Submitted:
25 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Extraction and Characterization of Seaweed Biopolymers
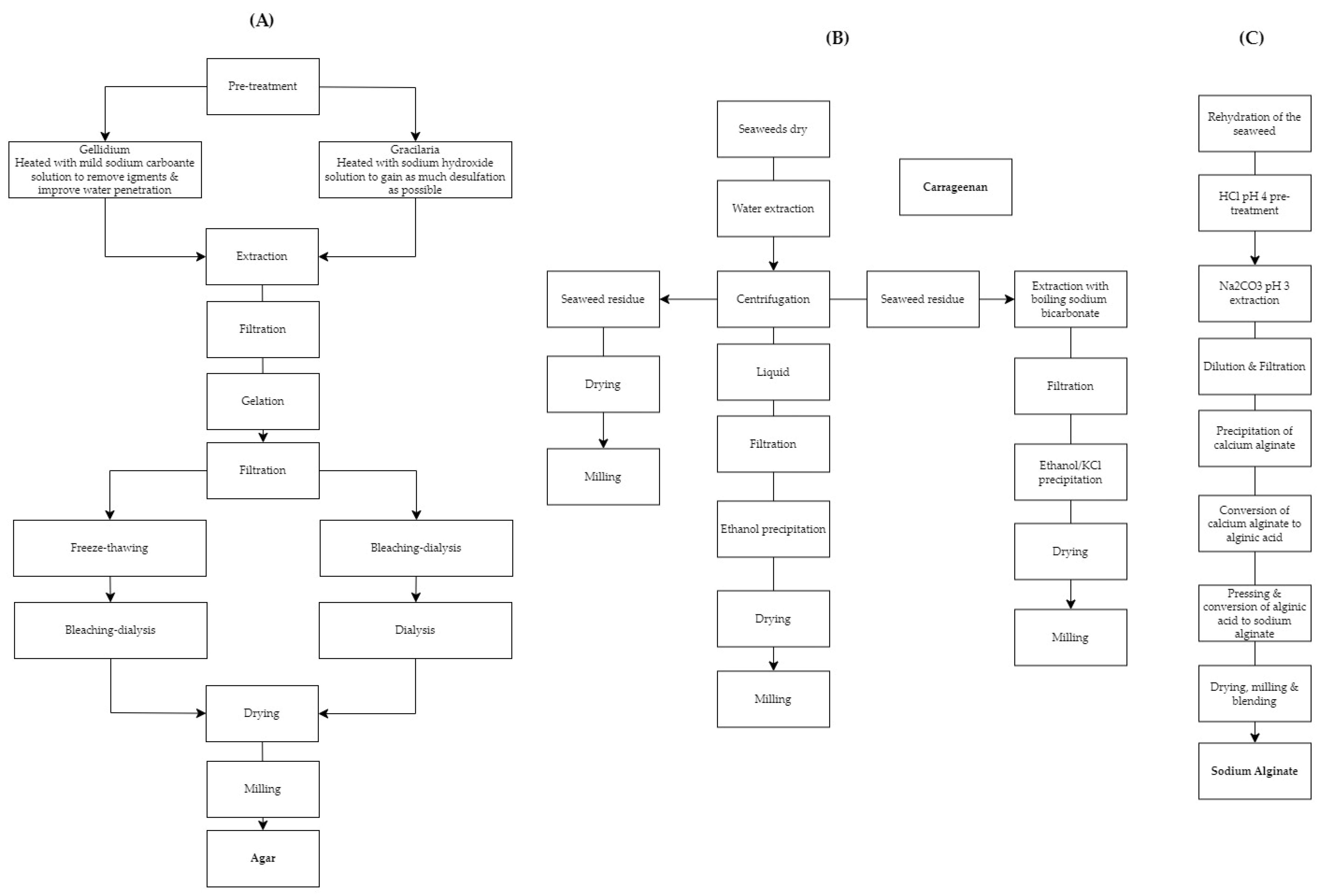
2.1. Extraction Methods
2.1.1. Traditional Methods
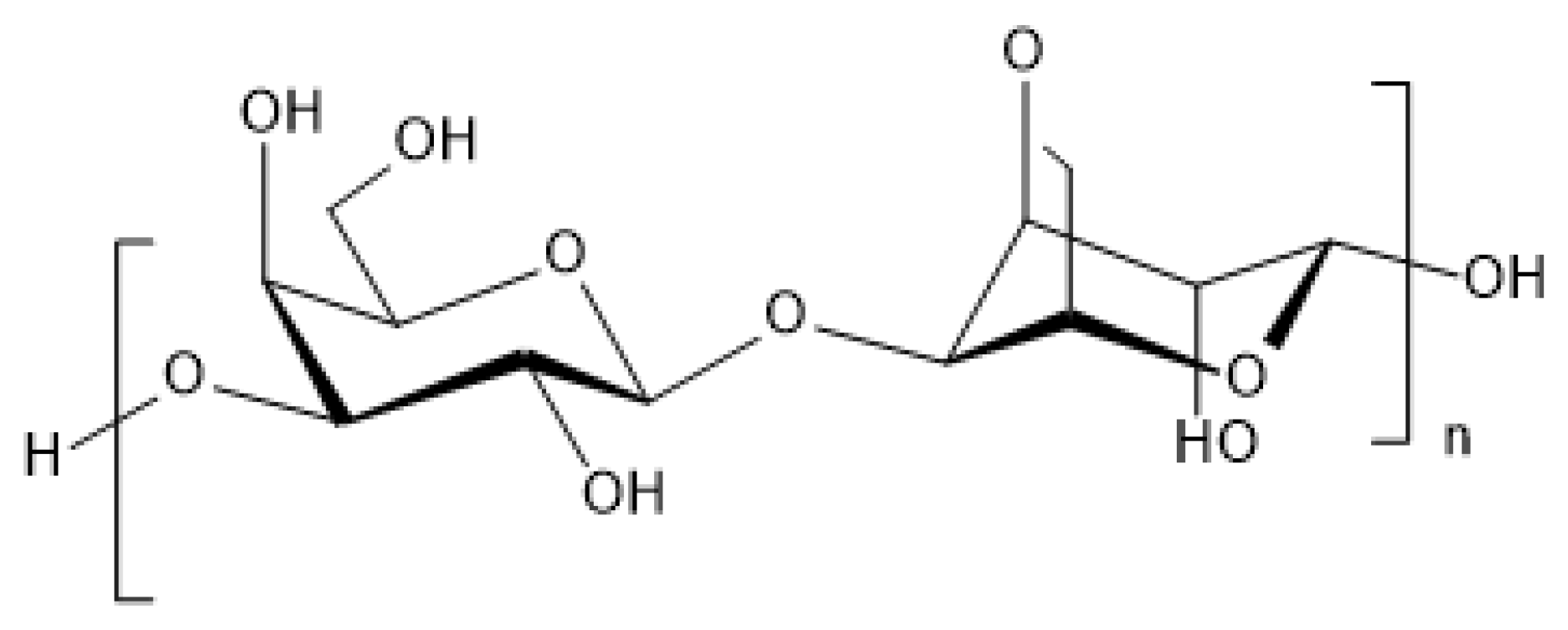
2.1.1.1. Agar
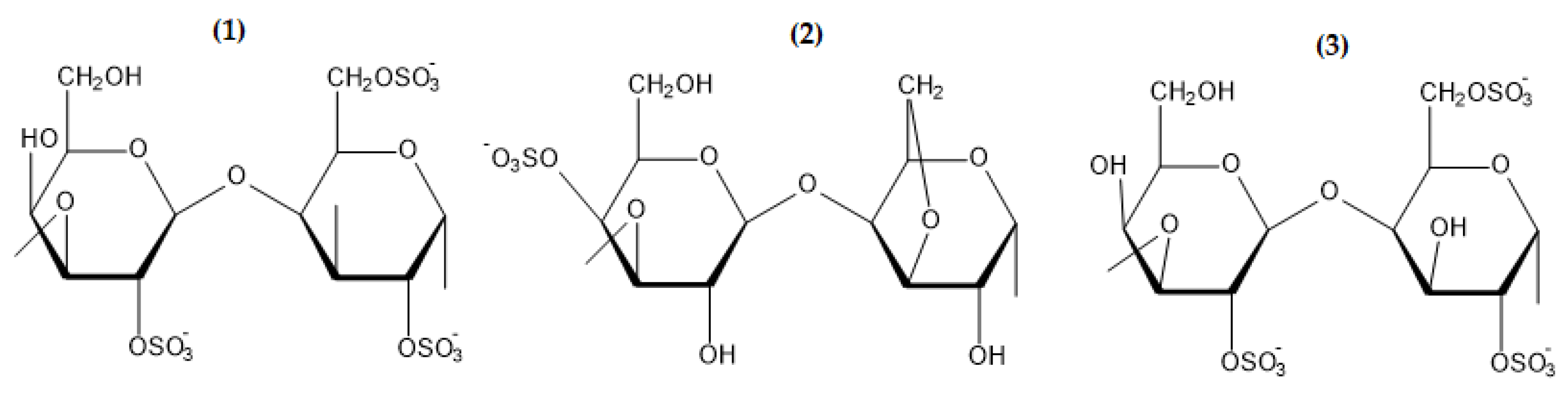
2.1.1.2. Carrageenan
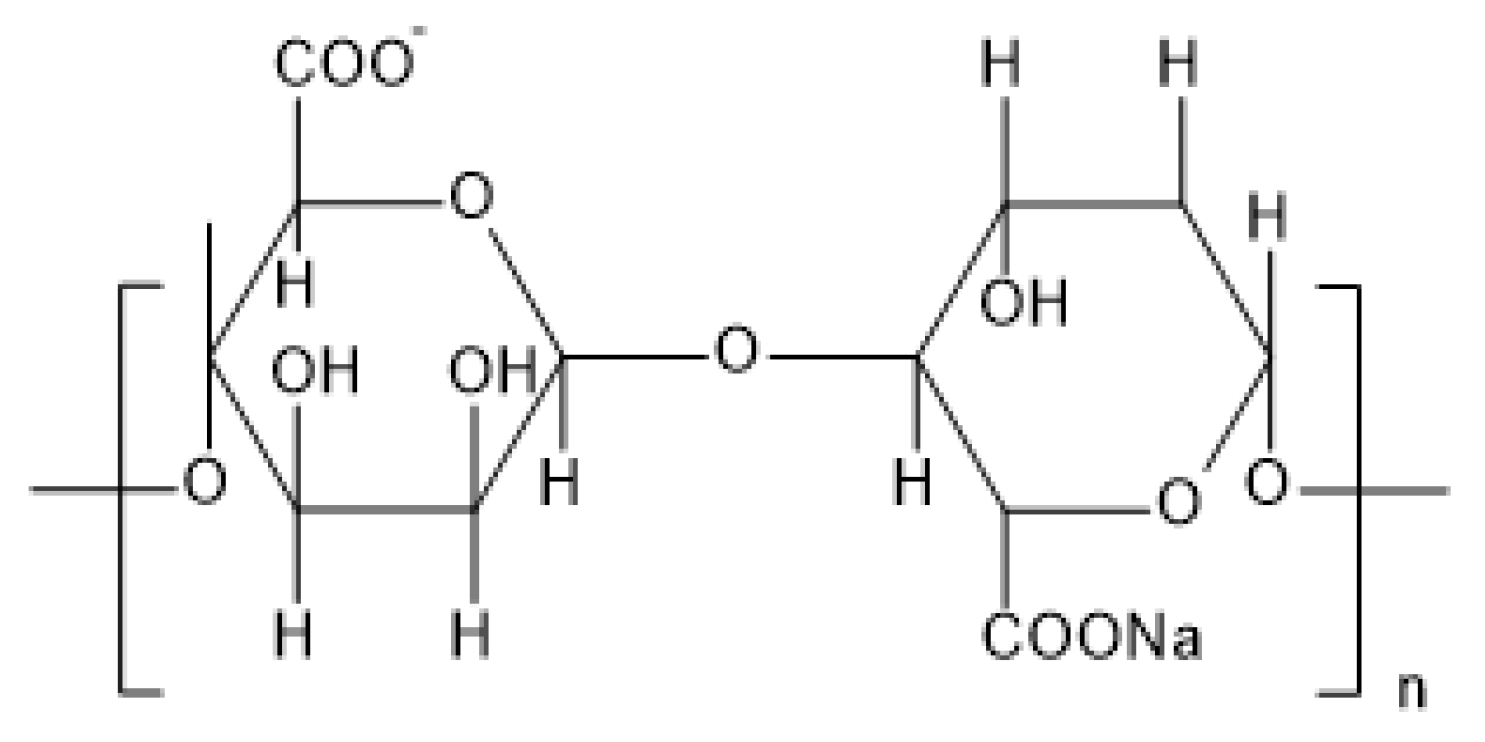
2.1.1. 3 Sodium Alginate
2.1.2. Enzyme-Assisted Extraction
2.1.3. Microwave-Assisted Extraction
2.1.4. Ultrasound-Assisted Extraction
2.1.5. Supercritical Fluid Extraction
2.1.6. Subcritical Water Extraction
2.1.7. Bead Milling
2.2. Characterization Techniques
2.2.1. Molecular Weight Determination
2.2.2. Spectroscopic Analysis
2.2.3. Thermal Analysis
3. Properties of Seaweed Biopolymers
3.1. Biodegradability
3.2. Mechanical Strength
3.3. Barrier Properties
3.4. Compatibility with Other Materials
4. Applications in Packaging
4.1. Food Packaging
4.1.1. Innovations in Seaweed Biopolymer-Based Food Packaging
4.1.2. Performance Evaluation
4.1.3. Consumer and Environmental Benefits
4.2. Edible Films & Coatings
4.2.1. Formulation and Development
4.2.2. Properties and Performance
4.2.3. Commercial Viability and Challenges
5. Challenges & Future Directions
5.1. Scalability and Cost-Effectiveness
5.2. Performance Under Diverse Conditions
5.2. Regulatory Approval and Consumer Acceptance
5.4. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alves, B. Plastic waste worldwide - statistics & facts. Statista 2024. https://www.statista.com/topics/5401/global-plastic-waste/ (accessed April 15, 2024).
