Submitted:
01 June 2025
Posted:
09 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
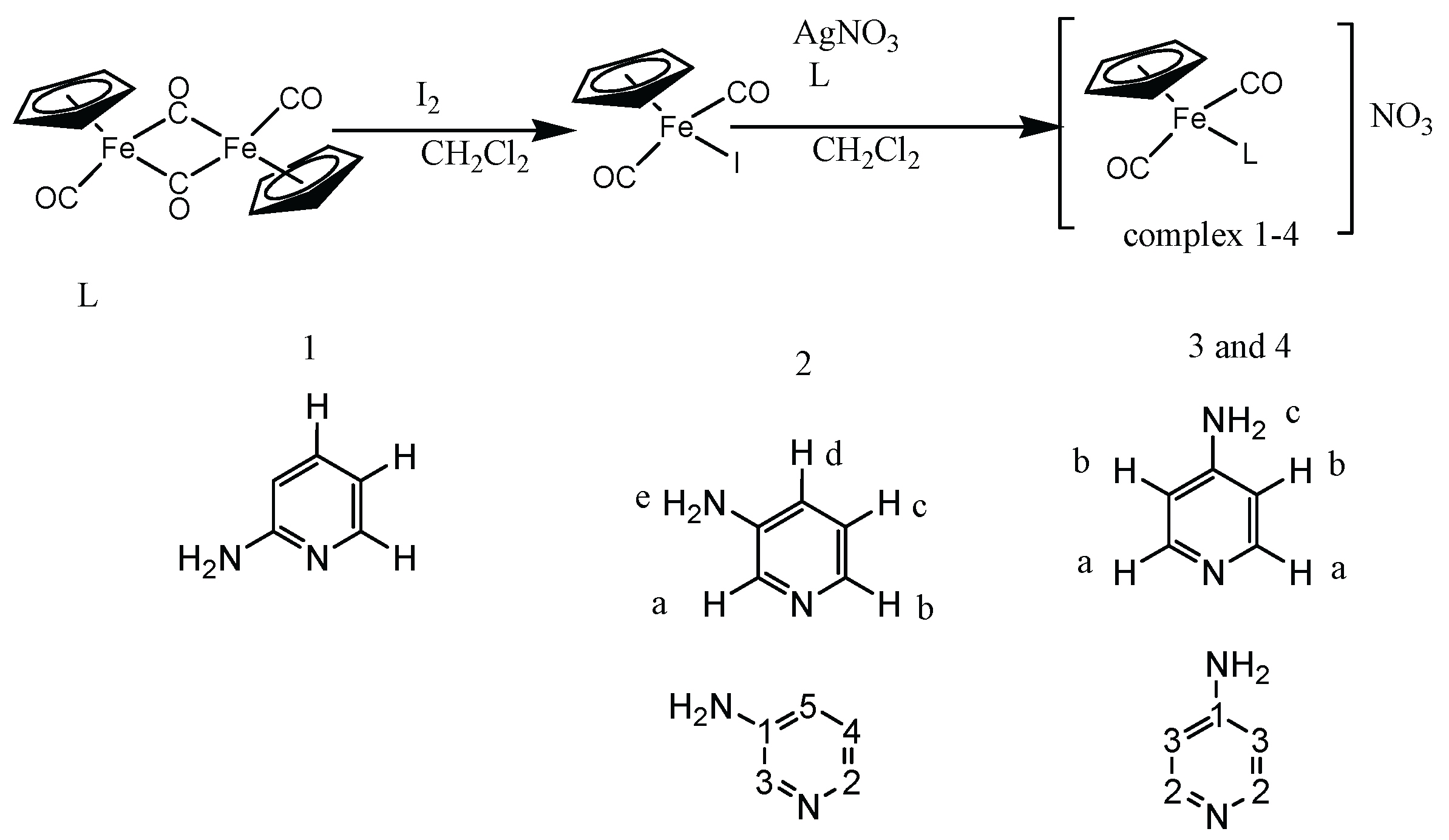
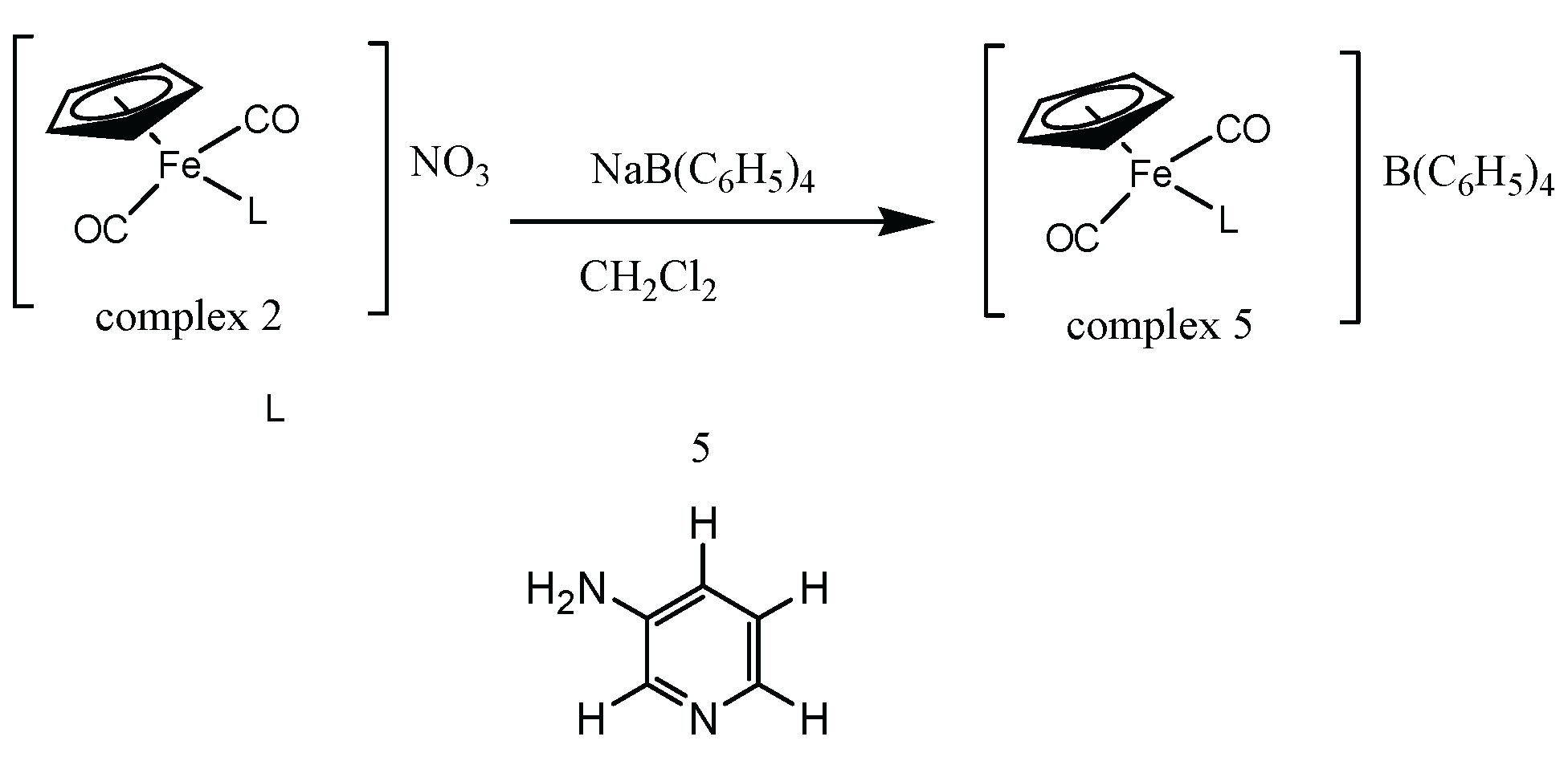
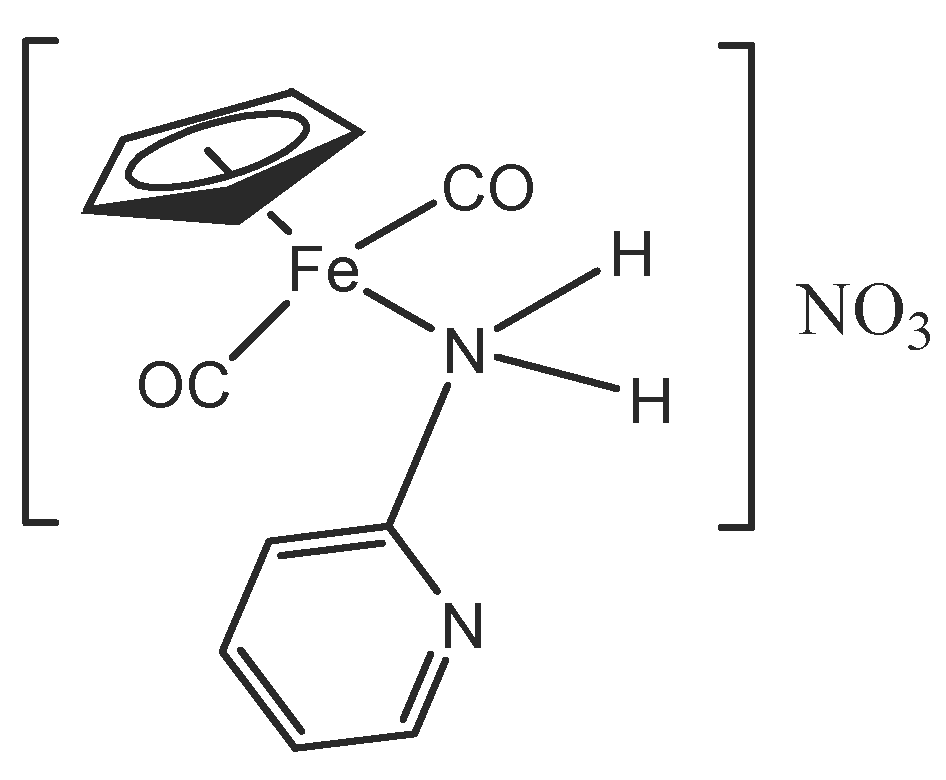
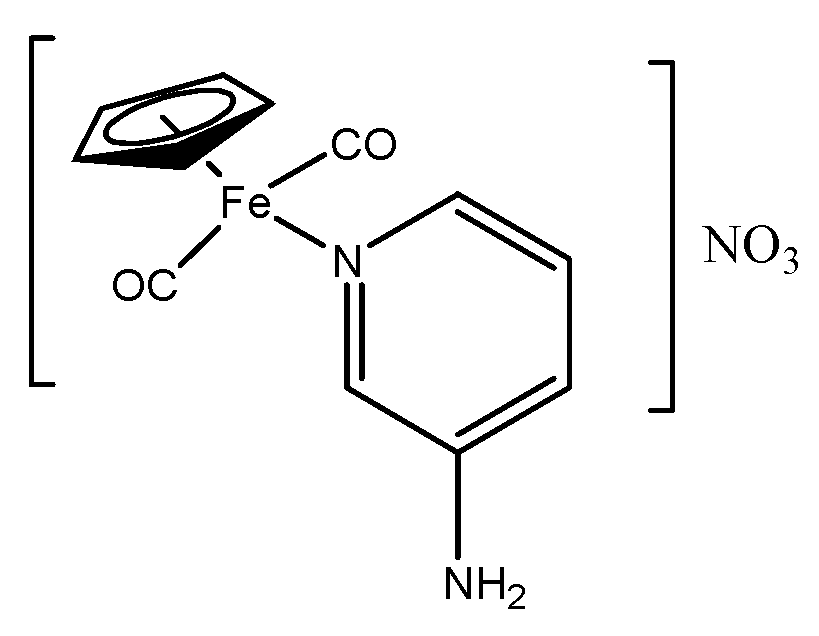
2.1. Synthesis and Characterization of Aminopyridine Complexes
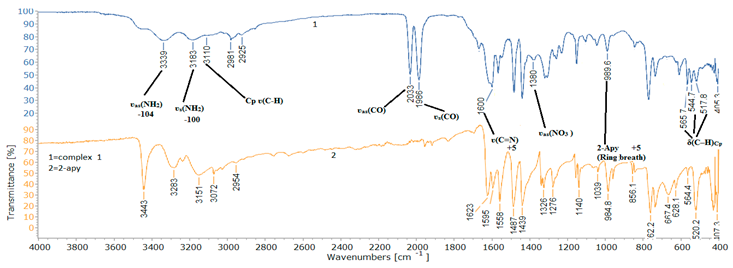
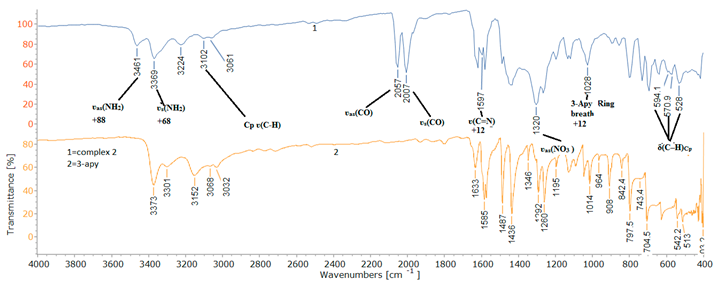
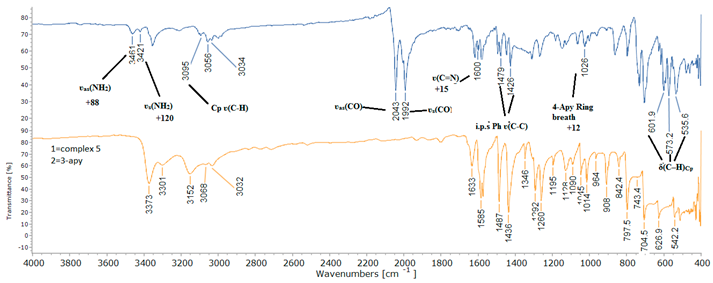
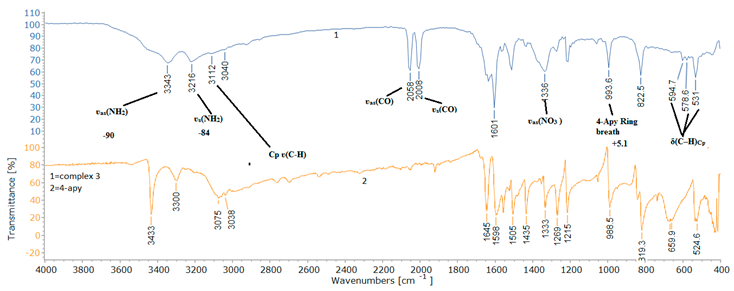
2.2. Spectroscopic Characterization
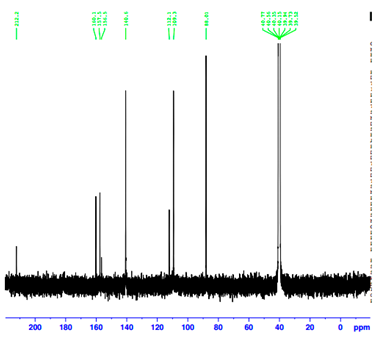
| Assignment | 4-Apy | [(η5-C5H5)Fe(CO)2(4-Apy)]NO3(B) | ∆ |
|---|---|---|---|
| C(1) | 154.2 | 160.2 | +6.0 |
| C(2) | 149.4 | 156.5 | +7.1 |
| C(3) | 108.8 | 112.2 | +2.4 |
| CCp | - | 88.0 | - |
| CO | - | 212.2 | - |
3. Materials and Methods
3.1. Materials for Synthetic Work
3.2. Experimental Methods3.3. General
3.4. Synthesis of the Iodo Complex [(ɳ5-C5H5)Fe(CO)2I]
3.5. Synthesis of [(ɳ5-C5H5)Fe(CO)2(2-Apy)]NO3
3.6. Synthesis of [(ɳ5-C5H5)Fe(CO)2(3-Apy)]NO3
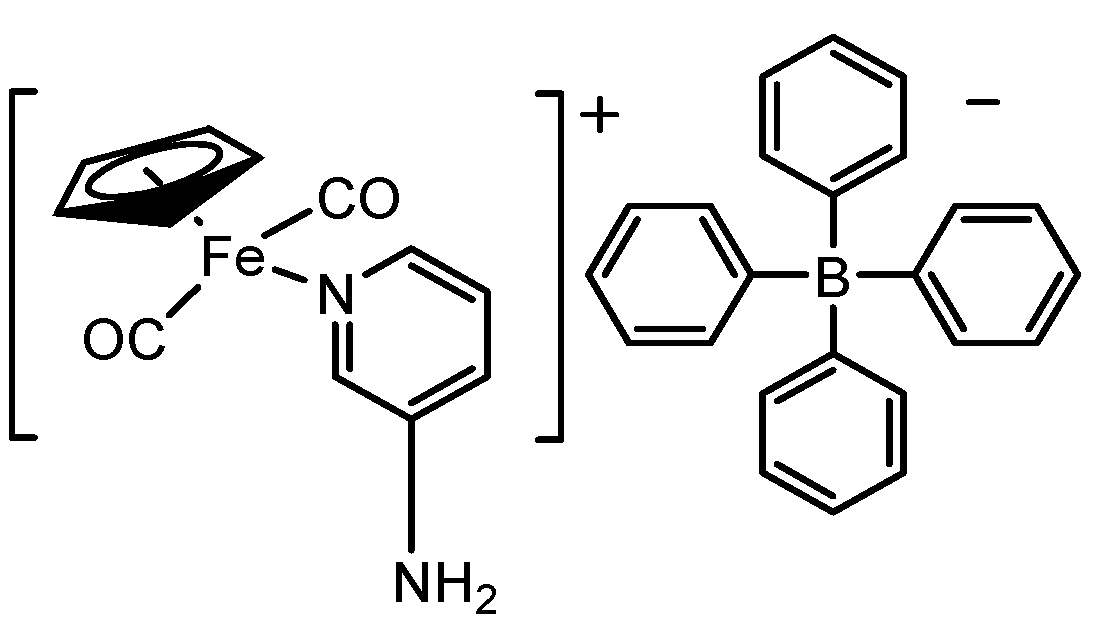
3.7. Synthesis of [(ɳ5-C5H5)Fe(CO)2(3-Apy)]BPH4 complex
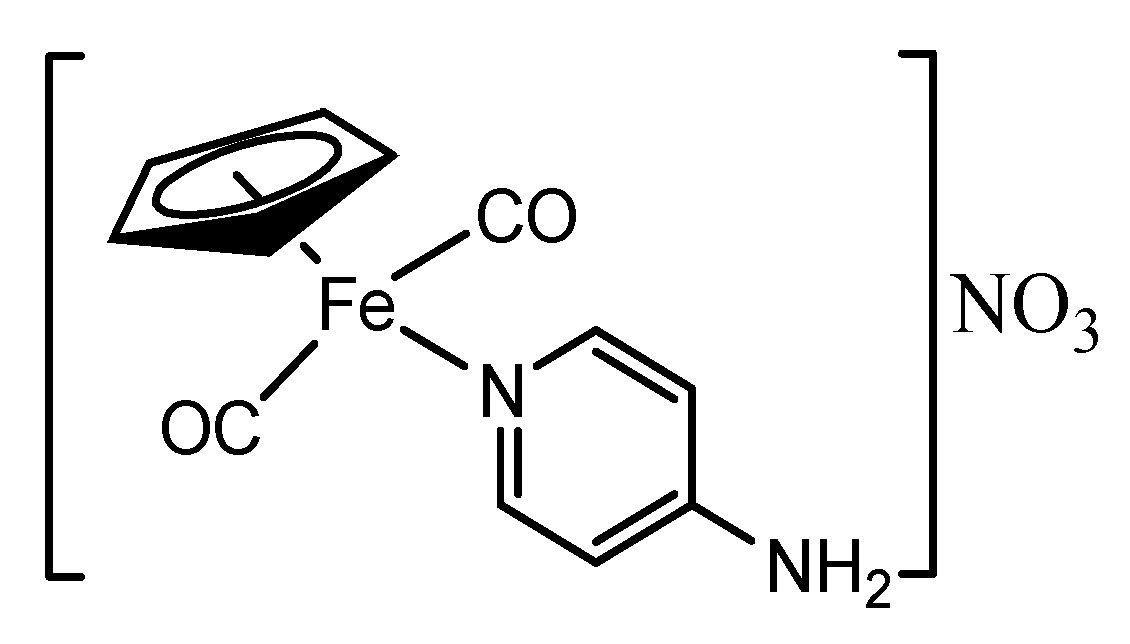
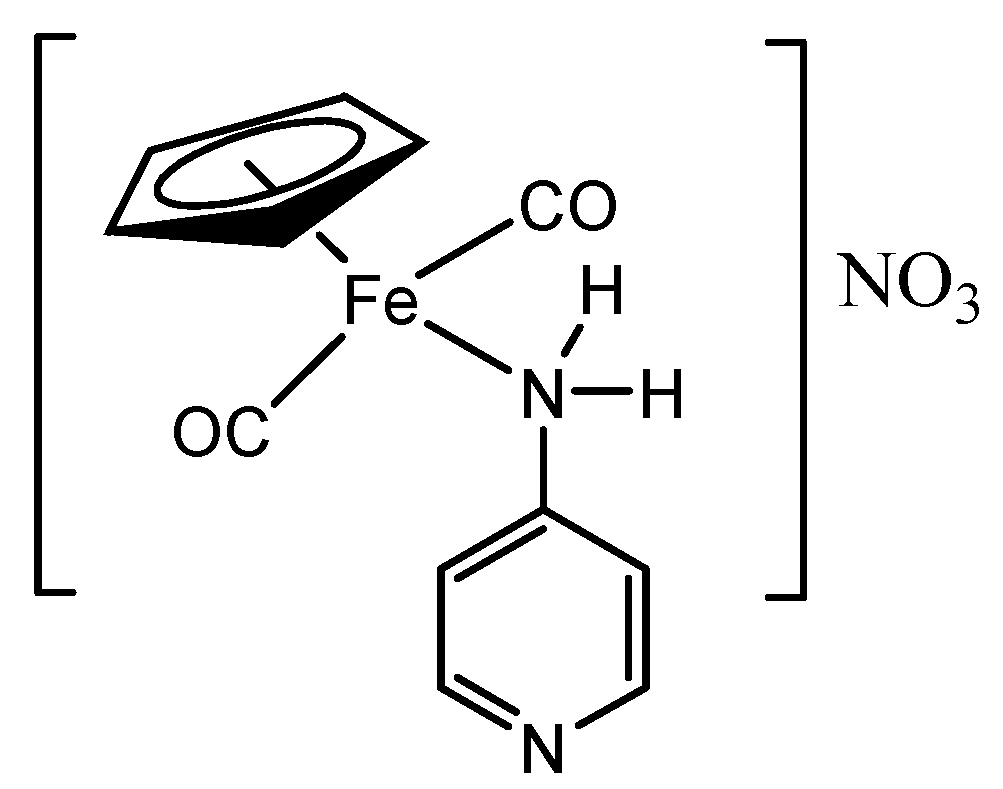
3.8. Synthesis of [(ɳ5-C5H5)Fe(CO)2(4-Apy)]NO3
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. Pyridine and pyridine derivatives. Ullmann’s Encyclopedia of Industrial Chemistry 2000. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, K.-I.; Doi, T.; Takimoto, Y. High-Sensitivity Detection and Postsource Decay of 2-Aminopyridine-Derivatized Oligosaccharides with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Analytical Chemistry 1997, 69, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Salgia, S. R.; Thompson, W. B.; Dillingham, E. O.; Bond, Stephen. E.; Feng, Z.; Prasad, K. R.; Gollamudi, R. Design and synthesis of piperidine-3-carboxamides as human platelet aggregation inhibitors. Journal of Medicinal Chemistry 1995, 38, 180–188. [Google Scholar] [CrossRef]
