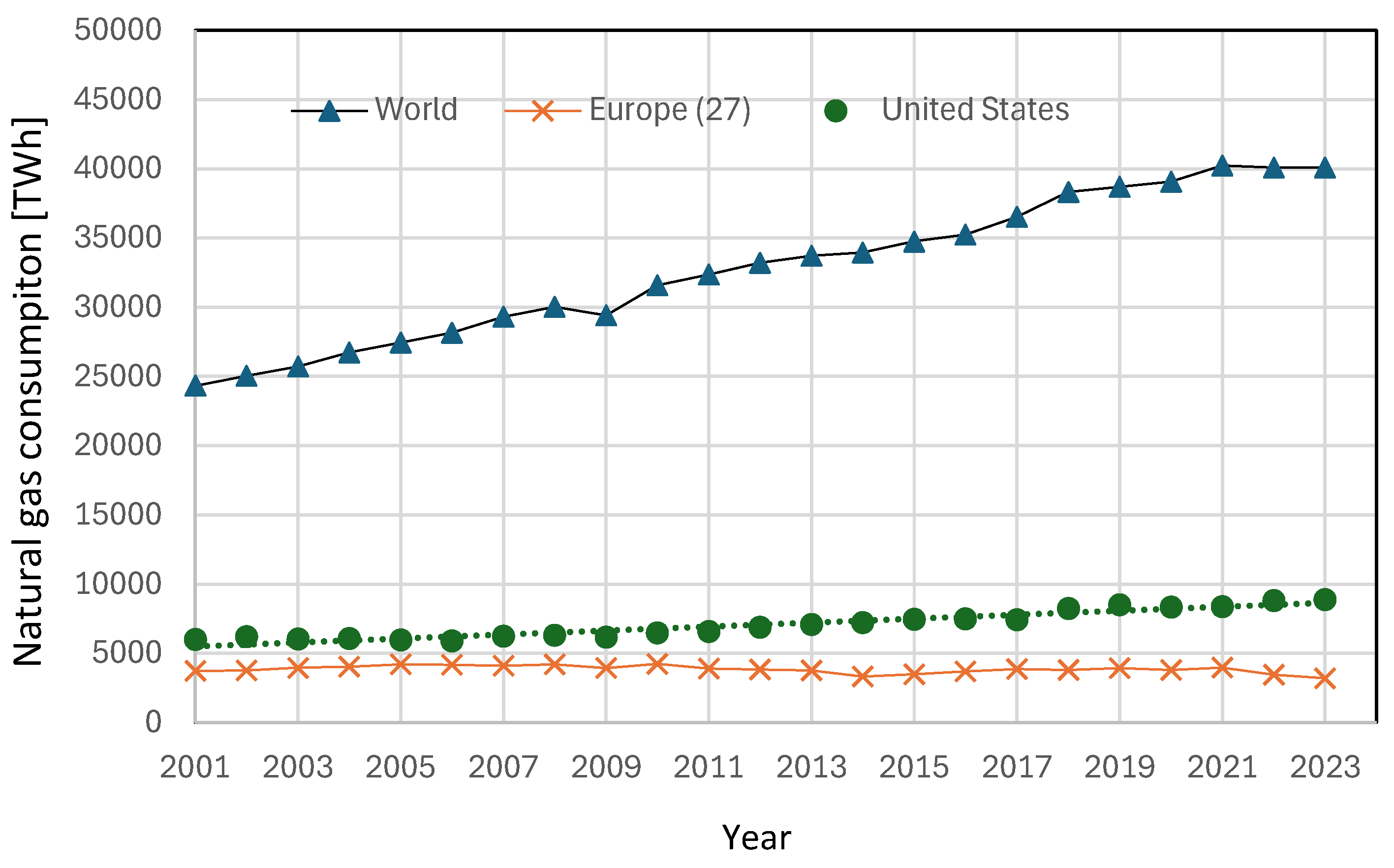1. Introduction
The topic of energy transition is one of the most relevant today. There are many studies and projects addressing this issue, but it is often presented in an overly monothematic way, frequently focusing only on the use of electricity. However, just as energy uses are complex and varied, the solutions to the problem must also encompass a wide spectrum of possibilities. These solutions particularly concern two main areas: civil uses and mobility. A sector that is somewhat underestimated in its potential is industrial energy use. This sector involves significant energy consumption, and since most of the energy used is of fossil origin, it has strong environmental impacts.
Referring to the industrial sector, data from the IEA report indicates that approximately 9 Gigatons of carbon dioxide (CO
2) were emitted by industries, primarily energy-intensive ones. [
1].
The utilization of hydrogen in the industrial sector could play a pivotal role in the energy transition and decarbonization efforts, especially in hard-to-abate sectors. Hydrogen produced through renewable sources holds promise as both a chemical element integrated into industrial processes and as a fuel, either as a partial or complete substitute for natural gas. The adoption of new technologies often hinges on gradual rather than revolutionary shifts, with advancements built upon existing frameworks. Hydrogen can be produced from renewable energy sources via electrolysis. Blending renewable hydrogen with conventional fuels supports the integration of renewable energy into the energy mix, enhancing energy security and sustainability, [
2].
Blending hydrogen with existing fuels can be a transitional strategy to gradually shift towards a hydrogen-based energy system. It allows the use of existing infrastructure while reducing the reliance on fossil fuels. One of the methods for introducing hydrogen in these sectors is its use as a fuel. An interesting possibility is the use of hydrogen in blended combustions, for burners, boilers and furnaces [
3,
4,
5]. Hydrogen's role as a co-fuel mixed with methane presents distinct challenges due to its differing chemical and physical properties. When combined, hydrogen alters combustion dynamics and flame characteristics, such as increased flame speed leading to compact, turbulent flames and potential issues with backfire and flashbacks, [
6]. Adjustments in combustion management become necessary, including air staging to widen the flammability range and mitigate ignition energy requirements. Despite reduced CO
2 emissions, high temperatures can elevate NOX emissions, prompting recommendations for future burner configurations emphasizing Dry Low NOX or flameless designs. Additionally, hydrogen's small size and chemical reactivity with steel alloys pose challenges in piping and storage, necessitating careful consideration in infrastructure design. Ultimately, these factors highlight the complexity of harnessing hydrogen's potential while ensuring efficient heat release. When blending or replacing one fuel with another, the power range and stability of combustion are influenced. Furthermore, the two gas are at different densities, which influences the flow rate that passes through the same nozzle and the heat transfer rate. For any combustion system (boiler or furnace), one of the most important parameters is the heat release rate. Similar gas volume flow rates will give very different heat release rates for different gases. Furthermore, since most systems supply gas through a fixed nozzle, the flow rate varies as a function of density, which in the case of natural gas and hydrogen at atmospheric conditions is an order of magnitude different.
Some papers analyze the possibilities of using hydrogen in hard-to-abate industries (e.g., steel, glass, paper), focusing on hydrogen as an alternative fuel in burners, a reducing agent, and an energy storage system linked to process electrification and the use of renewable energy, [
2]. Several contributions provide support through CFD and Multiphysics modelling of combustion in industrial boilers using hydrogen-rich fuels and possible integration of catalytic units in hard-to-abate technologies, [
7]. Other articles try to analyze hydrogen use in specific industries (e.g., steel, glass, paper), focusing on hydrogen as an alternative fuel in burners, [
8].
Starting from an extensive review of existing literature, this study examines the concept of blended combustion, specifically focusing on hydrogen-methane mixtures in different percentage. Notably, numerous industrial burner manufacturers already offer products capable of accommodating such blends, with claims of up to 20% hydrogen by volume sustaining existing burner technologies. However, the theoretical underpinnings supporting this threshold remain elusive, given the distinct thermophysical properties and flammability characteristics of hydrogen and methane.
The paper is structured as follows. After the introductory section, section 2 delves into the significance of thermal energy use and its importance in global decarbonization efforts.
Section 3 provides an overview of blended combustion technology, reviewing current advancements and state-of-the-art practices. Then a thermodynamic analysis explores the potential of hydrogen as an alternative fuel in burners, focusing on mass and energy balances and employing idealized models of natural gas as methane and introducing the use of synthetic indicators. The following section evaluates the interchangeability of natural gas-hydrogen blends using synthetic indicators, considering the variations in natural gas composition. The discussion section summarizes the findings, emphasizing the potential impact of blended combustion on reducing natural gas usage. A final section that outlines the main conclusions drawn from the study is provided as usual.
