Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
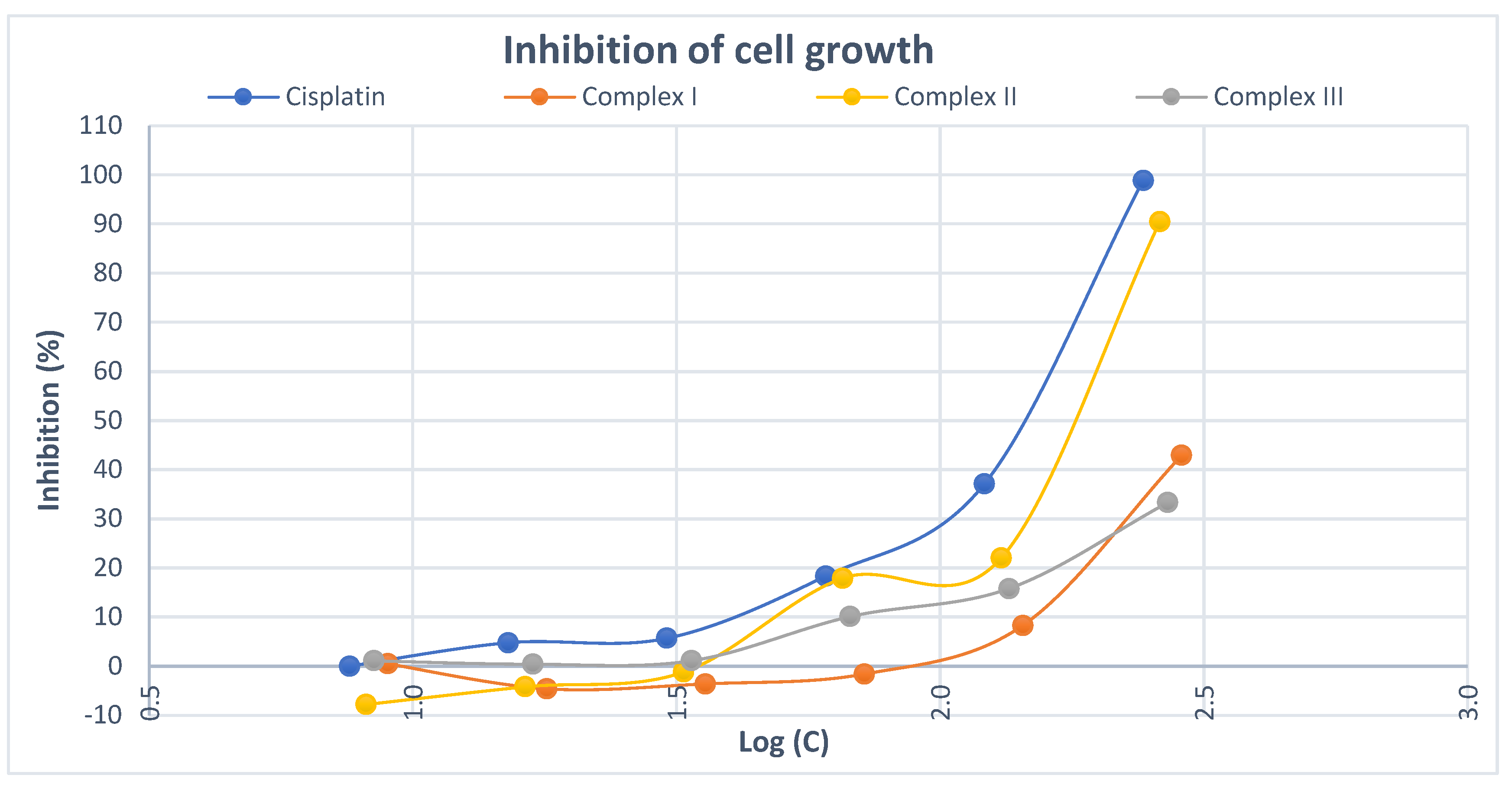
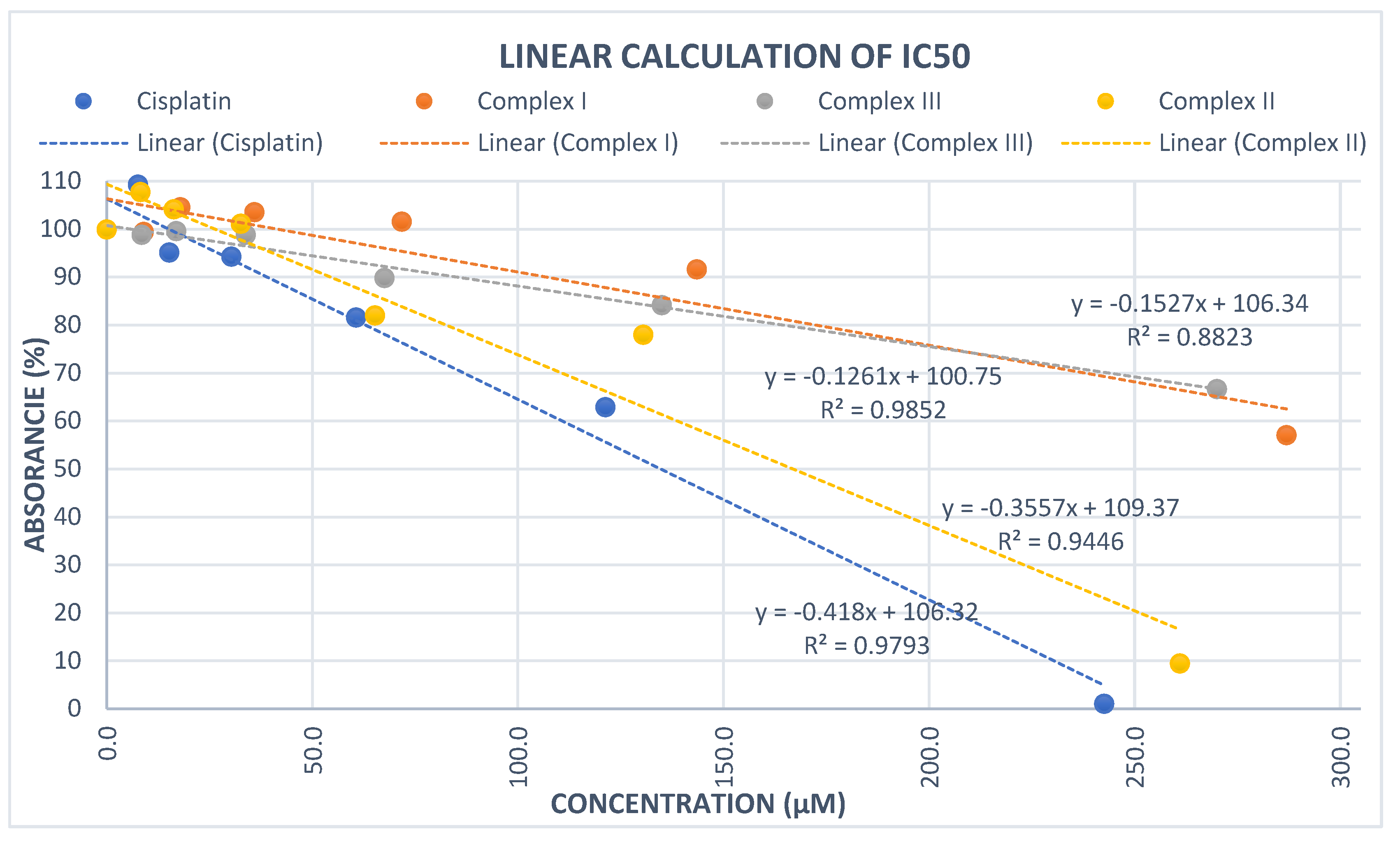
2. Results and discussion
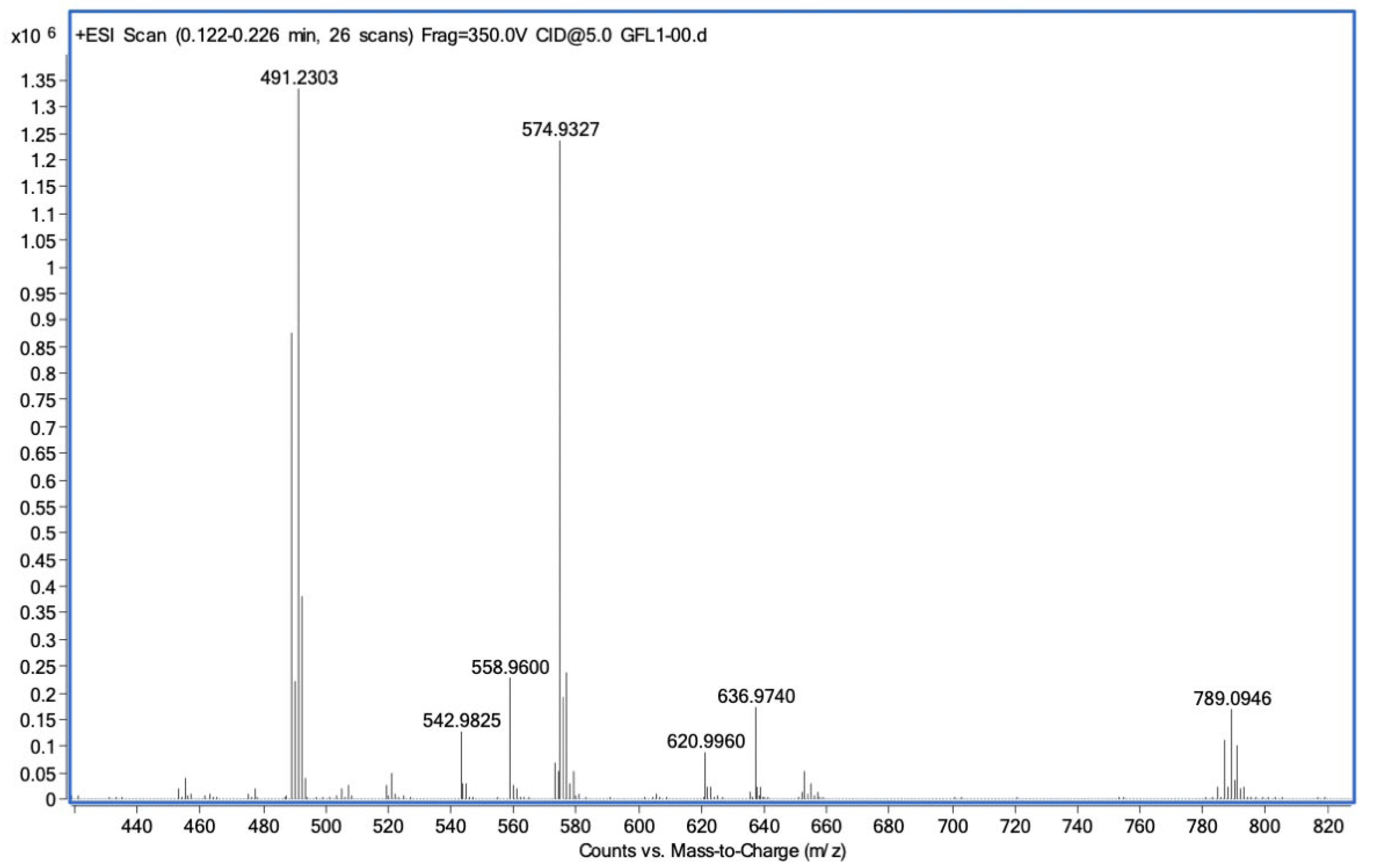
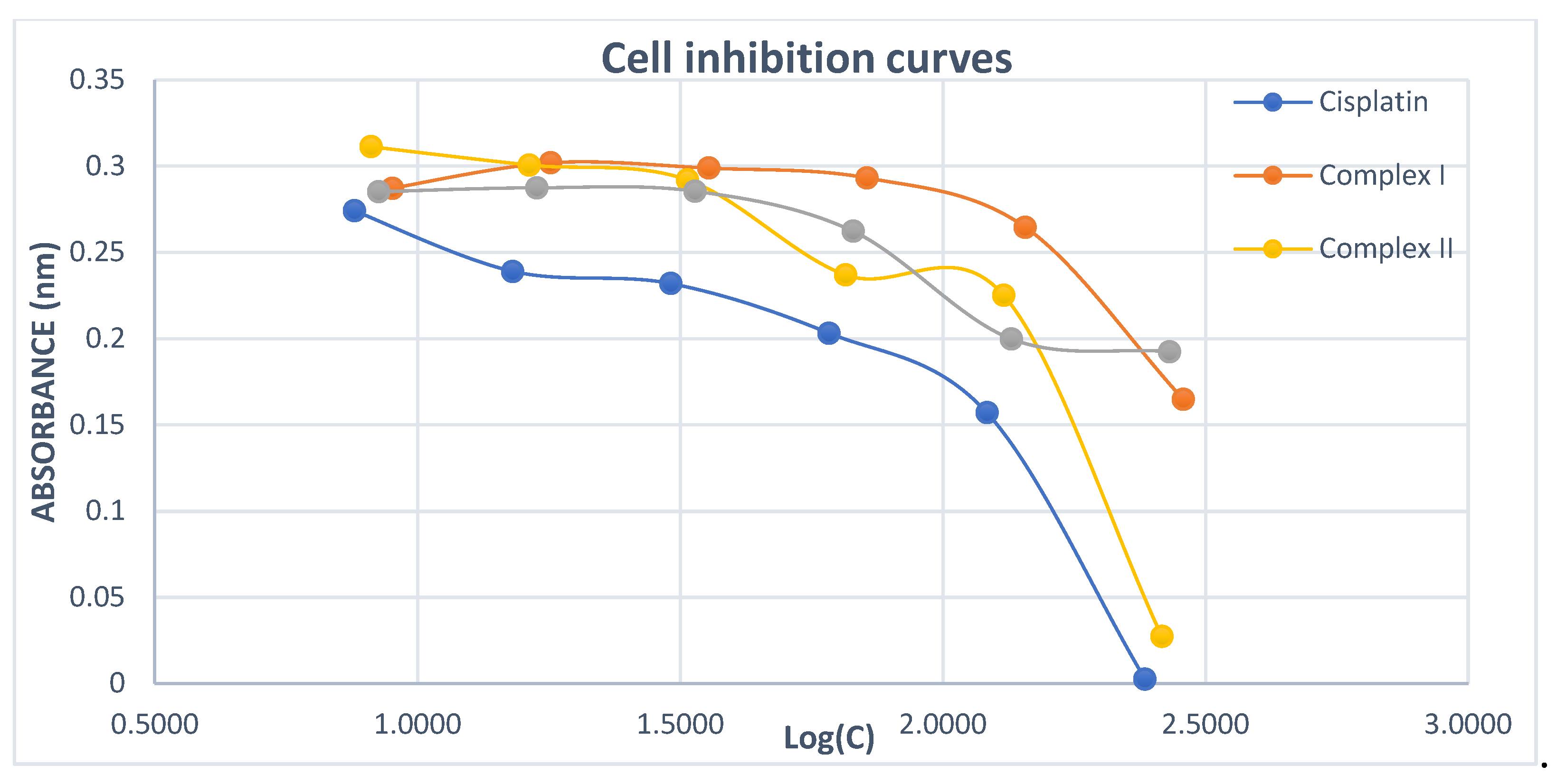
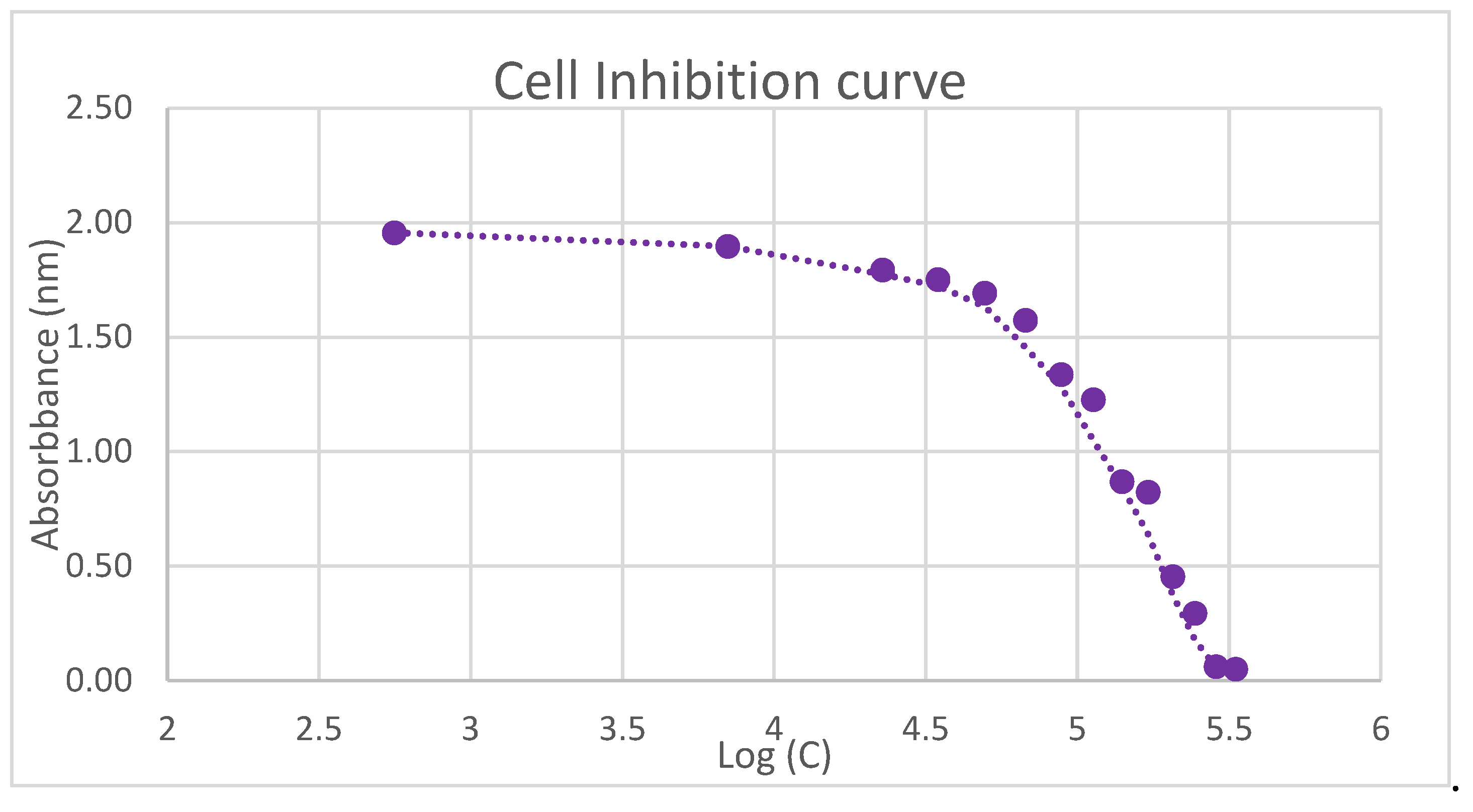
Cytotoxicity of Complex IV
| Concentration (μM) | Absorbance (%) |
|---|---|
| 250 | 2,7 |
| 234 | 3,3 |
| 218 | 15,4 |
| 203 | 23,8 |
| 187 | 43,1 |
| 172 | 45,6 |
| 156 | 64,4 |
| 140 | 70,0 |
| 125 | 82,5 |
| 109 | 88,7 |
| 94 | 91,9 |
| 78 | 94,0 |
| 47 | 99,5 |
| 16 | 100 |
| 0 | 100 |
