Submitted:
25 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanostructured Lipid Carriers (NLC)
2.3. Characterization of Unloaded and Bemotrizinol Loaded NLC
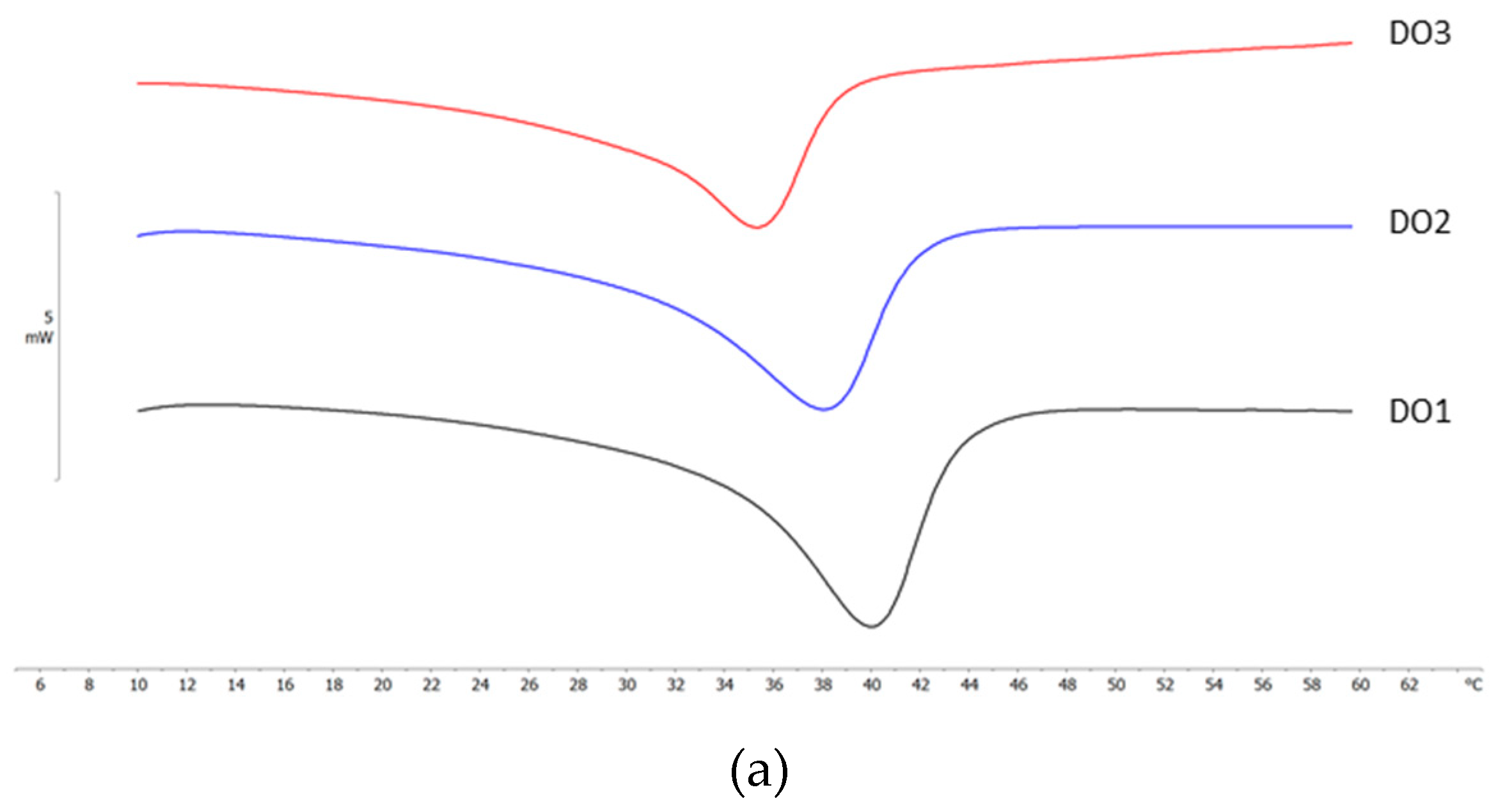
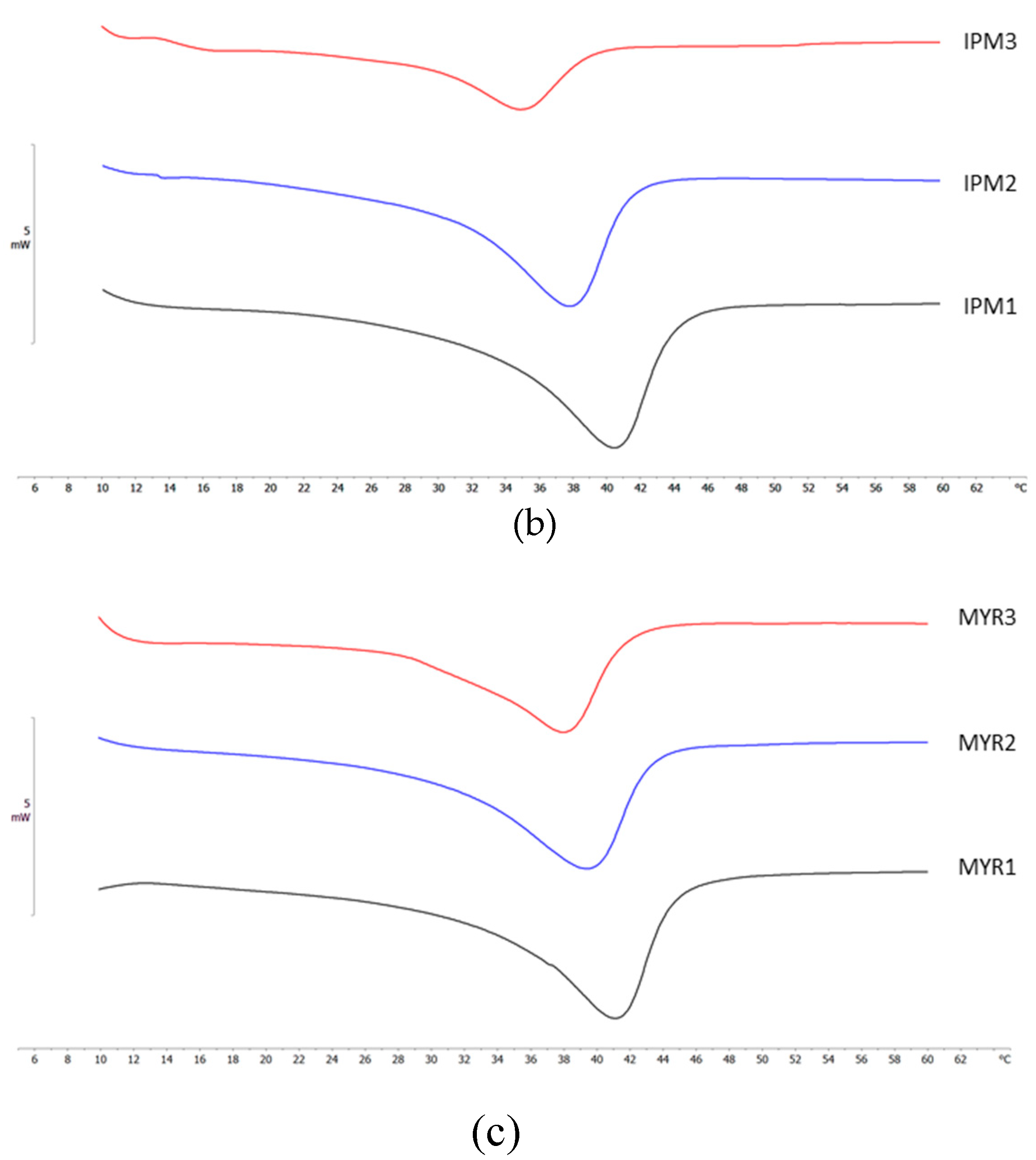
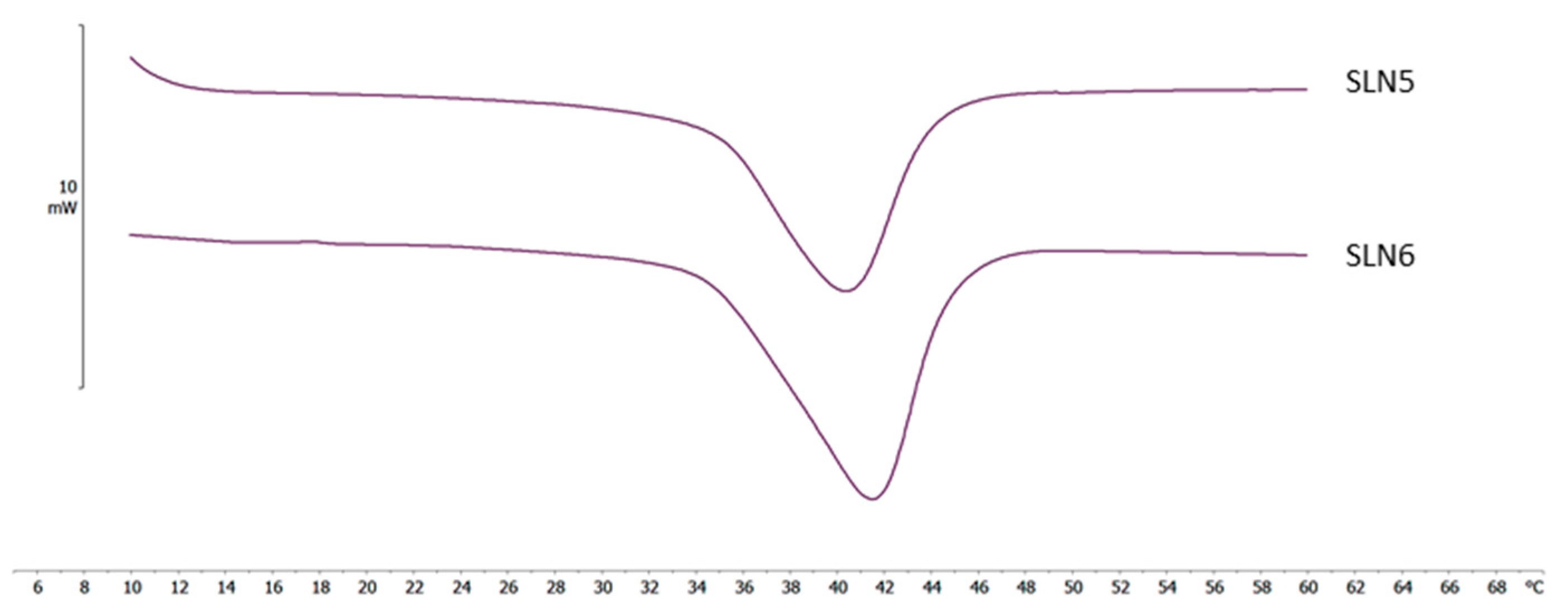
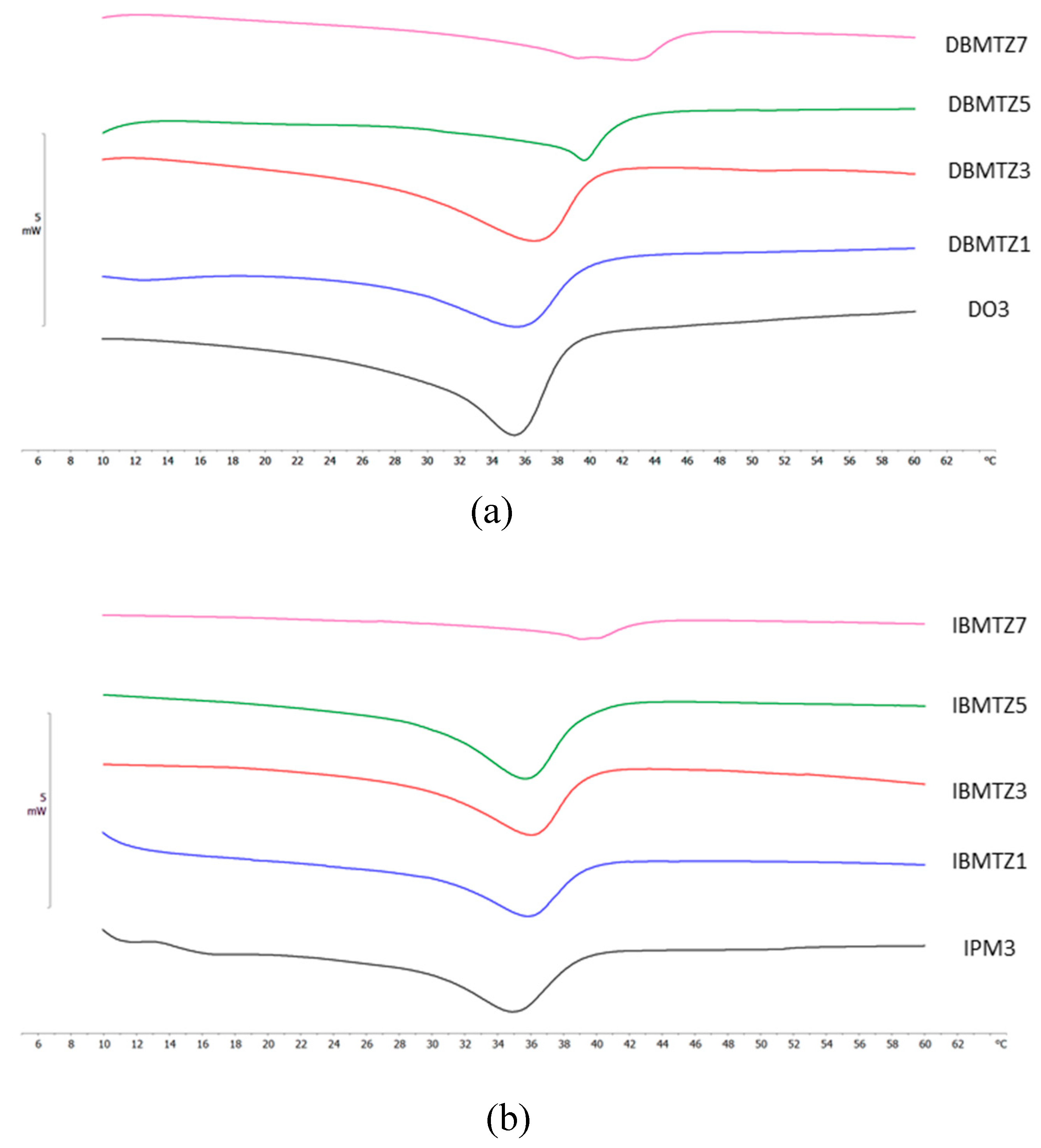
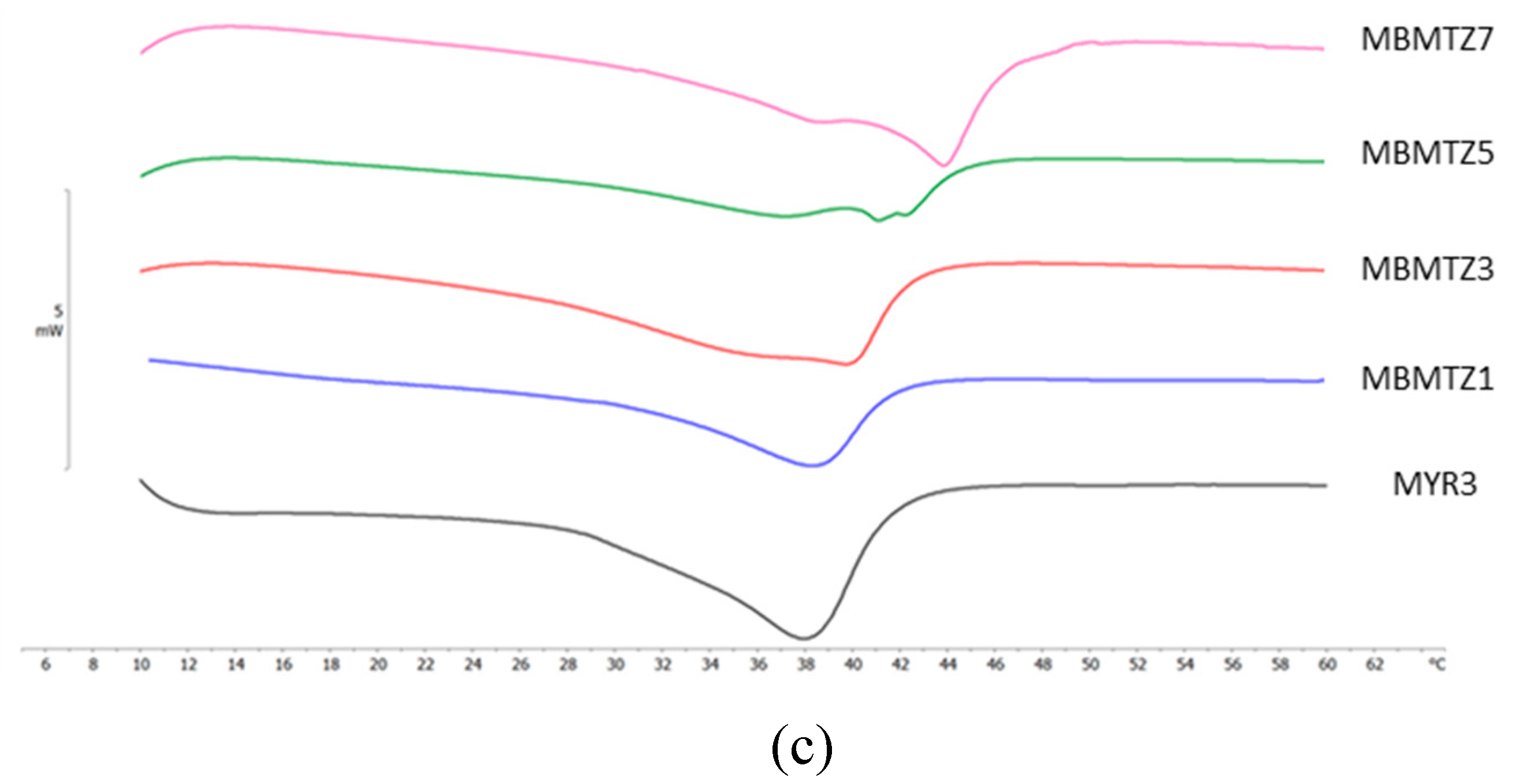
2.4. Differential Scanning Calorimetry (DSC) Analyses
2.5. Stability Studies on Nanostructured Lipid Carriers (NLC)
2.6. Preparation of O/W Emulsions
2.7. Stability Studies on O/W Emulsions
2.8. Spreadability
2.9. Occlusive Properties
2.10. Viscosity
2.11. In Vitro Release of Bemotrizinol
2.12. In Vitro Skin Permeation Experiments
2.13. Determination of In Vitro Sun Protection Factor (SPF)
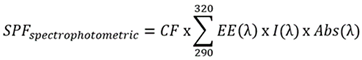
2.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lionetti, N.; Rigano, L. The new sunscreens among formulation strategy, stability issues, changing norms, safety and efficacy evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef]
- Li, H.; Colantonio, S.; Dawson, A.; Lin, X.; Beecker, J. Sunscreen application, safety, and sun protection: the evidence. Journal of Cutaneous Medicine and Surgery 2019, 23(4), 357–369. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ 2020, 192(50), E1802–E1808. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In vitro evaluation of sunscreen safety: effects of the vehicle and repeated applications on skin permeation from topical formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ouchene, L.; Litvinov, I.V.; Netchiporouk, E. Systemic absorption of common organic sunscreen ingredients raises possible safety concerns for patients. Journal of Cutaeous Medicine and Surgery 2019, 23(4), 449–450. [Google Scholar] [CrossRef] [PubMed]
- Oral, D.; Yirun, A.; Erkekoglu, P. Safety concerns of organic ultraviolet filters: special focus on endocrine-disrupting properties. Journal of Environmental Pathology, Toxicology, and Oncology 2020, 39, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Nesseem, D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. International Journal of Cosmetic Science 2011, 33(1), 70–79. [Google Scholar] [CrossRef]