2. Thermal Energy Use and Its Importance for Decarbonization
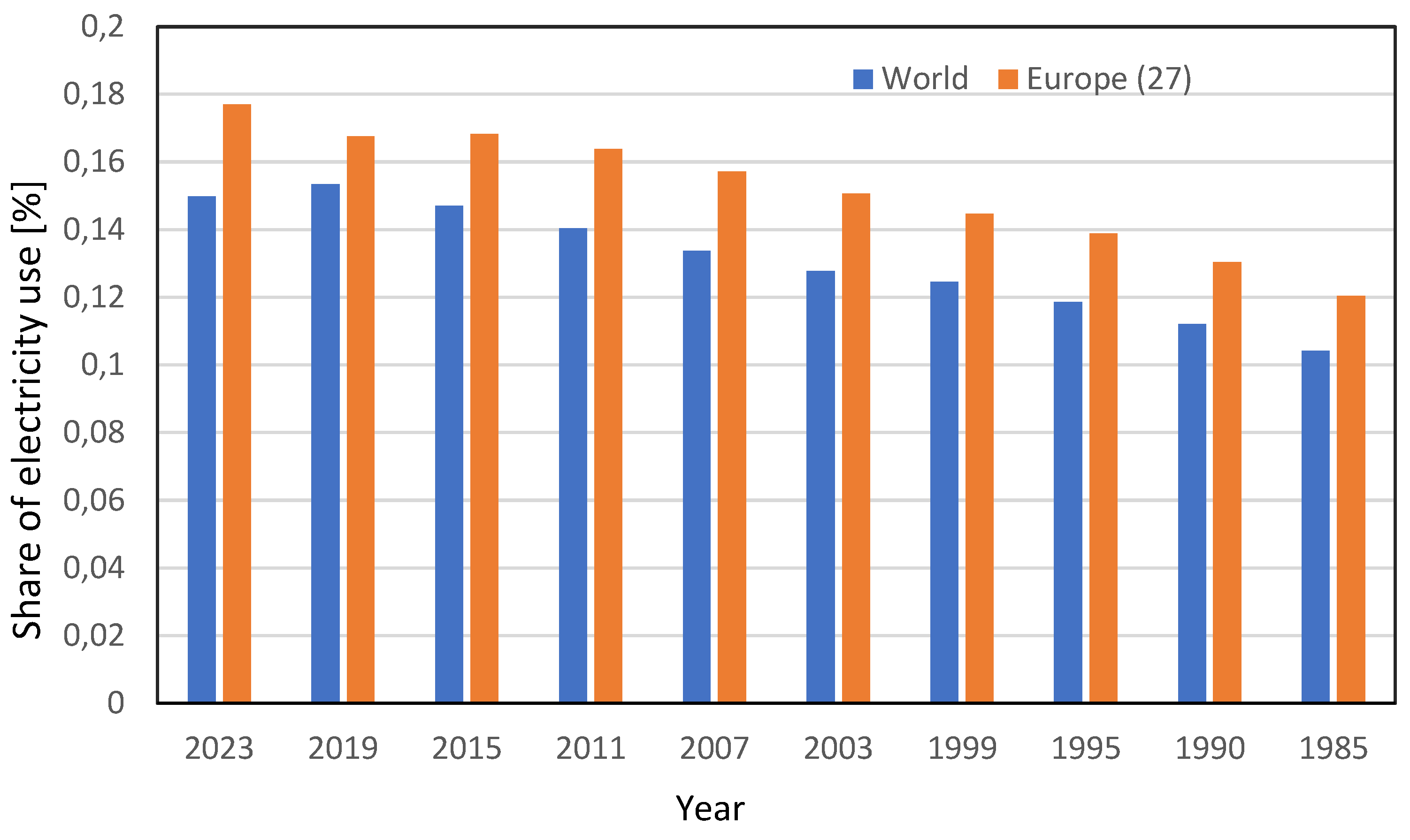
If we analyze the data related to global energy uses, a rather interesting fact stands out. The main uses of energy worldwide (about 50%) are for thermal energy. In terms of primary energy, the use of electricity is often much lower; in the most industrialized countries, it can be traced back to a share of less than 20%. It is rather curious that the bulk of the initiatives related to decarbonization focus on electricity uses. As clearly emerges from the data reported in
Table 1, if we take into consideration the data relating to the use of energy in the last 40 years at world level, there has certainly been a significant increase in the use of electricity. However, this has not greatly influenced the percentage of electricity compared to the total energy used, which has only gone from around 10.4% to about 15%, [
9]. The other energy uses are primarily related to mobility, mainly connected with petroleum, and thermal products, mainly connected to coal and natural gas.
The percentages increase slightly in some countries, but even considering the European Union, the percentage of electricity use does not increase significantly, as shown in
Table 2 if we consider that the share of electricity with respect to total energy use is less than 20% and the trend of increase is not so relevant, as it is clear from
Figure 1, that considers last 40 years and that a great part of non-electric energy uses is non-renewable energy use.
It is therefore clear that some ambitious objectives set by international organizations will not only be unachievable but not even approachable if only the contribution from renewable energy is considered with reference to the electrical use sector alone.
We can also move towards a progressive electrification of thermal uses. This is evident, for example, in the civil and residential sectors, where the penetration of heat pump systems is increasing, effectively transferring thermal uses into electrical ones. The whole topic of ZEB (Zero Energy Buildings) or n-ZEB (nearly ZEB), which is diffusely discussed in the recent literature, [
10], revolves around the powering of heat pump systems using renewable energy, usually photovoltaic systems. The topic, although interesting and noteworthy, nevertheless encounters a significant problem: in winter conditions, when there is a greater need for thermal energy to heat buildings, solar radiation is often reduced. Meanwhile, the integration of heat pump systems with photovoltaic systems is certainly an excellent solution for summer cooling. Anyway, the electricity use for air conditioning represents only 10% of the total (2200 TWh on a total of 27500 TWh as in
Table 1).
To overcome these structural limits, recent literature has given much space to the theme of energy storage in its various forms and to the integration of energy users. This theme has taken on various connotations (virtual power plants, microgrids, energy communities), even if it does not seem to address the real problem, which is the mismatch between energy use and renewable production.
Furthermore, the topic of renewable energy is often trivialized. It is often assumed that renewable energy resources cost nothing, but this is not true because all forms of energy have a cost. In the case of renewable sources, while it is true that the resource itself has no costs, the installed power does cost a lot, and this affects system costs.
Returning to the use of thermal energy, this is only partly linked to the civil sector. In many cases, energy is used for high-temperature industrial processes, where it is quite difficult to think simply about replacing this energy with renewable energy.
While there are alternatives such as solar for process heat at temperatures below 60 °C, which are nevertheless relevant—as also analyzed by one of the authors of this work in a recent article, [
11]—the use of solar heating is certainly not conceivable for numerous applications where the temperature exceeds 150-200 °C. This is particularly true in sectors conventionally referred to as hard-to-abate.
In those sectors energy transition could only happen by progressively electrifying thermal processes, but this is not always possible due to technological challenges. In certain energy sectors, hydrogen represents the only real alternative to allow renewable energy to penetrate significantly. Therefore, in this decarbonization process, the role of hydrogen produced from renewable sources (green hydrogen) can become very important, particularly its use as a fuel for high-temperature processes. Initially, this could be done by mixing it with natural gas, with increasingly higher percentages of hydrogen. Among other things, this approach would have the advantage of allowing a progressive penetration of hydrogen in the thermal energy sector.
3. Blended Combustion of Hydrogen and Natural Gas
The high thermal demands of industrial furnaces, mainly in the various hard-to-abate sectors (steel, chemical and petrochemical, cement, glass, paper and cardboard among the others) are traditionally met by fossil fuels, particularly natural gas. Hydrogen can be used as a fuel to produce thermal energy too.
Almost all the heat high-grade requirements are (T > 400 °C), with temperatures reaching up to 1500 °C or more, where any electrification of the heating sources is complex and requires a complete redesign of the equipment with high capital cost). In this case, the burners can be slightly modified to use hydrogen as a fuel, at a relatively low capital cost.
Ensuring that hydrogen can meet similar conditions without altering oven characteristics or final product quality is paramount. Transitioning from existing burners and ovens to gradually incorporate hydrogen blending before fully transitioning to hydrogen is an intriguing strategy. However, the chemical and physical properties of hydrogen (H
2) mixed with CH
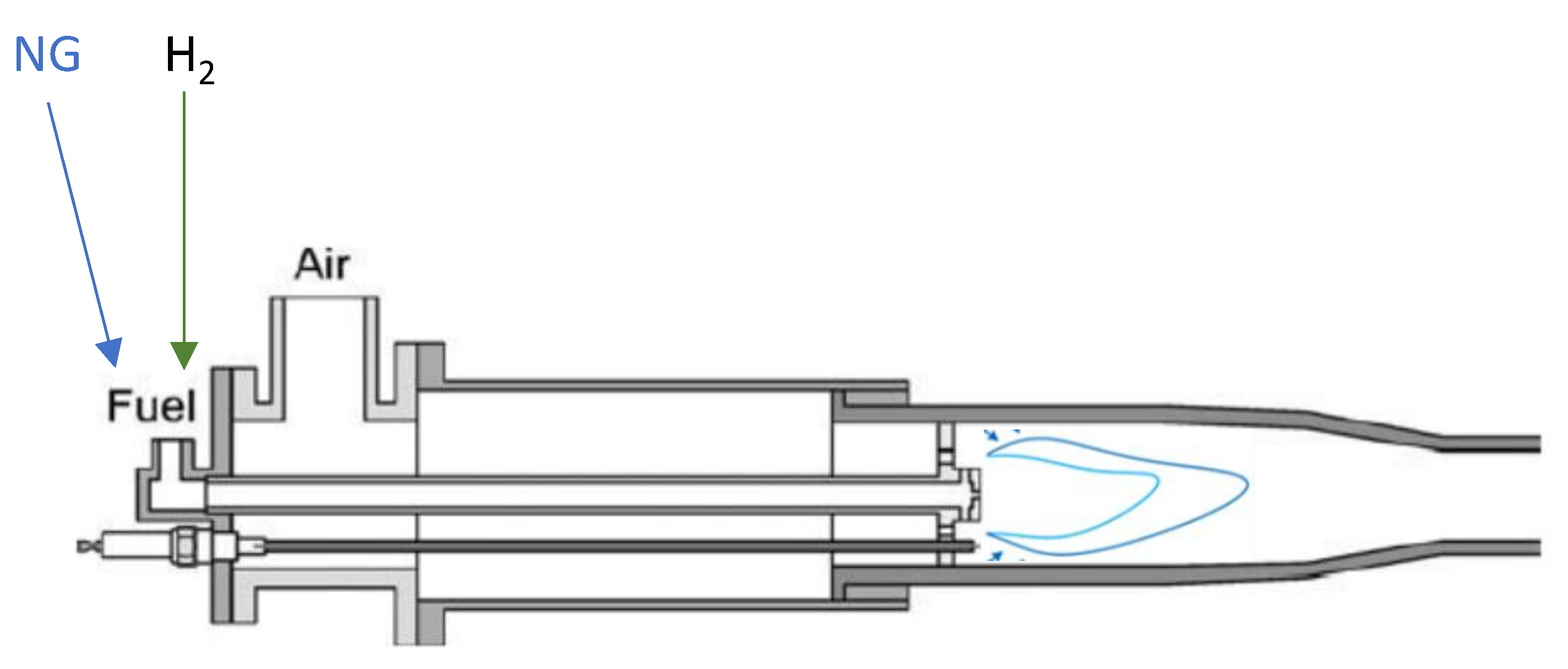
4 result in notable changes to flame characteristics compared to using methane alone, particularly as the hydrogen content increases. A simple schematic representation of an air furnace with blended combustion is provided in
Figure 2. When planning to implement blending techniques in production line burners, it's essential to anticipate potential changes in heat exchange methods based on the percentage of hydrogen in the mixture. This might require modifications to furnace design or exhaust recovery pathways. Considering its thermophysical properties, [
12], the presence of hydrogen alters combustion for several different reasons:
hydrogen delivers about 2.5 times more heat per unit mass than CH4 but low density: Higher Heating Value (HHV) and Lower Heating Valus (LHV) are 2.4-2.6 times the corresponding of Methane;
hydrogen's faster flame speed results in shorter, more compact, and turbulent flames;
hydrogen raises combustion temperatures, which may exceed oven thermal specifications.
hydrogen expands the flammability range mixtures, increasing reactivity and the risk of flashbacks.
