Submitted:
24 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
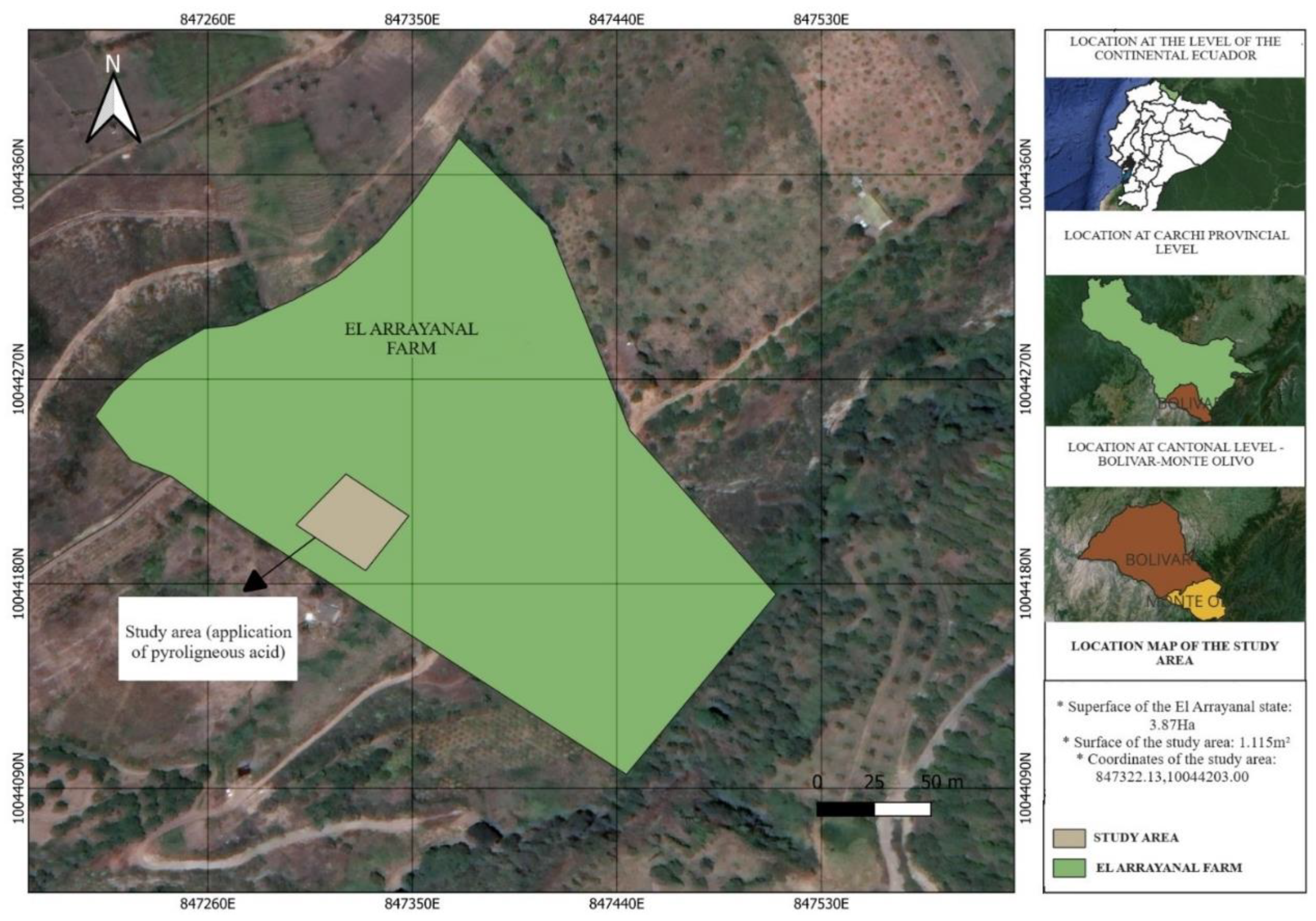
2.1. Study Area
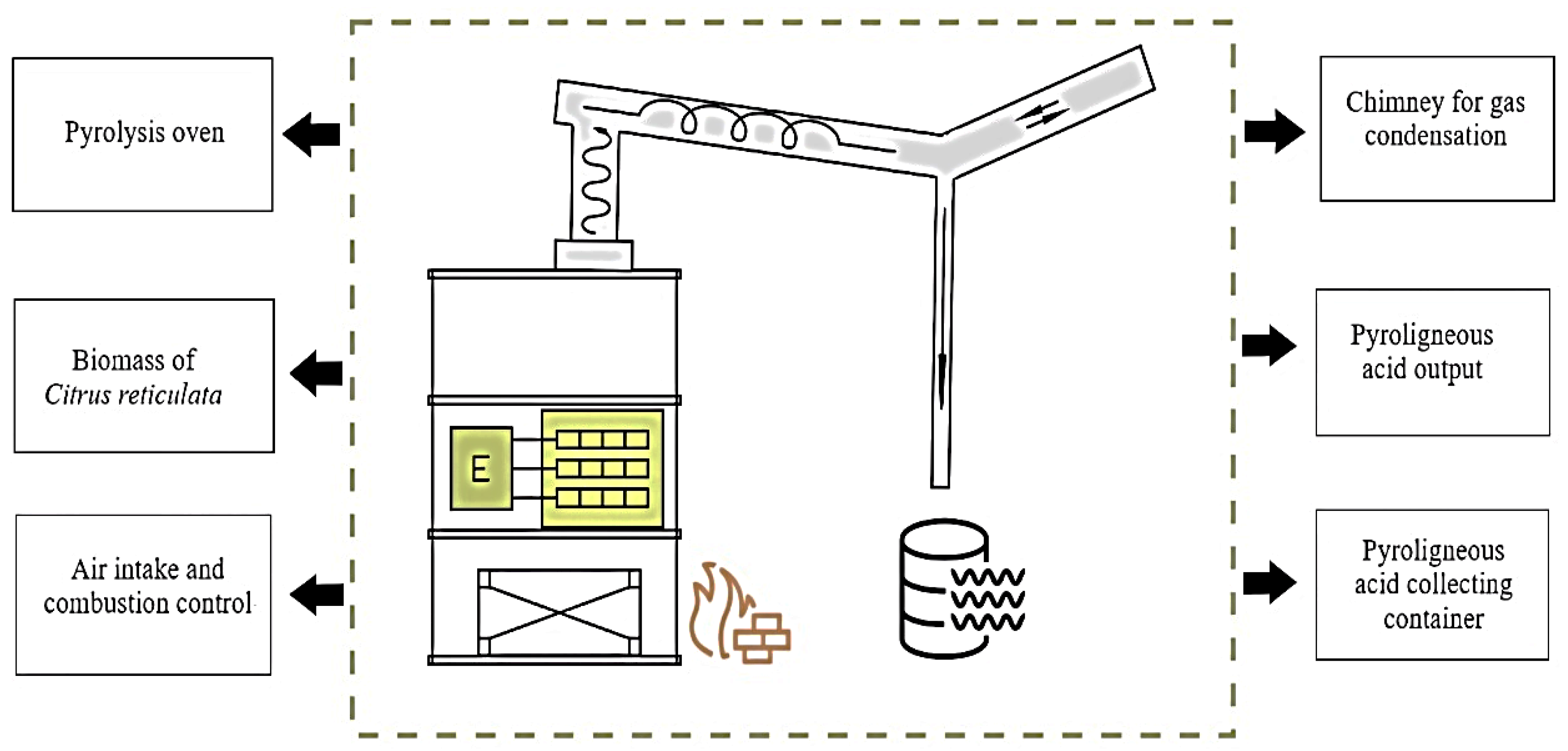
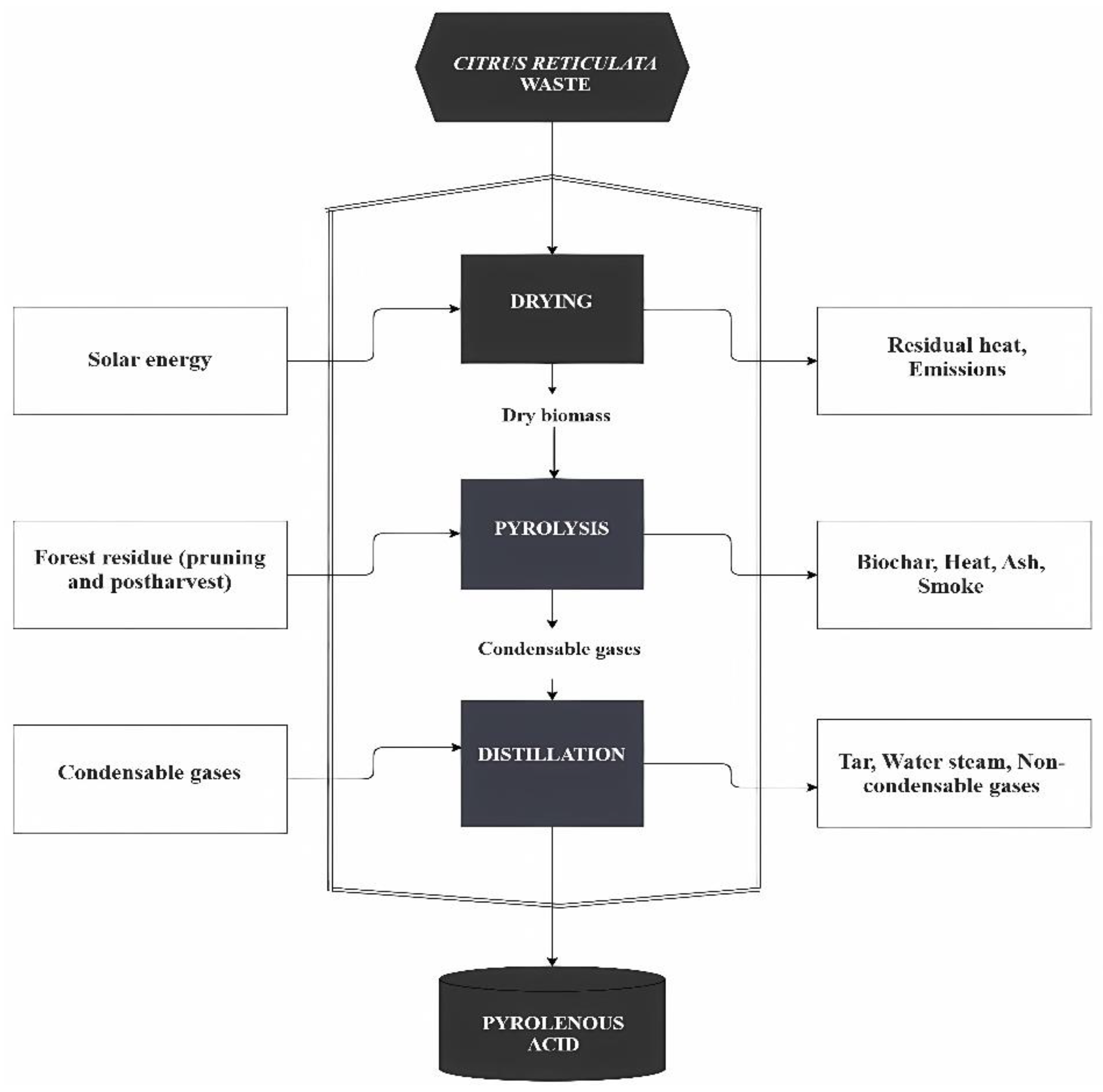
2.2. Extraction of Pyroligneous Acid from Mandarin Pruning Biomass
2.3. Characterization of Pyroligneous Acid
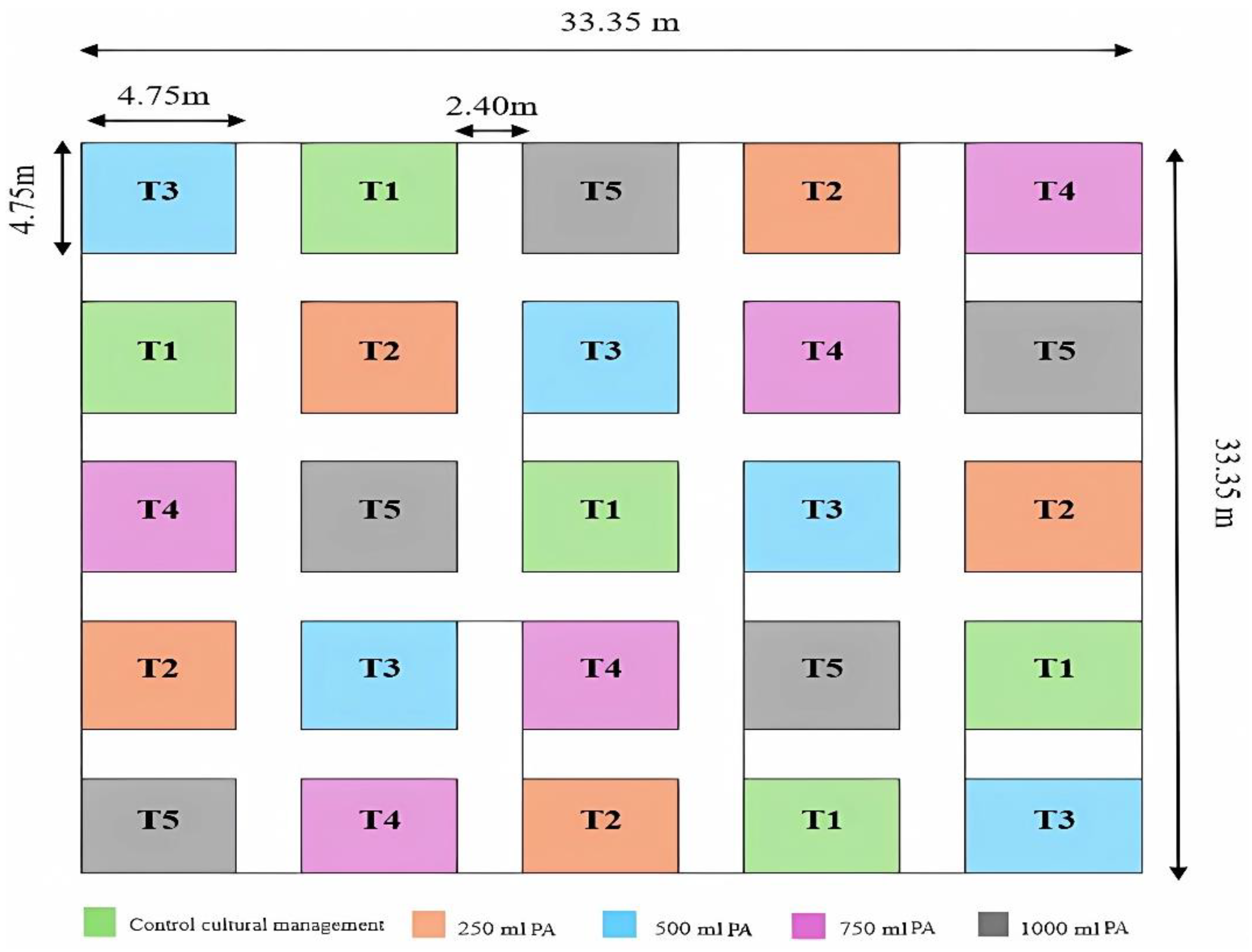
2.4. Experimental Design
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Pyroligneous Acid
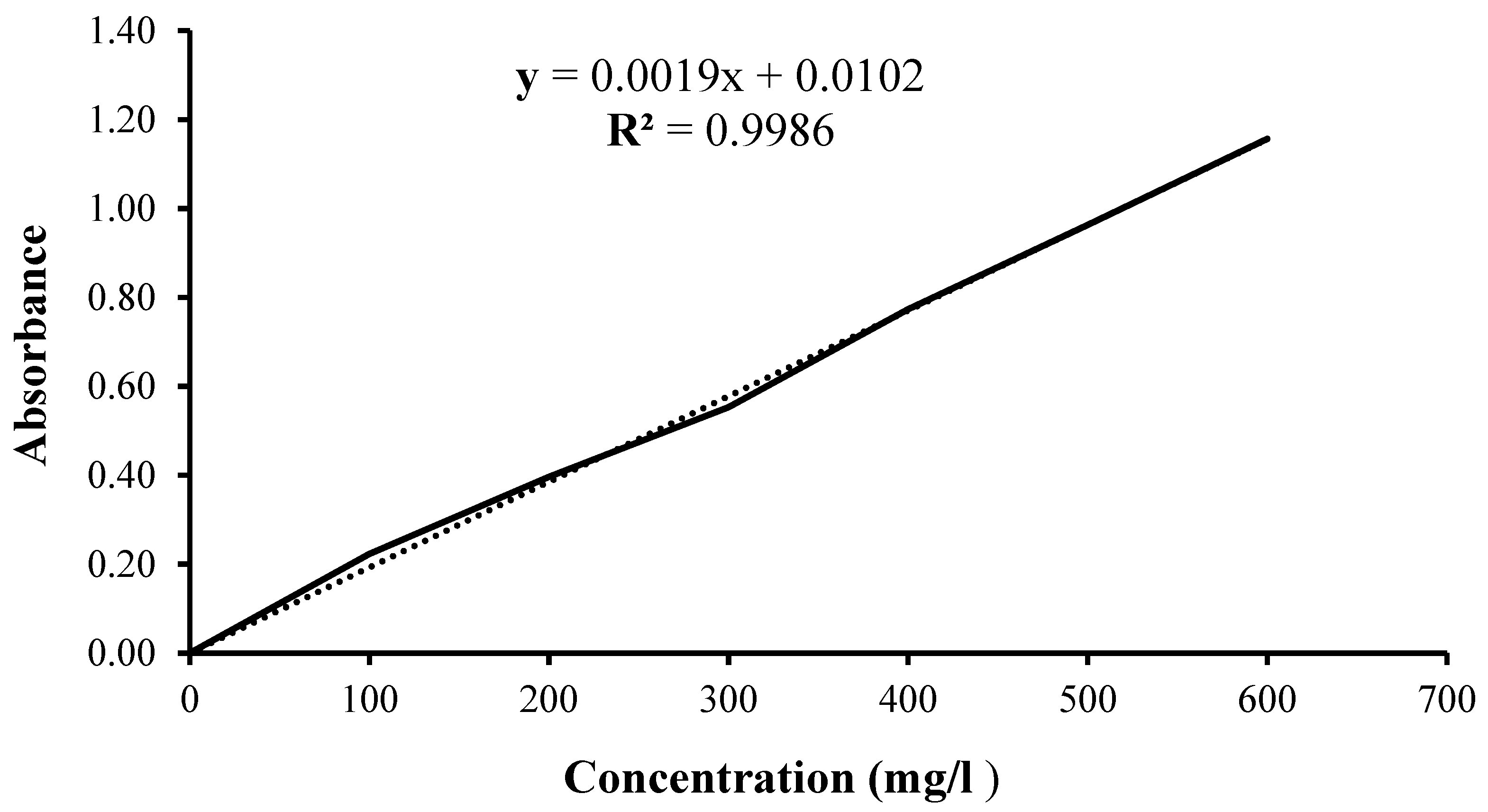
3.2. Analysis of Total Polyphenols
3.3. Efficiency of Pyroligneous Acid in Mandarin Crops
4. Discussion
Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alotaibi, SS, Darwish, H., Alzahrani, AK, Alharthi, S., Alghamdi, AS, Al- Barty, AM, Helal, M., Maghrabi, A., Baazeem, A., Alamari, HA, & Noureldeen, A. (2022). Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae ). Agronomy, 12 (9). [CrossRef]
- Autónomo, G., Parroquial, D., De, R., & Olivo, M. (2019). Monte Olivo Territorial Development and Planning Plan.
- Bouket, A.C., Narmani, A., Tavasolee, A., Elyasi, G., Abdi, A., Naeimi, S., Sharifi, K., Oszako, T., Alenezi, F.N., & Belbahri, L. (2022). In Vitro Evaluation of Wood Vinegar (Pyroligneous Acid) VOCs Inhibitory Effect against a Fungus-like Microorganism Ovatisporangium ( Phytopythium ) Isolate Recovered from Tomato Fields in Iran. Agronomy, 12 (7). [CrossRef]
- Burbano-Salas, D. (2018). Use of Kikuyu (Pennisetum Clandestinum L), residue from pruning areas greens for obtaining acid pyroligneous acid for agricultural purposes Use of Kikuyo (Pennisetum Clandestinum L), residue from the pruning of green areas for obtaining pyroligneous acid for agricultural purposes (Vol. 21).
- Cândido, NR, Modolo, LV, Pasa, VMD, & de Fátimaa, Â. (2023). Pyroligneous acids of coconut shell, black wattle and eucalyptus: physical-chemical characterization and in vitro evaluation as potential urease inhibitors. Quimica Nova , 46 (10), 961–971. [CrossRef]
- Carril, P., Bianchi, E., Cicchi, C., Coppi, A., Dainelli, M., Gonnelli, C., Loppi, S., Pazzagli, L., & Colzi, I. (2023). Effects of Wood Distillate (Pyroligneous Acid) on the Yield Parameters and Mineral Composition of Three Leguminous Crops. Environments - MDPI, 10 (7). [CrossRef]
- Castillo, B., & Dueñas, C. (2023). Exposure to pesticides in Latin America: Bibliographic Review. Journal of Forensic Sciences of Honduras ,9, 14–25. [CrossRef]
- Catacora-Pinazo, M., Quispe-Apaza, I., Julian -Laime, E., Zanabria-Mallqui, R., Roque-Cruz, M., & Zevallos-Pollito, P. (2019). Characterization of the chemical components of pyroligneous acid obtained from 3 forest species, for agricultural purposes in San Gaban, Puno (Perú) characterization of chemicals components of pyroleinous acid obtained from 3 forestry species, with agricultural purposes in San Gaban, Puno (Perú). 07 (2), 6–16. https://www.ceprosimad.com/.