- Nikolić, S.; Keck, C.M.; Anselmi, C.; Müller, R.H. Skin photoprotection improvement: synergistic interaction between lipid nanoparticles and organic UV filters. International Journal of Pharmaceutics 2011, 414, 276–284. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A comprehensive review with the application of nanotechnology. Journal of Drug Delivery Science and Technology 2023, 86, 104720. [Google Scholar] [CrossRef]
- de Araújo, M.M.; Schneid, A.C.; Oliveira, M.S.; Mussi, S.V.; de Freitas, M.N.; Carvalho, F.C.; Bernes Junior, E.A.; Faro, R.; Azevedo, H. NLC-Based Sunscreen Formulations with Optimized Proportion of Encapsulated and Free Filters Exhibit Enhanced UVA and UVB Photoprotection. Pharmaceutics 2024, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced. Drug Delivery Reviews 2002, 54 (Suppl. S1), S131–55. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. International Journal of Pharmaceutics 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, P.G.; Conway, B.R. Lipid nanoparticles for dermal drug delivery. Current Pharmaceutical Design 2015, 21(20), 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Jeon, Y.E.; Kang, S.; Lee, J.Y.; Lee, K.W.; Kim, K.T.; Kim, D.D. Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol. Pharmaceutics 2020, 12(9), 845. [Google Scholar] [CrossRef]
- Tran, P.; Lee, S.E.; Kim, D.H.; Pyo, Y.C.; Park, J.S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J Pharm Investig 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid lipid nanoparticles and nanostructured lipid carriers: emerging lipidbased drug delivery systems. Pharmaceutical Chemistry Journal 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochemistry and Photobiology 2001, 74(3), 401–406. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.S.; Moreira, L.M.C.C.; Oliveira, T.M.T.; Melo, D.F.; Azevedo, E.P.; Gadelha, A.E.G.; Fook, M.V.L.; Oshiro-Júnior, J.A.; Damasceno, B.P.G.L. Bemotrizinol-loaded carnauba wax-based nanostructured lipid carriers for sunscreen: optimization, characterization, and in vitro evaluation. AAPS PharmSciTech 2020, 21(8), 288. [Google Scholar] [CrossRef]
