Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Procedures
2.3. 16S rRNA Gene Sequencing and Data Processing
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
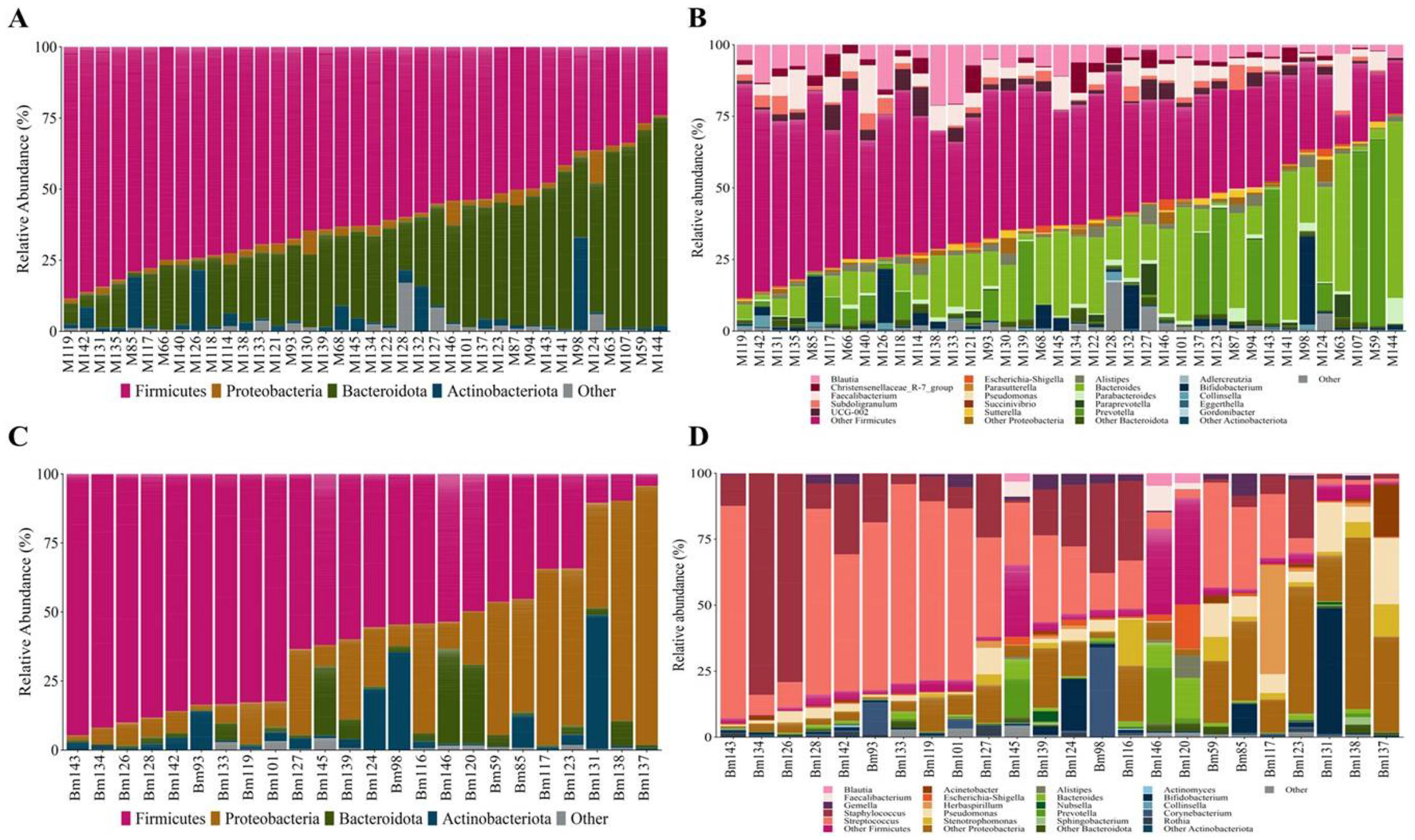
3.2. Microbial Composition
3.3. Factors Influencing Mother-Newborn Gut and BM Microbiota Composition
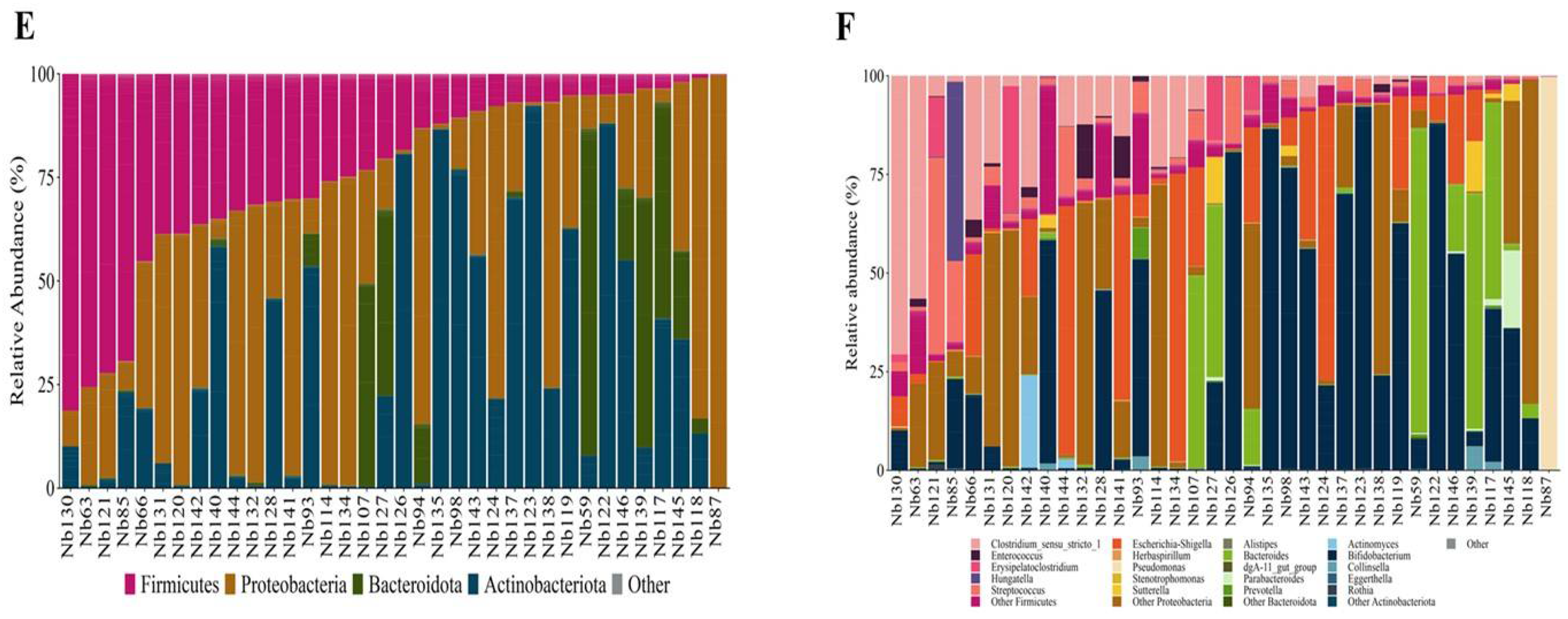
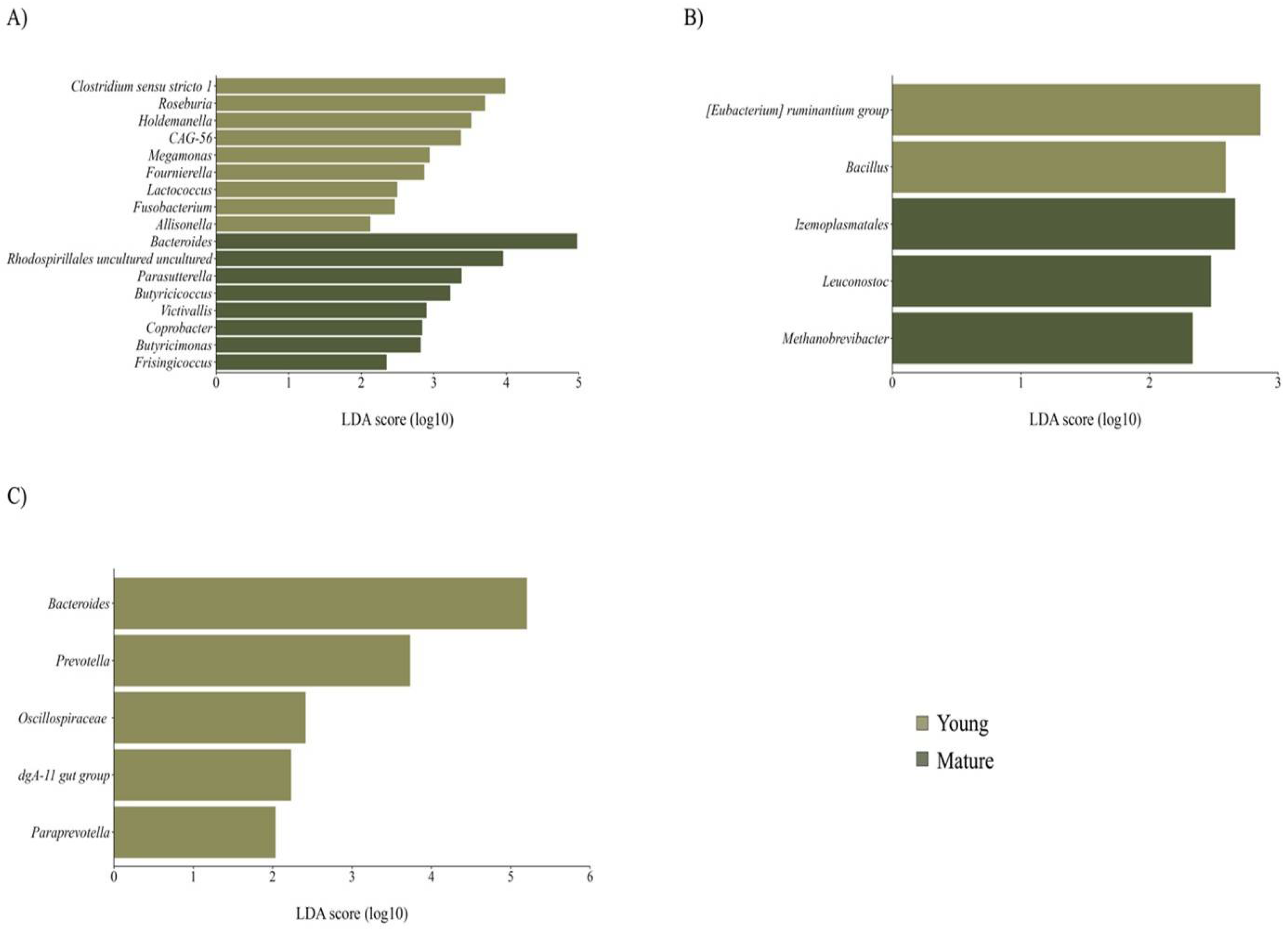
3.3.1. Maternal BMI and Total Body Fat
3.3.2. Maternal Age
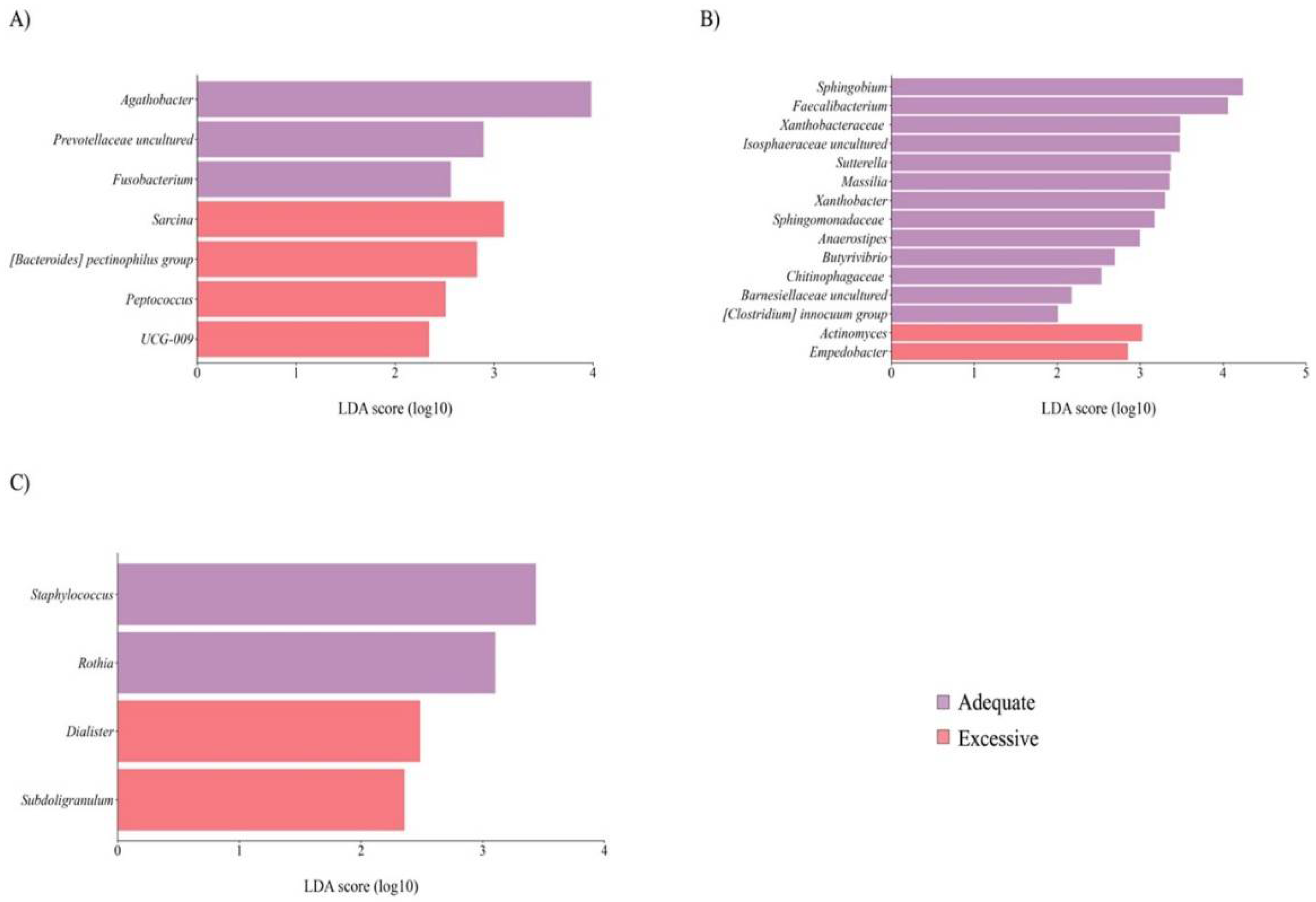
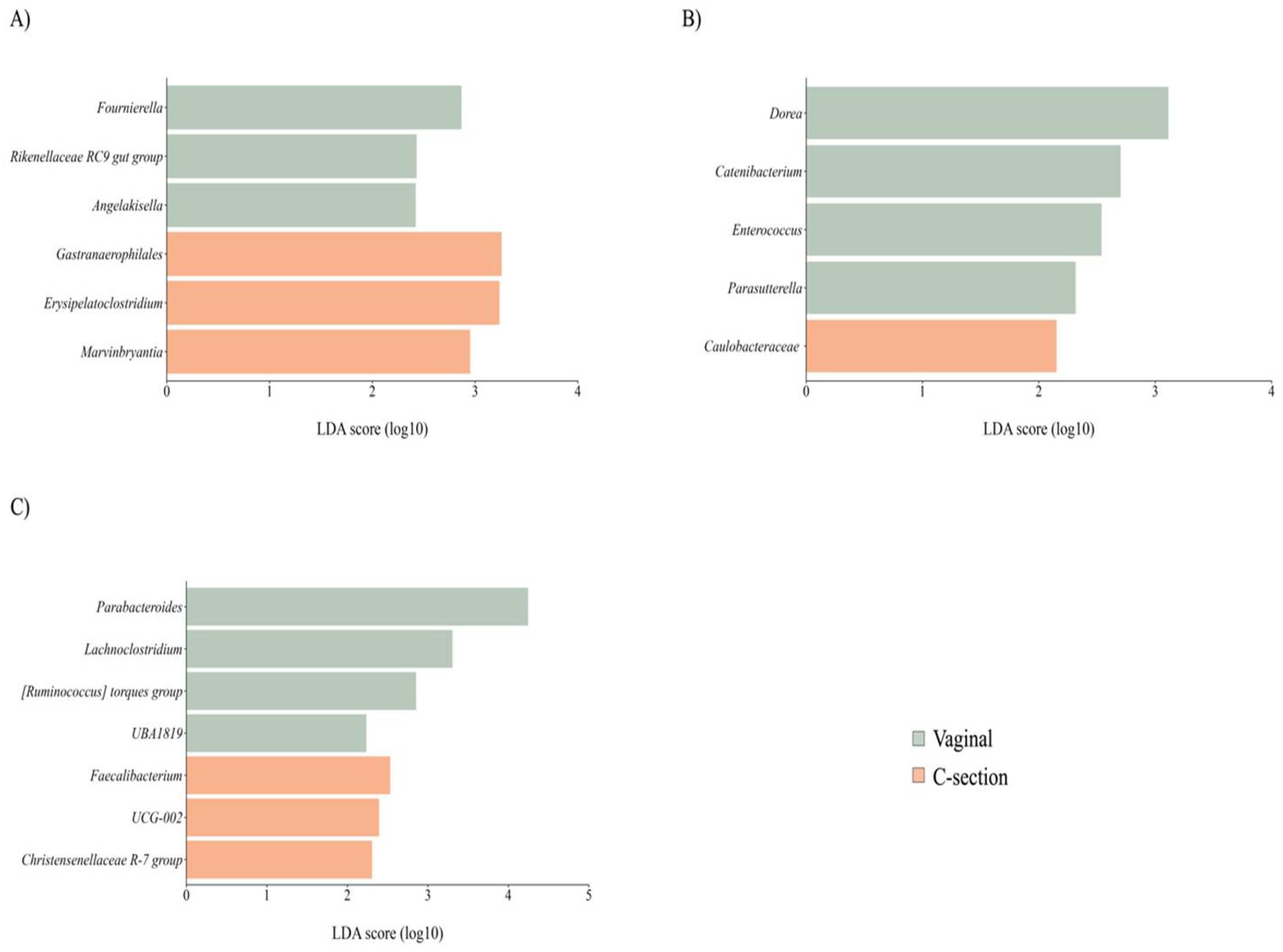
3.3.3. Delivery Mode
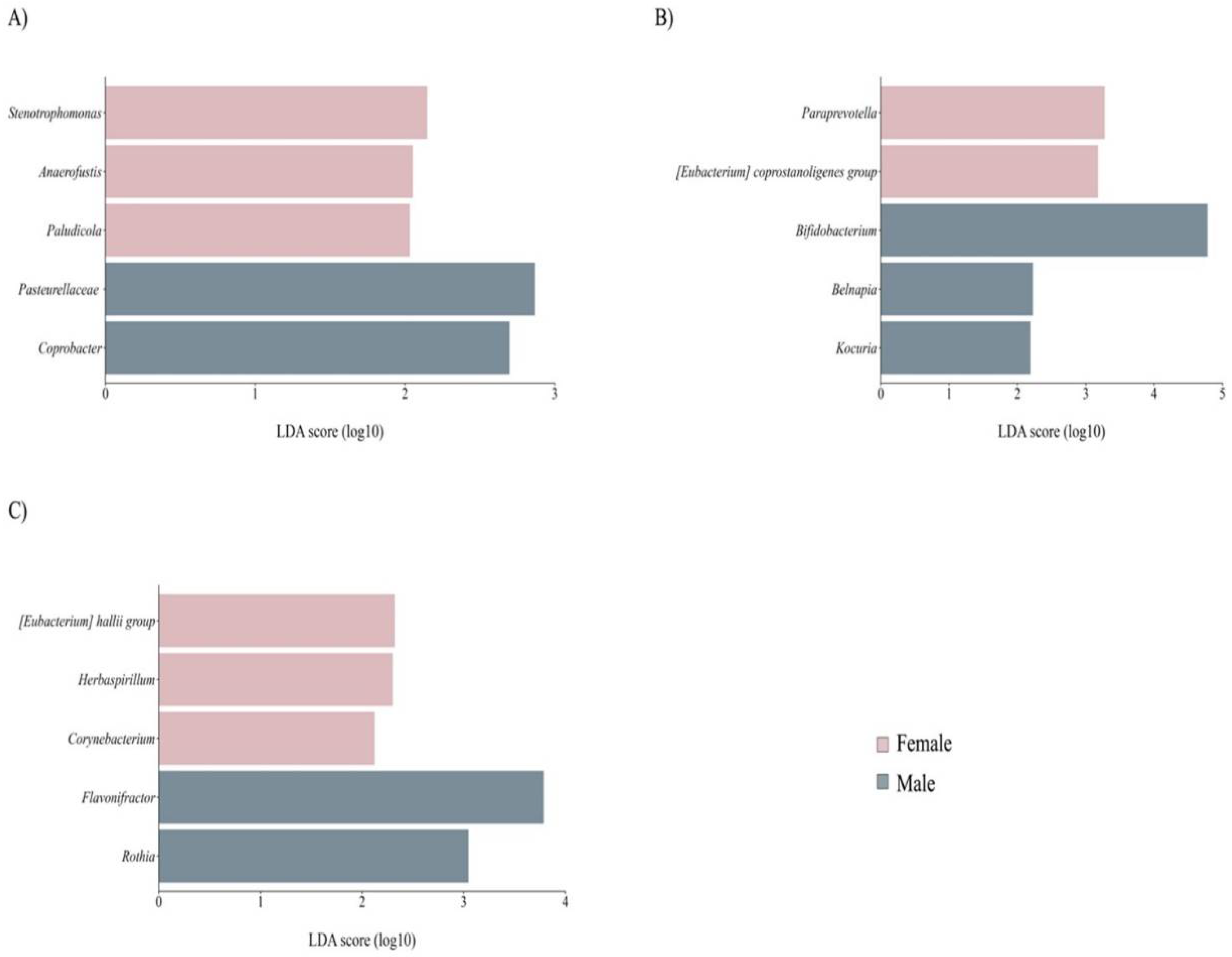
3.3.4. Newborn Sex