Burner manufacturers currently report the feasibility of blending with up to 20% H2 by volume without requiring changes to burner technology or operating conditions at temperatures exceeding 750°C, despite changes in combustion properties.
There are various types of boilers available on the market that allow hydrogen to be used even partially as a fuel. In detail, there are boilers (or furnaces) that can be converted from natural gas to hydrogen, partially converted boilers that can operate with a mixture of natural gas and hydrogen (generally up to 20%) and 100% hydrogen-ready boilers that can operate exclusively with hydrogen gas. To date, the actual use of boilers designed for hydrogen is rather limited, also due to the reduced availability of infrastructure for the production and transport of hydrogen, however it is likely that this sector will be strongly developed in the coming years. Fuel switching considerations are crucial when transitioning to hydrogen. A thorough evaluation of the entire system (boiler or furnace) is necessary to ensure a seamless and effective integration.
This involves assessing the compatibility of existing infrastructure with hydrogen, examining the combustion characteristics, and identifying any required modifications or upgrades. Factors such as burner design, fuel delivery systems, and safety measures must be carefully analyzed. Additionally, the impact on overall system efficiency, emissions, and operational costs needs to be considered. A comprehensive approach ensures that the shift to hydrogen not only supports decarbonization goals but also maintains the reliability and performance of the combustion system.
Table 3 and
Table 4 show some boilers (
Table 3) and burners (
Table 4) which allow the use of blends natural gas-hydrogen up to 100% H
2, respectively. As can be seen by observing the data reported in
Table 3 and
Table 4, two points of view are being compared: that of creating blended combustion with hydrogen fractions lower than 20% and that of commercialize systems capable of operating with 100% of hydrogen. The first type would try to modify the existing systems as little as possible and would guarantee a certain versatility of use, while the second type still requires a complete modification of the thermal generation system. Burner manufacturers generally indicate that up to 20% hydrogen by volume can be mixed with methane while maintaining the same burner technology, provided the process parameters meet the product specifications. In applications where direct flame contact with the product is necessary, such as many processes in the steel industry, the presence of hydrogen increases the flame temperature. This can result in the output product reaching excessively high temperatures, potentially causing mechanical issues. Additionally, hydrogen in the mixture results in shorter flames and expands the flammability range, increasing reactivity, diffusivity, and reaction speed compared to pure methane.
Hydrogen burners are still in the research phase, as complete replacement with hydrogen requires new gas lines, control systems and technology that is very different from the traditional one, involving some relevant additional problems, as evidenced in
Table 5. Combustion management is difficult and still under study, but as has happened in the past when faced with the use of new fuels, various analyzes are necessary for complete knowledge of the fuel.
A relevant obstacle to the diffusion of totally hydrogen energy conversion systems may be represented by the cost of H2 ready boilers which can be higher than traditional natural gas boilers and furnaces. Furthermore, we should not ignore the fact that in the case of devices totally powered by hydrogen there is a risk of losing flexibility and the supply of green hydrogen is certainly not simple if we consider the average power of industrial furnaces. Although evaluating the effectiveness of the two strategies must consider the specific application context, the next section aims to provide a more detailed analysis of the decarbonization potential of the blended-combustion strategy.
4. Thermodynamic Analysis of Hydrogen as an Alternative Fuel in Burners
The unique physical-chemical properties of hydrogen (higher diffusivity, larger flame speed, higher adiabatic flame temperature, T
ad,f , etc.) compared to hydrocarbon fuels present significant challenges for its use in burners and combustors, [
13].
Table 6, drawn up by the authors on the basis of various sources available in the literature, highlights, albeit in a fairly qualitative manner, some relevant differences between the two fuels, from which the relevant quantitative differences between some parameters, as Minimum Energy required for Ignition (MEI), flame velocity in air and diffusion coefficient in air can also be highlighted, which will obviously have an influence on the phenomenon of combustion.
A lot of research activity is conducted on this topic, providing experimental data and knowledge for the design of hydrogen-fueled burners and combustors, focusing mainly on the analysis of premixed hydrogen/air combustion in swirl burners. Key areas of investigation include the effects of swirl level and fuel injector configuration on flame structure and combustion stability (flashback and blow-off), and NOx emissions. Experimental studies of H
2 combustion in premixed and non-premixed atmospheric swirl burners are conducted too. It is also possible to find several modelling activities of domestic and industrial boilers, including CFD analysis. The enrichment of green fuels with hydrogen in variable fractions to assess performance and emissions of small-scale energy systems for stationary use is investigated too. But the first relevant evaluation concerns energy balance. For this reason, assimilating natural gas with methane, the equivalent composition of the fuel blend can be identified as a function of hydrogen percentage by mole or weight. Assuming an ideal combustion by using the properties of components in
Table 6, a model of combustion can be developed.
The analysis of hydrogen blends can be conducted basing on a preliminary energy analysis, based on a thermodynamic model. For a well-defined combustion device, the idea to achieve equivalent thermal input in the burners [
12]. Hydrogen and methane (the primary component of natural gas) not only have significantly different lower heating values (LHV) which is approximately 10 MJ/Nm³ for hydrogen versus 33 MJ/Nm³ for methane), but they also require different amounts of oxygen for complete combustion due to their different molecular weight and combustion characteristics.
According to the basic oxidation reaction, considering a combustion with air, the following stoichiometric equation can be considered. From the mass balance it means that for 1 mole of Methane, 2 mole of oxygen are required (approximately 9.52 mole of air):
From the balance expressed by Eq. (1) it is possible to observe the equivalence between molar production of CO2 with respect to molar use of CH4. Considering that the ratio between the molar weight is 2.75 (44 for CO2 and 16 for CH4), this means that for each kg of methane burned, 2.75 kg of CO2 are produced and consequently emitted because of the oxidation process. For this reason, we can appreciate how for each unit of energy saved, 2.75 units of CO2 are emitted, so that each reduction of mass flow rate of natural gas corresponds to a reduction of the carbon dioxide emissions.
Another relevant difference connected to the different combustion temperature is represented by the changes in flue gas composition with a reduction in the flue gas flow. The change in flue gas composition and flame temperature can affect the radiation flux, for two relevant reasons. The heat radiation varies according to the law:
Combustion of blended mixture of methane and hydrogen determines a variation of the temperature
and in the emission coefficient (
) of the flame gases depends upon the partial pressures of the gas composition (it mainly depends on the quantity of carbon dioxide and water, on the absorption path length and on the temperature.
The emissivity of the two substances, including pressure and overlap functions can be obtained from empirical relations derived from spectral data.
The flame temperature increases with increasing hydrogen content, the water fraction increases, and CO2 fraction in flue gas decreases with increasing hydrogen content. As a result, the estimated net radiation flux from the flame increases with increasing hydrogen content. As we have said and as can be appreciated by analyzing some of the characteristic parameters of the two fuels, the problem cannot be analyzed only from the point of view of mass and energy balances, while recognizing this as a relevant assumption.
A preliminary analysis of the combustion of methane-hydrogen mixtures can be carried out using some synthetic indicators. The concept of gas interchangeability using empirical indicators was developed since before the Second World War [
14]. Gas interchangeability can be defined as the degree of change in the performance of a combustion device when the fuel is varied. Two fuels can be considered interchangeable when they can be used in the same combustion device without the operating conditions and other operating parameters relating to both combustion performance and safety varying significantly. Meeting gas interchangeability criteria ensures that any gas-fired equipment using a replacement gas continues to meet the performance standards for which it was originally approved, [
15].
However, the approach based on the use of synthetic indicators appears to be closely linked to a commercial approach to the combustion problem. However, some of the indicators found in the literature appear rather interesting and worthy of consideration.
Among the others, for example the Wobbe index (WI), a parameter for defining fuel interchangeability. This indicator considers the idea that gases with similar Wobbe numbers produce equivalent heat release rates, facilitating comparisons and optimizing burner performance. According to the vision diffused in the literature, this preliminary analysis becomes crucial for ensuring optimal burner performance, including factors such as flashback prevention, flame propagation, and heat transfer capacity, [
6]. Wobbe index, which serves as a metric for fuel interchangeability, [
6] can be defined for a specific fuel, as natural gas, as:
where RD is the ratio between the density of natural gas,
and air,
. If the same concept is applied to a mixture of hydrogen and natural gas the Wobbe Index can be defined as:
where for each fuel HHV is the higher heating value, is density and x the volumetric fraction. The basic concept for blended combustion is that gases with similar Wobbe index will produce the same heat release in a furnace through the same nozzle at a pressure of similar power supply. Calculating WI of the hydrogen-methane mixture shows that it is not profitable to increase too much hydrogen percentage [
6].
Another metric for fuel interchangeability is the Heat Rate Ratio (HRR) that is defined as the ratio between the Wobbe Index of a gas selected for the substitution (for example a mixture natural gas-hydrogen) and the Wobbe Index of the natural gas (to replace).
The Wobbe index, although widely accepted as an indicator, is a highly qualitative parameter. Moreover, there are other similar indicators available. The Wobbe index is a parameter that is difficult to use practically in its basic form as described by the equations above, because we cannot generally consider having a mixture of just two gases. Natural gas is a multi-component fuel, in which methane is certainly the major component, but the fraction of other gases is also significant. Shkarovskiy et al., 2022 [
16] suggested that two gases can be considered interchangeable when the HHR is within the range 0.95-1.05 while a greater deviation is considered inadmissible as it implies a reduction in combustion efficiency and a loss of combustion stability. Other metrics for fuel interchangeability require the volumetric composition of the gas mixtures.
Another relevant indicator, used to appreciate the modification in combustion is the Maximum Combustion Potential (MCP). This is an index of the theoretical burning rate of a mixture of gases based on the burning rate of hydrogen and is defined as:
In the MCP indicator the volumetric fractions of hydrogen (H2), carbon monoxide (CO) and hydrocarbons are considered, in details in CmHn the fractions of all the hydrocarbons are included except CH4 which is considered separately.