- Uemura, K.; Kitagawa, S.; Kondo, M.; Fukui, K.; Kitaura, R.; Chang, H.-C.; Mizutani, T. Novel flexible frameworks of porous Cobalt(II) coordination polymers that show selective guest adsorption based on the switching of Hydrogen-Bond pairs of AMIde groups. Chemistry - a European Journal 2002, 8, 3586. [Google Scholar] [CrossRef]
- Uemura, K.; Kitagawa, S.; Fukui, K.; Saito, K. A contrivance for a dynamic porous framework: cooperative guest adsorption based on square grids connected by Amide−Amide hydrogen bonds. Journal of the American Chemical Society 2004, 126, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D. K.; Das, A.; Dastidar, P. Supramolecular structural diversities in the metal–organic frameworks derived from pyridylamide ligands: studying the effects of ligating topologies, hydrogen bonding backbone of the ligands and counter anions. CrystEngComm 2007, 9, 548–555. [Google Scholar] [CrossRef]
- Mohammadi, S.; Foroumadi, A. 4-Aminopyridine. In Elsevier eBooks; 2023; pp 393–397. [CrossRef]
- Akyüz, S. The FT-IR spectroscopic investigation of transition metal(II) 4-aminopyridine tetracyanonickelate complexes. Journal of Molecular Structure 1999, 482, 171–174. [Google Scholar] [CrossRef]
- Swiatkowski, M.; Sieranski, T.; Bogdan, M.; Kruszynski, R. Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System. Molecules 2019, 24, 3357. [Google Scholar] [CrossRef]
- Sadimenko, A. P. ChemInform Abstract: Organometallic complexes of Aminopyridines. ChemInform 2011, 42. [Google Scholar] [CrossRef]
- Kalidasan, M.; Forbes, S.; Mozharivskyj, Y.; Kollipara, M. R. Half-sandwich pentamethylcyclopentadienyl group 9 metal complexes of 2-aminopyridyl ligands: Synthesis, spectral and molecular study. Journal of Chemical Sciences 2015, 127, 1135–1144. [Google Scholar] [CrossRef]