- Stegmann, P.; Daioglou, V.; Londo, M.; van Vuuren, D.P.; Junginger, M. Plastic futures and their CO2 emissions. Nature 2022, 612, 272–276. [Google Scholar] [CrossRef]
- IUCN. Marine plastic pollution 2021.
- Kühn, S.; van Franeker, J.A. Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef]
- Laist, D.W. Impacts of Marine Debris: Entanglement of Marine Life in Marine Debris Including a Comprehensive List of Species with Entanglement and Ingestion Records. In: Coe JM, Rogers DB, editors. Marine Debris: Sources, Impacts, and Solutions, New York, NY: Springer; 1997, p. 99–139. [CrossRef]
- Bertolazzi, S.; Cuttitta, A.; Pipitone, V. Addressing marine plastic pollution: a systematic literature review. Curr. Opin. Environ. Sustain. 2024, 68. [Google Scholar] [CrossRef]
- Fletcher S, Roberts K, Shirian Y, Virdin J, Conesa Alcolea I, Brown A, et al. Policy Options to Eliminate Additional Marine Plastic Litter by 2050 under the G20 Osaka Blue Ocean Vision: An International Resource Panel Think Piece. United Nations Environment Programme; 2021.
- Krause, S.; Molari, M.; Gorb, E.V.; Gorb, S.N.; Kossel, E.; Haeckel, M. Persistence of plastic debris and its colonization by bacterial communities after two decades on the abyssal seafloor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer additives. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Tajeddin B, Arabkhedri M. Chapter 16 - Polymers and food packaging. In: AlMaadeed MAA, Ponnamma D, Carignano MA, editors. Polymer Science and Innovative Applications, Elsevier; 2020, p. 525–43. [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Smith, O.; Brisman, A. Plastic Waste and the Environmental Crisis Industry. Crit. Criminol. 2021, 29, 289–309. [Google Scholar] [CrossRef]
- Pilapitiya, P.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11. [Google Scholar] [CrossRef]
- Ritchie H, Samborska V, Roser M. Plastic Pollution. Our World in Data 2023.
- Lange, J.-P. Managing Plastic Waste─Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Arias-Nava, E.H.; Sullivan, B.P.; Valles-Rosales, D.J. Biopolymer Degradation Analysis: Accelerated Life Testing Study to Characterize Polylactic Acid Durability. Materials 2021, 14, 5730. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef]
- Tullberg, R.M.; Nguyen, H.P.; Wang, C.M. Review of the Status and Developments in Seaweed Farming Infrastructure. J. Mar. Sci. Eng. 2022, 10, 1447. [Google Scholar] [CrossRef]
- Hasselström, L.; Thomas, J.-B.; Nordström, J.; Cervin, G.; Nylund, G.M.; Pavia, H.; Gröndahl, F. Socioeconomic prospects of a seaweed bioeconomy in Sweden. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ruslan FS, Susanti D, Noor NM, Aminudin NI, Taher M. Bioactive Compounds, Cosmeceutical And Nutraceutical Applications of Green Seaweed Species (Chlorophyta). Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology 2021;16:41–55.