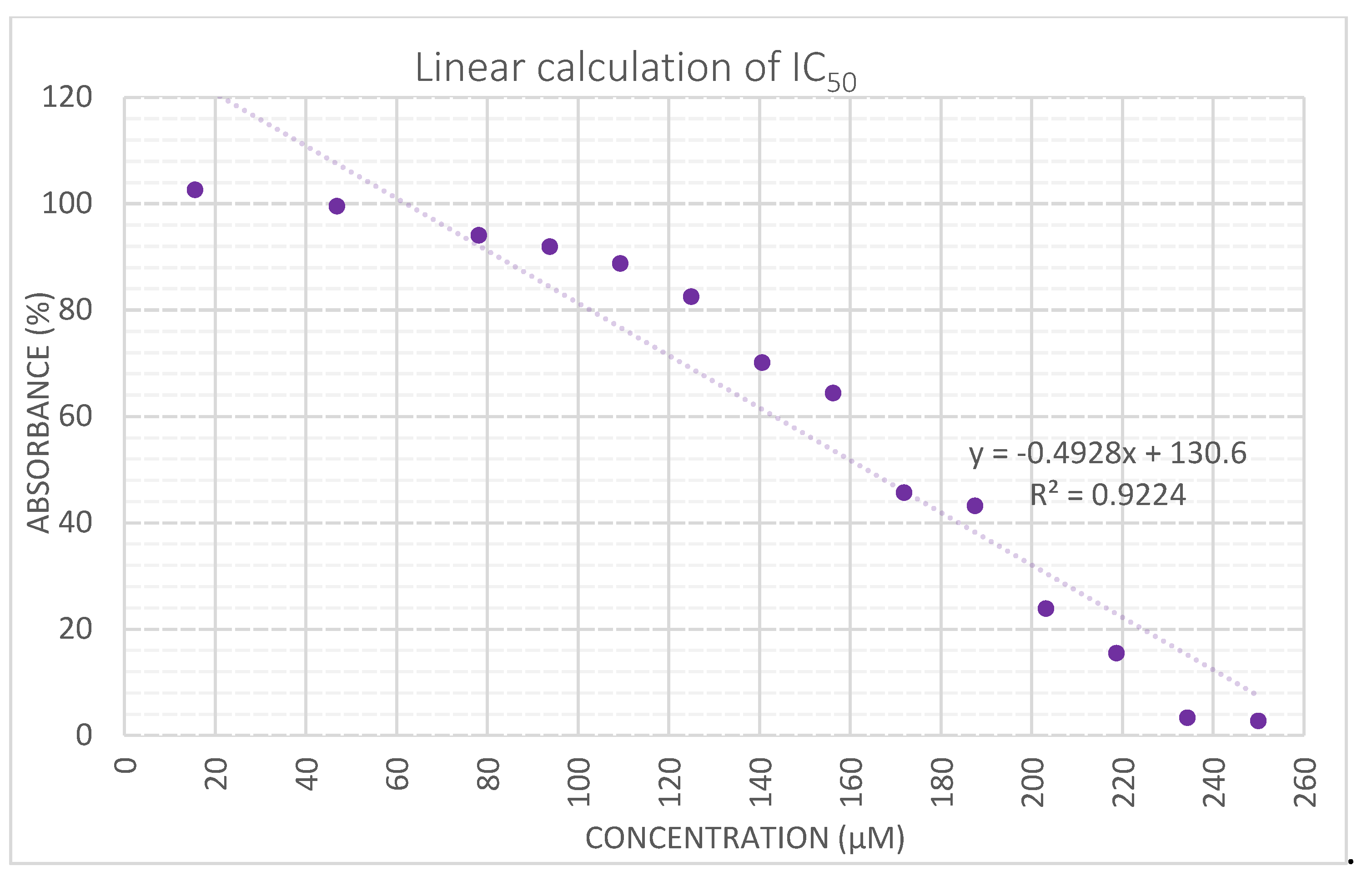
3. Materials and Methods.
Instrumentation:
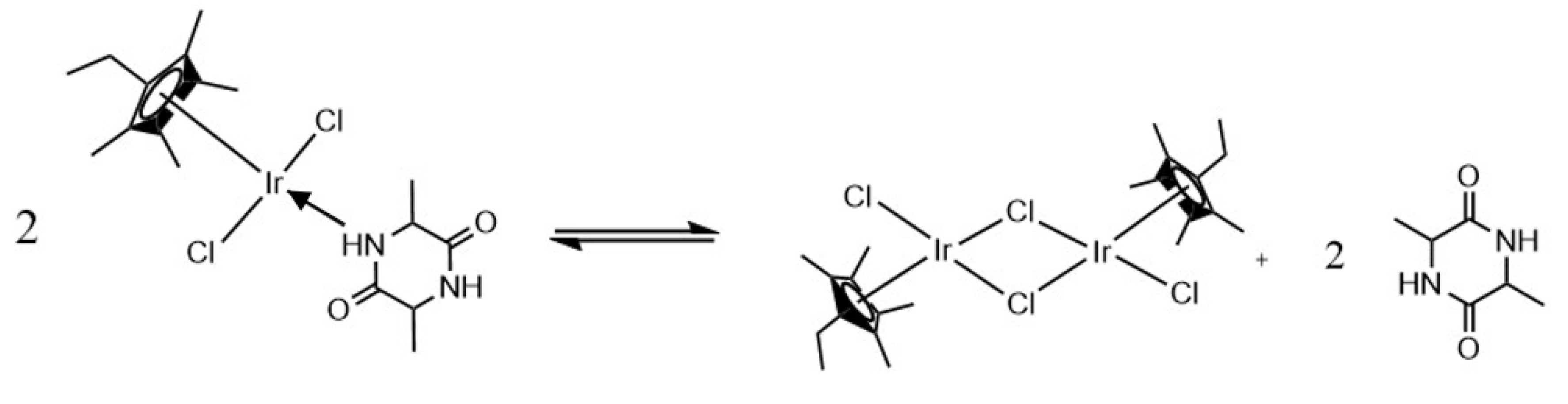
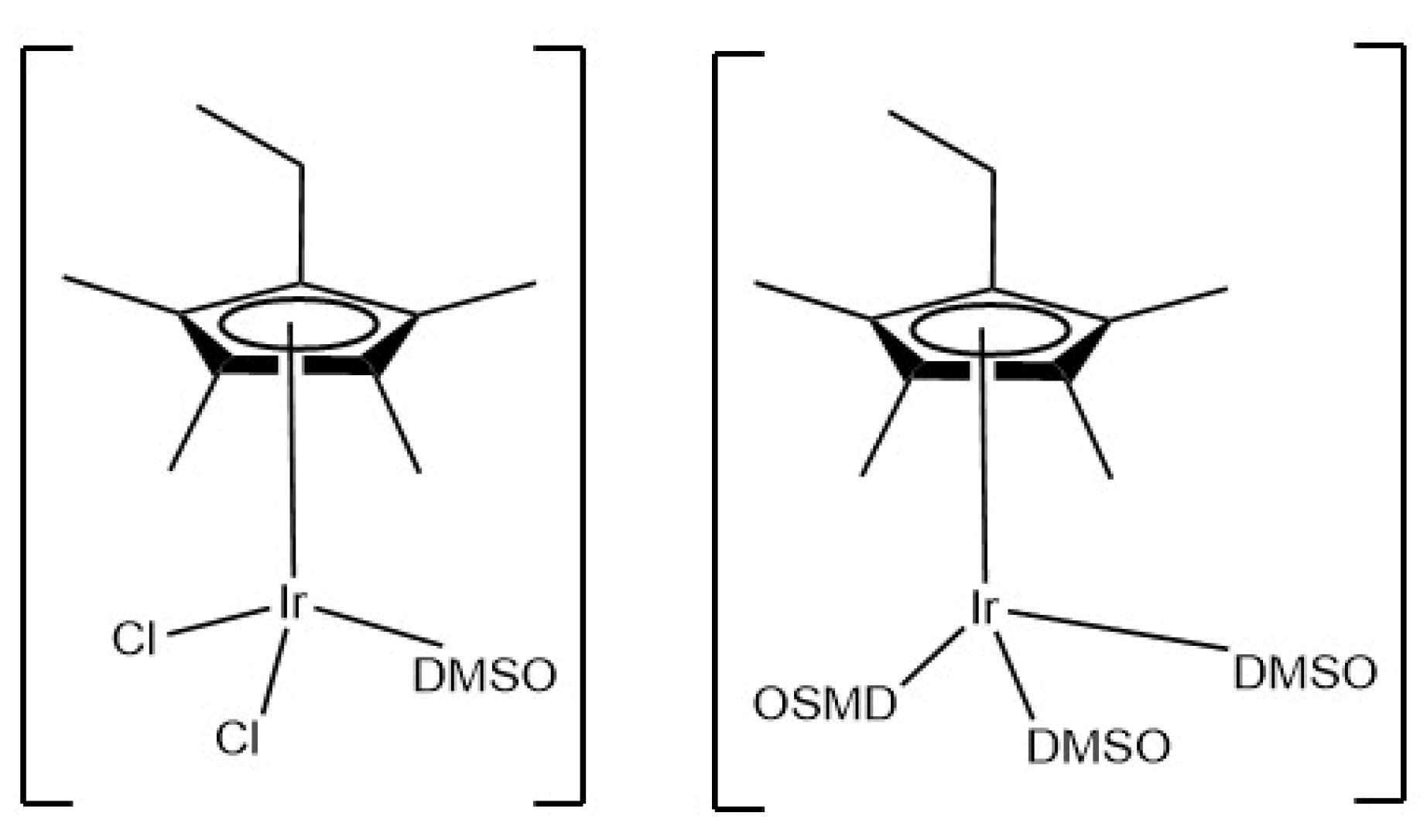
Synthesis of the Complexes
4. CONCLUSIONS
Funding
References
- L. Kelland, The resurgence of platinum-based cancer chemotherapy Nat. Rev. Cancer, 2007, 7, 573-584.
- D. Wang and S. J. Lippard, Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery, 2005, 4, 307–320.
- DL Ma, M Wang, Z Mao, C Yang, CT Ng, CH Leung. Rhodium complexes as therapeutic agents. Dalton Trans., 2016, 45, 2762–2771.