- Noor, A. Coordination Chemistry of Bulky Aminopryridinates with Main Group and Transition Metals. Topics in Current Chemistry 2021, 379. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-E.; Yuan, S.-F.; Tong, H.-B.; Bai, S.-D.; Wei, X.-H.; Liu, D.-S. Metal (Mg, Fe, Co, Zr and Ti) complexes derived from aminosilyl substituted aminopyridinato ligand: synthesis, structures and ethylene polymerization behaviors of the group 4 complexes. Dalton Transactions 2012, 41, 9460. [Google Scholar] [CrossRef]
- Hafeez, M.; Kretschmer, W. P.; Kempe, R. Hafnium trialkyls stabilized by bulky, Electron-Rich aminopyridinates. Zeitschrift Für Anorganische Und Allgemeine Chemie 2012, 638, 324–330. [Google Scholar] [CrossRef]
- Chelucci, G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coordination Chemistry Reviews 2013, 257, 1887–1932. [Google Scholar] [CrossRef]
- Thierer, L. M.; Jenny, S. E.; Shastri, V.; Donley, M. R.; Round, L. M.; Piro, N. A.; Kassel, W. S.; Brown, C. L.; Dudley, T. J.; Zubris, D. L. Amino pyridine iron(II) complexes: Characterization and catalytic application for atom transfer radical polymerization and catalytic chain transfer. Journal of Organometallic Chemistry 2020, 924, 121456. [Google Scholar] [CrossRef]
- Yuoh, A. C. B.; Agwara, M. O.; Yufanyi, D. M.; Conde, M. A.; Jagan, R.; Eyong, K. O. Synthesis, Crystal Structure, and Antimicrobial Properties of a Novel 1-D Cobalt Coordination Polymer with Dicyanamide and 2-Aminopyridine. International Journal of Inorganic Chemistry 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Brađan, G.; Čobeljić, B.; Pevec, A.; Turel, I.; Milenković, M.; Radanović, D.; Šumar-Ristović, M.; Adaila, K.; Milenković, M.; Anđelković, K. Synthesis, characterization and antimicrobial activity of pentagonal-bipyramidal isothiocyanato Co(II) and Ni(II) complexes with 2,6-diacetylpyridine bis(trimethylammoniumacetohydrazone). Journal of Coordination Chemistry 2016, 69, 801–811. [Google Scholar] [CrossRef]