- Júnior, A.I.M.; Soccol, C.R.; Camara, M.C.; Aulestia, D.T.M.; Vandenberghe, L.P.d.S.; de Carvalho, J.C. Challenges in the production of second-generation organic acids (potential monomers for application in biopolymers). Biomass- Bioenergy 2021, 149. [Google Scholar] [CrossRef]
- Verma, S.K.; Prasad, A.; Sonika; Katiyar, V. State of art review on sustainable biodegradable polymers with a market overview for sustainability packaging. Mater. Today Sustain. 2024, 26. [Google Scholar] [CrossRef]
- Al Mahmud, Z.; Mobarak, H.; Hossain, N. Emerging trends in biomaterials for sustainable food packaging: A comprehensive review. Heliyon 2024, 10, e24122. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Torres-Giner, S.; Ferrandiz-Bou, S.; Quiles-Carrillo, L.; Montanes, N. Development of Sustainable and Cost-Competitive Injection-Molded Pieces of Partially Bio-Based Polyethylene Terephthalate through the Valorization of Cotton Textile Waste. Int. J. Mol. Sci. 2019, 20, 1378. [Google Scholar] [CrossRef] [PubMed]
- Chavez, B.A.; Raghavan, V.; Tartakovsky, B. A comparative analysis of biopolymer production by microbial and bioelectrochemical technologies. RSC Adv. 2022, 12, 16105–16118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 1–29. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of seaweeds as healthy approach to formulate new low-salt meat products. Curr. Opin. Food Sci. 2020, 40, 20–25. [Google Scholar] [CrossRef]
- Lim, C.; Yusoff, S.; Ng, C.; Lim, P.; Ching, Y. Bioplastic made from seaweed polysaccharides with green production methods. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Liyanapathiranage, A.; Makehelwala, M.; Dassanayake, R.; Manamperi, A.; Merah, O.; Mani, S.; Koduru, J.R.; Madhujith, T. Algal polysaccharides: Structure, preparation and applications in food packaging. Food Chem. 2023, 405. [Google Scholar] [CrossRef]
- Contessa, C.R.; da Rosa, G.S.; Moraes, C.C. New Active Packaging Based on Biopolymeric Mixture Added with Bacteriocin as Active Compound. Int. J. Mol. Sci. 2021, 22, 10628. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Hosseini, H.M.; Fooladi, A.A.I. Antibacterial activity of agar-based films containing nisin, cinnamon EO, and ZnO nanoparticles. J. Food Saf. 2018, 38, e12440. [Google Scholar] [CrossRef]
- Martins, B.A.; de Albuquerque, P.B.S.; de Souza, M.P. Bio-based Films and Coatings: Sustainable Polysaccharide Packaging Alternatives for the Food Industry. J. Polym. Environ. 2022, 30, 4023–4039. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.-C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process. Biochem. 2017, 58, 289–297. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E. montassir; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties. Trends Food Sci. Technol. 2023, 140. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 243–269. [Google Scholar]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Meyer, A.S. Chemistry, gelation, and enzymatic modification of seaweed food hydrocolloids. Trends Food Sci. Technol. 2021, 109, 608–621. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Relation between structure and rheological/thermal properties of agar. A mini-review on the effect of alkali treatment and the role of agaropectin. Food Struct. 2017, 13, 24–34. [Google Scholar] [CrossRef]
- Bose, I.; Nousheen; Roy, S. ; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef]
- Lipnizki, F. Basic aspects and applications of membrane processes in agro-food and bulk biotech industry. Comprehensive Membrane Science and Engineering, vol. 4, Elsevier Science Publishers B.V.; 2010, p. 165–94.
- Venkatesan J, Nithya R, Sudha PN, Kim S-K. Chapter Four - Role of Alginate in Bone Tissue Engineering. In: Kim S-K, editor. Advances in Food and Nutrition Research, vol. 73, Academic Press; 2014, p. 45–57. [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S. New extraction technology and characterization of sodium alginate. IOP Conf. Series: Earth Environ. Sci. 2020, 474. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O'Doherty, J.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, A.; Ruiz-Matute, A.; Soria, A.; Sanz, M. Green techniques for extraction of bioactive carbohydrates. TrAC Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O'Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Domínguez, H.; Torres, M.D. Valorisation of edible brown seaweeds by the recovery of bioactive compounds from aqueous phase using MHG to develop innovative hydrogels. Process. Biochem. 2019, 78, 100–107. [Google Scholar] [CrossRef]
- Soria AC, Ruiz-Aceituno L, Ramos L, Sanz ML. Microwave assisted extraction of polysaccharide | DIGITAL.CSIC. Polysaccharides: Bioactivity and biotechnology, Springer Nature; 2015, p. 987–1008.
- Xu, Z. Solubility of Polysaccharides. BoD – Books on Demand; 2017.
- Mussatto, SL. Microwave-Assisted Extraction of Fucoidan from Marine Algae. Natural Products From Marine Algae, vol. 1308, 2015.