3.4. Exploring the Impact of Maternal and Neonatal Factors on GM and BM Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, T.S.; Shanahan, F.; O'Toole, P.W. The gut microbiome as a modulator of healthy aging. Nat. Rev. Gastroenterol. Hepatol. 2022, 19(9), 565–584. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell. Host Microbe 2015, 17(5), 690–703. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 2017, 82(3), 387–95. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Azad, MB. Origins of human milk microbiota: new evidence and arising questions. Gut Microbes 2020, 12(1), 1667722. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Pannaraj, P.S.; Rautava, S.; Rodríguez, J.M. The Microbiota of the human mammary ecosystem. Front. Cell Infect. Microbiol. 2020, 10, 586667. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96(3), 544–551. [Google Scholar] [CrossRef] [PubMed]
- Davé, V.; Street, K.; Francis, S.; Bradman, A.; Riley, L.; Eskenazi, B.; et al. Bacterial microbiome of breast milk and child saliva from low-income Mexican-American women and children. Pediatr. Res. 2016, 79(6), 846–854. [Google Scholar] [CrossRef] [PubMed]
- López-Leyva, L.; González, E.; Li, C.; Ajeeb, T.; Solomons, N.W.; Agellon, L.B.; et al. Human milk microbiota in an indigenous population is associated with maternal factors, stage of lactation, and breastfeeding practices. Curr. Dev. Nutr. 2021, 5(4), nzab013. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, C.; Prescott, S.; Alhusen, J. Influence of maternal prepregnancy obesity and excessive gestational weight gain on maternal and child gastrointestinal microbiome composition: A Systematic Review. Biol. Res. Nurs. 2020, 22(1), 114–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, D.; Cai, W.; Zhu, H.; Shane, M.I.; Liao, C.; et al. Distribution of vaginal and gut microbiome in advanced maternal age. Front. Cell Infect. Microbiol. 2022, 12, 819802. [Google Scholar] [CrossRef] [PubMed]
- Ojo-Okunola, A.; Claassen-Weitz, S.; Mwaikono, K.S.; Gardner-Lubbe, S.; Stein, D.J.; Zar, H.J; et al. Influence of socio-economic and psychosocial profiles on the human breast milk bacteriome of south African women. Nutrients 2019, 11(6), 1390. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Macías, E; Selma-Royo, M.; Martínez-Costa, C.; Collado, M.C. Breastfeeding practices influence the breast milk microbiota depending on pre-gestational maternal BMI and weight gain over pregnancy. Nutrients 2021,13(5), 1518.