- Wissing, S.; Müller, R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. International Journal of Pharmaceutics 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: structure, protection and skin effects. Journal of Biomedical Nanotechnoogy 2007, 3(4), 317–331. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. Journal of Nanoparticle Research 2020, 22, 141. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Safety assessment of alkyl esters as used in cosmetics. International Journal of Toxicology 2015, 34 (Suppl 2), 5S–69S. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Heldreth, B. Amended safety assessment of triglycerides as used in cosmetics. International Journal of Toxicology 2022, 41 (Suppl. S3), 22S–68S. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Santagati, L.M.; Sarpietro, M.G.; Castelli, F.; Panico, A.; Siciliano, E.A.; Lai, F.; Valenti, D.; Sinico, C. In vitro skin permeation of idebenone from lipid nanoparticles containing chemical penetration enhancers. Pharmaceutics 2021, 13, 1027. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Torrisi, C.; Pignatello, R.; Castelli, C.; Montenegro, L. Assessment of the technological properties of idebenone and tocopheryl acetate co-loaded lipid nanoparticles. Applied Sciences 2021, 11, 3553. [Google Scholar] [CrossRef]
- Ruktanonchai, U.; Limpakdee, S.; Meejoo, S.; Sakulkhu, U.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. The effect of cetyl palmitate crystallinity on physical properties of gamma-oryzanol encapsulated in solid lipid nanoparticles. Nanotechnology 2008, 9(9), 095701. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Rapisarda, L.; Ministeri, C.; Puglisi, G. Effects of lipids and emulsifiers on the physicochemical and sensory properties of cosmetic emulsions containing vitamin E. Cosmetics 2015, 2, 35–47. [Google Scholar] [CrossRef]
- Chaudhary, B.; Verma, S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Scientific WorldJournal 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Bakhrushina, E. O.; Anurova, M. N.; Zavalniy, M. S.; Demina, N. B.; Bardakov, A.I.; Krasnyuk, I.I. Dermatologic Gels Spreadability Measuring Methods Comparative Study. Int J Appl Pharm. 2022, 14, 164–168. [Google Scholar]