- Vázquez-Delfín, E.; Robledo, D.; Freile-Pelegrín, Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J. Appl. Phycol. 2013, 26, 901–907. [Google Scholar] [CrossRef]
- Nesic, A.; De Bonis, M.V.; Poggetto, G.D.; Ruocco, G.; Santagata, G. Microwave Assisted Extraction of Raw Alginate as a Sustainable and Cost-Effective Method to Treat Beach-Accumulated Sargassum Algae. Polymers 2023, 15, 2979. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Boffito DC, Van Gerven T. Process Intensification and Catalysis☆. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier; 2019. [CrossRef]
- Ostasevicius, V.; Jurenas, V.; Mikuckyte, S.; Vezys, J.; Stankevicius, E.; Bubulis, A.; Venslauskas, M.; Kizauskiene, L. Development of a Low-Frequency Piezoelectric Ultrasonic Transducer for Biological Tissue Sonication. Sensors 2023, 23, 3608. [Google Scholar] [CrossRef]
- Kadam, S.U.; O'Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Barrio, L.P.G.; Cabral, E.M.; Zhao, M.; García, C. .; Senthamaraikannan, R.; Padamati, R.B.; Tiwari, U.; Curtin, J.F.; Tiwari, B.K. Comparison Study of an Optimized Ultrasound-Based Method versus an Optimized Conventional Method for Agar Extraction, and Protein Co-Extraction, from Gelidium sesquipedale. Foods 2022, 11, 805. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Gomez, L.P.; Senthamaraikannan, R.; Padamati, R.B.; O'Donnell, C.P.; Tiwari, B.K. Investigation of enzyme-assisted methods combined with ultrasonication under a controlled alkali pretreatment for agar extraction from Gelidium sesquipedale. Food Hydrocoll. 2021, 120, 106905. [Google Scholar] [CrossRef]
- Clifford, A.A.; Williams, J.R. Introduction to Supercritical Fluids and Their Applications. In Supercritical Fluid Methods and Protocols; Springer Science and Business Media LLC, 2003; Volume 13, pp. 1–16.
- Uddin MdS, Sarker MdZI, Ferdosh S, Akanda MdJH, Easmin MstS, Shamsudin SHBt, et al. Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: a review. Journal of the Science of Food and Agriculture 2014;95:1385–94.
- Sahena, F.; Zaidul, I.; Jinap, S.; Karim, A.; Abbas, K.; Norulaini, N.; Omar, A. Application of supercritical CO2 in lipid extraction – A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Idris, S.A.; Rosli, N.R.; Aris, R.M.A.R. Supercritical carbon dioxide extraction of fatty acids compounds from tamarind seeds. Mater. Today: Proc. 2022, 63, S462–S466. [Google Scholar] [CrossRef]
- Patel A, Matsakas L, Sartaj K, Chandra R. Chapter 2 - Extraction of lipids from algae using supercritical carbon dioxide. In: Inamuddin, Asiri AM, Isloor AM, editors. Green Sustainable Process for Chemical and Environmental Engineering and Science, Elsevier; 2020, p. 17–39. [CrossRef]
- Ju, Z.; Howard, L.R. Subcritical Water and Sulfured Water Extraction of Anthocyanins and Other Phenolics from Dried Red Grape Skin. J. Food Sci. 2006, 70, S270–S276. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.; Brinkman, U. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.-S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Postma, P.; Suarez-Garcia, E.; Safi, C.; Yonathan, K.; Olivieri, G.; Barbosa, M.; Wijffels, R.; Eppink, M. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef]
- Firdayanti, L.; Yanti, R.; Rahayu, E.S.; Hidayat, C. Carrageenan extraction from red seaweed (Kappaphycopsis cottonii) using the bead mill method. Algal Res. 2023, 69. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Ci, S.X.; Huynh, T.H.; Louie, L.W.; Yang, A.; Beals, B.J.; Ron, N.; Tsang, W.-G.; Soon-Shiong, P.; Desai, N.P. Molecular mass distribution of sodium alginate by high-performance size-exclusion chromatography. J. Chromatogr. A 1999, 864, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Roomiani, L.; Tadayoni, M. Microwave-assisted extraction optimization, antimicrobial and antioxidant properties of carrageenan from red algae (Gracilaria acerosa). J. Food Meas. Charact. 2022, 17, 1156–1166. [Google Scholar] [CrossRef]
- Diogo AC, Alvarenga NB. Progress in Rheology of Biological and Synthetic Polymer Systems. Instituto Politécnico de Beja; 2004.
- Xu, R.; Zheng, L.; Zhang, S.; Huang, M.; Zhao, M. Complex coacervation of type II collagen with anionic polysaccharides: Effects of solution pH and molecular conformation of polysaccharide. Food Hydrocoll. 2023, 144. [Google Scholar] [CrossRef]
- Ureña, M.; Carullo, D.; Phùng, T.T.-T.; Fournier, P.; Farris, S.; Lagorce, A.; Karbowiak, T. Effect of polymer structure on the functional properties of alginate for film or coating applications. Food Hydrocoll. 2024, 149. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Madera-Santana, T.; Robledo, D.; Veleva, L.; Quintana, P.; Azamar, J. Degradation of agar films in a humid tropical climate: Thermal, mechanical, morphological and structural changes. Polym. Degrad. Stab. 2006, 92, 244–252. [Google Scholar] [CrossRef]
- Smith, B. A Process for Successful Infrared Spectral Interpretation 2016;31:14–21.
- Berna, F. Fourier Transform Infrared Spectroscopy (FTIR). In: Gilbert AS, editor. Encyclopedia of Geoarchaeology, Dordrecht: Springer Netherlands; 2017, p. 285–6. [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Mahmood, T.; Hussain, N.; Shahbaz, A.; Mulla, S.I.; Iqbal, H.M.; Bilal, M. Sustainable production of biofuels from the algae-derived biomass. Bioprocess Biosyst. Eng. 2022, 46, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Supercritical Water Gasification - an overview | ScienceDirect Topics n.d. https://www.sciencedirect.com/topics/engineering/supercritical-water-gasification (accessed , 2024). 9 July.
