Submitted:
21 July 2024
Posted:
23 July 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets and Experimental Design
2.3. Chemical Analyses and Calculations
2.4. Statistical Analysis
3. Results
3.1. Chemical and Amino Acid Composition
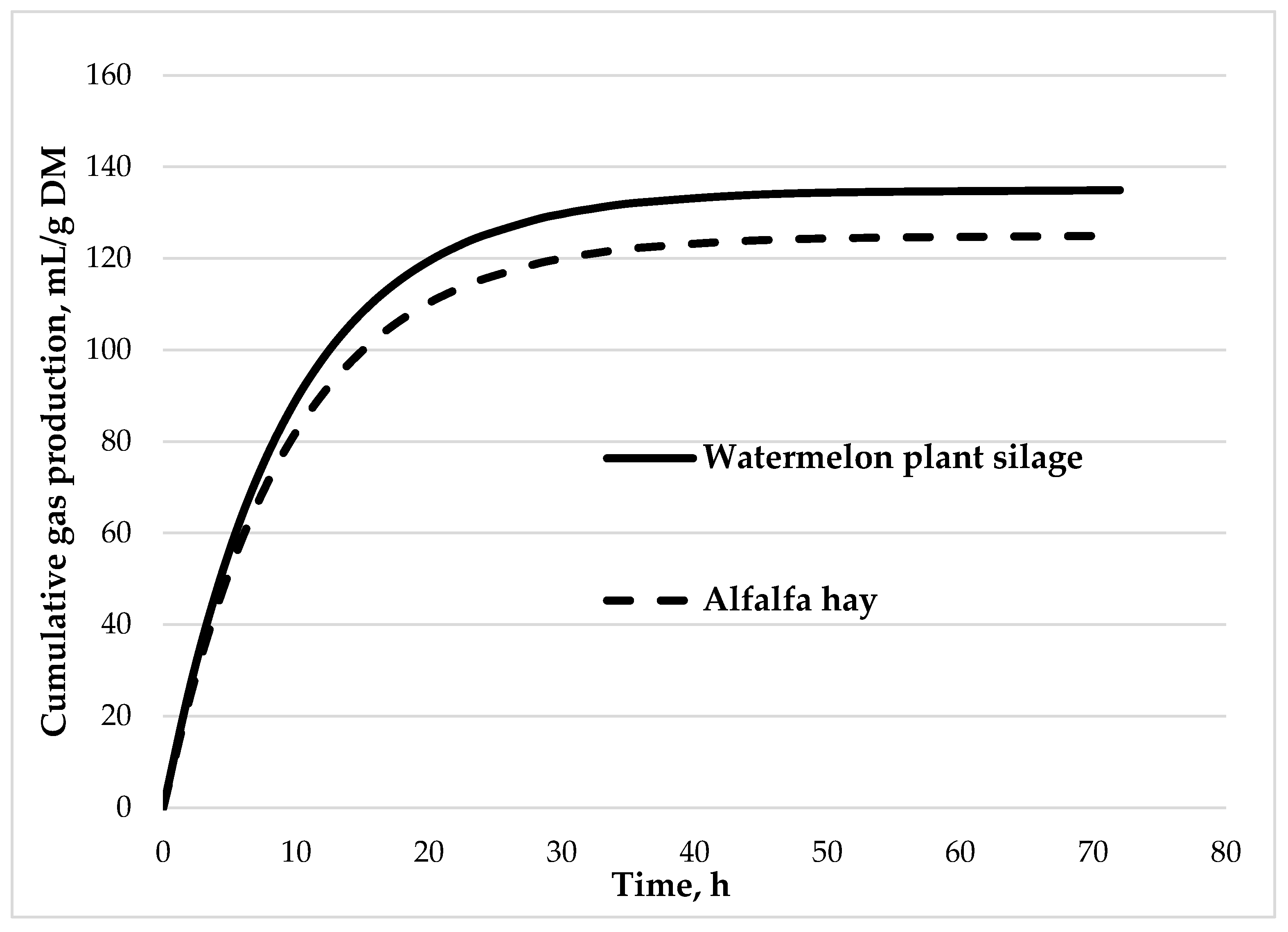
3.2. The Effect of Different Forages on Rumen Fermentation Parameters
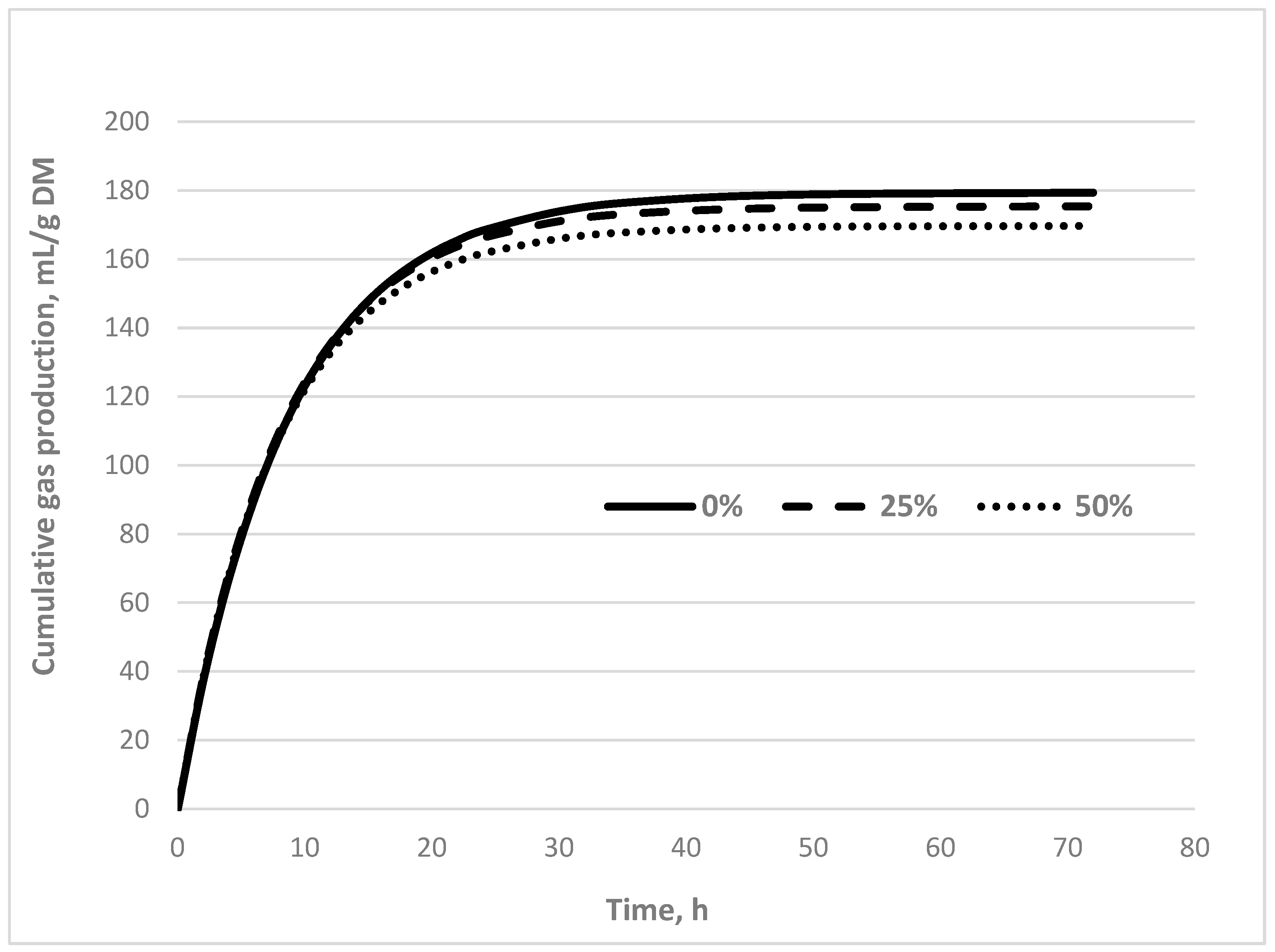
3.3. Effects of Replacing Alfalfa Hay with Watermelon Plant Silage on the Ruminal Fermentation Parameters of the Experimental Diet.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commision. A new Circular Economy Action Plan. For a cleaner and more competitive Europe (COM/2020/98 final) 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2020:98:FIN (accessed on 22 June 2024).
- FAO. How to Feed the World in 2050. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 21 June 2024).
- FAO. The future of food and agriculture:Trends and challenges. Rome 2017. Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 22 June 2024).
- United Nations. Department of Economic and social affairs 2019. Available online: https://population.un.org/wpp/ (accessed on 22 June 2024).