Lin et al., 2018 [
17] suggested that two gases can be considered interchangeable when the variation of MCP remains below of ±10%. Several risks are impacted in the use of this indicator and the results obtained are influenced by the presence of unburned gas, combustion process and combustion products.
Although the use of indicators such as the Wobbe index and Maximum Combustion Potential, appears to be prevalent in the literature, this approach tends to be quite qualitative and does not adequately address the problem from an energy and reduction of pollutant emissions perspective. The Wobbe index, while useful for certain comparisons, falls short in providing a comprehensive energy analysis of blended combustion. Therefore, let us delve deeper into the issue from an energy standpoint to gain a more accurate understanding. A mass and energy balance of the combustion systems appears to be essential. In the case of blended combustion, it is essential to maintain the required thermal power input to the system. Therefore, when transitioning from pure natural gas to a mixture of natural gas and hydrogen, increasing the hydrogen fraction necessitates an increase in the inlet volumetric flow rate due to hydrogen's lower energy density. Moreover, considering that in general the combustion processes are at high temperature, it is better to consider the Lower Heating Value, instead of Higher Heating Value.
In this way, the equivalent calorific value of the fuel mix, LHV
MIX, can be estimated by the weighted average of the heating values of fuels present in the blend according to the following model, in which x is the fraction (in mass or volumetric) considered:
In general, the volumetric fraction can be considered, so that, for a given input power in the burner, P
burner, the volumetric flow rate of fuel, q
MIX in m
3/s, can be estimated as:
The volumetric flow rate of hydrogen can be estimated as:
while the volumetric flow rate of methane results to be:
Increasing the fraction of hydrogen in the fuel, the volumetric flow rate q
MIX increases.
Table 7 provides a trend of volumetric flow rates for a reference burner with input power of 1 MW. Increasing the fraction of hydrogen in the fuel, the volumetric flow rate q
MIX increases.
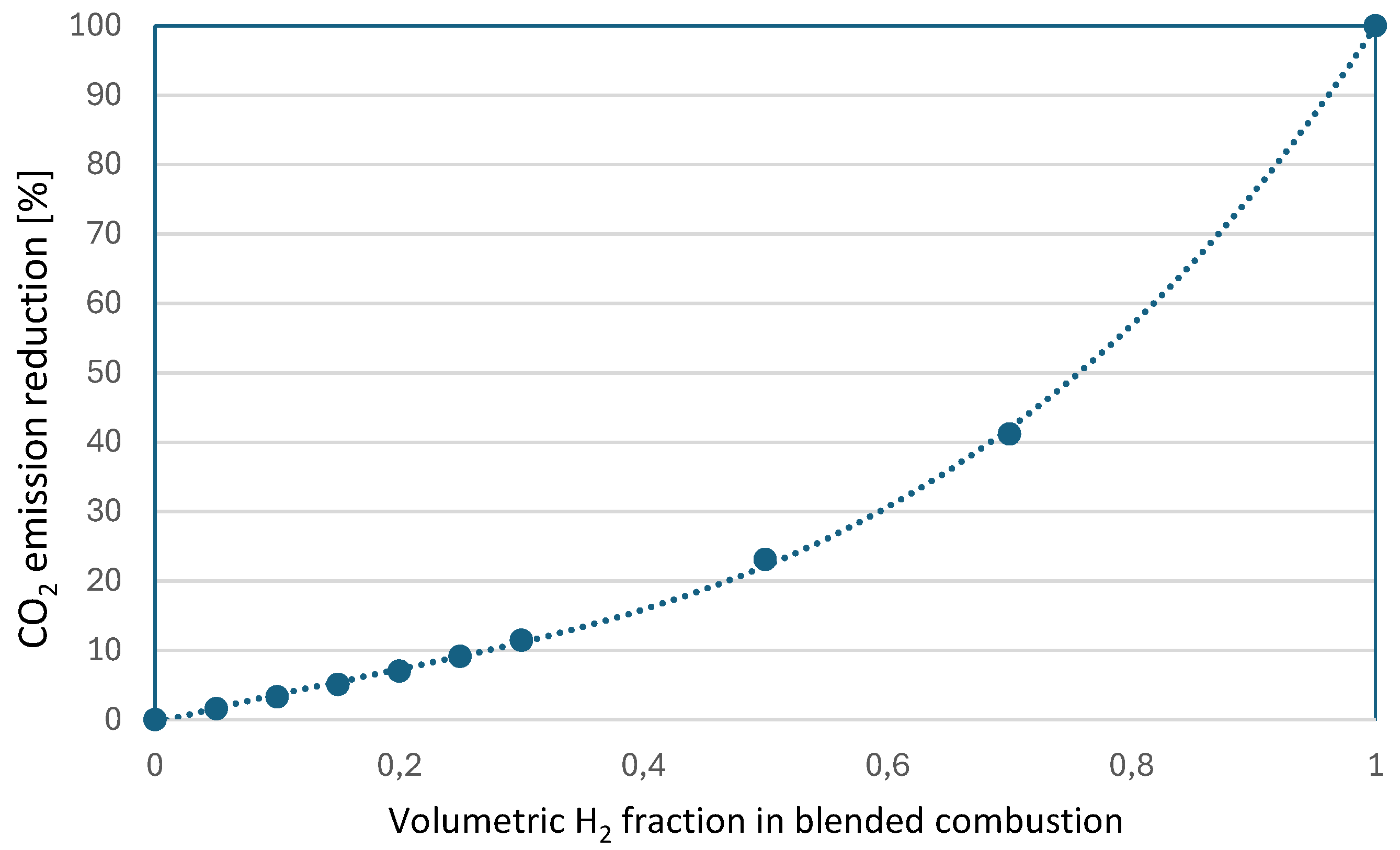
Table 8 instead, starting from the volumetric percentage of hydrogen in the mixture, shows the corresponding variations in the mass flow rates of the two fuels and an evaluation of the potential reduction of carbon dioxide emissions. Analyzing the results in the table, it is evident that the commercially utilized levels of blended combustion (around 20% hydrogen by volume) are not particularly effective from a decarbonization perspective. A 20% hydrogen blend results in an estimated specific CO2 emissions reduction of only about 7%, while increasing the blend to 30% barely surpasses a 10% reduction (11.3%) of CO2 emissions (
Figure 3).
In this section, we have examined the issue of hydrogen-natural gas blended combustion, starting with the idealization of natural gas as methane. In practice, however, natural gas has a composition that is quite different from methane and can vary significantly from case to case. This variability complicates the situation further, making it more challenging to address the problem of blended combustion. In the next section, we will explore the potential effects of these variations.
5. Results of Interchangeability Metrics for Natural Gas-Hydrogen Blends Using Synthetic Indicators
From a conceptual standpoint, the problem of blended combustion of natural gas with hydrogen is relatively straightforward. However, there are two approaches to address the issue: purchasing boilers or furnaces specifically designed for pure hydrogen use or assessing the limits of hydrogen utilization in conventional systems. The latter appears to be the simpler and more intermediate approach. However, identifying the upper limits of hydrogen usage is not always easy. It is crucial to keep in mind that the composition of natural gas varies significantly depending on the region of the world from which it originates. In the previous section we have already discussed the topic of blended combustion from an energy point of view, seeing how the problem could be addressed by combining the use of synthetic parameters such as WI and MCP and the use of mass and energy balances. This approach turns out to be quite simple in the case of a methane-hydrogen mixture, however it must be considered that natural gas has an often-variable composition in terms of chemical composition. This complicates the perspective a bit and makes it more difficult to control the properties of the mixture as a function of the percentage fraction of hydrogen because natural gas may contain small percentages of hydrogen too.
For example, the natural gas injected into the Italian national distribution network is imported from various countries. In 2021 Italy has imported natural gas mainly from (data published by the Italian Ministry of Ecological Transition): Russia (40.0%), Algeria (30.8%), Azerbaijan (9.9%), Qatar (9.4%), Libia (4.4%), Norway (2.7%), U.S.A. (1.5%), The Netherlands (0.4%), Nigeria (0.4%), Egypt (0.3%), Spain (0.1%). The percentages have changed drastically since 2022, following the outbreak of the Russia-Ukraine war. Although the main element is methane (CH
4), natural gas is also composed of secondary elements such as ethane, propane and butane. Depending on the origin of extraction, natural gas can be characterized a specific wellhead composition [
16]. Consequently, the values of higher heating value (HHV), Wobbe index (WI) and relative density (RD) cannot be considered invariable. Average values are frequently used, however depending on the type of investigation it may be more correct to consider the actual variability of these characteristics.
Table 9 shows the main characteristics of the natural gas injected into the national distribution network for some countries of origin. At a national level, each country establishes the acceptability ranges of Lower and Higher Calorific Value, chemical composition ranges and relative density (density of the gas compared to that of the air) that must be satisfied by the natural gas injected into the distribution network. In Italy, these requirements (
Table 10) are indicated in [
19].
