1. Introduction
The "hard to abate" sector refers to industries or sectors that face significant challenges in reducing their greenhouse gas emissions and transitioning to more sustainable practices. These sectors typically involve processes or activities that rely heavily on fossil fuels or emit substantial amounts of carbon dioxide (CO
2), making it difficult to implement traditional decarbonization measures [
1].
The term “hard-to-abate” highlights the complexities and barriers faced by these sectors in achieving emission reduction targets. Some examples of hard-to-abate sectors include steel production, cement, glass and paper manufacturing, and heavy transportation [2, 3].
The challenges in decarbonizing these sectors stem from the intrinsic nature of their operations, which often require high-temperature heat sources or involve energy-intensive processes. Finding viable alternatives to fossil fuels and developing technologies capable of reducing emissions without compromising productivity and competitiveness are key challenges [
4].
Innovative solutions, such as the use of hydrogen, carbon capture and storage (CCS), electrification, and circular economy approaches, are being explored to tackle these challenges [
5].
The role of hydrogen in decarbonization should not be underestimated, as it offers versatility and compatibility with existing industrial processes [6, 7]. However, it is crucial to recognize that hydrogen serves as a carrier of energy and must be produced sustainably. Therefore, it is essential to establish a clear understanding of the energy balance within each process and carefully consider this aspect in all new projects. Failing to do so may result in missed opportunities for cost-effectiveness and environmental benefits. However, it is equally important to assess the energy sources used for hydrogen production. Green hydrogen, produced from renewable sources, ensures minimal carbon emissions throughout its lifecycle and aligns with the objectives of decarbonization [
8]. It offers a compelling solution for decarbonization, but an inherent challenge arises from the relatively low energy density of renewable sources such as solar. This poses difficulties in effectively harnessing and integrating green energy, hydrogen, and the hard-to-abate sectors [
9].
The objective of this paper is to provide a comprehensive and elucidating analysis of the previously discussed aspects. Building upon the previous research and discussions, we aim to offer clarity and a deeper understanding of the key elements associated with hydrogen utilization in hard-to-abate sectors. By synthesizing the existing knowledge and insights, we seek to shed light on the intricate details and interconnections between various facets of hydrogen integration. This includes the evaluation of different technologies, the energy balance considerations, the potential applications, and the challenges related to implementing hydrogen solutions in hard-to-abate industries. Strictly speaking, the idea of the authors is to provide a comprehensive and insightful clarification of the previously examined aspects, contributing to a deeper understanding of the role and potential of hydrogen in addressing the decarbonization challenges of hard-to-abate industries.
The existing literature offers extensive material on hydrogen and related technologies. However, the focus tends to be primarily on the analysis of specific technologies and specific aspects. While this is valuable, it is equally important to construct a broader perspective rooted in fundamental physical elements that are often overlooked in the literature.
One critical aspect that is often neglected is the holistic consideration of the entire cycle, encompassing the energy source to the final utilization of hydrogen. This comprehensive perspective forms the original contribution of our work. By taking into account the entire energy pathway, including production, storage, distribution, and utilization, we can better understand the interconnectedness and implications of different stages within the hydrogen value chain.
This approach enables a more accurate assessment of the overall efficiency, environmental impact, and economic feasibility of hydrogen technologies.
Our work seeks to bridge the gap between detailed technological analyses and the larger energy system context. By incorporating the broader perspective and considering the complete energy cycle, we aim to provide a comprehensive and insightful contribution to the existing body of knowledge on hydrogen and its potential applications.
The paper follows a well-structured organization. Firstly, in
Section 2, a comprehensive analysis of the diverse hard-to-abate sectors is conducted, setting the stage for understanding the unique challenges and characteristics of each sector.
In
Section 3, key elements associated with hydrogen production through water electrolysis are outlined. This section focuses on crucial aspects related to technology, scalability, and efficiency, shedding light on the central considerations of hydrogen generation.
Moving forward,
Section 4 critically examines the potential implementation of hydrogen in hard-to-abate industries, considering three major routes that hold significant relevance. This analysis delves into the technical, economic, and environmental aspects, providing insights into the feasibility and benefits of adopting hydrogen solutions.