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Maternal microbiota, cortisol concentration, and post-partum weight recovery are dependent on mode of delivery. Nutrients 2020, 12(6), 1779. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81(1); 17–47.
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019, 25(2), 324–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; et al. The effects of delivery mode on the gut microbiota and health: State of Art. Front. Microbiol. 2021, 12, 4084. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; et al. Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J. Nutr. 2017, 147(9), 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Hird, S.M.; Chen, M.H.; Xu, W.; Maas, K.; et al. Sex differences in gut microbial development of preterm infant twins in early life: A longitudinal analysis. Front. Cell Infect. Microbiol. 2021, 11, 671074. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012, 7(4), e3308. [Google Scholar] [CrossRef] [PubMed]
- Hausman Kedem, M.; Mandel, D.; Domani, K.A.; Mimouni, F.B.; Shay, V.; Maron, R.; et al. The effect of advanced maternal age upon human milk fat content. Breastfeed Med, 2013, 8(1), 116–119.
- Lubetzky, R.; Sever, O.; Mimouni, F.B.; Mandel, D. Human milk macronutrients content: Effect of advanced maternal age. Breastfeed Med. 2015, 10(9), 433–436. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7(1), 14. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21(12), E607–E615. [Google Scholar] [CrossRef] [PubMed]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; et al. Fecal microbial community structure is stable over time and related to variation in macronutrient and micronutrient intakes in lactating women1-3. J. Nutr. 2015, 145(10), 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.N.; Ferro, L.E.; Russell, K.E.B.; Sugino, K.Y.; Kerver, J.M.; Comstock, S.S. Fecal bacterial communities differ by lactation status in post-partum women and their infants. J. Hum. Lact. 2022, 38(2), 270–280. [Google Scholar] [CrossRef] [PubMed]
- Weerasuriya, W.; Saunders, J.E.; Markel, L.; et al. Maternal gut microbiota in the post-partum Period: A Systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 285, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Cao, X.; Zhang, K.; et al. 16S rRNA gene sequencing revealed changes in gut microbiota composition during pregnancy and lactation in mice model. Vet. Sci. 2022, 9(4), 169. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5(6), 779–784. [Google Scholar] [CrossRef] [PubMed]
- Franco-Esquivias, A.P.; Peña, C.G.; Torres-Lozano, C.; Vaca-Paniagua, F.; Díaz-Velásquez, C.; Ortega-Cisneros, M.; et al. Gut microbiota in Mexican patients with common variable immunodeficiency. Gac. Med. Mex. 2019,155(5),447–452.
- Takada, T.; Kurakawa, T.; Tsuji, H.; Nomoto, K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Piquer-Esteban, S.; Ruiz-Ruiz, S.; Arnau, V.; Diaz. W.; Moya, A. Exploring the universal healthy human gut microbiota around the World. Comput. Struct. Biotechnol. J. 2022, 20, 421–433.
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; et al. GABA Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2019, 4(3), 396–403. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Odisho, A.S.; Lewis, K.; et al. Brexanolone, a GABAA modulator, in the treatment of post-partum depression in adults: A comprehensive review. Front. Psychiatry 2021, 2, 699740. [Google Scholar]
- Civardi, E.; Garofoli, F.; Tzialla, C.; Paolillo, P.; Bollani, L.; Stronati, M. Microorganisms in human milk: lights and shadows. J. Matern-Fetal Neonatal Med. 2013, 26, Suppl 2, 30–34.