Considering the limit values reported in [
16], the results of Wobbe Index (WI) for natural gas-hydrogen blends are shown in different cases and composition. The analyzed mixtures were obtained considering the different properties of the natural gas injected into the Italian national distribution network (
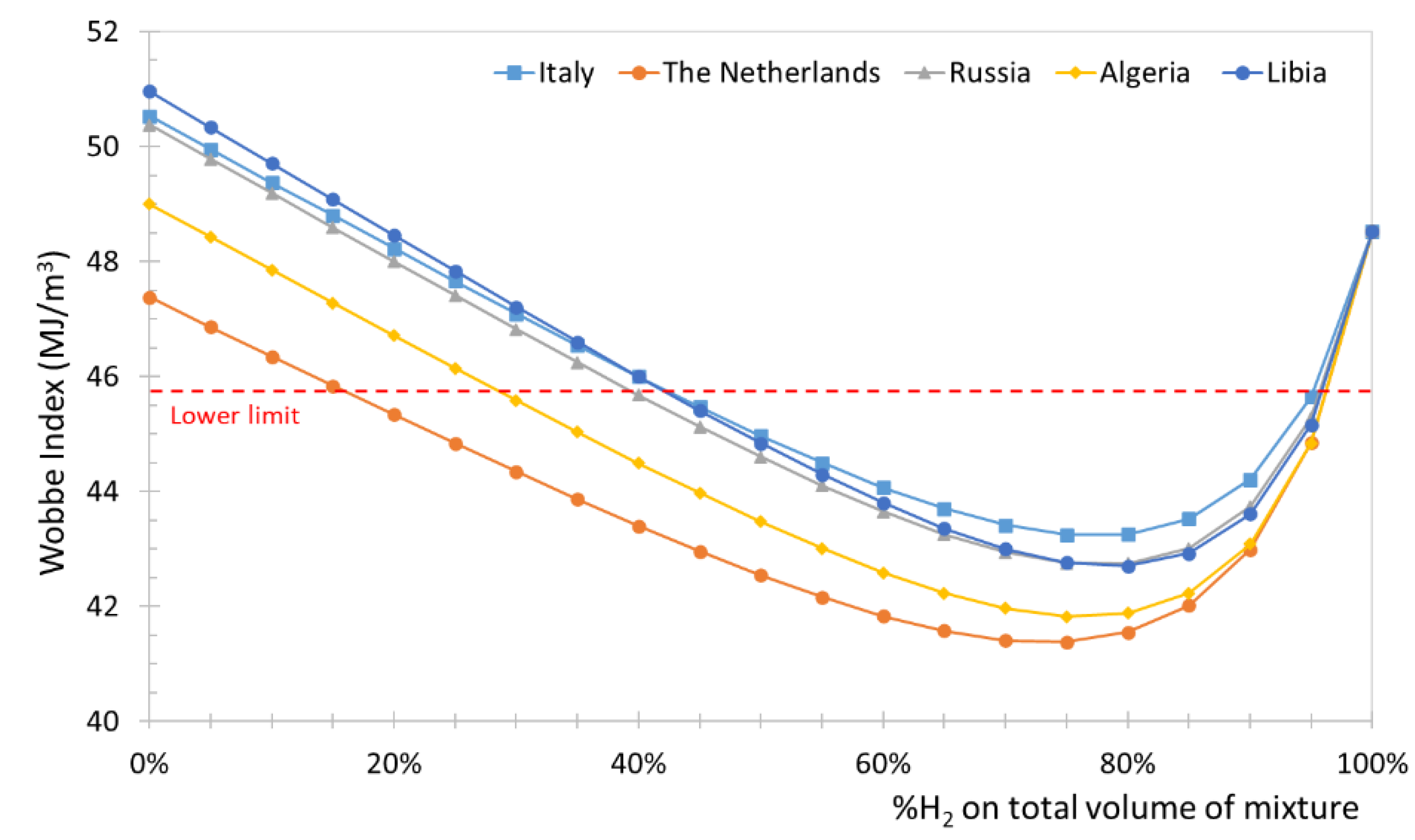
Table 9) and volumetric fractions of hydrogen increasing from 0% to 100%. In
Figure 4 is shown the variation of WI with the percentage by volume of hydrogen for natural gas extracted in Russia, The Netherlands, Algeria, and Libia, while with a dotted red line is represented the lower limit level of the Wobbe index (WI=45.7 MJ/Sm
3, as described in
Section 3) introduced by [
19].
From the obtained results it is possible to observe that the percentage of hydrogen that can be considered acceptable (i.e. which corresponds to a Wobbe index of at least 45.7 MJ/Sm3) varies significantly depending on the origin of the natural gas. In detail, for the gas extracted in Italy, Russia and Libia, hydrogen volume concentrations up to 45% can be accepted, while for the gas coming from Holland and Algeria the percentage of acceptable hydrogen is lower and equal to approx. 15% and 30%. In all cases, in relation to the WI, natural gas-hydrogen blends with a volumetric percentage of hydrogen greater than 95% are permitted. Considering the wide variability of the characteristics of natural gas and the acceptability ranges required on Italian territory, natural gas-hydrogen blends was studied assuming different types of natural gas created using the HHV and RD limit values reported in
Table 10. In detail, the following gases were hypothesized: a natural gas characterized by values maximums of both HHV and RD (NG1), a natural gas with the minimum values of both HHV and RD (NG4), and gases with the maximum of HHV and the minimum of RD and vice versa (NG2, NG3).
Finally, an "average" natural gas characterized by the average of the acceptable limit values of HHV and RD (NG5) was assumed. The characteristics of the studied natural gases are shown in
Table 11.
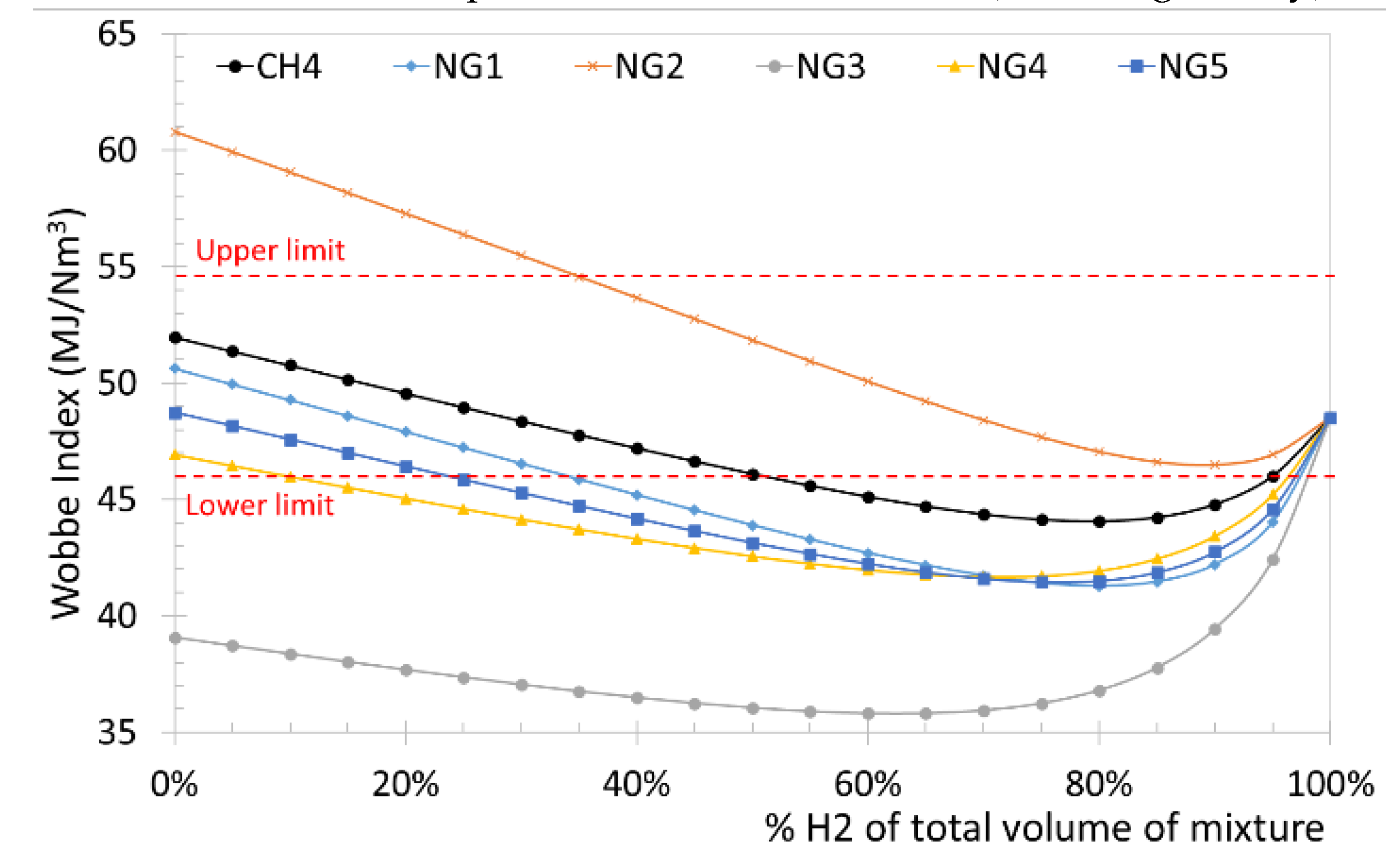
Figure 5 shows the variations of WI as the percentage (by volume) of hydrogen varies for different natural gases, for the pure methane-hydrogen blends. In
Figure 5, the lower and upper limits of the Wobbe index for boilers in accordance with the EN 437 standard are also represented with horizontal dotted lines. From the results reported in
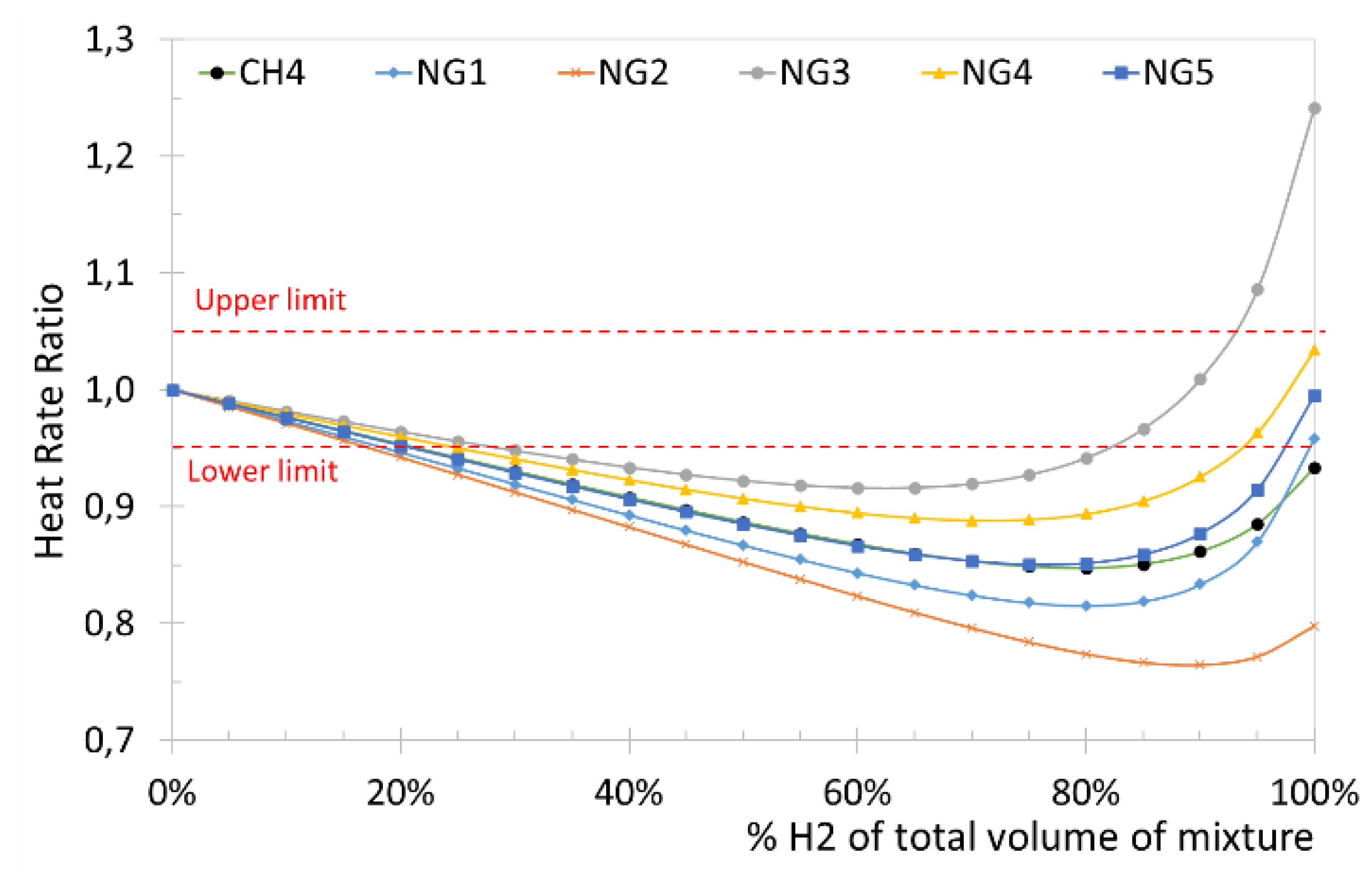
Figure 5, for a pure methane-hydrogen blend it is possible to reach up to 50% hydrogen by volume. As regards NG5 (average HHV and average RD) natural gas, it is possible to introduce up to approximately 25% by volume of hydrogen, while for NG4 (Minimum acceptable HHV and minimum acceptable RD), only 10% by volume is permitted. Finally, the NG3 (Minimum acceptable HHV and maximum acceptable RD) does not allow mixing with hydrogen because WI is always lower than the minimum required. The same natural gases used for the evaluation of WI as a function of the percentage of hydrogen were used to analyze HRR. In
Figure 6 the results of HHR and the lower and upper acceptability limits (according to [
18]) corresponding to a variation of ± 5% (two dashed red lines) are represented too. From the results of
Figure 5 and
Figure 6 it is evident that a maximum volume percentage of hydrogen of between 20% and 30% is admitted depending on the type of natural gas, but some relevant variations can be appreciated.