- Chimaine, F. T.; Yufanyi, D. M.; Yuoh, A. C. B.; Eni, D. B.; Agwara, M. O. Synthesis, crystal structure, photoluminescent and antimicrobial properties of a thiocyanato-bridged copper(II) coordination polymer. Cogent Chemistry 2016, 2, 1253905. [Google Scholar] [CrossRef]
- Mohammed, H. S. Synthesis, characterization, structure determination from powder X-ray diffraction data, and biological activity of azo dye of 3-aminopyridine and its complexes of Ni(II) and Cu(II). Bulletin of the Chemical Society of Ethiopia 2021, 34, 523–532. [Google Scholar] [CrossRef]
- J, Jisha. M. Synthesis and characterization of Schiff base complexes of Zr(IV) and Th(IV) complexes of Schiff base derived from furan 3- carboxaldehyde and 3- amino pyridine. International Journal of Emerging Trends in Science and Technology 2017, 4. [CrossRef]
- Guo, L.; Hu, X.; Yang, Y.; An, W.; Gao, J.; Liu, Q.; Liu, Z. Synthesis and biological evaluation of zwitterionic half-sandwich Rhodium(III) and Ruthenium(II) organometallic complexes. Bioorganic Chemistry 2021, 116, 105311. [Google Scholar] [CrossRef]
- M’thiruaine, C. M.; Friedrich, H. B.; Changamu, E. O.; Bala, M. D. Reactions of N-heterocyclic ligands with substitutionally labile organometallic complexes, [(η5-C5R5)Fe(CO)2E]BF4. Inorganica Chimica Acta 2012, 390, 83–94. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in toxicological research of the anticancer drug cisplatin. Chemical Research in Toxicology 2019, 32, 1469–1486. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief review. Advanced Pharmaceutical Bulletin 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Standfest-Hauser, C. M.; Mereiter, K.; Schmid, R.; Kirchner, K. Some binding modes of 2-aminopyridine to ruthenium(ii) fragments. Dalton Transactions 2003, No. 11, 2329. [Google Scholar] [CrossRef]