Section 5 provides detailed insights into the specific routes of implementing hydrogen in the steel sector. By focusing on this sector, a crucial industry within the hard-to-abate landscape, the paper offers a comprehensive understanding of the challenges, opportunities, and implications associated with integrating green hydrogen.
Finally, the Conclusions section serves as a synthesis of the paper's findings, highlighting the genuine perspectives and potential of green hydrogen in the challenging hard-to-abate sectors. This concluding section encapsulates the key takeaways, implications, and prospects, offering a comprehensive overview of the role and potential impact of green hydrogen in driving decarbonization efforts.
2. Hard-to-Abate Sectors: Energy Analysis and the Possible Role of Hydrogen
Hard-to-abate industrial processes encompass a range of activities within sectors such as cement, metallurgy, glass, paper and chemicals (these sectors include both traditional applications in chemical production and refineries, as well as innovative uses in green chemistry). While electrification serves as an effective pathway for decarbonization in many sectors, it may not be a straightforward solution for all industries.
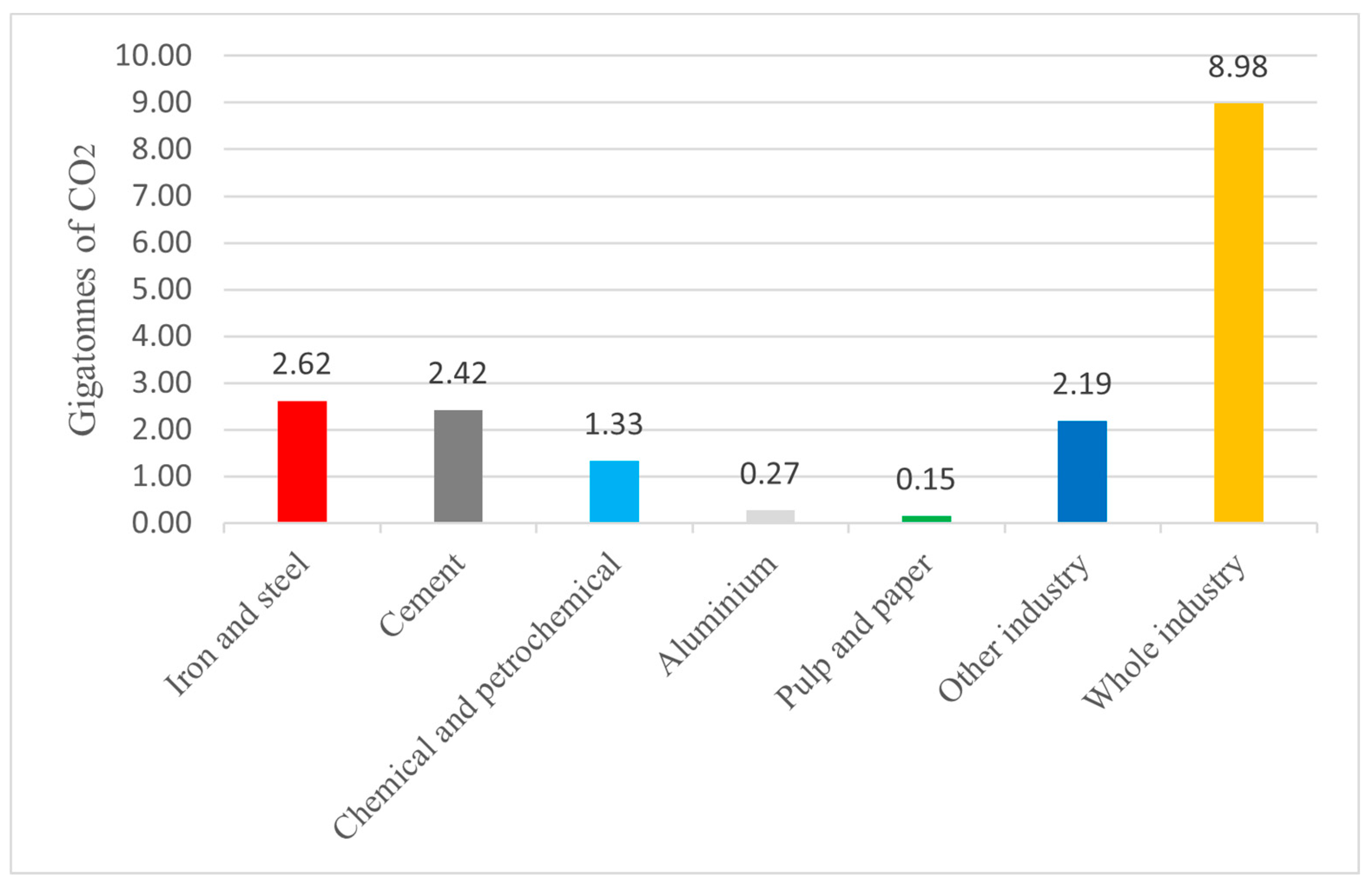
Certain sectors, referred to as hard-to-abate sectors, face unique challenges in achieving their sustainability objectives. For these sectors, hydrogen emerges as a promising alternative. Hydrogen can play a pivotal role in enabling the decarbonization of these industries. The adoption of hydrogen technologies offers opportunities for these sectors to diversify their energy sources, optimize their operations, and contribute to overall decarbonization efforts. It presents a pathway that complements electrification and enables the decarbonization of processes. The IEA report highlights the significant role of hard-to-abate sectors as major contributors to global CO
2 emissions, as depicted in
Figure 1 [
10]. The industrial sector stands out as a substantial emitter, accounting for approximately 9 gigatons of CO
2 emissions. Within the industrial sector, energy-intensive industries play a prominent role, with the iron and steel sector alone contributing to almost 30% of the global industrial CO
2 emissions.
However, it is important to note that other industries also make substantial contributions to CO2 emissions, including the cement sector and various other sectors such as glass and paper. These industries are widespread worldwide and play a significant role in the overall emissions profile.