(*) mean value between maximum and minimum acceptable values.
To analyze the behavior of a different index like MCP for evaluating the interchangeability of gases, it is necessary to know the volumetric composition of the gas mixtures. While, on the one hand, the density and higher calorific value values are available, the volumetric compositions are more difficult to obtain. The data presented in this section have been obtained considering the typical composition of natural gas, as indicated in [
20] and within [
21] and reported in
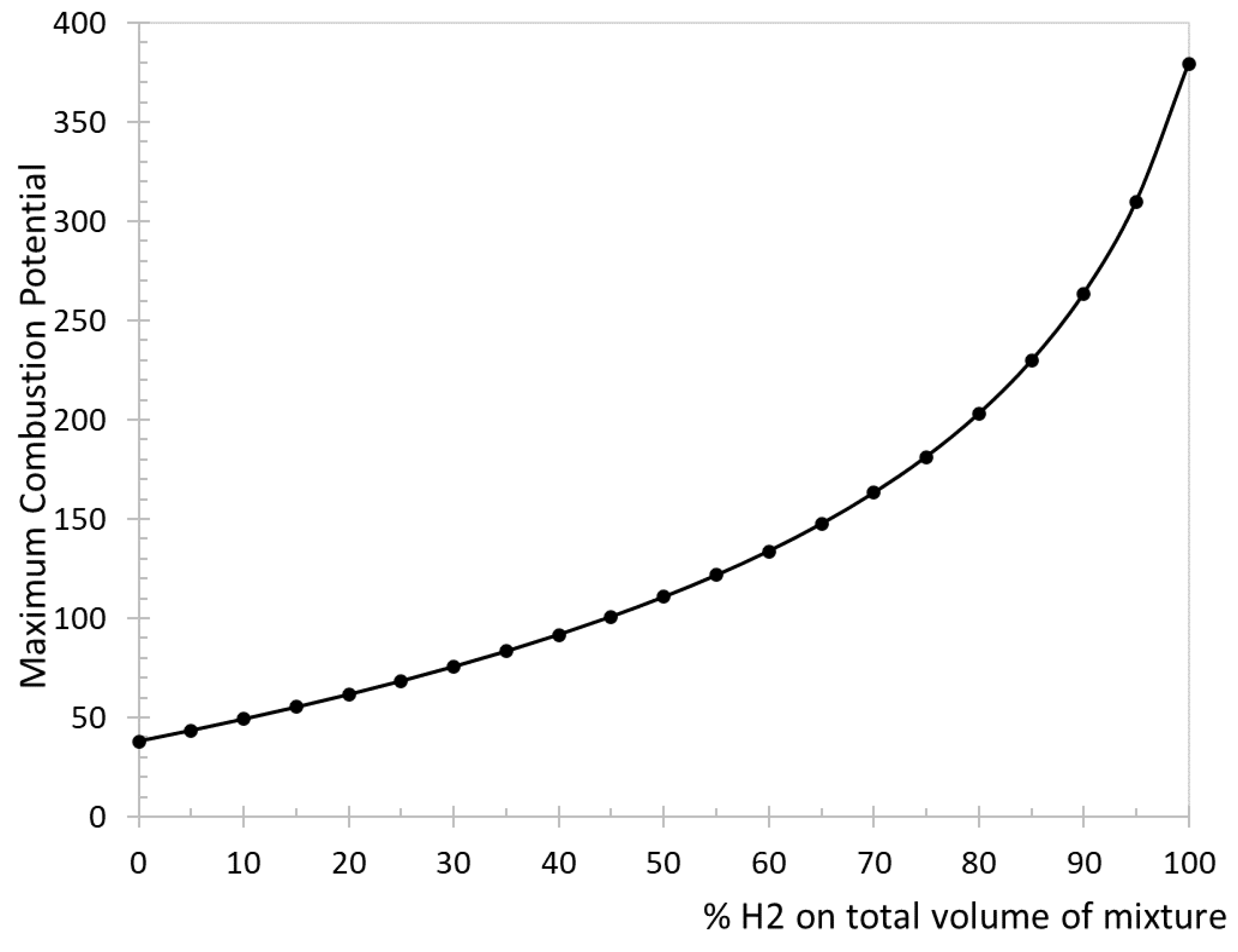
Table 12. The variation of MCP as a function of the volumetric percentage of hydrogen in the natural gas-hydrogen blend is represented in
Figure 7. This trend is always increasing as hydrogen percentage increases. Currently no specific limit values for this index are recognized, so the analysis is limited to evaluating the variation of the index in comparison its reference value (natural gas only).
In this sense, the indicator requires a careful interpretation. For example, it can be observed that MCP varies even for small percentages of hydrogen. A percentage of hydrogen of 5% corresponds to an increase in MCP of approximately 15%, while it is doubled for a percentage of hydrogen of 30%. Anyway, the usage limits of this indicator are not actually clear.
6. Discussion
The behavior of hydrogen as a fuel is well known, and resorting to systems with blended combustion can represent one of the first steps towards decarbonization, before considering the systematic use of hydrogen as a pure fuel. While blended combustion is not a long-term strategy, gradually increasing hydrogen content in mixtures should not be underestimated as a transitional measure. The study of gas interchangeability in the context of natural gas-hydrogen blends is extremely relevant, especially considering future developments in the transition to cleaner energy sources.
Implementing hydrogen blending requires addressing several technical challenges connected to the differences introduced by hydrogen in terms of lower volumetric heating value (10 MJ/Nm³), higher flame temperature, shorter flame length, broader flammability range and higher reactivity. These differences necessitate careful consideration when blending hydrogen with natural gas to ensure compatibility and efficiency in combustion systems.
While a blending level of up to 20% by volume is technically achievable, the feasibility of different blending levels depends on factors such as the origin of the natural gas to be blended with hydrogen. Additionally, uncertainties remain regarding the long-term material sensitivities (e.g., pipes and devices) and potential reductions in lifespan due to hydrogen presence, necessitating further investigation. The natural gas distributed in the national network does not have constant characteristics and can vary significantly depending on its origin. Nationally, acceptability ranges are provided for some characteristics of natural gas, such as relative density or higher calorific value; however, these ranges are quite wide and allow for gaseous mixtures with different characteristics. This variability must be considered when evaluating the possibility of mixing natural gas with hydrogen, as the properties of these mixtures can vary significantly based on the percentage of hydrogen introduced and the initial characteristics of the natural gas.
The technical effort required to blend hydrogen into the natural gas supply is substantial, particularly when substituting 20% by volume of natural gas with green hydrogen. This process demands significant modifications to infrastructure, including adjustments to pipelines, storage facilities, and end-user equipment to accommodate the different properties of hydrogen. Hydrogen has a lower heating value compared to natural gas, meaning that, energy-wise, 20% hydrogen by volume contributes less energy than the equivalent volume of natural gas. Despite this considerable technical undertaking, the substitution of 20% natural gas with green hydrogen results in relatively modest greenhouse gas (GHG) savings—approximately 6 to 7%. This modest reduction is due to hydrogen's lower energy density, which requires more hydrogen to achieve the same energy output as natural gas. Consequently, while hydrogen blending is a step towards reducing GHG emissions, its impact is limited unless coupled with other decarbonization measures. The pursuit of hydrogen blending should be seen as part of a broader strategy to start decarbonization of thermal energy uses, reducing the economic impact on the complete subsystem.
Considering that natural gas consumption at a global level is still growing, as is clearly demonstrated in
Figure 8 and that this corresponds to about 40000 TWh globally, about 21-22% of the total energy use, as discussed in section 2, even if a reduction of a few percentage points could have a positive effect in terms of decarbonization, [
22].