- G. S. Yellol, A. Donaire, V. Vasylyeva, C. Janiak, and J. Ruiz, On the antitumor properties of novel cyclometalated benzimidazole Ru (II), Ir (III) and Rh (III) complexes Chem. Commun., 2013, 49, 11533–11535.
- C. H. Leung, H. J. Zhong, D. S. H. Chan and D. L. Ma, Bioactive iridium and rhodium complexes as therapeutic agentsCoord. Chem. Rev., 2013, 257, 1764–1776.
- J. P. C. Coverdale, I. Romero-Canelon, C. Sanchez-Cano, G. J. Clarkson, A. Habtemarian, M. Wills and P. J. Sandler. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells, Nat. Chem., 2018, 10, 347–354.
- J. M. Hearn, I. Romero-Canelon, B. Qamar, Z. Liu, I. Hands-Portman and P. J. Sandler, The Potent Oxidant Anticancer Activity of Organoiridium, Catalysts, ACS Chem. Biol, 2013, 8, 1335–1343.
- W. Liu and R. Gust, Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs, Chem. Soc. Rev., 2013, 42, 755–773.
- G. Gasser, I. Ott and N. Metzler-Nolte, Organometallic Anticancer Compounds, J. Med. Chem., 2013, 56, 1291–1300.
- C. G. Hartinger, N. Metzler-Nolte and P. J. Dyson, Ruthenium(II)−Arene RAPTA Type Complexes Containing Curcuminand Bisdemethoxycurcumin Display Potent and Selective Anticancer Activity Organometallics, 2012, 31, 5677–5685.
- Romero-Canelon, L. Salassa and P. J. Sadler. The Contrasting Activity of Iodido versus Chlorido Ruthenium andOsmium Arene Azo- and Imino-pyridine Anticancer Complexes: Control of Cell Selectivity, Cross-Resistance, p53 Dependence, andApoptosis. Pathway J. Med. Chem., 2013, 56, 1291–1300. [Google Scholar] [CrossRef]
- F. J. Ballester, E. Ortega, V. Porto, H. Kostrhunova, N. Davila-Ferreira, D. Bautista, V. Brabec, F. Dominguez, M. D. Santana and J. Ruiz, New half-sandwich ruthenium(II) complexes as proteosynthesis inhibitors in cancer cells, Chem. Commun., 2019, 55, 1140–1143.