- Wissing, S.A.; Lippacher, A.; Muller, R.H. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). Journal of Cosmetic Science 2001, 52(5), 313–324. [Google Scholar]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-loaded lipid nanocarriers: correlation between in vitro occlusion factor and in vivo skin hydrating effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef]
- Fallica, F.; Leonardi, C.; Toscano, V.; Santonocito, D.; Leonardi, P.; Puglia, C. Assessment of Alcohol-Based Hand Sanitizers for Long-Term Use, Formulated with Addition of Natural Ingredients in Comparison to WHO Formulation 1. Pharmaceutics 2021, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Santonocito, D.; Bonaccorso, A.; Musumeci, T.; Ruozi, B.; Pignatello, R.; Carbone, C.; Parenti, C.; Chiechio, S. Lipid Nanoparticle Inclusion Prevents Capsaicin-Induced TRPV1 Defunctionalization. Pharmaceutics 2020, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. Anais Brasileiros de Dermatologia 1986, 61(3), 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreen formulas. Photochemistry and Photobiology 1979, 29(3), 559–566. [Google Scholar] [CrossRef]
- Gonzalez Solveyra, E.; Szleifer, I. What is the role of curvature on the properties of nanomaterials for biomedical applications? Wires Nanomedicine and Nanobiotechnology 2016, 8(3), 334–354. [Google Scholar] [CrossRef] [PubMed]
- Alajami, H.N.; Fouad, E.A.; Ashour, A.E.; Kumar, A.; Yassin, A.E.B. Celecoxib-loaded solid lipid nanoparticles for colon delivery: formulation optimization and in vitro assessment of anti-cancer activity. Pharmaceutics 2022, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics 2000, 50(1), 161–77. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: an overview. Acta Pharmaceutica 2012, 62(4), 433–472. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. International Journal of Pharmaceutics 2000, 199(2), 167–177. [Google Scholar] [CrossRef]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. International Journal of Pharmaceutics 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. Eds. Micro and Nano Technologies, Characterization and Biology of Nanomaterials for Drug Delivery. Amsterdam: Elsevier 2019, 47–76. [Google Scholar]