- Das, P.; V. P., C.; Mathimani, T.; Pugazhendhi, A. Recent advances in thermochemical methods for the conversion of algal biomass to energy. Sci. Total. Environ. 2021, 766, 144608. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; Oconnor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Hii S-L, Lim J-Y, Ong W-T, Wong C-L. AGAR FROM MALAYSIAN RED SEAWEED AS POTENTIAL MATERIAL FOR SYNTHESIS OF BIOPLASTIC FILM 2016.
- Sari WM, Supartono W, Suharno. Characterization of Biodegradable Films from Raw Seaweed (Kappaphycus Alvarezii) and Glycerol. IOP Conf Ser: Earth Environ Sci 2024;1364:012081. [CrossRef]
- Jost, V. Packaging related properties of commercially available biopolymers – An overview of the status quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Elhrari, W.; Shebani, A.; Klash, A.; Elhabishi, R.; Abdsalam, S.; Elbreki, H. The Influence of LDPE Content on the Mechanical Properties of HDPE/LDPE Blends. Res. Dev. Mater. Sci. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Castro-Landinez, J.F.; Salcedo-Galan, F.; Medina-Perilla, J.A. Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability. Polymers 2021, 13, 705. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.B.; Mishra, S. The Compatibilizing Effect of Maleic Anhydride on Swelling Properties of Plant-Fiber-Reinforced Polystyrene Composites. Polym. Technol. Eng. 2006, 45, 923–927. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Liu, C.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. Effect of chlorinated phosphate ester based on castor oil on thermal degradation of poly (vinyl chloride) blends and its flame retardant mechanism as secondary plasticizer. RSC Adv. 2015, 5, 41169–41178. [Google Scholar] [CrossRef]
- Dardmeh, N.; Khosrowshahi, A.; Almasi, H.; Zandi, M. Study on Effect of the Polyethylene Terephthalate/Nanoclay Nanocomposite Film on the Migration of Terephthalic Acid into the Yoghurt Drinks Simulant. J. Food Process. Eng. 2015, 40. [Google Scholar] [CrossRef]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Enhancing Tensile Strength, Thermal Stability, and Antioxidant Characteristics of Transparent Kappa Carrageenan Films Using Grapefruit Essential Oil for Food Packaging Applications. ACS Omega 2024, 9, 9003–9012. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, W.A.W.; Azman, N.A.M.; Adam, F.; Subramaniam, S.D.; Hamid, K.H.A.; Almajano, M.P. Exploring the Potential of Seaweed Derivatives for the Development of Biodegradable Plastics: A Comparative Study. Polymers 2023, 15, 2884. [Google Scholar] [CrossRef] [PubMed]
- M, H.; Chong, E.; Jafarzadeh, S.; Paridah, M.; Gopakumar, D.A.; Tajarudin, H.; Thomas, S.; Khalil, H.A. Enhancement in the Physico-Mechanical Functions of Seaweed Biopolymer Film via Embedding Fillers for Plasticulture Application—A Comparison with Conventional Biodegradable Mulch Film. Polymers 2019, 11, 210. [Google Scholar] [CrossRef]
- Abdul Khalil HPS, Tye YY, Chow ST, Saurabh CK, Paridah MT, Dungani R, et al. Cellulosic pulp fiber as reinforcement materials in seaweed-based film. BioRes 2017;12:29–42.
- Khalil, H.P.S.A.; Lai, T.K.; Tye, Y.Y.; Paridah, M.T.; Fazita, M.R.N.; Azniwati, A.A.; Dungani, R.; Rizal, S. Preparation and Characterization of Microcrystalline Cellulose from Sacred Bali Bamboo as Reinforcing Filler in Seaweed-based Composite Film. Fibers Polym. 2018, 19, 423–434. [Google Scholar] [CrossRef]
- Abdul Khalil HPS, Yap SW, Tye YY, Tahir PM, Rizal S, Nurul Fazita MR. Effects of corn starch and Kappaphycus alvarezii seaweed blend concentration on the optical, mechanical, and water vapor barrier properties of composite films. BioRes 2018;13:1157–73.
- Abdul Khalil HPS, Tye YY, Ismail Z, Leong JY, Saurabh CK, Lai TK, et al. Oil palm shell nanofiller in seaweed-based composite film: Mechanical, physical,and morphological properties. BioRes 2017;12:5996–6010.
- Kumar, U.S.U.; Paridah, M.T.; Owolabi, F.A.T.; Gopakumar, D.A.; Rizal, S.; Amirul, A.A.; Rahman, A.A.; Alfatah, T.; Mistar, E.M.; Aprilia, N.A.S.; et al. Neem leaves extract based seaweed bio-degradable composite films with excellent antimicrobial activity for sustainable packaging material. BioResources 2018, 14, 700–713. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials 2020, 13, 2895. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Membr. Sci. 2015, 493, 46–57. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Rhim, J.-W. Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Joshi, G.V.; Patel, H.A.; Ingole, P.G.; Mody, H.M.; Bajaj, H.C. Montmorillonite-Alginate Nanocomposites as a Drug Delivery System: Intercalation and In Vitro Release of Vitamin B1 and Vitamin B6. J. Biomater. Appl. 2009, 25, 161–177. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. PLA/algae composites: Morphology and mechanical properties. Compos. Part A: Appl. Sci. Manuf. 2015, 73, 109–115. [Google Scholar] [CrossRef]
- Mak, T.M.; Xiong, X.; Tsang, D.C.; Yu, I.K.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2019, 297, 122497. [Google Scholar] [CrossRef]
- Prezkop L, Stone A, Baker GA, Johnson LK, Deutsch J. Challenges and initiatives in reducing food losses and waste: United States. Preventing food losses and waste to achieve food security and sustainability, Burleigh Dodds Science Publishing; 2019.