Despite the fact that the use of blended natural gas-hydrogen combustion does not appear to be very relevant, it is also true that on a general level even a penetration of a few percentage points of hydrogen use, in the event that this is produced by renewable energy, it could still produce a non-negligible medium-term impact, both in terms of penetration of renewable sources and in terms of potential decarbonisation.
7. Conclusions
In this paper, we have analyzed the potential use of hydrogen as a co-combustible in industrial burners and furnaces. Our study examined the feasibility and effectiveness of hydrogen-natural gas blended combustion in industrial furnaces, beginning with a general energy analysis and incorporating the use of various indicators. From the analysis, it becomes clear that while blended combustion may seem like a practical short- to medium-term strategy for achieving decarbonization, its long-term impact is limited. Industrial practices suggest that blends containing up to 20-30% hydrogen by volume are feasible, but the theoretical limitations of such blends become apparent beyond the 25% threshold. A hydrogen-methane blend with 20% hydrogen by volume results in a CO2 emissions reduction of approximately 7%. Increasing the hydrogen content to 30% yields a reduction of just over 10%.
Furthermore, the natural gas circulating in the network often contains variable percentages of hydrogen, typically around 2%, which further limits the potential for additional hydrogen blending. This variability complicates the application of blended combustion in real-world industrial scenarios, where the composition of natural gas can vary significantly depending on its origin. The power range and combustion stability are influenced by the change of fuel, necessitating design calculations and experiments at each stage to efficiently utilize the burners and achieve greater controllability while minimizing pollutant emissions. This can be addressed either on an energy basis or by employing synthetic indicators.
The interchangeability of fuels was analyzed using both energy balance methods and synthetic indicators. Various indices are present in the literature; however, there is no international consensus on which indices and related limit values should be used. In certain cases, the evaluation is limited to comparing the value of the index obtained with the new mixture to the value obtained for the original mixture. The Wobbe index is the most used indicator, as it requires less data and is easier to calculate. However, it does not consider the volumetric composition of the mixture. The Heat Release Rate (HRR) is another easy-to-use index, but it only provides relative information by considering the variation of the Wobbe Index compared to its initial value.
The findings underscore the thermodynamic complexities associated with high hydrogen concentrations in blended combustion. Beyond a certain threshold, transitioning directly to pure hydrogen combustion appears more advantageous, indicating a paradigm shift in burner design and operation.
In conclusion, while blended combustion can be a viable medium-term strategy, the penetration of hydrogen is limited to relatively low mass fractions, resulting in modest CO2 emission reductions. Therefore, while the transition to pure hydrogen combustion appears to be more expensive and far to be realized due to the required technological changes. For this reason, the use of hydrogen in blended combustion in industrial sector, mainly in the hard to abate sector, appears to be an important step. Another advantage of blended combustion systems is that they allow for the maintenance of existing infrastructure. This approach also offers the flexibility to operate the facilities even in the absence of hydrogen, which is not possible with systems designed exclusively for hydrogen combustion. By integrating hydrogen into the existing natural gas framework, industries can gradually transition to cleaner energy sources without the immediate need for extensive and costly infrastructure upgrades. This dual capability ensures continuous operation and energy supply reliability, making blended combustion a practical interim solution during the transition to a fully hydrogen-based energy system.
Author Contributions
“Conceptualization, A.F. and M.R.; methodology, A.F and M.R..; software, A.F. and M.R; validation, A.F. and M.R.; formal analysis, A.F.; investigation, M.R.; resources, A.F.; data curation, A.F and M.R..; writing—original draft preparation, A.F.; writing—review and editing, A.F and M.R.; visualization, A.F.; supervision, A.F.; project administration, A.F.; funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have con-tributed substantially to the work reported.
Funding
This work was supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 - Call for tender No. 1561 of 11.10.2022 of Ministero dell’Università e della Ricerca (MUR); project funded by the European Union—NextGenerationEU. Award Number: Project code PE0000021, Concession Decree No. 1561 of 11.10.2022 adopted by Ministero dell’Università e della Ricerca (MUR), CUP I53C22001450006, according to attachment E of Decree No. 1561/2022, Project title “Network 4 Energy Sustainable Transition—NEST”.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directly directed to the corresponding authors.
Acknowledgements
In this section, you can acknowledge any support given which is not covered by the author con-tribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
“The authors declare no conflicts of interest.”
References
- International Energy Agency (IEA), CO2 Emissions in 2023, available at https://iea.blob.core.windows.net/assets/33e2badc-b839-4c18-84ce-f6387b3c008f/CO2Emissionsin2023.pdf.
- Franco, A.; Giovannini, C. Routes for Hydrogen Introduction in the Industrial Hard-to-Abate Sectors for Promoting Energy Transition. Energies 2023, 16, 6098. [Google Scholar] [CrossRef]
- Hydrogen newsletter 2023. Hydrogen-ready boilers: A corner in the industry to connect with net-zero initiatives. available at: https://www.hydrogennewsletter.com/hydrogen-ready-boilers-a-corner-in-theindustry-to-connect-wit/) (accessed on 15 June 2024).
- Raghavan, V. Combustion Technology: essentials of flame and burners, 2nd ed., Springer Cham, Switzerland, 2021.
- von Schéele, J. Advancing Use of hydrogen as fuel in Steelmaking. Millenium Steel 2022, 20–22. [Google Scholar]
- Amaduzzi, R.; Ferrarotti, M.; Parente, A. Strategies for hydrogen-enriched methane flameless combustion in a quasi-industrial furnace. Frontiers in Energy Research 2021, 8, 590300. [Google Scholar] [CrossRef]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.; Habib, M. A.; Ghoniem, A.F. Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: Technology, fundamentals, and numerical simulations. Applied energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Zhan, X. , Chen, Z. , & Qin, C. Effect of hydrogen-blended natural gas on combustion stability and emission of water heater burner. Case Studies in Thermal Engineering, 2022, 37, 102246. [Google Scholar]
- Ritchie, H., Rosado P. and Roser M. (2023) – Energy, Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.
- Jaysawal, R. K. , Chakraborty, S. , Elangovan, D., & Padmanaban, S.. Concept of net zero energy buildings (NZEB)-A literature review. Cleaner Engineering and Technology 2022, 11, 100582. [Google Scholar]
- Franco, A. (). Methods for the sustainable design of solar energy systems for industrial process heat. Sustainability 2020, 12, 5127. [Google Scholar]
- NIST, National Institute of Standards and Technology, Thermophysical Properties of Fluid Systems, available at https://webbook.nist.gov/chemistry/fluid/ (accessed on 15 June 2024).
- Sankowski, L. , Kaiser F. , Schmitz N., Schwotzer C., Pfeifer H., CO2-neutral Process Heating for Carburizing Furnaces – an Ecological Analysis, HTM Journal of Heat Treatment and Materials, 2023, 78, 3–16. [Google Scholar]
- Serrato D, Zapata-Mina J, Restrepo A and Torres J, Assessment of liquefied natural gas (LNG) regasified through gas interchangeability in energy consumption sectors. Energy Reports 2021, 7, 2526–2533.
- Park C, Oh S, Kim C, Choi Y and Ha Y, Effect of natural gas composition and gas interchangeability on performance and emission characteristics in an air–fuel controlled natural gas engine. Fuel 2021, 287, 119501.
- Shkarovskiy, A.; Koliienko, A.; Turchenko, V. Interchangeability and Standardization of the parameters of combustible gases when using hydrogen. Architecture and Engineering 2022, 7, 695–703. [Google Scholar] [CrossRef]
- Lin, X.L.; Ma, H.; Liu, C.; Zhang, J.; Zhang, Y.; Miao, Z. Experimental research on gas interchangeability indices for domestic fully premixed burners. Fuel 2018, 233, 695–703. [Google Scholar] [CrossRef]
- Decree of the Italian Ministry of Economic Development of 19/02/2007 "Approval of the technical regulation on the chemical-physical characteristics and the presence of other elements in combustible gas".
- Zachariah-Wolff, J.L.; Egyedi, T.; Hemmes, K. ; From natural gas to hydrogen via the Wobbe index: The role of standardized gateways in sustainable infrastructure transitions. International Journal of Hydrogen Energy 2007, 32, 1235–1245. [Google Scholar] [CrossRef]
- Kakaee, A.H.; Paykani, A.; Ghajar, M. The influence of fuel composition on the combustion and emission characteristics of natural gas fueled engines. Renewable and Sustainable Energy Reviews 2014, 38, 64–78. [Google Scholar] [CrossRef]
- International Gas Union. Guidebook to Gas Interchangeability and Gas Quality. Norway, Oslo, 2011.
- Energy Institute - Statistical Review of World Energy (2024) – with major processing by Our World in Data. “Gas consumption” [dataset].
Figure 1.
Percentage of electricity with respect to total energy uses in the World and in Europe (27).
Figure 1.
Percentage of electricity with respect to total energy uses in the World and in Europe (27).
Figure 2.
Air Furnaces with premixed blended combustion (schematic view).
Figure 2.
Air Furnaces with premixed blended combustion (schematic view).
Figure 3.
Potential emission reduction as a function of hydrogen volumetric fraction in the fuel.
Figure 3.
Potential emission reduction as a function of hydrogen volumetric fraction in the fuel.
Figure 4.
Variation of WI of NG-H2 blends in relation to the % by volume of hydrogen for natural gases with different origins.
Figure 4.
Variation of WI of NG-H2 blends in relation to the % by volume of hydrogen for natural gases with different origins.
Figure 5.
Variation of WI of NG-H2 blends in relation to the % by volume of hydrogen.
Figure 5.
Variation of WI of NG-H2 blends in relation to the % by volume of hydrogen.
Figure 6.
Variation of HHR of NG-H2 blends in relation to the % by volume of hydrogen.
Figure 6.
Variation of HHR of NG-H2 blends in relation to the % by volume of hydrogen.
Figure 7.
Variation of MCP in relation to the % of H2 in hydrogen-natural gas mixtures.
Figure 7.
Variation of MCP in relation to the % of H2 in hydrogen-natural gas mixtures.
Figure 8.
Natural gas consumption in the current century: comparison among world, Europe (27) and United States.
Figure 8.
Natural gas consumption in the current century: comparison among world, Europe (27) and United States.
Table 1.
Energy consumption in the world: data rearranged from [
9].
Table 1.