- Choi, JY, Shinde, PL, Kwon, IK, Song, YH, & Chae, BJ (2009). Effect of Wood Vinegar on the Performance, Nutrient Digestibility and Intestinal Microflora in Weanling Pigs. Asian-Australas J Anim Sci,22 (2), 267–274. [CrossRef]
- Gomez, JP, Velez, JPA, Pinzon, MA, Arango, JAM, & Muriel, AP (2021). Chemical Characterization and Antiradical Properties of Pyroligneous Acid from a Preserved Bamboo, Guadua angustifolia Kunth. Brazilian Archives of Biology and Technology, 64, 1–13. [CrossRef]
- Gómez-arroyo, S., Martínez- valenzuela, C., Carbajal- lópez, Y., Martínez-arroyo, A., Elena CALDERÓN-SEGURA, M., Villalobos- pietrini, R., & Waliszewski, SM (nd). Genotoxic risk due to occupational exposure to pesticides in Latin America.
- Guo, G., Wang, Q., Huang, Q., Fu, Q., Liu, Y., Wang, J., Hu, S., Mašek, O., Wang, L., & Zhang, J. (2021). Effect of pyrolysis temperature on the characterization of dissolved organic matter from pyroligneous acid. Molecules, 26 (11). [CrossRef]
- Iacomino, G., Idbella, M., Staropoli, A., Nanni, B., Bertoli, T., Vinale, F., & Bonanomi, G. (2024). Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy, 14 (1). [CrossRef]
- Ibrahim, D., Kassim, J., Lim, SH., & Rusli, W. (2014). Evaluation of antibacterial effects of Rhizophora apiculata pyroligneous acid on pathogenic bacteria. Malaysian Journal of Microbiology. [CrossRef]
- Jindo, K., Goron, T.L., Kurebito, S., Matsumoto, K., Masunaga, T., Mori, K., Miyakawa, K., Nagao, S., & Tokunari, T. (2022). Sustainable Plant Growth Promotion and Chemical Composition of Pyroligneous Acid When Applied with Biochar as a Soil Amendment. Molecules, 27 (11). [CrossRef]
- Lee, CL, Chin, KL, Khoo, PS, Hafizuddin, MS, & H'ng, PS (2022). Production and Potential Application of Pyroligneous Acids from Rubberwood and Oil Palm Trunk as Wood Preservatives through Vacuum-Pressure Impregnation Treatment. Polymers, 14 (18). [CrossRef]
- María Ocampo González, C. 17. María Ocampo González, C. (2015). Acid compounds pyrolean from residual coniferous biomass cypress (Cupressus lusitanica Mill) and patula (Pinus pátula) 1 Compounds of Pyroligneous acid produced from residual biomass of conifers Cypress (Cupressus lusitanica Mill) and Patula pine (Pinus patula) (Vol. 28).
- Morales, MM, Sartori, WW, Silva, BR, Spera, ST, Mendes, ABD, & Ambrosio-Albuquerque, EP (2022). Wood vinegar: chemical characteristics, phytotoxic effects, and impacts on greenhouse gas emissions. Native, 10 (3), 400–409. [CrossRef]
- Ofoe, R., Qin, D., Gunupuru, L.R., Thomas, R.H., & Abbey, Lord. (2022). Effect of Pyroligneous Acid on the Productivity and Nutritional Quality of Greenhouse Tomato. Plants, 11 (13). [CrossRef]
- Othman, N., Elias, N.H., & Zainalabidin, N. (2023). Wood Vinegar as an Alternative Insecticide in Controlling Rice Weevil, Sitophylus oryzae (Coleoptera: Curculionidae). Advances in Agricultural and Food Research Journal, 4 (1). [CrossRef]
- Paiva, PEB, Neto, LM, Marques, NT, Duarte, BZ, & Duarte, AM (2024). Citrus Aphids in Algarve Region (Portugal): Species, Hosts, and Biological Control. Ecologies, 5 (1), 101–115. [CrossRef]
- Panwar, N., Thirumurugan, S., & Kumar, S. (2023). Host Plant Resistance in Brassicaceae against Aphids. In S. Kumar (Ed.), Brassica (p. Ch. 5). IntechOpen. [CrossRef]
- Pimenta, AS, Fasciotti, M., Monteiro, TVC, & Lima, KMG (2018). Chemical composition of pyroligneous acid obtained from eucalyptus GG100 clone. Molecules, 23 (2). [CrossRef]
- Pisa, L., Goulson, D., Yang, E.-C., Gibbons, D., Sánchez-Bayo, F., Mitchell, E., Aebi, A., van Der Sluijs, J., MacQuarrie, C.J.K., & Giorio, C. (2021). An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environmental Science and Pollution Research, 28 , 11749–11797.
- Sánchez, VH, & Mendoza, JLZ (2019). Adoption and impact of agricultural technologies developed in Ecuador. In Granja (Vol. 30, Issue 2, pp. 27–36). Polytechnic University Salesian. [CrossRef]
- Souza, JBG, Ré -Poppi, N., & Raposo Jr, JL (2012). Characterization of pyroligneous acid used in agriculture by gas chromatography-mass spectrometry. Journal of the Brazilian Chemical Society, 23, 610–617.