- Schaefer, D.; Cheung, W.M. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Kerry, J.; O’grady, M.; Hogan, S. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: a review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Dwivany, F.M.; Aprilyandi, A.N.; Suendo, V.; Sukriandi, N. Carrageenan Edible Coating Application Prolongs Cavendish Banana Shelf Life. Int. J. Food Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Guitián, M.V.; Ibarguren, C.; Soria, M.C.; Hovanyecz, P.; Banchio, C.; Audisio, M.C. Anti-Listeria monocytogenes effect of bacteriocin-incorporated agar edible coatings applied on cheese. Int. Dairy J. 2019, 97, 92–98. [Google Scholar] [CrossRef]
- Alexandre, S.; Vital, A.C.P.; Mottin, C.; Prado, R.M.D.; Ornaghi, M.G.; Ramos, T.R.; Guerrero, A.; Pilau, E.J.; Prado, I.N.D. Use of alginate edible coating and basil (Ocimum spp) extracts on beef characteristics during storage. J. Food Sci. Technol. 2020, 58, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Avalos, M.; Minjares-Fuentes, R.; Femenia, A.; Contreras-Esquivel, J.; Quintero-Ramos, A.; Esparza-Rivera, J.; Meza-Velázquez, J. Application of an Alginate–Chitosan Edible Film on Figs (Ficus carica): Effect on Bioactive Compounds and Antioxidant Capacity. Food Bioprocess Technol. 2019, 12, 499–511. [Google Scholar] [CrossRef]
- Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; McClements, D.J.; Liu, F.; Cheng, C.; Xiong, J.; Zhu, M.; Chen, S. Investigation of a novel smart and active packaging materials: Nanoparticle-filled carrageenan-based composite films. Carbohydr. Polym. 2023, 301, 120331. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost competitiveness of sustainable bioplastic feedstocks – A Monte Carlo analysis for polylactic acid. Clean. Eng. Technol. 2022, 6. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.-X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Garrido, T.; Leceta, I.; de la Caba, K. Films based on proteins and polysaccharides: Preparation and physical–chemical characterization. Eur. Polym. J. 2013, 49, 3713–3721. [Google Scholar] [CrossRef]
- Sonchaeng, U.; Wongphan, P.; Pan-Utai, W.; Paopun, Y.; Kansandee, W.; Satmalee, P.; Tamtin, M.; Kosawatpat, P.; Harnkarnsujarit, N. Preparation and Characterization of Novel Green Seaweed Films from Ulva rigida. Polymers 2023, 15, 3342. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Sondak, C.F.A.; Beardall, J. The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 2017, 52, 495–505. [Google Scholar] [CrossRef]
- Jagtap AS, Meena SN. Chapter 23 - Seaweed farming: A perspective of sustainable agriculture and socio-economic development. In: Jhariya MK, Meena RS, Banerjee A, Meena SN, editors. Natural Resources Conservation and Advances for Sustainability, Elsevier; 2022, p. 493–501. [CrossRef]
- Behera, D.P.; Vadodariya, V.; Veeragurunathan, V.; Sigamani, S.; Moovendhan, M.; Srinivasan, R.; Kolandhasamy, P.; Ingle, K.N. Seaweeds cultivation methods and their role in climate mitigation and environmental cleanup. Total. Environ. Res. Themes 2022, 3-4. [Google Scholar] [CrossRef]
- Global CO2 emissions by year 1940-2023. Statista n.d. https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed February 27, 2024).
- Banerjee A, Jhariya MK, Raj A, Yadav DK, Khan N, Meena RS. Energy and Climate Footprint Towards the Environmental Sustainability. In: Banerjee A, Meena RS, Jhariya MK, Yadav DK, editors. Agroecological Footprints Management for Sustainable Food System, Singapore: Springer; 2021, p. 415–43. [CrossRef]
- Duarte CM, Wu J, Xiao X, Bruhn A, Krause-Jensen D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Frontiers in Marine Science 2017;4.
- (Puscaselu), R.G.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Chiabrando V, Giacalone G. Effects of alginate edible coating on quality and antioxidant properties in sweet cherry during postharvest storage. Italian Journal of Food Science 2015;27:173–80. [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Nurul Fazita, M.R.; Hafiidz, J.M.; Banerjee, A.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Wu, Y.; Weller, C.; Hamouz, F.; Cuppett, S.; Schnepf, M. Moisture Loss and Lipid Oxidation for Precooked Ground-Beef Patties Packaged in Edible Starch-Alginate-Based Composite Films. J. Food Sci. 2001, 66, 486–493. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Chaudhary, S. Seaweed polysaccharide coatings/films for meat based foods. Food Humanit. 2023, 1, 777–792. [Google Scholar] [CrossRef]
- Singh, A.; Prajapati, P.; Vyas, S.; Gaur, V.K.; Sindhu, R.; Binod, P.; Kumar, V.; Singhania, R.R.; Awasthi, M.K.; Zhang, Z.; et al. A Comprehensive Review of Feedstocks as Sustainable Substrates for Next-Generation Biofuels. BioEnergy Res. 2022, 16, 105–122. [Google Scholar] [CrossRef]
- Third-generation bioethanol: status, scope, and challenges n.d. https://wgbis.ces.iisc.ac.in/energy/paper/Third-generation%20bioethanol%20status,%20scope,%20and%20challenges/introduction.htm (accessed July 12, 2024).