- Fuster, M. G.; Moulefera, I.; Montalbán, M. G.; Pérez, J.; Víllora, G.; García, G. Synthesis and Characterization of New Ruthenium (II) Complexes of Stoichiometry [Ru(p-Cymene)Cl2L] and Their Cytotoxicity against HeLa-Type Cancer Cells. Molecules 2022, 27, 7264. [Google Scholar] [CrossRef]
- Marszaukowski, F.; Guimarães, I. D. L.; Da Silva, J. P.; Da Silveira Lacerda, L. H.; De Lazaro, S. R.; De Araujo, M. P.; Castellen, P.; Tominaga, T. T.; Boeré, R. T.; Wohnrath, K. Ruthenium(II)-arene complexes with monodentate aminopyridine ligands: Insights into redox stability and electronic structures and biological activity. Journal of Organometallic Chemistry 2018, 881, 66–78. [Google Scholar] [CrossRef]
- M’thiruaine, C. M.; Friedrich, H. B.; Changamu, E. O.; Bala, M. D. Synthesis and characterization of amine complexes of the cyclopentadienyliron dicarbonyl complex cation, [Cp(CO)2Fe]+. Inorganica Chimica Acta 2010, 366, 105–115. [Google Scholar] [CrossRef]
- M’thiruaine, C. M.; Friedrich, H. B.; Changamu, E. O.; Omondi, B. Regioselective reactions of electrophilic iron dicarbonyl cations, [(η5-C5R5)(CO)2Fe]+ (R = H, CH3) with heterofunctional amine ligands. Journal of Organometallic Chemistry 2012, 717, 52–60. [Google Scholar] [CrossRef]
- Chengo, K. Synthesis, Characterization, bioassay and density Functional Theory studies of cationic iron half sandwich complexes of selected heterofunctional active pharmaceutical agents. Doctor of Philosophy Degree (In Applied Chemistry), Kenyatta University, Kenya, 2020. https://ir-library.ku.ac.ke/handle/123456789/21492.
