S. V., Namirembe, C., Okoche, L., Ferrenberg, J., Towlerton, A., Larsen, A., Orem, J., Casper, C., Frenkel, L., & Uldrick, T. S. Multiple High-Risk HPV Types Contribute to Cervical Dysplasia in Ugandan Women Living With HIV on Antiretroviral Therapy. Journal of acquired immune deficiency syndromes (1999). 2022, 90(3), 333–342. [CrossRef]
- Borella, F., Preti, M., Bertero, L., Collemi, G., Castellano, I., Cassoni, P., Cosma, S., Carosso, A. R., Bevilacqua, F., Gallio, N., Benedetto, C., & Micheletti, L. (2020). Is There a Place for Immune Checkpoint Inhibitors in Vulvar Neoplasms? A State of the Art Review. International journal of molecular sciences. 2020, 22(1), 190. [CrossRef]
- Groves, I. J., Drane, E. L. A., Michalski, M., Monahan, J. M., Scarpini, C. G., Smith, S. P., Bussotti, G., Várnai, C., Schoenfelder, S., Fraser, P., Enright, A. J., & Coleman, N. (2021). Short- and long-range cis interactions between integrated HPV genomes and cellular chromatin dysregulate host gene expression in early cervical carcinogenesis. PLoS pathogens. 2021, 17(8), e1009875. [CrossRef]
- Pérez-González, A., Cachay, E., Ocampo, A., & Poveda, E. (2022). Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms. 2022, 10(5), 1047. [CrossRef]
- Basukala O., Banks L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses. 2021, 13, 1892.
- Lien, K., Mayer, W., Herrera, R., Padilla, N. T., Cai, X., Lin, V., Pholcharoenchit, R., Palefsky, J., & Tugizov, S. M. HIV-1 Proteins gp120 and Tat Promote Epithelial-Mesenchymal Transition and Invasiveness of HPV-Positive and HPV-Negative Neoplastic Genital and Oral Epithelial Cells. Microbiology spectrum. 2022, 10(6), e0362222. [CrossRef]
- Li, S., Yim, M. K., Yip, K. L., Xiao, C., Luk, H. Y., Xiao, S., Chen, Z., Chan, P. K. S., & Boon, S. S. E6-Encoded by Cancer-Causing Human Papillomavirus Interacts with Aurora Kinase A To Promote HPV-Mediated Carcinogenesis. Journal of virology. 2023, 97(2), e0187222. [CrossRef]
- Sekaran, K., Varghese, R. P., Krishnan, S., Zayed, H., El Allali, A., & Doss, G. P. C. (2024). Dissecting Crucial Gene Markers Involved in HPV-Associated Oropharyngeal Squamous Cell Carcinoma from RNA-Sequencing Data through Explainable Artificial Intelligence. Frontiers in bioscience (Landmark edition). 2024, 29(6), 220. [CrossRef]
- Justiz-Vaillant, A.; Gopaul, D.; Soodeen, S.; Unakal, C.; Thompson, R.; Pooransingh, S.; Arozarena-Fundora, R.; Asin-Milan, O.; Akpaka, P.E. Advancements in Immunology and Microbiology Research: A Comprehensive Exploration of Key Areas. Microorganisms 2024, 12, 1672. [Google Scholar] [CrossRef]
Table 1.
Key Epidemiological Data for Cervical Cancer and HPV for SSA regions [
84].
Table 1.
Key Epidemiological Data for Cervical Cancer and HPV for SSA regions [
84].
| Burden of cervical cancer and HPV infection |
SSA |
Eastern Africa |
Central Africa |
Western Africa |
Southern Africa |
| Annual number of new cervical cancer cases |
93,225 |
45,707 |
11,540 |
8652 |
8652 |
| Age-standardized incidence rate per 100,000 women |
34.8 |
42.7 |
30.6 |
31.5 |
31.5 |
| Annual number of cervical cancer deaths |
57,381 |
28,197 |
7917 |
4721 |
4721 |
| Age-standardized mortality rate from cervical cancer per 100,000 women |
22.5 |
27.6 |
22.2 |
17.9 |
17.9 |
| HPV prevalence (%) in the general population |
18.6 |
20.5 |
9.8 |
17.9 |
17.9 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).