- Lardy, F.; Vennat, B.; Pouget, M.P.; Pourrat, A. Functionalization of hydrocolloids: principal component analysis applied to the study of correlations between parameters describing the consistency of hydrogels. Drug Development and Industrial Pharmacy 2000, 26(7), 715–721. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of semisolid formulations: An update. Pharmaceutical Technology 2002, 26(9), 84–105. [Google Scholar]
- Ahmad, J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Chu, C.C.; Hasan, Z.A.A.; Tan, C.P.; Nyam, K.L. In vitro safety evaluation of sunscreen formulation from nanostructured lipid carriers using human cells and skin model. Toxicology in Vitro 2022, 84, 105431. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Zaid, A. Features, applications, and sustainability of lipid nanoparticles in cosmeceuticals. Saudi Pharmaceutical Journal 2022, 30, 53–65. [Google Scholar] [CrossRef]
- Santos, E.P.; Freitas, Z.M.; Souza, K.R.; Garcia, S.; Vergnanini, A. In vitro and in vivo determinations of sun protection factors of sunscreen lotions with octylmethoxycinnamate. International Journal of Cosmetic Science 1999, 21(1), 1–5. [Google Scholar] [CrossRef]
- Sheu, M.T.; Lin, C.W.; Huang, M.C.; Shen, C.H. Correlation of in vivo and in vitro measurements of sun protection factor. Journal of Food and Drug Analysis 2003, 11(2), 12. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacognosy Research 2010, 2(1), 22–25. [Google Scholar]
- Pissavini, M.; Tricaud, C.; Wiener, G.; Lauer, A.; Contier, M.; Kolbe, L.; Trullás Cabanas, C.; Boyer, F.; Meredith, E.; de Lapuente, J.; Dietrich, E.; Matts, P.J. Validation of a new in vitro Sun Protection Factor method to include a wide range of sunscreen product emulsion types. International Journal of Cosmetic Science 2020, 42(5), 421–428. [Google Scholar] [CrossRef]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. Journal of Cosmetic Dermatology 2019, 18(1), 315–321. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Janotková, L.; Bendová, H.; Jedličková, L.; Hamranová, V.; Martiniaková, S. The Real UVB Photoprotective Efficacy of Vegetable Oils: In Vitro and in Vivo Studies. Photochemical & Photobiological Sciences 2021, 20, 139–151. [Google Scholar]
- Yang, S.I.; Liu, S.; Brooks, G.J.; Lanctot, Y.; Gruber, J. V Reliable and Simple Spectrophotometric Determination of Sun Protection Factor: A Case Study Using Organic <scp>UV</Scp> Filter-based Sunscreen Products. J Cosmet Dermatol 2018, 17, 518–522. [Google Scholar] [PubMed]
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae Fucus Vesiculosus with Antioxidant and Photo-Protecting Properties. Mar Drugs 2022, 20, 687. [Google Scholar] [CrossRef] [PubMed]
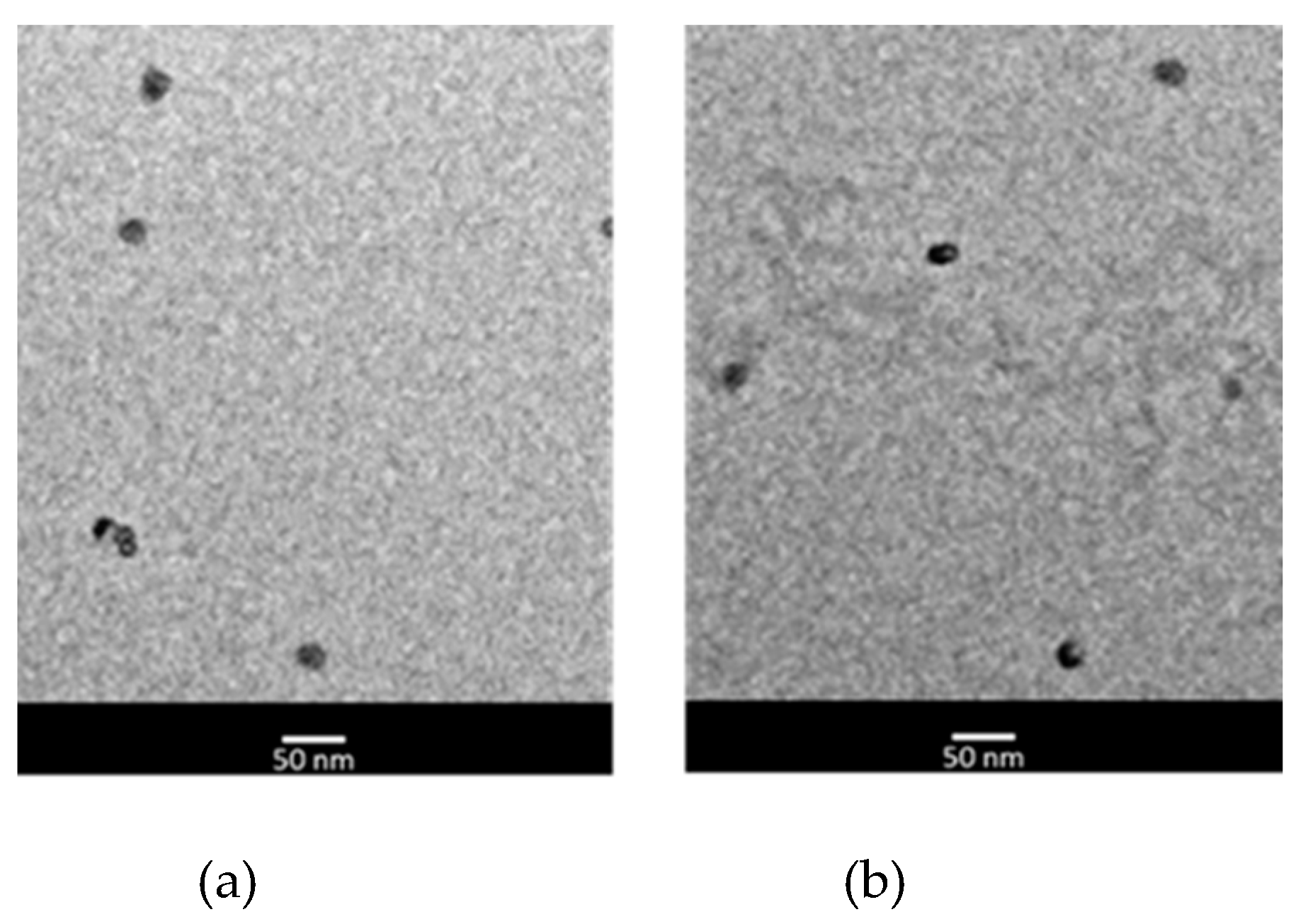
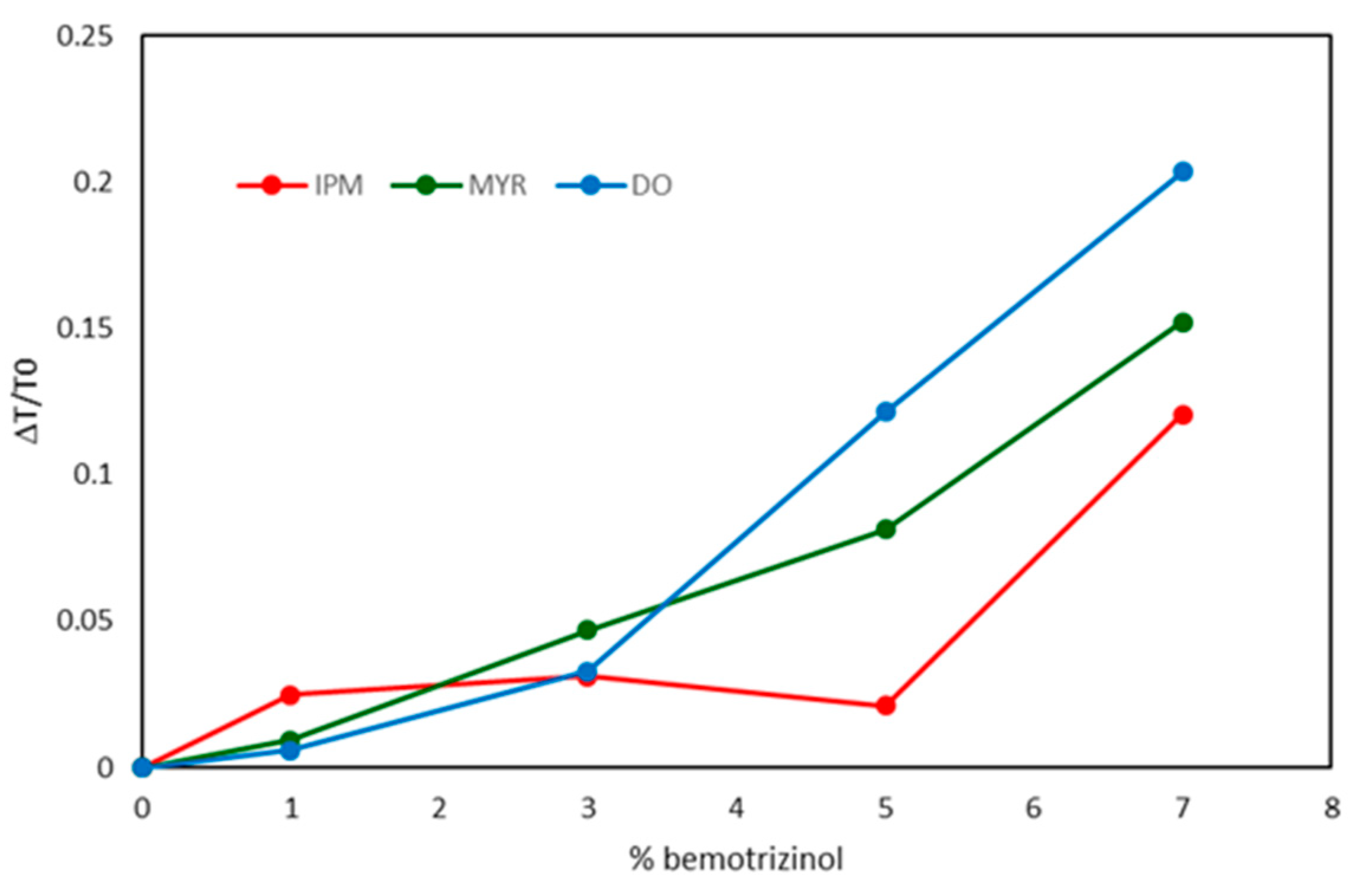
| NLC code | Oleth-20 | GOa | CPb | IPMc | MYRd | DOe |
|---|---|---|---|---|---|---|
| IPM1 | 8.7 | 4.4 | 6.0 | 1.0 | --- | --- |
| IPM2 | 8.7 | 4.4 | 5.0 | 2.0 | --- | --- |
| IPM3 | 8.7 | 4.4 | 4.0 | 3.0 | --- | --- |
| MYR1 | 8.7 | 4.4 | 6.0 | --- | 1.0 | --- |
| MYR2 | 8.7 | 4.4 | 5.0 | --- | 2.0 | --- |
| MYR3 | 8.7 | 4.4 | 4.0 | --- | 3.0 | --- |
| DO1 | 8.7 | 4.4 | 6.0 | --- | --- | 1.0 |
| DO2 | 8.7 | 4.4 | 5.0 | --- | --- | 2.0 |
| DO3 | 8.7 | 4.4 | 4.0 | --- | --- | 3.0 |
| NLC code | Oleth-20 | GOa | CPb | IPMc | MYRd | DOe | BMTZf |
|---|---|---|---|---|---|---|---|
| IBMTZ1 | 8.7 | 4.4 | 4.0 | 3.0 | --- | --- | 1.0 |
| IBMTZ3 | 8.7 | 4.4 | 4.0 | 3.0 | --- | --- | 3.0 |
| IBMTZ5 | 8.7 | 4.4 | 4.0 | 3.0 | --- | --- | 5.0 |
| IBMTZ7 | 8.7 | 4.4 | 4.0 | 3.0 | --- | --- | 7.0 |
| MBMTZ1 | 8.7 | 4.4 | 4.0 | --- | 3.0 | --- | 1.0 |
| MBMTZ3 | 8.7 | 4.4 | 4.0 | --- | 3.0 | --- | 3.0 |
| MBMTZ5 | 8.7 | 4.4 | 4.0 | --- | 3.0 | --- | 5.0 |
| MBMTZ7 | 8.7 | 4.4 | 4.0 | --- | 3.0 | --- | 7.0 |
| DBMTZ1 | 8.7 | 4.4 | 4.0 | --- | --- | 3.0 | 1.0 |
| DBMTZ3 | 8.7 | 4.4 | 4.0 | --- | --- | 3.0 | 3.0 |
| DBMTZ5 | 8.7 | 4.4 | 4.0 | --- | --- | 3.0 | 5.0 |
| DBMTZ7 | 8.7 | 4.4 | 4.0 | --- | --- | 3.0 | 7.0 |