- Khan, T.; Dixit, S.; Ahmad, R.; Raza, S.; Azad, I.; Joshi, S.; Khan, A. R. Molecular docking, PASS analysis, bioactivity score prediction, synthesis, characterization and biological activity evaluation of a functionalized 2-butanone thiosemicarbazone ligand and its complexes. Journal of Chemical Biology 2017, 10, 91–104. [Google Scholar] [CrossRef]
- Dörr, M.; Meggers, E. Metal complexes as structural templates for targeting proteins. Current Opinion in Chemical Biology 2014, 19, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Phiri, Ll. Spectroscopic studies-Cyclopentadienyl iron dicarbonyl ketone complexes. Masters of Science in chemistry, university of Zambia, Zambia, 199. https://oatd.org/oatd/record?record=oai\:dspace.unza.zm\:123456789\%2F274.
- M’thiruaine, C. M.; Friedrich, H. B.; Changamu, E. O.; Bala, M. D. Synthesis and characterization of amine complexes of the cyclopentadienyliron dicarbonyl complex cation, [Cp(CO)2Fe]+. Inorganica Chimica Acta 2010, 366, 105–115. [Google Scholar] [CrossRef]
- Kennedy, W. Synthesis, Characterization And Screening Of Selected Amine Complexes Of The Organometallic Moiety [(ŋ5-C5H5)(CO)(PPh3)Fe]+ For Antibacterial Activity. MA Thesis, kenyatta university, Kenya 2016. https://Https://Ir-Library.Ku.Ac.Ke/Bitstream/Handle/123456789/15344/Synthesis%2c%20characterization%20and%20screening.Pdf?Sequence=1&Isallowed=Y.
- Pilon, A.; Brás, A. R.; Côrte-Real, L.; Avecilla, F.; Costa, P. J.; Preto, A.; Garcia, M. H.; Valente, A. A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells. Molecules 2020, 25, 1592. [Google Scholar] [CrossRef] [PubMed]
- Sall, A. S.; Tamboura, F. B.; Gaye, M. Spectroscopic studies of some lanthanide(III) nitrate complexes synthesized from a new ligand 2,6-bis-(salicylaldehyde hydrazone)-4-chlorophenol. https://doaj.org/article/29a4704908e941e69cbf3a97d8cf8e8b.