- Sparks, TC, Crossthwaite, AJ, Nauen, R., Banba, S., Cordova, D., Earley, F., Ebbinghaus- Kintscher, U., Fujioka, S., Hirao, A., & Karmon, D. (2020). Insecticides, biologics and nematicides: Updates to IRAC's mode of action classification-a tool for resistance management. Pesticide Biochemistry and Physiology, 167, 104587.
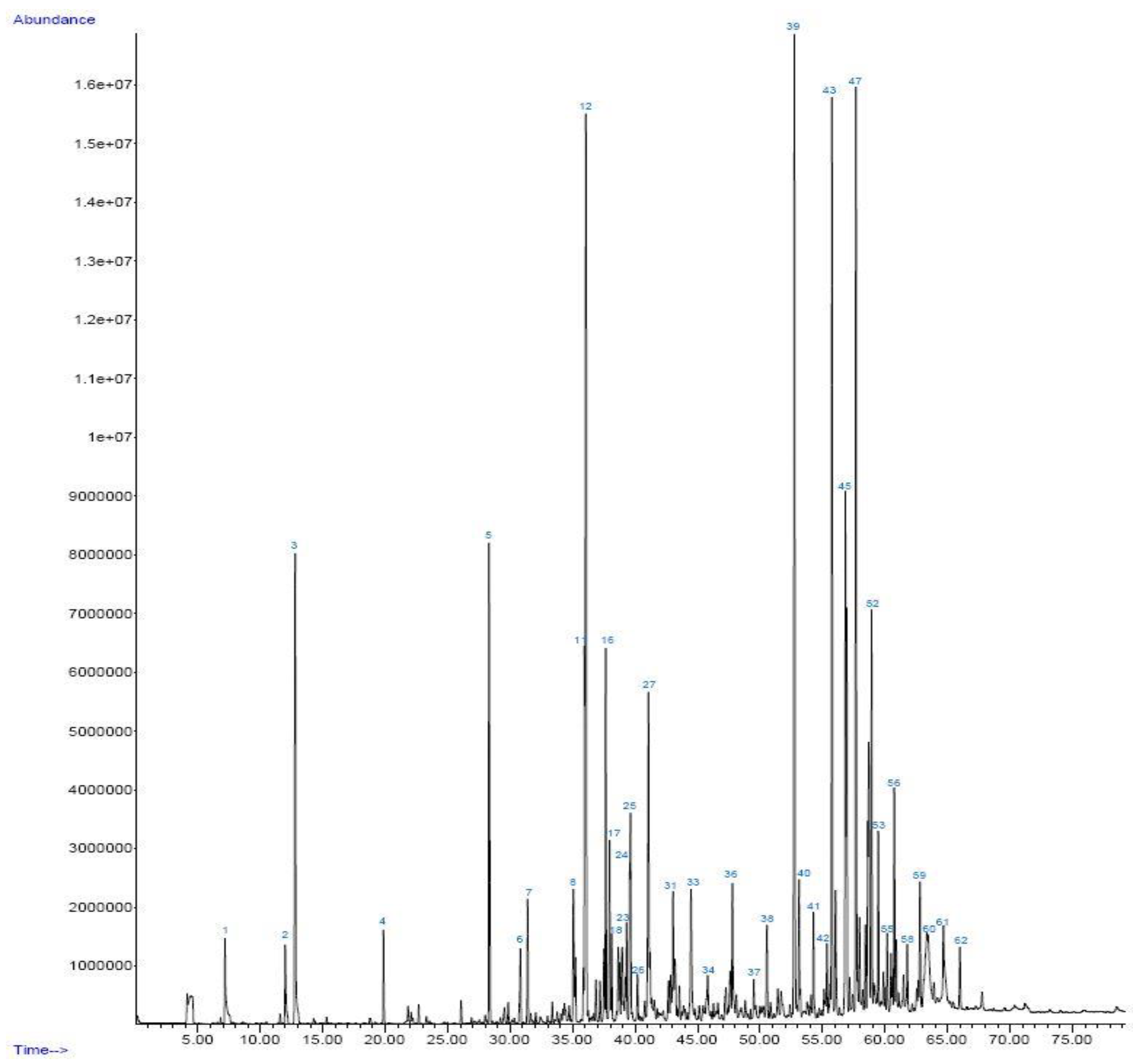
| No. | Compound | Retention time (min) | Area (%) |
|---|---|---|---|
| 1 | Cyclotrisiloxane, hexamethyl- | 7.172 | 0.74 |
| 2 | Cyclotetrasiloxane, octamethyl- | 11.993 | 0.74 |
| 3 | Acetonitrile | 12.791 | 4.39 |
| 4 | Cyclopentasiloxane, decamethyl- | 19.858 | 0.54 |
| 5 | Cyclohexasiloxane, dodecamethyl- | 28.295 | 4.03 |
| 6 | 2-Cyclopenten-1-one | 30.815 | 0.77 |
| 7 | 2-Cyclopenten-1-one, 2-methyl- | 31.397 | 0.98 |
| 8 | Acetic acid | 35.058 | 1.29 |
| 9 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 35.224 | 0.52 |
| 10 | 2-Propanone, 1- (acetyloxy)- | 35.869 | 0.37 |
| 11 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetratris (trimethylsiloxy) tetra | 35.945 | 1.68 |
| 12 | Furfural | 36.062 | 9.48 |
| 13 | 2-Cyclopenten-1-one, 3,4-dimethyl- | 36.861 | 0.33 |
| 14 | Acridine, 9-methyl- | 37.191 | 0.32 |
| 15 | Ethanone, 1-(2-methyl-1-cyclopenten-1-yl)- | 37.511 | 0.58 |
| 16 | 1,2,4-Triazol-4-amine, 5-ethyl-3-(3-methyl-5- phenylpyrazol-1-yl)- | 37.646 | 2.24 |
| 17 | Ethanone, 1-(2-furanyl)- | 37.955 | 1.50 |
| 18 | 4-Thiazoleacetic acid, 2- (p- chlorophenyl)-, hydrazide | 38.118 | 0.65 |
| 19 | 2-Cyclopenten-1-one, 3-methyl- | 38.645 | 0.62 |
| 20 | 3-Pentanone, 2-methyl- | 38.766 | 0.44 |
| 21 | 2-Butanone, 1- (acetyloxy)- | 38.971 | 1.00 |
| 22 | 2-Furanmethanol, acetate | 39.214 | 0.36 |
| 23 | Phosphonoacetic Acid, 3TMS derivative | 39.311 | 0.56 |
| 24 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 39.570 | 1.00 |