| Ingredient | Formulation code | ||||||
|---|---|---|---|---|---|---|---|
| A | B | BNLC | C | CNLC | D | DNLC | |
| Phase A | |||||||
| Cetiol Sa | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| MYRb | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Greensylc | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Crodamol CAPd | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Argan oil | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Wheat oil | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Shea oil | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| TAe | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Brij 72f | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Brij 721g | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Cetyl palmitate | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Cetyl alcohol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| BMTZh | --- | 0.4 | --- | 0.8 | --- | 1.6 | --- |
| Phase B | |||||||
| EDTAi | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Water | q.s.l | q.s.l | q.s.l | q.s.l | q.s.l | q.s.l | q.s.l |
| Phase C | |||||||
| Kemipur 100m | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Benzyl alcohol | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Phase D | |||||||
| Parfum | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Phase E | |||||||
| BMTZ-NLCn | --- | --- | 5.0 | --- | 10.0 | --- | 20.0 |
| NLC code | Z-average ± S.D. (nm) | PDI | Zeta ± S.D. (mV) |
|---|---|---|---|
| IPM1 | 38.5 ± 1.5 | 0.102 ± 0.008 | -10.3 ± 1.1 |
| IPM2 | 37.4 ± 1.8 | 0.111 ± 0.007 | -9.2 ± 1.2 |
| IPM3 | 37.9 ± 2.0 | 0.107 ± 0.011 | -11.3 ± 0.9 |
| MYR1 | 47.9 ± 2.8 | 0.115 ± 0.008 | -8.9 ± 1.0 |
| MYR2 | 45.6 ± 1.6 | 0.105 ± 0.009 | -10.4 ± 1.7 |
| MYR3 | 48.1 ± 1.9 | 0.109 ± 0.007 | -9.4 ± 0.8 |
| DO1 | 45.3 ± 1.4 | 0.153 ± 0.009 | -11.5 ± 1.6 |
| DO2 | 41.1 ± 2.0 | 0.171 ± 0.012 | -9.9 ± 1.2 |
| DO3 | 41.7 ± 2.3 | 0.166 ± 0.008 | -10.6 ± 1.8 |
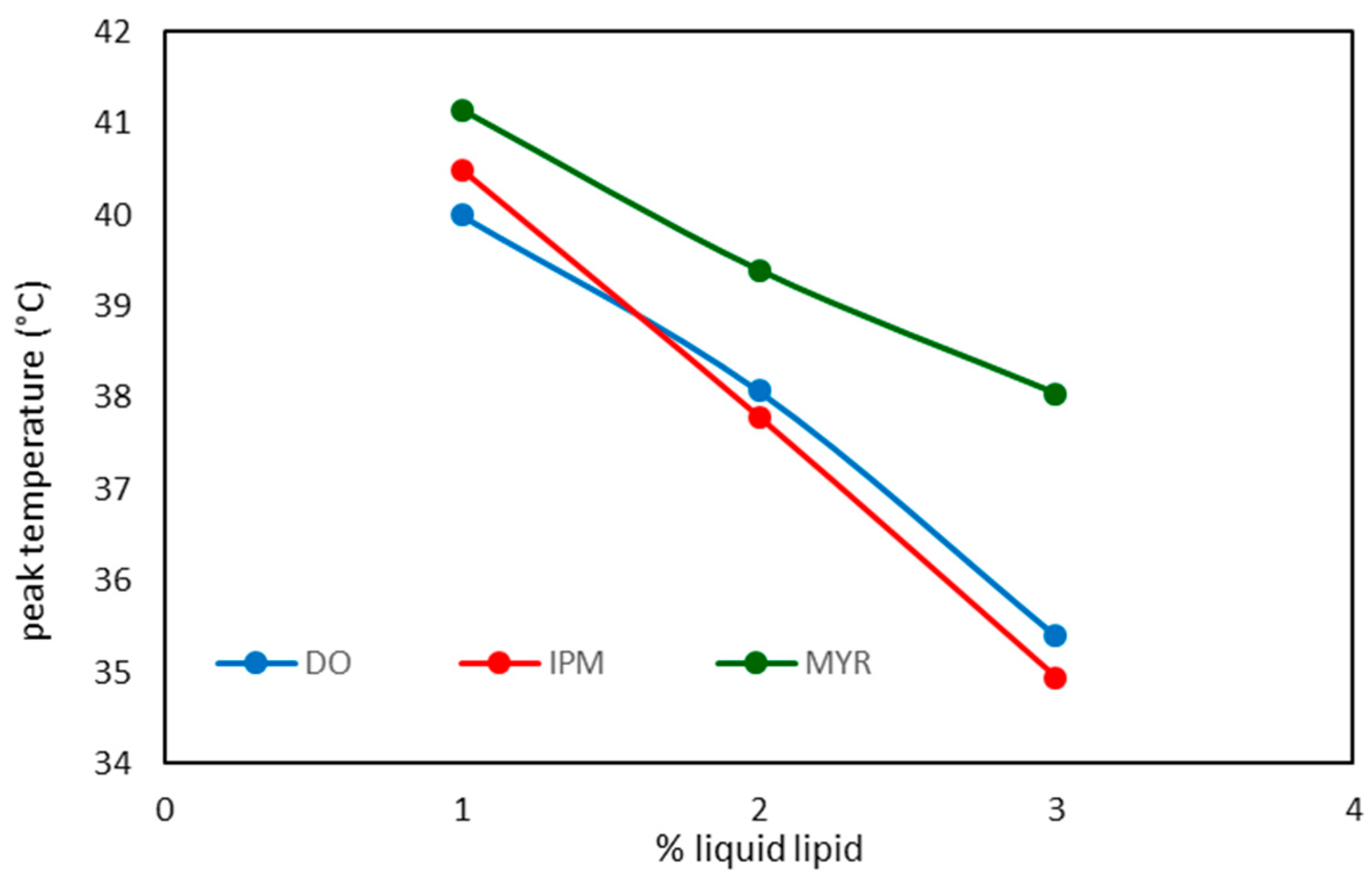
| NLC code | ΔH ± S.D. (J/g) |
RI % |
|---|---|---|
| DO 1 | 9.46 ± 0.38 | 63.9 |
| DO 2 | 7.22 ± 0.14 | 58.5 |
| DO 3 | 5.01 ± 0.05 | 50.8 |