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12(4), 1039. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.A.; Mancabelli, L.; Milani, C.; et al. Comprehensive insights from composition to functional microbe-based biodiversity of the infant human gut microbiota. Npj Biofilms Microbiomes 2023, 9(1), 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini A. Proteobacteria: A common factor in human diseases. Biomed Res. Int. 2017, 2017, ID 9351507.
- Camacho-Morales, A; Caba, M.; García-Juárez, M.; Caba-Flores, M.D.; Viveros-Contreras, R.; Martínez-Valenzuela, C. Breastfeeding contributes to physiological immune programming in the newborn. Front. Pediatr. 2021, 9, 744104.
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; et al. Commensal enterobacteriaceae protect against Salmonella colonization through oxygen competition. Cell Host Microbe 2019, 25(1), 128–139. [Google Scholar] [CrossRef] [PubMed]
- Stuivenberg, G.A.; Burton, J.P.; Bron, P.A.; Reid, G. Why are bifidobacteria important for infants? Microorganisms 2022, 10(2), 278. [Google Scholar] [CrossRef] [PubMed]
- Zsálig, D.; Berta, A.; Tóth, V.; et al. A review of the relationship between gut microbiome and obesity. Appl Sci. 2023, 13(1), 610. [Google Scholar] [CrossRef]
- Tang, M.; Marroquin, E. The role of the gut microbiome in the intergenerational transmission of the obesity phenotype: A narrative review. Front. Med. 2022, 9, 1057424. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: a systematic review. Eur. J. Clin. Nutr. 2020, 74(9), 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10(1), 19417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Sun, J.H.; Wang, W.J. Gut microbiota in gastrointestinal diseases during pregnancy. World J. Clin. Cases 2022, 10(10), 2976–2989. [Google Scholar] [CrossRef] [PubMed]
- James Booth, S. Fusobacterium Infections. In: Reference Module in Biomedical Sciences. Elsevier; 2014.
- Mark, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA. 2016, 113(6), E791–800. [Google Scholar]
- Balan, P.; Chong, Y.S.; Umashankar, S.; et al. Keystone Species in Pregnancy Gingivitis: A snapshot of oral microbiome during pregnancy and post-partum period. Front. Microbiol. 2018, 9, 2360. [Google Scholar] [CrossRef] [PubMed]
- Dessí, A.; Bosco, A.; Pintus, R.; Orrú, G.; Fanos, V. Fusobacterium nucleatum and alteration of the oral microbiome: from pregnancy to SARS-COV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25(13), 4579–4596. [Google Scholar] [PubMed]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; et al. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers 2021, 13(9), 2124. [Google Scholar] [CrossRef] [PubMed]
- Daiy, K.; Harries, V.; Nyhan, K.; Marcinkowska, U.M. Maternal weight status and the composition of the human milk microbiome: A scoping review. PloS One 2022, 17(10), e0274950. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Carpén, N.; Helve, O.; De Vos, W.M.; Korpela, K.; Salonen, A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. eBioMedicine 2021, 69, 103475. [Google Scholar] [CrossRef] [PubMed]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17(9), e12921. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; et al. Human milk from atopic mothers has lower levels of short chain fatty acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Grossi, E.; Drago, L. Role of the human breast milk-associated microbiota on the newborns’ immune system: A mini review. Front. Microbiol. 2017, 8, 2100. [Google Scholar] [CrossRef]
- Wang, L.C.; Huang, Y.M.; Lu, C.; et al. Lower caprylate and acetate levels in the breast milk is associated with atopic dermatitis in infancy. Pediatr. Allergy Immunol. 2022, 33(2), e13744. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88(4), 894–899. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87(3), 534–538. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.;C.; García-Valdés, L.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104(1), 83–92.
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol 2014, 16(9), 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11(2), 357–368. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.G.; Coyne, M.J.; Gentyala, R.R.; Chatzidaki-Livanis, M.; Comstock, L.E. Bacteroidales secreted antimicrobial proteins target surface molecules necessary for gut colonization and mediate competition in vivo. MBio 2016, 7(4), 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ginger, M.R.; Rosen, J.M. Pregnancy-induced changes in cell-fate in the mammary gland. Breast Cancer Res. 2003, 5(4), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.P.; Boe, D. M, et al. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021, 109(6), 1045–1061. [Google Scholar] [CrossRef]
- Bosco, N.; Noti ,M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22(5), 289–303.
- Sepp, E.; Smidt, I.; Rööp, T.; Štšepetova, J.; Kõljalg, S.; Mikelsaar. M.; et al. Comparative analysis of gut microbiota in centenarians and young people: Impact of eating habits and childhood living environment. Front. Cell Infect. Microbiol. 2022, 12, 851404.