Hydrogen has the potential to play a significant role in the decarbonization of the "hard to abate" sector. Hydrogen offers the advantage of being a versatile energy carrier, capable of being produced from renewable sources and used in various applications and with different roles. The understanding that hydrogen serves as an intermediate carrier necessitates a comprehensive and thoughtful analysis. While hydrogen is often discussed in the literature with a focus on specific details, there appears to be a lack of holistic and general analysis. It is important to recognize that hydrogen is not readily available; it must be produced through various methods. Merely examining the intricacies of hydrogen without considering the broader context can result in an incomplete understanding of its role in the energy transition. A comprehensive analysis should encompass the entire value chain of hydrogen, including at least its production, storage and utilization.
By examining the interdependencies and complexities associated with hydrogen, a more robust understanding of its potential and challenges can be achieved, enabling informed decision-making and the development of effective strategies for its integration into energy systems.
3. Hydrogen Production through Electrolysis
The main methods for hydrogen production are steam reforming and electrolysis.
Steam reforming, also known as steam methane reforming (SMR), is the most common method for producing hydrogen on an industrial scale. It involves reacting natural gas (methane) with steam at high temperatures (700-1,000°C) in the presence of a catalyst. This process produces a mixture of hydrogen and carbon monoxide, known as syngas, which is then further processed to separate and purify the hydrogen.
Water electrolysis is a process that uses an electrical current to split water molecules into hydrogen and oxygen. It requires the use of an electrolyser, which mainly consists of two electrodes separated by an electrolyte. When sufficient electrical current is provided, hydrogen gas is produced at the cathode, and oxygen gas is produced at the anode.
In the absence of external energy sources or catalysts, the natural tendency of water is to remain in its stable molecular form. The decomposition of water into hydrogen and oxygen requires energy input to overcome the energetic barrier and drive the reaction in the forward direction. In the process of hydrogen production through electrolysis, energy is indeed required. The energy consumption for electrolysis can vary depending on several factors, including the electrolysis technology used and the operating conditions.