| IPM 1 | 8.96 ± 0.24 | 60.5 |
| IPM 2 | 6.59 ± 0.20 | 53.4 |
| IPM 3 | 2.23 ± 0.08 | 22.6 |
| MYR 1 | 10.37 ± 0.45 | 70.0 |
| MYR 2 | 7.80 ± 0.15 | 63.0 |
| MYR 3 | 4.21 ± 0.17 | 42.6 |
| NLC code | Z-average ± S.D. (nm) |
PDI | Zeta ± S.D. (mV) |
|---|---|---|---|
| IBMTZ1 | 33.2 ± 2.1 | 0.217 ± 0.015 | -10.4± 1.3 |
| IBMTZ3 | 35.5 ± 1.9 | 0.090 ± 0.004 | -9.9 ± 1.7 |
| IBMTZ5 | 45.3 ± 2.3 | 0.115 ± 0.009 | -11.8 ± 1.4 |
| IBMTZ7 | 72.5 ± 6.3 | 0.149 ± 0.014 | -9.9 ± 1.3 |
| MBMTZ1 | 49.8 ± 2.9 | 0.127 ± 0.011 | -11.2 ± 1.4 |
| MBMTZ3 | 58.4 ± 2.1 | 0.136 ± 0.009 | -10.2 ± 1.9 |
| MBMTZ5 | 78.1 ± 3.9 | 0.191 ± 0.018 | -11.5 ± 0.9 |
| MBMTZ7 | 99.3 ± 2.5 | 0.289 ± 0.019 | -9.9 ± 1.2 |
| DBMTZ1 | 45.0 ± 4.1 | 0.129 ± 0.013 | -10.7 ± 1.5 |
| DBMTZ3 | 50.8 ± 4.3 | 0.167 ± 0.015 | -11.2 ± 1.4 |
| DBMTZ5 | 66.9 ± 3.3 | 0.181 ± 0.018 | -11.3 ± 1.8 |
| DBMTZ7 | 81.5 ± 2.8 | 0.264 ± 0.019 | -10.8 ± 0.7 |
| NLC code | ΔH ± S.D. (J/g) | RI % |
|---|---|---|
| DBMTZ1 | 2.72 ± 0.12 | 27.5 |
| DBMTZ3 | 3.67 ± 0.13 | 37.1 |
| DBMTZ5 | 2.03 ± 0.04 | 20.6 |
| DBMTZ7 | 1.94 ± 0.09 | 19.6 |
| IBMTZ1 | 2.00 ± 0.04 | 20.2 |
| IBMTZ3 | 2.46 ± 0.11 | 24.8 |
| IBMTZ5 | 2.93 ± 0.06 | 29.6 |
| IBMTZ7 | 0.83 ± 0.03 | 8.4 |
| MBMTZ1 | 2.10 ± 0.10 | 21.2 |
| MBMTZ3 | 4.04 ± 0.12 | 40.9 |
| MBMTZ5 | 2.58 ± 0.05 | 26.1 |
| MBMTZ7 | 4.37 ± 0.21 | 44.2 |
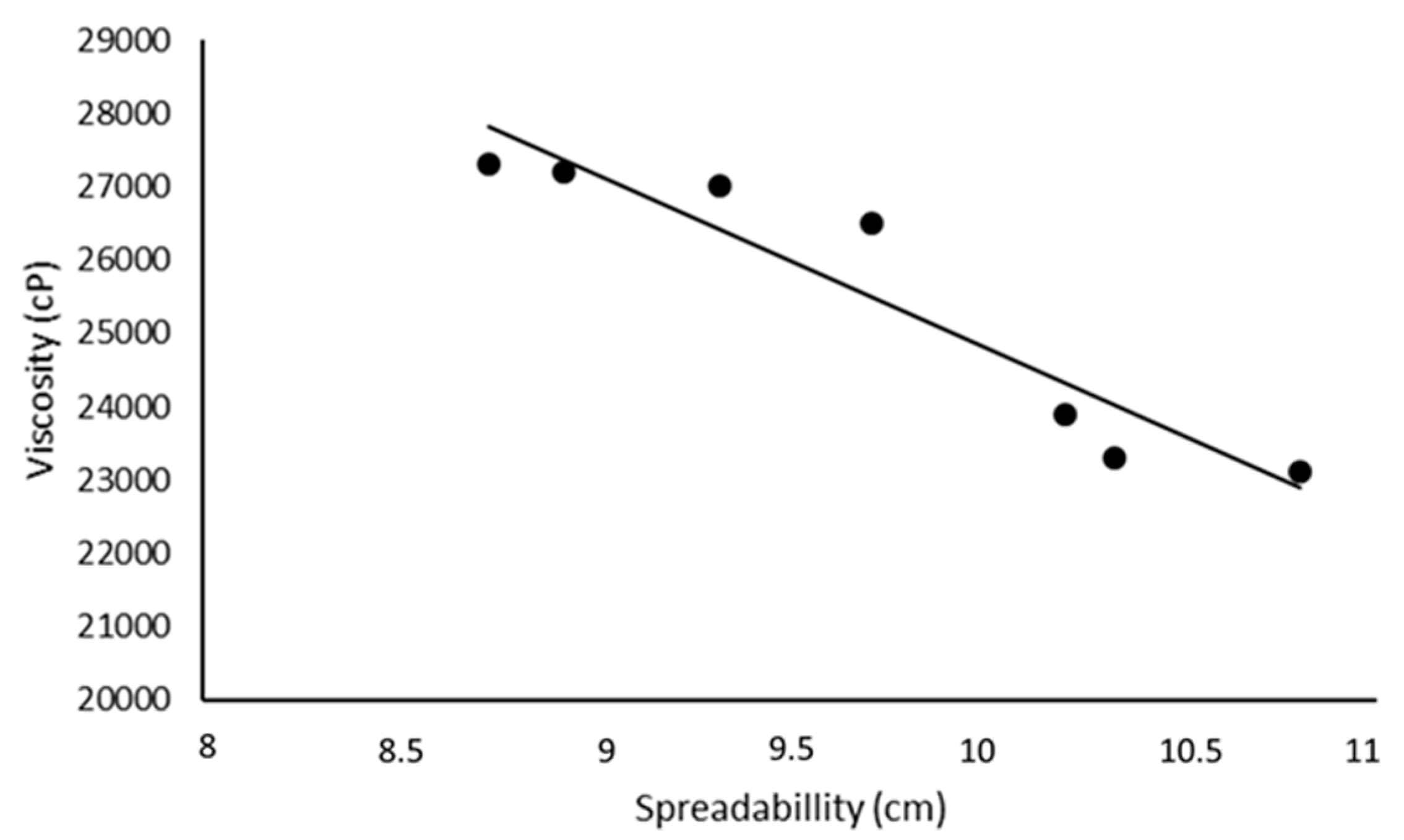
| Emulsion code | pH | F | S (cm) | V (cP) | Q (μg) | SPF |
|---|---|---|---|---|---|---|
| A | 6.6 ± 0.1 | 50.41 ± 1.52 | 8.92 ± 0.31 | 27200 ± 1200 | --- | 1.22 ± 0.04 |
| B | 7.5 ± 0.2 | 53.31 ± 1.43 | 9.71 ± 0.22 | 26500 ± 1400 | 0.60 ± 0.12 | 3.03 ± 0.11 |
| BNLC | 7.0 ± 0.1 | 51.45 ± 0.99 | 10.33 ± 0.40 | 23300 ± 900 | 0.98 ± 0.23 | 3.54 ± 0.12 |
| C | 7.4 ± 0.1 | 51.70 ± 1.32 | 8.73 ± 0.38 | 27300 ± 1500 | 0.88 ± 0.17 | 4.14 ± 0.18 |
| CNLC | 7.4 ± 0.2 | 49.83 ± 1.44 | 10.2 ± 0.14 | 23900 ± 1100 | 0.87 ± 0.19 | 4.78 ± 0.21 |
| D | 7.5 ± 0.1 | 50.66 ± 0.98 | 9.32 ± 0.02 | 27000 ±1400 | 0.71 ± 0.11 | 5.26 ± 0.28 |
| DNLC | 7.4 ± 0.1 | 51.07 ± 1.02 | 10.8 ± 0.28 | 23100 ± 1000 | 0.66 ± 0.13 | 6.34 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