- Kim, G.; Bae, J.; Kim, M.J.; Kwon, H.; Park, G.; Kim, S.J.; et al. Delayed establishment of gut microbiota in infants delivered by cesarean section. Front. Microbiol. 2020, 11, 2099. [Google Scholar] [CrossRef] [PubMed]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15(4), 197–205. [Google Scholar] [CrossRef] [PubMed]
- Busi, S.B.; De Nies, L.; Habier, J.; Wampach, L.; Fritz, J.V.; Heintz-Buschart, A.; et al. Persistence of birth mode-dependent effects on gut microbiome composition, immune system stimulation and antimicrobial resistance during the first year of life. ISME Commun. 2021, 1(1), 8. Word Health Organization. Complementary Feeding: Family foods for breastfed children. Dep. Nutr. Heal. Dev. , 1–56.
- Yao, Y.; Cai, X.; Fei, W.; Ren, F.; Wang, F.; Luan, X.; et al. Regulating gut microbiome: Therapeutic strategy for rheumatoid arthritis during pregnancy and lactation. Front. Pharmacol. 2020, 11:594042.
- Ramiro-Cortijo, D.; Gila-Diaz, A.; Herranz Carrillo, G.; Cañas, S.; Gil-Ramírez, A.; Ruvira, S.; et al. Influence of neonatal sex on breast milk protein and antioxidant content in Spanish women in the first month of lactation. Antioxidants 2022, 11(8), 1472. [Google Scholar] [CrossRef] [PubMed]
- Galante, L.; Milan, A.M.; Reynolds, C.M.; Cameron-Smith, D.; Vickers, M.H.; Pundir, S. Sex-specific human milk composition: The role of infant sex in determining early life nutrition. Nutrients 2018, 10(9), 1194. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Tras. Med. 2012, 10(1), 253. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.R.; Wei, S.T.S.; Wang, P.H.; Wu, P.H.; Yu, C.P. Microbial degradation of steroid sex hormones: implications for environmental and ecological studies. Microb. Biotechnol. 2020, 13(4), 926–949. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Mora-Ortiz, M.; Tena-Sempere, M.; Lopez-Miranda, J.; Camargo, A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol. Sex Differ. 2023, 14(1), 4. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD or median (minimum – maximum) |
|---|---|
| Maternal | |
| Age (y) | 29.60 (19 – 35) |
| Height (m) | 1.59 ± 0.06 |
| Body weight (kg) | |
| Pre-gestational | 63.74 ± 10.51 |
| 20-28PPD | 64.75 ± 10.64 |
| Last gestational | 74.25 ± 10.39 |
| Gain during pregnancy | 10.51 ± 4.59 |
| BMI (kg/m2) | |
| Pre-gestational | 25.3 ± 3.99 |
| 20-28PPD | 25.71 ± 4.07 |
| Total body fat (%) | 33.03 ± 6.44 |
| Delivery mode (V/C, %) | 53%/47% |
| Newborn | |
| Gestational age (weeks) | 39.3 ± 1.08 |
| Length (cm) | |
| At birth | 50 (48 – 53) |
| At 20-28PND | 52.32 ± 1.79 |
| Gain at 20 20-28PND | 2.27 ± 1.67 |
| Body weight (kg) | |
| At birth | 3.07 ± 0.33 |
| At 20-28PND | 3.81 ± 0.46 |
| Gain at 20-28PND 1 | 0.74 ± 0.47 |
| Head circumference (cm) | 36.55 ± 1.01 |
| Newborn sex F/M (%) | 45/55 |
| r2 | p-Value | |
|---|---|---|
| Mother gut | ||
| Unweighted UniFrac | ||
| Age, y | 0.209 | 0.022 |
| Total body fat, % | 0.004 | 0.944 |
| Delivery mode | 0.010 | 0.830 |
| Sex | 0.021 | 0.707 |
| Weighted UniFrac | ||
| Age, y | 0.144 | 0.075 |
| Total body fat, % | 0.002 | 0.971 |
| Delivery mode | 0.033 | 0.593 |
| Sex | 0.041 | 0.499 |
| Breast milk | ||
| Unweighted UniFrac | ||
| Age, y | 0.030 | 0.719 |
| Total body fat, % | 0.016 | 0.850 |
| Delivery mode | 0.163 | 0.173 |
| Sex | 0.139 | 0.230 |
| Weighted UniFrac | ||
| Age, y | 0.086 | 0.378 |
| Total body fat, % | 0.049 | 0.623 |
| Delivery mode | 0.059 | 0.530 |
| Sex | 0.293 | 0.028 |
| Newborn gut | ||
| Unweighted UniFrac | ||
| Age, y | 0.194 | 0.038 |
| Total body fat, % | 0.004 | 0.945 |
| Delivery mode | 0.163 | 0.060 |
| Sex | 0.019 | 0.743 |
| Weighted UniFrac | ||
| Age, y | 0.048 | 0.465 |
| Total body fat, % | 0.018 | 0.740 |
| Delivery mode | 0.084 | 0.231 |
| Sex | 0.030 | 0.614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