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; Balzer, C.; Bennett, E.M.; Carpenter, S.R.; Hill, J.; Monfreda, C.; Polasky, S.; Rockström, J.; Sheehan, J.; Siebert, S.; Zaks, D.P.M.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob Food Sec 2021, 28, 100488. [Google Scholar] [CrossRef]
- Arco-Pérez, A.; Ramos-Morales, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Martín-García, A.I. Nutritive evaluation and milk quality of including of tomato or olive by-products silages with sunflower oil in the diet of dairy goats. Anim Feed Sci Technol 2017, 232, 57–70. [Google Scholar] [CrossRef]
- Méndez, D.A.; Martínez-Abad, A.; Martínez-Sanz, M.; López-Rubio, A.; Fabra, M.J. Tailoring structural, rheological and gelling properties of watermelon rind pectin by enzymatic treatments. Food Hydrocoll 2023, 135, 108119. [Google Scholar] [CrossRef]
- Chharang, D.; Tribhuwan, S.; Vijay, P.; Hemant, J.; Surendra, S.S. Effect of Replacement of Conventional Feeds by Prosopis juliflora Pods and Citrullus lanatus Seed Cake on Nutrient Utilization in Marwari Goats. J Anim Res 2020, 10, 551–555. [Google Scholar] [CrossRef]
- Hassan, M.; Belanche, A.; Jiménez, E.; Rivelli, I.; Martín-García, A.I.; Margolles, A.; Yáñez-Ruiz, D.R. Evaluation of the nutritional value and presence of minerals and pesticides residues in agro-industrial by-products to replace conventional ingredients of small ruminant diets. Small Rumin Res 2023, 229, 107117. [Google Scholar] [CrossRef]
- Martı́n-Garcı́a, A.I.; Moumen, A.; Yáñez-Ruiz, D.R.; Molina-Alcaide, E. Chemical composition and nutrients availability for goats and sheep of two-stage olive cake and olive leaves. Anim Feed Sci Technol 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Ruiz, D.R.Y.; Moumen, A.; Garcı́a, A.I.M. Ruminal degradability and in vitro intestinal digestibility of sunflower meal and in vitro digestibility of olive by-products supplemented with urea or sunflower meal. Anim Feed Sci Technol 2003, 110, 3–15. [Google Scholar] [CrossRef]
- Meneses, M.; Megías, M.D.; Madrid, J.; Martínez-Teruel, A.; Hernández, F.; Oliva, J. Evaluation of the phytosanitary, fermentative and nutritive characteristics of the silage made from crude artichoke (Cynara scolymus L.) by-product feeding for ruminants. Small Rumin. Res 2007, 70, 292–296. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Rural Develop 1988, 28, 7–55. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fígares, I.; Prieto, C.; Nieto, R.; Aguilera, J.F. Free amino acid concentrations in plasma, muscle and liver as indirect measures of protein adequacy in growing chickens. Anim. Sci 1997, 64, 529–539. [Google Scholar] [CrossRef]
- Kheddouma, A.; Arhab, R.; Martín-García, AI.; Aouidane, L.; Bouraiou, A. Effects of the methane-inhibitors Nitrophenol, 5-Nitrobenzimidazol and two new synthetic nitrocompounds on in vitro ruminal fermentation. Biocatal Agric Biotechnol 2018, 14, 160–165. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br. J. Nutr 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Al-Sagheer, A.A.; El-Waziry, A.M.; El-Zarkouny, S.Z.; Elwakeel, E.A. Ensiling Characteristics, In Vitro Rumen Fermentation Patterns, Feed Degradability, and Methane and Ammonia Production of Berseem (Trifolium alexandrinum L.) Co-Ensiled with Artichoke Bracts (Cynara cardunculus L.). Animals 2023, 131543. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Gao, Z.; Xu, J.; Liu, B.; Guo, M.; Yang, X.; Niu, J.; Zhu, X.; Ma, S.; Li, D.; Sun, Y.; Shi, Y. Whole-plant corn silage improves rumen fermentation and growth performance of beef cattle by altering rumen microbiota. Appl. Microbiol. Biotechnol 2022, 106, 4187–4198. [Google Scholar] [CrossRef] [PubMed]