- W. X. Ni, W. L. Man, S. M. Yiu, M. Ho, M. T. W. Cheung, C. C. Ko, C. M. Che, Y. W. Lam and T. C. Lau, Halide Control of N, N-Coordination versus N, C-Cyclometalation and Stereospecific Phenyl Ring Deuteration of Osmium(II) p-CymenePhenylazobenzothiazole Complexes, Chem. Sci., 2012, 3, 1582–1588.
- E. Ortega, F. Ballester, A. Hernandez-Garcia, S. Hernandez-Garcia, M. A. Guerrero-Rubio, D. Bautista, M. D. Santana and J. Ruiz, Inorg. Chem. Front., 2021, 8, 141–155.
- J. C. Dabroviak, “Metals in Medicine”, John, Wiley and Sons Ltd, Chichester, 2009, p. 149.
- O. Domotorr, V. F. S Pape, N. V. May, G. Szakac and E. A. Enyedy, Comparative solution equilibrium studies of antitumor ruthenium (η6 -p-cymene) and rhodium (η5 -C5Me5) complexes of 8-hydroxyquinolines, Dalton Trans., 2017, 46, 4382–4396.
- Z. Liu, L. Salassa, A. Habtemarian, A. M. Pizarro, G. J. Clarkson and P. J. Sadler, Potent Half-Sandwich Iridium(III) Anticancer Complexes Containing C∧N-Chelated and Pyridine Ligands, Inorg. Chem., 2011, 50, 5777–5783.
- Z. Zhu, Z. Wang, Y. Hao, C. Zhu, J. Jiao, H. Chen, Y. –M. Wang, J. Yan, Z. Guo and X. Wang, Glutathione boosting the cytotoxicity of a magnetic platinum(IV) nano-prodrug in tumor cells, Chem. Sci., 2016, 7, 2864–2869.
- S. Dilruba and G. V. Kalayda, Platinum-based drugs: past, present and future, Cancer Chemother. Pharmacol, 2016, 77, 1103–1124.
- C. Johnstone T, K. Suntharalingam and J. S. Lippard, The next generation of platinum drugs: targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs, Chem. Rev., 2016, 116, 3436–3486.
- C. G. Hartinger, N. Metzler-Nolte and P. J. Dyson, Challenges and opportunities in the development of organometallic anticancer drugs, Organometallics, 2012, 31, 5677–5685.
- Z. Liu, I. Romero-Canelon, B. Qamar, J. M. Hearn, A. Habtemariam, N. P. Barry, A. M. Pizarro, G. K. Clarkson and P. J. Sadler, The Potent Oxidant Anticancer Activity of Organoiridium Catalysts, Angew. Chem., Int. Ed., 2014, 53, 3941–3946.
- Y. Li, C. P. Tan, W. Zhang, L. He, L. N. Ji and Z. W. Mao, Phosphorescent iridium(III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents, Biomaterials, 2015, 39, 95–104.
- Z. Liu, A. Habtemariam, A. M. Pizarro, S.a. Fletcher, A. Kisova, O. Vrana, L. Salassa, P. C. A. Buijninex, G. J. Clarkson, V. Brabec, and P. J. Sadler, Organometallic Half-Sandwich Iridium Anticancer Complexes, J. Med. Chem., 2011, 54, 3011–3026.
- C. Wang, J. Liu, Z. Tian, M. Tian, L. Tian, W. Zhao and Z. Liu, Half-sandwich iridium N-heterocyclic carbene anticancer complexes, Dalton Trans., 2017, 46, 6870–6883.
- L. He, C. P. Tan, R. R. Ye, Y. Z. Yao, Y. H. Liu, Q. Zhao, L. N. Ji and Z. W. Mao, L. He, C. P. Tan, R. R. Ye, Y. Z. Yao, Y. H. Liu, Q. Zhao, L. N. Ji and Z. W. Mao, Half-sandwich iridium N-heterocyclic carbene anticancer complexes, Angew. Chem., Int. Ed., 2014, 53, 12137–12141.
- J. M. Hearn, I. Romero-Canelon, B. Qamar, Z. Liu, I. Hands-Portman and P. J. Sadler, Organometallic Iridium(III) Anticancer Complexes with New Mechanisms of Action: NCI-60 Screening, Mitochondrial Targeting, and Apoptosis, ACS Chem. Biol., 2013, 8, 1335–1343.
- V. Novohradsky, L. Zerzankova, J. Stepankova, A. Kisova, H. Kostrhunova, Z. Liu, P. J. Sadler, J. Kasparkova and V. Brabec, A dual-targeting, apoptosis-inducing organometallic half-sandwich iridium anticancer complex, Metallomics, 2014, 6, 1491–1501.
- Z. Liu, A. Habtemarian, A. Pizarro, G. J. Clarkson and P. J. Sadler, Organometallic iridium (III) cyclopentadienyl anticancer complexes containing C, N-chelating ligands, Organometallics, 2011, 30, 4702–4710.
- Z. Liu, V. Lebrun, T. Kitanosono, H. Mallin, V. Köler, D. Häussinger, D. Hilvert, S. Kobayashi and T. R. Ward, Upregulation of an Artificial Zymogen by Proteolysis, Angew. Chem., Int. Ed., 2016, 11587-11590.
- Z. Liu, L. Salassa, A. Habtemariam, A. M. Pizarro, G. J. Clarkson and P. J. Sadler, Contrasting Reactivity and Cancer Cell Cytotoxicity of Isoelectronic Organometallic Iridium(III) Complexes, Inorg. Chem., 2011, 50, 5777–5783.
- L. He, Y. Li, C.-P. Tan, R.-R. Ye, M.-Chen, J.-J. Cao, L.-N. Ji and Z.-W. Mao, Cyclometalated iridium(III) complexes as lysosome-targeted photodynamic anticancer and real-time tracking agents, Chem. Sci, 2015, 2015, 6–5401.
- Z. Liu and P. J. Sadler, Formation of glutathione sulfenate and sulfinate complexes by an organoiridium (III) anticancer complex, Inorg. Chem. Front., 2014, 1, 668–672.
- Z. Liu, Z. Romero-Canelon, A. Habtemariam, G. J. Clarkson and P. J. Sadler, Potent Half-Sandwich Iridium(III) Anticancer Complexes Containing C∧N-Chelated and Pyridine Ligands, Organometallics, 2014, 33, 5324–5333.