| 25 | Fluoren-9-ol, 3,6-dimethoxy-9-(2-phenylethynyl)- | 39.626 | 1.30 |
| 26 | Phenol, 2-methoxy- | 40.193 | 0.38 |
| 27 | 2-Furancarboxaldehyde, 5-methyl- | 41.062 | 3.20 |
| 28 | Methyl 2-furoate | 41.193 | 0.39 |
| 29 | Benzonitrile | 42.701 | 0.49 |
| 30 | 2-Acetyl-5-methylfuran | 42.850 | 0.40 |
| 31 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | 43.034 | 0.85 |
| 32 | 2-Cyclopenten-1-one, 3,4,4-trimethyl- | 43.192 | 0.68 |
| 33 | 2-Furanmethanol | 44.471 | 1.63 |
| 34 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 45.810 | 0.52 |
| 35 | 2-Hexene, 3,4,4-trimethyl- | 47.599 | 0.54 |
| 36 | Oxime -, methoxy-phenyl -_ | 47.787 | 1.19 |
| 37 | Cyclotrisiloxane, hexamethyl- | 49.496 | 0.22 |
| 38 | 3,4-Dimethoxytoluene | 50.546 | 0.89 |
| 39 | Phenol, 2-methoxy- | 52.743 | 8.87 |
| 40 | Phenol, 2-methoxy-3-methyl- | 53.120 | 0.90 |
| 41 | Phenol, 2,6-dimethyl- | 54.274 | 0.90 |
| 42 | 2-Methoxy-5-methylphenol | 55.354 | 0.57 |
| 43 | Creosol | 55.753 | 7.00 |
| 44 | 1,4-Benzenediol, 2,3,5-trimethy | 56.037 | 1.20 |
| 45 | Phenol, 2-methyl- | 56.832 | 3.99 |
| 46 | Phenol | 56.937 | 3.13 |
| 47 | Phenol, 4-ethyl-2-methoxy- | 57.670 | 6.94 |
| 48 | Benzene, 1,2,3-trimethoxy-5-methyl | 57.962 | 0.75 |
| 49 | Phenol, 2-ethyl- | 58.426 | 0.62 |
| 50 | Phenol, 2,4-dimethyl- | 58.615 | 1.15 |
| 51 | Phenol, 2,4-dimethyl- | 58.694 | 3.15 |
| 52 | p- Cresol | 58.923 | 2.87 |
| 53 | Phenol, 2-methoxy-4-propyl- | 59.459 | 1.12 |
| 54 | Phenol, 2,3-dimethyl- | 60.171 | 0.60 |
| 55 | Phenol, 2-ethyl-6-methyl- | 60.480 | 0.45 |
| 56 | 3-Ethylphenol, isopropyl ether | 60.740 | 1.63 |
| 57 | Phenol, 3-ethyl- | 60.899 | 0.47 |
| 58 | Phenol, 2-ethyl- | 61.780 | 0.56 |
| 59 | Phenol, 2,6-dimethoxy- | 62.796 | 1.03 |
| 60 | Caffeine | 63.316 | 2.55 |