- Monllor, P.; Romero, G.; Sendra, E.; Atzori, A.S.; Díaz, J.R. Short-term effect of the inclusion of silage artichoke by-products in diets of dairy goats on milk quality. Animals 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.R.d.; Edvan, R.L.; Nascimento, K.d.S.; Alves Barros, D.M.; Barros, L.d.S.; Camboim, L.F.R.; Dias e Silva, T.P.; Miranda, R.d.S.; Araújo, M.J.d.; Lima Neto, A.F.; et al. Characterization of Melon, (Cucumis melo L.) Silage with Different Biomass Mixtures and Dry Matter Contents. Agriculture 2023, 13, 1536. [Google Scholar] [CrossRef]
- Limin, K.R.D.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy. Sci 2018, 5, 4020–4033. [Google Scholar]
- Lin, J.; Li, G.; Sun, L.; Wang, S.; Meng, X.; Sun, L. Varieties and ensiling: Impact on chemical composition, fermentation quality and bacterial community of alfalfa. Front Microbiol 2023, 13. [Google Scholar] [CrossRef]
- Ibrahim, S.E.; Sulieman, A.M.E.; Hassan, E.N.; Ali, N.A.; Hakeem, B.S.A.E.; Muhsin, A.M.A.A. Proximae chemical composition of watermelon (Citrullus vulgaris). Plant. Cell. Biotechnol. Mol. Bio 2021, 22, 114–121. [Google Scholar]
- Kazemi, M. In vitro ruminal-microbial fermentation pattern: nutritional insights about some agricultural crop mesocarps (peel) in ruminant nutrition. AMB. Express 2023, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Prater, T.A.; Miller, D.D. Calcium carbonate depresses iron bioavailability in rats more than calcium sulfate or sodium carbonate. J Nutr 1992, 122, 327–332. [Google Scholar]
- Wu, G. Amino Acids: Biochemistry and Nutrition (2nd ed.); CRC Press, 2021. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv Nutr 2018, 9, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S. E.; Sulieman, A.M.E.; Ali, N.A.; Bothaina.; Hakeem, S.A.; Amin, H.B.; Abdelmuhsin, A.A.; Veettil, V.N. Amino acid Profile of the Watermelon, Citrullus vulgaris and Detection of its Antimicrobial Activity. Biotech. Res. Comm 2019, 12. [Google Scholar] [CrossRef]
- Belanche, A.; Palma-Hidalgo, J.M.; Nejjam, I.; Serrano, R.; Jiménez, E.; Martín-García, I.; Yáñez-Ruiz, D.R. In vitro assessment of the factors that determine the activity of the rumen microbiota for further applications as inoculum. J Sci Food Agric 2019, 99, 163–172. [Google Scholar] [CrossRef]
- Pardo, Z.; Mateos, I.; Campos, R.; Francisco, A.; Lachica, M.; Ranilla, M.J.; Fernández-Fígares, I. Heat stress increases in vitro hindgut fermentation of distinct substrates in iberian pigs. Animals 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amanzougarene, Z.; Fondevila, M. Fitting of the in vitro gas production technique to the study of high concentrate diets. Animals 2020, 10, 1935. [Google Scholar] [CrossRef]
- E.N. Bergman. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Baldwin, R.L.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J Dairy Sci 2004, 87, E55–E65. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Huang, Y.X.; Dong, K.H.; Yang, W.Z.; Zhang, S.L.; Wang, H. Effects of isovalerate on ruminal fermentation, urinary excretion of purine derivatives and digestibility in steers. J. Anim. Physiol. Anim. Nutr 2009, 93, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; El-Morsy, A.; El-Shinnawy, A.; El-Ashry, G.; Osman, M. Utilization of Biologically Treated Watermelon Vine in Rations for Dairy Cows. J. Anim. Poult. Prod 2020, 11, 117–123. [Google Scholar] [CrossRef]