- G. Giambastiani, L. Tuconi, R. L. Kuhlman and P. D. Hustad, “Imino-and Amido-Pyridinate d-Block Metal Complexes in Polymerization/Oligomerization Catalysis, in Olefin Upgrading Catalysis by Nitrogen-based Metal Complexes I. Springer 2011, 34, 197–281.
- X. Xiong, L.-Y. Liu, Z.-W. Mao, T. Zou, Approaches Towards Understanding the Mechanism-of-action of Metallodrugs, Coord. Chem. Rev., 2022, 453, 214311.
- K. Peng, Y. Zheng, W. Xia and Z.-W. Mao, Organometallic anti-tumor agents: targeting from biomolecules to dynamic bioprocesses, Chem. Soc. Rev., 2023, 52, 2790–2832.
- Kastner, T. Mendrina, F. Bachmann, W. Berger, B. K. Keppler, P. Heffeter and C. R. Kowol. Tumor-Targeted Dual-Action NSAID-Platinum(IV) Anticancer prodrugs. Inorg. Chem. Front., 2023, 10, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- G H. Ribeiro, A. R. Costa, A. R. de Souza, F. V. da Silva, F. T. Martins, A. M. Plutin and A. A. Batista, An Overview on the Anticancer Activity of Ru(II)/Acylthiourea Complexes, Coord. Chem. Rev., 2023, 488, 215161.
- J. Li and T. Chen, Transition Metal Complexes as Photosensitizers for Integrated Cancer Theranostic Applications, Coord. Chem. Rev., 2020, 418, 213355.
- X. He, L. Wei, J. Chen, S. Ge, M. Kandawa-Shultz, G. Shao and Y. Wang, Folate-targeted Iridium Complexes Amplify Photodynamic Therapy Efficacy Through Ferroptosis, Inorg. Chem. Front., 2023, 10, 4780–4788.
- H. Yuan, Z. Han, Y. Chen, F. Qi, H. Fang, Z. Guo, S. Zhang and W. He, Ferroptosis Photoinduced by New Cyclometalated Iridium(III) Complexes and Its Synergism with Apoptosis in Tumor Cell Inhibition, Angew. Chem., Int. Ed., 2021, 60, 8174–8181.
- X. Liu, A. Lv, P. Zhang, J. Chang, R. Dong, M. Liu, J. Liu, X. Huang, X-A. Yuan, Z. Liu. Dalton Trans., 2024, 53, 552–563.
- María Angeles Pujante-Galián, Sergio A. Pérez, Mercedes G. Montalbán, Guzmán Carissimi, Marta G. Fuster, Gloria Víllora, Gabriel García. P-Cymene Complexes of Ruthenium (II) as Antitumor agents. Molecules, 2020, 25, 5063–5077. [Google Scholar] [CrossRef] [PubMed]
- Marta G. Fuster, Imane Moulefera, Mercedes G. Montalban, José Pérez, Gloria Víllora and Gabriel García. Synthesis and Characterization of New Ruthenium (II) Complexes of Stoichiometry [Ru(p-Cymene) Cl2L] and Their Cytotoxicity against HeLa-Type Cancer Cells. Molecules, 2022, 27, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Natalia Sáez, Alfonso Canales-Martínez, Marta G. Fuster, Imane Moulefera, Delia Bautista, José Pérez,Gloria Víllora and Gabriel García, Synthesis and characterization of new iridium(III) complexes containing the fragment [Cp*IrCl2] and the ligands 2- and 4-aminobenzonitryl and 2- and 4-aminopyridine. J. Coord. Chem., 2024, 516-524. doi.org/10.1080/00958972.2024.
- Alfonso Canales-Martínez, Rosa María Pérez Pastor, Gloria Víllora and Gabriel García. Pure Appl Chem., 2024 (in the press).
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds (Part B), Sixth Edition, 2009, John Wiley & Sons, INC., Hooboken, New Jersey, USA.
- W. G. Geary, The Use of Conductivity Measurements in Organic Solvents for the Characterisation Compounds, Coord. Chem. Rew., 1971, 7, 81–122.
- Stockert, J. C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R. W.; Villanueva, A. ; MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochemica, 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- G Eisenbrand, B Pool-Zobel, V Baker, M Balls, B J Blaauboer, A Boobis, A Carere, S Kevekordes, J-C Lhuguenot, R Pieters, J Kleiner. ; Methods of in vitro toxicology, Food Chem Toxicol, 2002, 40, 193–236.
- Zoehler, A. Melo de Aguiar, and G. Ferreira Silveira; SAEDC: Development of a technological solution for exploratory data analysis and statistics in cytotoxicity. Pharmaceut. Statist. 2003, 2, 167–174. [Google Scholar]
- AAT Bioquest, Inc. (2022, June 26). Quest GrafTM IC50 Calculator. AAT Bioquest. Accessed 25 June 2022.
- Dooley, T.; Fairhurst, G.; Tiza, C. T.; Tabataian, K. ; Blanco. C.; Ethyltetramethylcyclopentadienyl complexes of cobalt, rhodium, iridium and ruthenium. Trans. Metal Chem. 1978, 3, 299–302. [Google Scholar]
- Bennett, M. A.; Huang, T. N.; Matheson, T. W.; Smith, K.; Inorganic Synthesis; John Wiley & Sons, Hoboken, NJ, USA, 1982, Volume 21, pp. 74-77.