| 61 | 3,5-Dimethoxy-4-hydroxytoluene | 64.674 | 1.18 |
| 62 | 5-tert-Butylpyrogallol | 65.992 | 0.57 |
| C mg/l |
Abs λ=750nm |
A |
|---|---|---|
| 100 | 0.21 | 0.22 |
| 0.24 | ||
| 0.22 | ||
| 200 | 0.41 | 0.40 |
| 0.40 | ||
| 0.38 | ||
| 300 | 0.55 | 0.55 |
| 0.55 | ||
| 0.56 | ||
| 400 | 0.77 | 0.77 |
| 0.77 | ||
| 0.78 | ||
| 500 | 0.96 | 0.96 |
| 0.97 | ||
| 0.96 | ||
| 600 | 1.17 | 1.16 |
| 1.14 | ||
| 1.16 |
| Sample | Laboratory code | Abs λ=750nm | C (mg/l) |
F.D. | mg Eq . AG/l sample | A |
|---|---|---|---|---|---|---|
| pyroligneous acid | INV 154 | 0.52 | 267.96 | 61.00 | 16345.56 | 16345.56 |
| 0.52 | 267.96 | 61.00 | 16345.56 | |||
| 0.52 | 267.96 | 61.00 | 16345.56 |
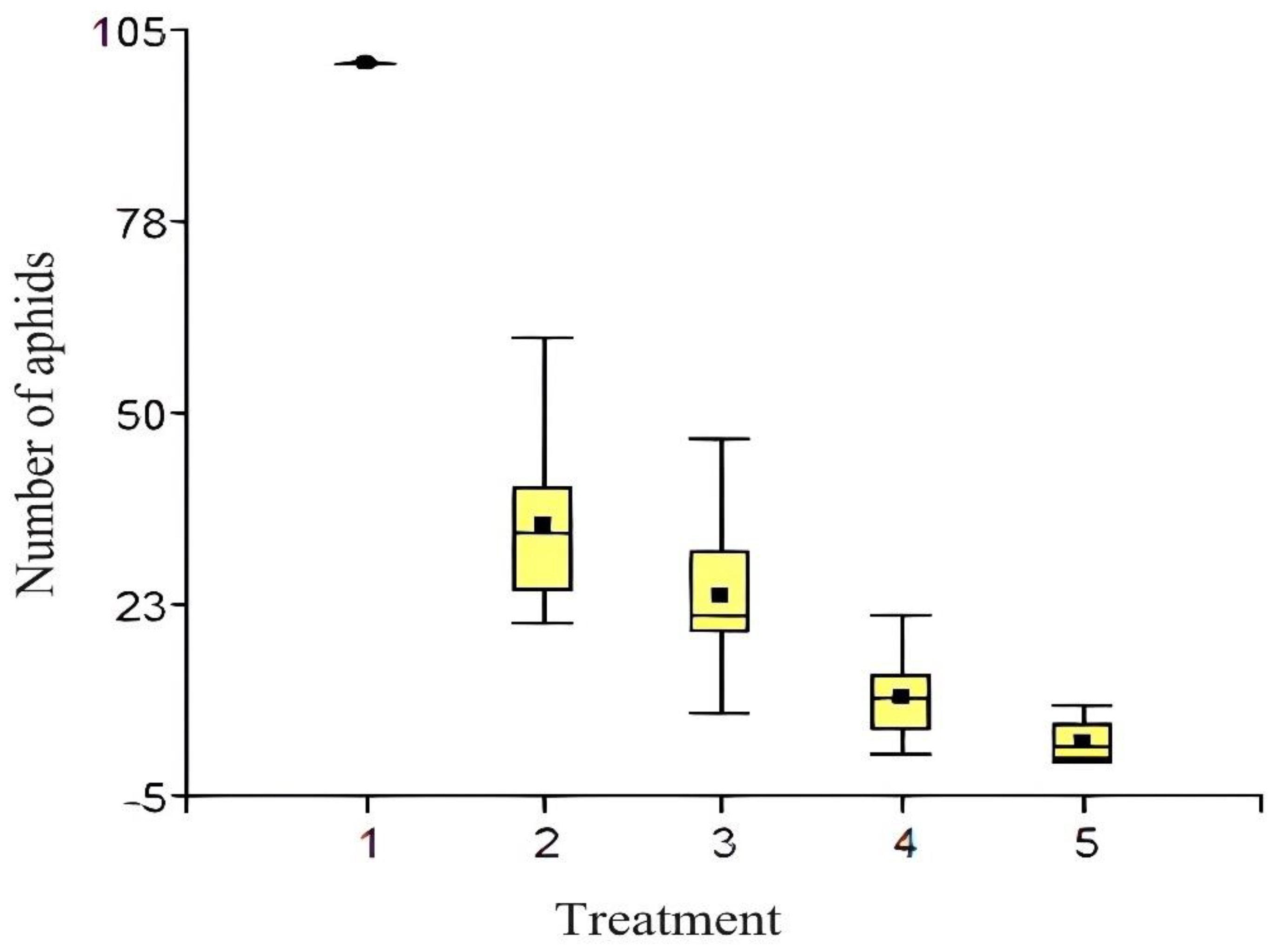
| Treatment | Number of aphids | Number of outbreaks evaluated | ||||
|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 4 | Block 5 | ||
| 1 | 100 | 100 | 100 | 100 | 100 | 4 |
| 2 | 8.5 | 6.91 | 10 | 10.5 | 6.66 | 4 |
| 3 | 4.75 | 5.75 | 7.5 | 6.83 | 3.91 | 4 |
| 4 | 2.66 | 2.58 | 2.41 | 1.66 | 1.91 | 4 |
| 5 | 0.33 | 0.83 | 0.75 | 0.58 | 0.75 | 4 |
| Variance analysis | |||||
| Variable | N | R2 | R2Acd | CV | |
| Number of aphids | 75 | 0.96 | 0.96 | 22.02 | |
| Analysis of Variance Table (SC type III) | |||||
| F.V. | S.C. | df | CM | F | p-value |
| Model | 91166.48 | 4 | 22791.62 | 410.95 | <0.0001 |
| Treatment | 91166.48 | 4 | 22791.62 | 410.95 | <0.0001 |
| Mistake | 3882.27 | 70 | 55.46 | ||
| Total | 95048.75 | 74 | |||
| Treatment | Measures | n | SE | ||||
|---|---|---|---|---|---|---|---|
| 5 | 2.60 | 15 | 1.92 | a | |||
| 4 | 9.00 | 15 | 1.92 | a | |||
| 3 | 23.47 | 15 | 1.92 | b | |||
| 2 | 34.07 | 15 | 1.92 | c | |||
| 1 | 100.00 | 15 | 1.92 | d | |||
| Means with a common letter are not significantly different (p > 0.05) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