| Composition | Watermelon plant | Watermelon plant silage | Alfalfa hay | Concentrate |
|---|---|---|---|---|
| DM 1, g/100 g FM 2 | 14.7 | 15.7 | 92.8 | 91.3 |
| Nutrients, g/100 g DM | ||||
| OM 3 | 79.8 | 77.4 | 89.6 | 91.6 |
| CP 4 | 22.6 | 21.1 | 18.9 | 19.7 |
| CF 5 | 1.40 | 3.09 | 1.29 | 3.29 |
| NDF 6 | 36.7 | 36.3 | 49.4 | 30.3 |
| ADF 7 | 24.2 | 26.4 | 34.9 | 15.3 |
| ADL 8 | 7.86 | 6.40 | 8.01 | 4.75 |
| Hemicellulose | 12.6 | 9.91 | 14.6 | 15.0 |
| Cellulose | 16.3 | 20.0 | 26.9 | 10.6 |
| Total Carbohydrates | 55,8 | 53,2 | 69,4 | 68,6 |
| Non Fibrous Carbohydrates | 19.1 | 16.9 | 20.0 | 38.3 |
| CE 11, MJ/kg DM | 16.0 | 15.5 | 17.6 | 17.1 |
| Macrominerals, g/kg DM | ||||
| Ca | 44.7 | 4.40 | ||
| K | 22.5 | 11.1 | ||
| Mg | 6.07 | 2.30 | ||
| P | 2.91 | 2.00 | ||
| S | 2.28 | 1.30 | ||
| Microminerals, mg/kg DM | ||||
| Na | 198 | 40.0 | ||
| Fe | 119 | 180 | ||
| Al | 103 | 227 | ||
| Mn | 39.6 | 43.1 | ||
| Zn | 14.6 | 46.8 | ||
| Cu | 5.62 | 5.33 | ||
| Ti | 3.42 | 10.6 | ||
| As | n.d. 12 | 0.340 | ||
| B | n.d. 12 | 11.9 | ||
| Si | n.d. 12 | 129 | ||
| Sr | n.d. 12 | 46.0 |
| g AA/100 g AA | Watermelon plant silage | Alfalfa Hay |
|---|---|---|
| Aspartic acid | 8.56 | 12.5 |
| Serine | 5.86 | 4.69 |
| Glutamic acid | 14.9 | 9.66 |
| Glycine+Histidine* | 4.96 | 8.15 |
| Arginine | 5.67 | 6.15 |
| Threonine* | 5.38 | 4.63 |
| Alanine | 8.10 | 6.20 |
| Proline | 8.10 | 10.5 |
| Tyrosine* | 3.22 | 3.92 |
| Valine* | 6.61 | 6.67 |
| Lysine* | 5.88 | 7.40 |
| Isoleucine* | 4.59 | 4.84 |
| Leucine* | 9.16 | 8.66 |
| Phenylalanine* | 9.03 | 5.98 |
| EAA 1 | 48.8 | 50.3 |
| NEAA 2 | 51.2 | 49.7 |
| g AA/kg DM | 151 | 165 |
| g AA/kg N | 716 | 874 |
| Watermelon plant silage | Alfalfa hay | SEM 1 | p-Value | |
|---|---|---|---|---|
| A 2, ml | 135 | 125 | 6.71 | 0.679 |
| c 3, h-1 | 0.108 | 0.107 | 0.006 | 0.901 |
| pH | 7.00 | 7.04 | 0.015 | 0.017 |
| GP 4 24h, ml/g DM | 120 | 111 | 5.07 | 0.506 |
| ME 5, MJ/kg DM | 6.67 | 5.72 | 0.167 | 0.058 |
| OMD 6, g/kg | 471 | 410 | 13.8 | 0.126 |
| CH4 µl/ml GP | 47.1 | 43.4 | 1.27 | 0.287 |
| Total VFA 7, mM | 66.6 | 115 | 6.70 | 0.011 |
| Acetate, % | 70.1 | 70.2 | 0.809 | 0.963 |
| Propionate, % | 17.3 | 19.0 | 0.283 | 0.022 |
| Butyrate, % | 7.40 | 6.17 | 0.381 | 0.170 |
| Isobutyrate, % | 1.78 | 1.17 | 0.042 | 0.001 |
| Valerate, % | 1.35 | 1.57 | 0.047 | 0.014 |
| Isovalerate, % | 2.07 | 1.77 | 0.102 | 0.210 |
| Acetate/Propionate | 4.08 | 3.51 | 0.103 | 0.057 |
| Inclusion rate, % | 0 | 25 | 50 | SEM 1 | p-value |
|---|---|---|---|---|---|
| A 2, ml | 179 | 175 | 170 | 9.01 | 0.549 |
| c 3, h-1 | 0.116 | 0.123 | 0.127 | 0.01 | 0.088 |
| pH | 6.71 | 6.72 | 6.72 | 0.16 | 0.469 |
| GP 4 24h, ml/g DM | 162 | 169 | 170 | 4.65 | 0.558 |
| ME 5, MJ/kg DM | 6.81 | 6.80 | 6.80 | 0.28 | 0.436 |
| OMD 6, g/kg | 645 | 692 | 769 | 59.9 | 0.067 |
| CH4 µl/ml GP | 48.3 | 54.4 | 55.3 | 1.00 | 0.094 |
| CH4, µl/mol VFA | 723 | 710 | 799 | 39.8 | 0.595 |
| Total VFA 7, mM | 66.8 | 81.1 | 70.5 | 5.14 | 0.529 |
| Acetate, % | 66.3 | 66.6 | 66.5 | 0.33 | 0.594 |
| Propionate, % | 20.5 | 20.7 | 20.9 | 0.24 | 0.183 |
| Butyrate, % | 8.16 | 7.89 | 7.73 | 0.32 | 0.895 |
| Isobutyrate, % | 1.42 | 1.39 | 1.38 | 0.03 | 0.345 |
| Valerate, % | 1.53 | 1.52 | 1.51 | 0.02 | 0.183 |
| Isovalerate, % | 1.86 | 1.82 | 1.81 | 0.05 | 0.531 |
| Acetate/Propionate | 3.22 | 3.22 | 3.18 | 0.03 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