Energy consumption in the world: data rearranged from [
9].
| year |
Total energy use
[TWh] |
Electricity generation
[TWh] |
Share of electricity generation [%] |
| 2023 |
183320 |
27479 |
0,150 |
| 2019 |
174458 |
26771 |
0,153 |
| 2015 |
163146 |
24006 |
0,147 |
| 2011 |
156261 |
21957 |
0,141 |
| 2007 |
147434 |
19712 |
0,134 |
| 2003 |
130139 |
16627 |
0,128 |
| 1999 |
119843 |
14926 |
0,125 |
| 1995 |
112842 |
13382 |
0,119 |
| 1990 |
106656 |
11961 |
0,112 |
| 1985 |
94876 |
9886 |
0,104 |
Table 2.
Energy consumption in Europe (27): data rearranged from [
9].
Table 2.
Energy consumption in Europe (27): data rearranged from [
9].
| year |
Total energy use
[TWh] |
Electricity generation
[TWh] |
Share of electricity generation [%] |
| 2023 |
15662 |
2773 |
0,177 |
| 2019 |
17155 |
2875 |
0,168 |
| 2015 |
17049 |
2870 |
0,168 |
| 2011 |
17745 |
2908 |
0,164 |
| 2007 |
18754 |
2947 |
0,157 |
| 2003 |
18582 |
2799 |
0,151 |
| 1999 |
17877 |
2587 |
0,145 |
| 1995 |
17345 |
2409 |
0,139 |
| 1990 |
17442 |
2273 |
0,130 |
| 1985 |
16793 |
2023 |
0,120 |
Table 3.
Some “H2 ready” boilers available on the market.
Table 3.
Some “H2 ready” boilers available on the market.
| Manufacturer |
Series |
Typology |
Power
[MW] |
Max H2 allowed |
| Viessman |
VITOMAX |
Boilers-Hot water |
0.65-22 |
100% |
| Bosch industrial |
UNIMAT |
Boilers-Hot water |
0.65-38 |
100% |
| UNICAL |
MODULEX |
Boilers-Hot water |
< 1.5 |
20% |
Table 4.
Some “H2 ready” burners available on the market.
Table 4.
Some “H2 ready” burners available on the market.
| Manufacturer |
Series |
Typology |
Power
[MW] |
Max H2 allowed |
| MACCHI ABS |
- |
Gas burners |
35 |
100% |
| SAAKE |
TERMINOX |
Gas burners |
3-28 |
20% |
| SAAKE |
ATONOX |
Gas burners |
7-100 |
20% |
| SAAKE |
SKVG |
Gas burners |
1-55 |
100% |
| SAAKE |
SSBG |
Gas burners |
1.5-90 |
100% |
| Bloom Engineering |
|
Premix burners |
0.073-41 |
8% |
| Bloom Engineering |
|
HTR burners |
0.012-1.3 |
100% |
| Bloom Engineering |
|
Regenerative burners |
0.7-15 |
100% |
| Bloom Engineering |
|
Radiant tube burners |
n.a. |
100% |
| Bloom Engineering |
|
Baffle burners |
0.075-117 |
100% |
| Bloom Engineering |
|
Air Stage burners |
0.050-10 |
100% |
| GF-ELTI |
H2BURN |
Auto-recuperative burners |
0.1 |
100% |
| GF-ELTI |
H2BURN |
Regenerative burners |
0.3 |
100% |
Table 5.
Problems connected to totally hydrogen combustion and possible solutions.
Table 5.
Problems connected to totally hydrogen combustion and possible solutions.
| Cause/Problem |
Effect |
Solution |
| High flame speed |
Flashback, flame detachment problems, overheating burner surface |
Higher gas injection pressure |
| High flame temperature |
Increase in thermal NOx, difficult to keep below the minimum emission limits |
Special materials in the construction of burner or furnace |
| Extremely flammable |
Risks of leaks and explosions |
Significant design modifications and advanced safety protocols |
| Short and compact flame shape |
Higher temperatures in localized areas: heat distribution throughout the boiler or furnace may be difficult |
Enhanced Combustion Control Systems, use of flame stabilizers and spreaders |
| Flame is not bright |
incomplete combustion, less radiative heat transfer, but greater convective heat transfer |
Optimize Air-Fuel Ratio, Use Flame Retention Heads, Install Flame Stabilizers |
| Small size of the H2 molecule |
Hydrogen embrittlement |
Special materials less sensitive to hydrogen embrittlement |
| Hydrogen combustion has a high noise intensity |
High noise intensity |
Sound Insulation and Dampening, Use of Acoustic Enclosures |
Table 6.
Properties of the two fuels considered in blended combustion (at p=1 bar and T=298 K).
Table 6.
Properties of the two fuels considered in blended combustion (at p=1 bar and T=298 K).
| Fuel |
LHV [MJ/kg] |
HHV
[MJ/kg] |
LHV
[MJ/m3] |
Density (ρ) [kg/m3] |
Tad,f
[K] |
Min. energy for ignition
[mJ] |
Flame velocity in air
[m/s] |
Diffusion coefficient in air
[cm2/s] |
| Methane (CH4) |
50 |
55.8 |
32.289 |
0.646 |
2220 |
0.29 |
0.4 |
0.16 |
| Hydrogen (H2) |
120 |
144 |
9.687 |
0.08076 |
2400 |
0.02 |
3.2 |
0.61 |
Table 7.
Volumetric flow rates (V) in blended combustion for burner of 1 MW power thermal power.
Table 7.
Volumetric flow rates (V) in blended combustion for burner of 1 MW power thermal power.
x H2
[% vol] |
LHVMIX
[MJ/m3] |
VH2
[m3/s] |
VCH4
[m3/s] |
| 0 |
32.289 |
0 |
0.0309 |
| 5 |
31.159 |
0.0016 |
0.0305 |
| 10 |
30.029 |
0.0033 |
0.0300 |
| 15 |
28.899 |
0.0052 |
0.0294 |
| 20 |
27.769 |
0.0072 |
0.0288 |
| 25 |
26.639 |
0.0094 |
0.0281 |
| 30 |
25.509 |
0.0118 |
0.0274 |
| 50 |
20.988 |
0.0238 |
0.0238 |
| 70 |
16.468 |
0.0425 |
0.0182 |
| 100 |
9.687 |
0.1032 |
0 |
Table 8.
Mass flow rates in blended combustion for a burner of thermal power (Qth) 1 MW power.
Table 8.
Mass flow rates in blended combustion for a burner of thermal power (Qth) 1 MW power.
x H2
[% vol] |
m (CH4)
[kg/s] |
m (H2)
[kg/s] |
Qth (CH4)
[MW] |
Qth (H2)
[MW] |
CO2 emitted
[kg/s MW] |
%CO2
reduction |
| 0 |
0.02 |
0 |
1 |
0 |
0.055 |
0 |
| 5 |
0.0197 |
0.000129534 |
0.984455959 |
0.015544041 |
0.054145078 |
0.015544041 |
| 10 |
0.0194 |
0.000268817 |
0.967741935 |
0.032258065 |
0.053225806 |
0.032258065 |
| 15 |
0.0190 |
0.000418994 |
0.94972067 |
0.05027933 |
0.052234637 |
0.05027933 |
| 20 |
0.0186 |
0.000581395 |
0.930232558 |
0.069767442 |
0.051162791 |
0.069767442 |
| 25 |
0.0182 |
0.000757576 |
0.909090909 |
0.090909091 |
0.05 |
0.090909091 |
| 30 |
0.0177 |
0.000949367 |
0.886075949 |
0.113924051 |
0.048734177 |
0.113924051 |
| 50 |
0.01538 |
0.001923077 |
0.769230769 |
0.230769231 |
0.042307692 |
0.230769231 |
| 70 |
0.01177 |
0.003431373 |
0.588235294 |
0.411764706 |
0.032352941 |
0.411764706 |
| 100 |
0 |
0.008333333 |
0 |
1 |
0 |
1 |
Table 9.
Characteristics of natural gas from some countries of origin.
Table 9.
Characteristics of natural gas from some countries of origin.
| Region of origin of natural gas |
HHV
(MJ/Sm3) |
Density
(kg/Sm3) |
Wobbe Index
(MJ/Sm3) |
| Italy |
37.70 |
0.682 |
50.53 |
| The Netherlands |
37.12 |
0.752 |
47.38 |
| Russia |
39.21 |
0.742 |
50.38 |
| Algeria |
39.35 |
0.790 |
49.00 |
| Libia |
40.61 |
0.778 |
50.96 |
Table 10.
Characteristics of natural gas from some countries of origin.
Table 10.
Characteristics of natural gas from some countries of origin.
| Properties/Index |
Acceptability range |
Unit |
| Higher Heating Value (HHV) |
34.95 - 45.28 |
MJ/Sm3
|
| Wobbe Index (WI) |
47.31 - 52.33 |
MJ/Sm3
|
| Relative Density (RD) |
0.56 - 0.8 |
- |
Table 11.
Characteristics of natural gases used for the study of hydrogen-natural gases blends based on HHV (at atmospheric pressure).
Table 11.
Characteristics of natural gases used for the study of hydrogen-natural gases blends based on HHV (at atmospheric pressure).
| Name |
Description |
HHV
(MJ/Sm3) |
RD |
Density
(kg/Sm3) |
| H2
|
Hydrogen |
12.5 |
- |
0.081 |
| CH4
|
Pure methane |
37.8 |
- |
0.648 |
| NG1 |
Max. acceptable HHV and RD |
45.28 |
0.80 |
0.98 |
| NG2 |
Max. acceptable HHV and min. RD |
45.28 |
0.555 |
0.68 |
| NG3 |
Min. acceptable HHV and max. RD |
34.95 |
0.80 |
0.98 |
| NG4 |
Min. acceptable HHV and min. RD |
34.95 |
0.555 |
0.68 |
| NG5 |
Average HHV* and averaged* |
40.11 |
0.677 |
0.83 |
Table 12.
Typical natural gas composition by volume [
21].
Table 12.
Typical natural gas composition by volume [
21].
| Component |
Symbol |
% Vol. |
| Methane |
CH4
|
88.10 |
| Ethane |
C2H6
|
4.20 |
| Propane |
C3H8
|
1.36 |
| Butane |
C4H10
|
0.30 |
| Pentane |
C5H12
|
0.06 |
| Carbon Dioxide |
CO2
|
0.78 |
| Nitrogen |
N2
|
5.20 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).