| COMPLEX | COLOUR | YIELD | ANALYTICAL DATAA | MASS DATA | M.P.B | ||
| (%) | C | H | N | Fragments | |||
| I | Dark Orane |
63 | 35.39 (35.54) | 4.59 (4.70) | 8.17 (8.36) |
332 |
|
| II | Dark Orange | 45 | 38.90 (39.16) | 4.41 (4.58) | 2.53 (2.69) | 380 |
|
| III | Dark Orange | 41 | 38.94 (39.16) | 4.38 (4.58) | 2.67 (2.69) | 434 | |
| IV | Yellow | 92 | 31.06 (31.20) |
3.59 (3.73) | 4.53 (4.54) | 435.1399 [M-Cl]+ 421.1159 [M-PF6]+ |
377 |
| COMPLEX | V(N-H) | V(M-CL) | V(C=O) | V(O-H) | V(P-F) |
| I | 3317 s, 3190 s | 317 s, 278 s | 1690 s | - | - |
| II | 3365 s, 3196 s | 314 s, 281 s | - | 3601 s | - |
| III | 3456 s, 3359 s | 299 s, 278 s | - | 3601 s | - |
| IV | 3284 s, 3176 s | 287 s | - | - | 844 s, 559 s |
| COMPLEX | 1H δ(CDCL3) | LIGAND STRUCTURE |
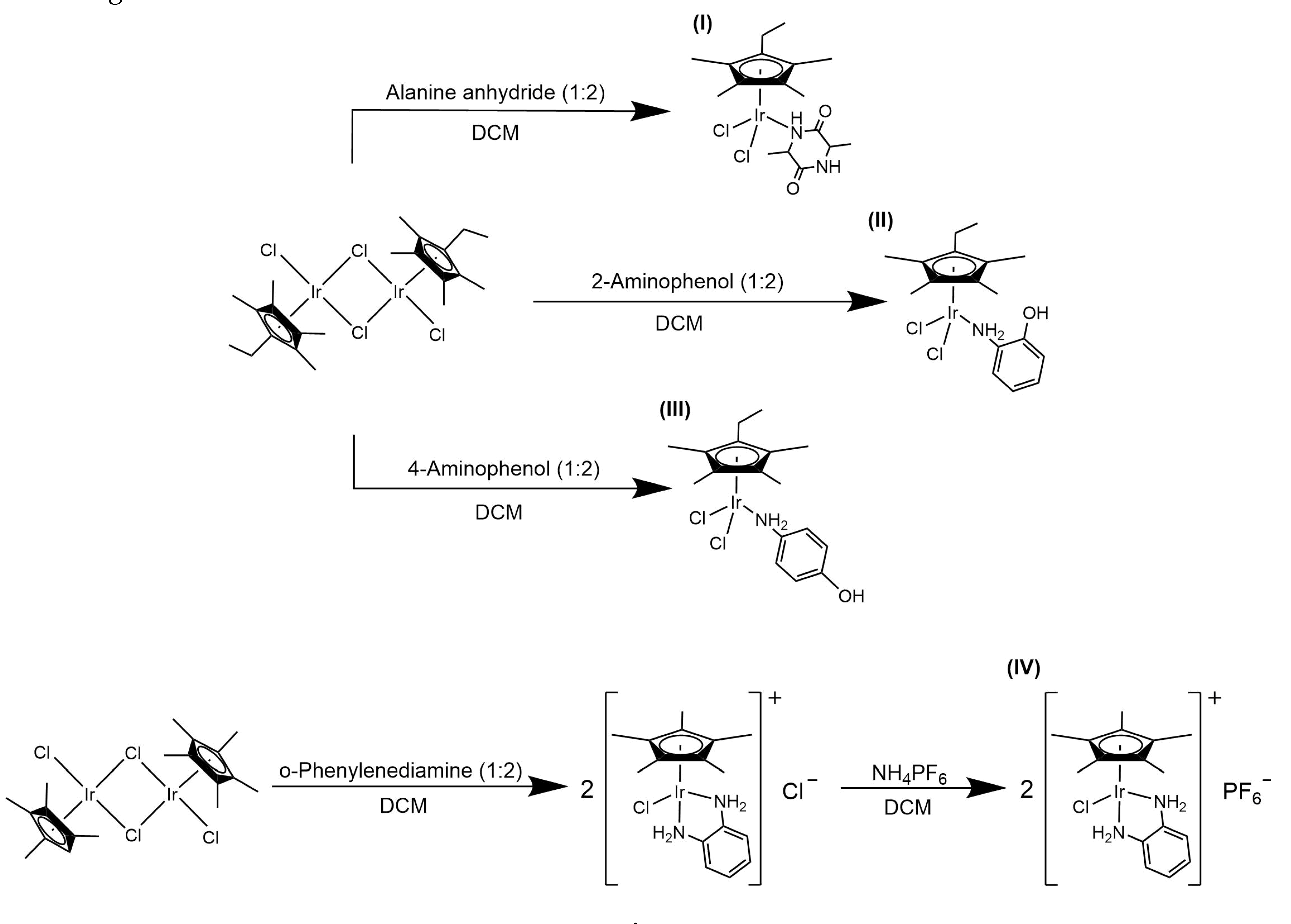
| I | 5.81 (d, 2H, J = 13.5 Hz, Ala-HNH) 4.11 (qt, 1H, J = 7.5 Hz, Ala-HF) 2.5 (qt, 2H, J = 7.5 Hz, Cpet-HB(-CH2(CH3)) 1.62 (s, 6H, Cpet-HD(-CH3)) 1.59 (s, 6H, Cpet-HC(-CH3)) 1.50 (d, 3H, J = 7.2 Hz, Ala-HE(-CH3)) 1.08 (t, 3H, J = 7.5 Hz, Cpet-HA(-CH2(CH3)) |
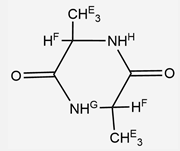 |
| II | 10.24 (s, 2H, H-NH2) 7.8 (s, 1H, H-OH) 7.03 (m, 2H, -HF) 6.89 (d, 1H, J = 7.2 Hz, -HI) 6.8 (t, 1H, J = 7.2 Hz, -HH) 2.08 (qt, 2H, J = 7.2 Hz, Cpet-HB(-CH2(CH3)) 1.82 (s, 6H, Cpet-HD(-CH3)) 1.66(s, 6H, Cpet-HC(-CH3)) 1.06 (t, 3H, J = 8 Hz, Cpet-HA(-CH2(CH3)) |
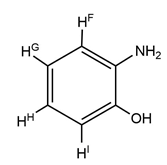 |
| III | 9.39 (s, 1H, H-OH) 8.44 (s, 2H, H-NH2) 7.03 (d, 2H, J = 8.8 Hz, -HF) 6.71 (d, 2H, J = 8.4 Hz, -HE) 2.35 (q, 2H, J = 7.2 Hz, HB(-CH2(CH3)) 1.81 (s, 6H, HD) 1.79 (s, 6H, HC) 1.03 (t, 3H, J = 3.6 Hz, HA(-CH2(CH3)) |
 |
| IV | 7.31 (m, 2H, HA) 7.20(m, 2H, HB) 4.52 (s, 2H, -NH2) 1.83 (s, 15H, -HCp*) |
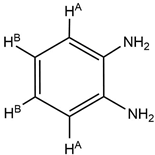 |
| Complex | IC50 (μM) |
| I | 368.9587 |
| II | 166.9103 |
| III | 402.4583 |
| cisplatin | 134.7368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