- Mihaylov, M. Y.; Zdravkova, V. R.; Ivanova, E. Z.; Aleksandrov, H. A.; Petkov, P. St.; Vayssilov, G. N.; Hadjiivanov, K. I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. Journal of Catalysis 2020, 394, 245–258. [Google Scholar] [CrossRef]
- Yenikaya, C.; Poyraz, M.; Sarı, M.; Demirci, F.; İlkimen, H.; Büyükgüngör, O. Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron 2009, 28, 3526–3532. [Google Scholar] [CrossRef]
- Büyükmurat, Y.; Akalin, E.; Özel, A. E.; Akyüz, S. Calculation and analysis of IR spectrum of 2-aminopyridine. Journal of Molecular Structure 1999, 482, 579–584. [Google Scholar] [CrossRef]
- Buyukmurat, Y.; Akyuz, S. Theoretical and experimental studies of IR spectra of 4-aminopyridine metal(II) complexes. Journal of Molecular Structure 2003, 651–653, 533–539. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds; 2008. [CrossRef]
- Dhaveethu, K.; Ramachandramoorthy, T.; Thirunavukkarasu, K. Spectroscopic, Thermal and Biological Studies of Zn(II), Cd(II) and Hg(II) Complexes Derived from 3-Aminopyridine and Nitrite Ion. Journal of the Korean Chemical Society 2013, 57, 712–720. [Google Scholar] [CrossRef]
- Lovely, K.; Christudhas, M. Synthesis, Characterization and Antimicrobial Studies of Co(II), Ni(II), Cu(II) and Zn(II) Complexes of 3-Pyridine Carboxaldehyde and L-Tryptophan. Journal of Chemical and Pharmaceutical Research 2013, 5, 154–159. [Google Scholar]
- Kartal, Z. Synthesis, spectroscopic, thermal and structural properties of [M(3-aminopyridine)2Ni(μ-CN)2(CN)2]n (M(II)=Co and Cu) heteropolynuclear cyano-bridged complexes. Spectrochimica Acta Part a Molecular and Biomolecular Spectroscopy 2015, 152, 577–583. [Google Scholar] [CrossRef]
- Mautner, F. A.; Jantscher, P. V.; Fischer, R. C.; Torvisco, A.; Reichmann, K.; Massoud, S. S. Syntheses, structural characterization, and thermal behaviour of metal complexes with 3-aminopyridine as co-ligands. Transition Metal Chemistry 2020, 46, 191–200. [Google Scholar] [CrossRef]
- Kartal, Z.; Şahi̇N, O. Synthesis, spectroscopic, thermal, crystal structure properties, and characterization of new Hofmann-Td-type complexes with 3-aminopyridine. Turkish Journal of Chemistry 2021, 45, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Büyükmurat, Y.; Akyüz, S. Theoretical and experimental IR spectra and assignments of 3-aminopyridine. Journal of Molecular Structure 2001, 563, 545–550. [Google Scholar] [CrossRef]
- Templeton, J. L. Hexakis(pyridine)ruthenium(II) tetrafluoroborate. Molecular structure and spectroscopic properties. Journal of the American Chemical Society 1979, 101, 4906–4917. [Google Scholar] [CrossRef]
- Pal, S. Pyridine: a useful ligand in transition metal complexes. In InTech eBooks; 2018. [CrossRef]
- Côrte-Real, L.; Robalo, M. P.; Marques, F.; Nogueira, G.; Avecilla, F.; Silva, T. J. L.; Santos, F. C.; Tomaz, A. I.; Garcia, M. H.; Valente, A. The key role of coligands in novel ruthenium(II)-cyclopentadienyl bipyridine derivatives: Ranging from non-cytotoxic to highly cytotoxic compounds. Journal of Inorganic Biochemistry 2015, 150, 148–159. [Google Scholar] [CrossRef]
- Schrock, R. R.; Osborn, J. A. .pi.-Bonded complexes of the tetraphenylborate ion with rhodium(I) and iridium(I). Inorganic Chemistry 1970, 9, 2339–2343. [Google Scholar] [CrossRef]
- Xu, H.; Wolf, C. Efficient copper-catalyzed coupling of aryl chlorides, bromides and iodides with aqueous ammonia. Chemical Communications 2009, 3035. [Google Scholar] [CrossRef]
- Fryzuk, M. D.; Johnson, S. A. The continuing story of dinitrogen activation. Coordination Chemistry Reviews 2000, 200, 379–409. [Google Scholar] [CrossRef]