- Souza, P.R.; de Oliveira, A.C.; Vilsinski, B.H.; Kipper, M.J.; Martins, A.F. Polysaccharide-Based Materials Created by Physical Processes: From Preparation to Biomedical Applications. Pharmaceutics 2021, 13, 621. [Google Scholar] [CrossRef]
- Nosrati, H.; Khodaei, M.; Alizadeh, Z.; Banitalebi-Dehkordi, M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int. J. Biol. Macromol. 2021, 192, 298–322. [Google Scholar] [CrossRef]
- Vorwald, C.E.; Gonzalez-Fernandez, T.; Joshee, S.; Sikorski, P.; Leach, J.K. Tunable fibrin-alginate interpenetrating network hydrogels to support cell spreading and network formation. Acta Biomater. 2020, 108, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Liu, T.; Zhang, X.; Wang, X.; Wei, Y.; Hu, Y.; Lian, X.; Zhao, L.; Chen, W.; Huang, D. Engineered bone tissues using biomineralized gelatin methacryloyl/sodium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2021, 33, 137–154. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2020, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: a critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Collén, P.N.; Lemoine, M.; Daniellou, R.; Guégan, J.-P.; Paoletti, S.; Helbert, W. Enzymatic Degradation of κ-Carrageenan in Aqueous Solution. Biomacromolecules 2009, 10, 1757–1767. [Google Scholar] [CrossRef]
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Chi, W.-J.; Chang, Y.-K.; Hong, S.-K. Agar degradation by microorganisms and agar-degrading enzymes. Appl. Microbiol. Biotechnol. 2012, 94, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Quincey, J. The Coca-Cola Company 2022 Business & Sustainability Report 2022.
- Circular Economy and Waste Statistics Highlights Report 2021. Food Waste n.d.
- Skoczinski P, Carus M, Tweddle G, Ruiz P, Hark N, Zhang A, et al. Bio-based Building Blocks and Polymers – Global Capacities, Production and Trends 2023–2028. nova-Institut GmbH; 2024. [CrossRef]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the Therapeutic Applications of Algal Polysaccharides. J. Polym. Environ. 2021, 30, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 1–29. [Google Scholar] [CrossRef]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in Extraction Methods to Recover Added-Value Compounds from Seaweeds: Sustainability and Functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, S.; Stuart, P.R. Systematic assessment of triticale-based biorefinery strategies: techno-economic analysis to identify investment opportunities. Biofuels, Bioprod. Biorefining 2018, 12, S46–S59. [Google Scholar] [CrossRef]
- Alamri, M.; Qasem, A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- pw2017_flexible_playbook_v6_opt.pdf n.d.
- Li, Y.; Li, H.; Huang, J.; Huang, L.; Chen, L.; Ni, Y.; Zheng, Q. An environmentally friendly and highly transparent ZnO/cellulose nanocomposite membrane for UV sensing and shielding. Cellulose 2022, 29, 4439–4453. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz. Recent progress on UV-light barrier food packaging films – a systematic review. Innov. Food Sci. Emerg. Technol. 2024, 91. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Hambleton, A.; Debeaufort, F.; Bonnotte, A.; Voilley, A. Influence of alginate emulsion-based films structure on its barrier properties and on the protection of microencapsulated aroma compound. Food Hydrocoll. 2009, 23, 2116–2124. [Google Scholar] [CrossRef]
- Hanry E, Surugau N. Characterization of bioplastics developed from Kappaphycus alvarezii crosslinked with commercial sodium alginate. 2023. [CrossRef]
- Hanry, E.L.; Surugau, N. Characteristics and Properties of Biofilms Made from Pure Carrageenan Powder and Whole Seaweed (Kappaphycus sp.). J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 76, 99–110. [Google Scholar] [CrossRef]
- Paoletti, S.; Donati, I. pH Effects on the Conformations of Galacturonan in Solution: Conformational Transition and Loosening, Extension and Stiffness. Polysaccharides 2023, 4, 271–324. [Google Scholar] [CrossRef]
- Regulation - 1935/2004 - EN - EUR-Lex, n.d. https://eur-lex.europa.eu/eli/reg/2004/1935/oj (accessed July 18, 2024).
- Uehara, T.; Nakatani, J.; Tsuge, T.; Asari, M. Consumer preferences and understanding of bio-based and biodegradable plastics. J. Clean. Prod. 2023, 417. [Google Scholar] [CrossRef]
- Ruf, J.; Emberger-Klein, A.; Menrad, K. Consumer response to bio-based products – A systematic review. Sustain. Prod. Consum. 2022, 34, 353–370. [Google Scholar] [CrossRef]
- Chummun I, Ramphul H, Jhurry D, Bhaw-Luximon A. Chapter 8 - Bionanomaterials for wound healing applications. In: Barhoum A, Jeevanandam J, Danquah MK, editors. Bionanotechnology : Emerging Applications of Bionanomaterials, Elsevier; 2022, p. 259–304. [CrossRef]
- Carvalho DN, Inácio AR, Sousa RO, Reis RL, Silva TH. Chapter 18 - Seaweed polysaccharides as sustainable building blocks for biomaterials in tissue engineering. In: Torres MD, Kraan S, Dominguez H, editors. Sustainable Seaweed Technologies, Elsevier; 2020, p. 543–87. [CrossRef]
| Polymer | Elongation at break (%) | Tensile strength (MPa) | Ref |
|---|---|---|---|
| HDPE | 2131.1 | 27.93 | [96] |
| LDPE | 349.0 | 9.93 | [96] |
| PP | 690 | 22.3 | [97] |
| PS | 3.35 | 20.64 | [98] |
| PVC | 180.37 | 30.33 | [99] |
| PET | 1.87 | 40.02 | [100] |
| Material | Filler/Additive | Elongation at break (%) | Tensile strength (MPa) | Ref |
|---|---|---|---|---|
| Seaweed | Cellulosic pulp fiber | 2.5-5.4 | 45-81 | [105] |
| Seaweed | Microcrystalline cellulose | 13.57-19.17 | 20.21-29.76 | [106] |
| Seaweed/Starch | - | 6.17-18.4 | 41.37-65.73 | [107] |
| Seaweed | Oil palm shell nanofiller | 2.08-3.30 | 31.4-44.8 | [108] |
| Seaweed | Neem leaves | 17.64-20.73 | 34.55-39.95 | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