- King, R. B.; Stone, F. G. A.; Jolly, W. L.; Austin, G.; Covey, W.; Rabinovich, D.; Steinberg, H.; Tsugawa, R. Cyclopentadienyl metal carbonyls and some derivatives. Inorganic Syntheses 1963, 99–115. [Google Scholar] [CrossRef]
| Compound/Band position | ||||
|---|---|---|---|---|
| Band Assignment | 2-Apy | [(η5-C5H5)Fe(CO)22-Apy]NO3 | Ref | |
| Cp ʋ(C-H) | - | 3110 | - | 34 |
| ʋas(CO) | - | 2033 | - | 31,37 |
| ʋs(CO) | - | 1986 | - | 31,37 |
| ʋs(NH2) | 3283 | 3183 | -100 | 29, 35, 41 |
| ʋas (NH2) | 3443 | 3339 | -104 | 29, 35, 41 |
| ʋ(C=N) | 1595 | 1600 | +5 | 40 |
| 2-Apy(Ring breath) | 985 | 989 | +4 | 41 |
| ʋas(NO3 ) | - | 1380 | - | 38, 39 |
| Assignment | 3-Apy | [(η5-C5H5)Fe(CO)23-Apy] NO3 | Ref | |
|---|---|---|---|---|
| Cp ʋ(C-H) | - | 3102 | 34 | |
|
ʋ(CO) ʋs(CO) |
- - |
2057 2007 |
- - |
23, 29, 30 23, 29, 30 |
| ʋs(NH2) | 3301 | 3369 | +68 | 46-48 |
| ʋas(NH2) | 3373 | 3461 | +88 | 46-48 |
| ʋ(C=N) | 1585 | 1597 | +12 | 45, 49 |
| 3-Apy (Ring breath) | 1014 | 1028 | +14 | 45, 49 |
| ʋas(NO3 ) | - | 1320 | - | 38, 39 |
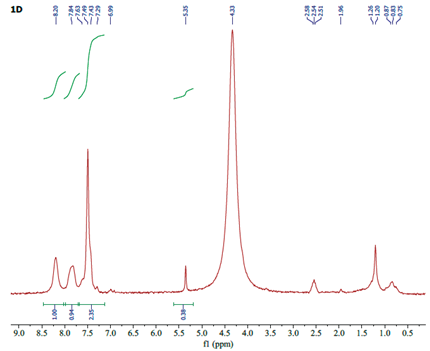
| Assignment | 3-Apy | [(η5-C5H5)Fe(CO)23-Apy]NO3 | ∆ |
|---|---|---|---|
| Ha | 8.08 | 8.20 | +0.12 |
| Hb | 7.99 | 7.84 | -0.15 |
| Hc | 7.03 | 7.49 | +0.46 |
| Hd | 6.97 | 6.99 | +0.02 |
| He | 3.89 | 4.33 | +0.44 |
| HCp | - | 5.35 | - |
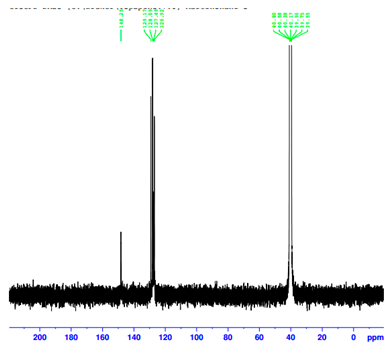
| Assignment | 3-Apy | [(η5-C5H5)Fe(CO)23-Apy]NO3 | ∆ |
|---|---|---|---|
| C(1) | 142.5 | 148.3 | +5.8 |
| C(2) | 139.8 | 129.2 | -10.2 |
| C(3) | 137.3 | 128.1 | -9.2 |
| C(4) | 123.6 | 127.5 | +3.9 |
| C(5) | 121.3 | 126.9 | +5.6 |
| Ccp | - | 88.4 | - |
| CO | - | 210.1 | - |
| Assignment | 3-Apy | [(η5-C5H5)Fe(CO)23-Apy] BPh4 | Ref | |
|---|---|---|---|---|
| Cpʋs(C-H) | - | 3095 | - | 37 |
|
ʋas(CO) ʋs(CO) |
- - |
2043 1992 |
- - |
29, 30, 35, 36 |
| ʋs(NH2) | 3306 | 3421 | +120 | 42,44 |
| ʋas(NH2) | 3374 | 3461 | +88 | 42, 44 |
| 3-Apy Ring breath | 1014 | 1026 | +12 | 44 |
| ʋ(C=N) | 1585 | 1600 | +15 | 44 |
| i.p.s Ph ʋ(C-C) | - | 1479, 1426 | - | 53 |
| Assignment | 4-Apy | [(η5-C5H5)Fe(CO)24-Apy]NO3 (A) | Ref | |
|---|---|---|---|---|
| Cpʋs(C-H) | 3095 | 34 | ||
|
ʋas(CO) ʋs(CO) |
- - |
2038 1976 |
- - |
29, 30 29,30 |
| ʋs(NH2) | 3300 | 3214 | -84 | 46-48 |
| ʋas(NH2) | 3433 | 3337 | -96 | 46-48 |
| ʋ(C=N) | 1598 | 1624 | +26 | 40,42 |
| 4-Apy Ring breath | 988 | 1025 | +37 | 40,42 |
| ʋas(NO3) | - | 1326 | - | 38,39 |
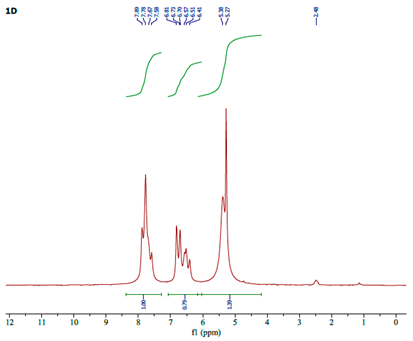
| Assignment | (4-Apy) | [(η5-C5H5)Fe(CO)2(4-Apy)]NO3(A) | ∆ |
|---|---|---|---|
| Ha | 7.98 | 7.78 | -0.20 |
| Hb | 6.47 | 6.70 | +0.23 |
| Hc | 6.04 | 6.41 | +0.37 |
| HCp | - | 5.38 | - |
| Assignment | 4-Apy | [(η5-C5H5)Fe(CO)24-Apy NO3(A) | ∆ |
|---|---|---|---|
| C(1) | 154.2 | 157.5 | +4.3 |
| C(2) | 149.4 | 140.6 | -8.8 |
| C(3) | 108.8 | 109.3 | 0.5 |
| CCp | - | 88.0 | - |
| CO | - | 212.2 | - |
| Assignment | 4-Apy | [(η5-C5H5)Fe(CO)2(4-Apy)]NO3(B) | Ref | |
|---|---|---|---|---|
| Cp ʋ(C-H) | - | 3112 | - | 34 |
|
ʋas(CO) ʋs(CO) |
- - |
2058 2008 |
- - |
29, 35 |
| ʋs(NH2) | 3300 | 3216 | -84 | 31 |
| ʋas(NH2) | 3433 | 3343 | -90 | 31 |
| ʋ(C=N) | 1598 | 1601 | +3 | 40,42 |
| Pyridine Ring breath | 988 | 993 | +3 | 40,42 |
| ʋas(NO3 ) | - | 1336 | - | 38,39 |
| Assignment | 4-Apy | [(η5-C5H5)Fe(CO)24-Apy]NO3(B) | ∆ |
|---|---|---|---|
| Ha | 7.98 | 7.89 | -0.09 |
| Hb | 6.57 | 6.81 | 0.24 |
| Hc | 6.04 | 6.73 | 0.69 |
| HCp | 5.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