The production of electrolytic hydrogen from water stands out as one of the most practical and promising technologies for the large-scale production of "green" hydrogen, allowing the utilization of renewable energy sources, such as solar or wind power, to generate electrical power for hydrogen production without carbon dioxide emissions.
The hydrogen produced can be used as feedstock, alternative fuel or to generate electricity as needed, once it is stored.
Fuel cells operate by electrochemically reacting hydrogen with oxygen from the air to produce electricity, and heat and water as byproducts. This electrochemical process offers a clean and efficient way to convert the chemical energy stored in hydrogen into usable electrical power, with significantly higher efficiencies compared to traditional combustion-based power generation.
Extensive research efforts are being conducted to develop functional components and systems for electrochemical technologies in hydrogen production.
These efforts encompass both low-temperature technologies (50-80 °C) such as proton exchange membrane (PEM), alkaline (ALK), and anion exchange membrane (AEM) electrolysers, as well as high-temperature technologies (500-1,000 °C) like solid oxide (SO) cells, molten carbonate (MC) cells, and proton conductive cells (PCC).
Each of these electrochemical technologies offers unique advantages in terms of operational temperature, efficiency, scalability, and suitability for specific applications. Research activities focus on improving the performance, durability, and cost-effectiveness of these technologies to enable their widespread adoption in hydrogen production and utilization systems.
3.1. Theoretical Elements of Electrolysis
Low-temperature electrolysis is described from the splitting reaction of the liquid water molecule:
whose enthalpy change in standard conditions (1 atm and 25 °C), ΔH°, equals 285.8 kJ per mol of reaction [
11].
ΔH° of the reverse reaction, H
2 combustion and steam condensation, is a positive value corresponding to the higher calorific value of hydrogen 39.4 kWh/kg (about 2.016 g
H2/mol
reac). Electrolysis performed at high temperatures uses steam according to the reaction in which water is in gaseous form:
whose standard enthalpy change is 241.8 kJ/mol
reac [
11].
ΔH° of the reverse reaction, H2 combustion, corresponds to the lower calorific value (LHV) of hydrogen, about 33.3 kWh/kg.
Water electrolysis is a nonspontaneous process and the reaction free energy change is a large positive quantity. The change in free energy of a system for a constant-temperature process is the difference between the enthalpy change and the product of the absolute temperature and the entropy change, Equation (3):
ΔG corresponds with the electrical energy demand of the electrolytic process, TΔS the thermal energy demand, and ΔH the total energy demand required.
Figure 2 shows the mass and energy flows of the electrolytic process.
The electrical energy demand in standard conditions, ΔG°, of liquid water electrolysis equals 232.7 kWh per mol of reaction [
11] (= 32.7 kWh per kg of hydrogen), instead for steam electrolysis 228.6 kJ/mol
reac [
11] (= 31.5 kWh/kg
H2) [
11] are necessary.
Bi et al. [
12] represented graphically how the electrical demand decreases considerably with increasing the temperature at which electrolysis is carried out, especially when the process happens with steam instead of liquid water. For temperatures above 100 °C, the total energy demand increases slightly as the temperature increases, because the increase in the heat demand is greater than the decrease in the required electricity.
The change in free energy represents the amount of electrical work that can be obtained or must be provided in a reaction [
11], Equation (4):
where n = 2 is the number of moles of electrons, e
−, transferred per mole of hydrogen in the overall redox equation of the electrochemical process, F = 96,485.3 C/mol
e− is the Faraday constant, and E
cell is the cell voltage, i.e. the difference between the electrodes reduction potential. By the respective standard free-energy change value, E°
cell is calculated to be 1.23 V for liquid water electrolysis, and 1.18 V for steam electrolysis.
Assuming the reaction is reversible and carrying out the electrolytic process at constant temperature and pressure, E°cell corresponds with the reversible cell voltage, Urev, which is defined as the minimum voltage to be applied between the electrodes for electrolysis to take place.
In not ideal conditions, the cell voltage is always higher than U
rev due to the irreversibilities of the real electrolytic process. These efficiency losses lead to an increase of the voltage (overpotentials, η
˅) required for water electrolysis compared to the theoretical one, as shown in Equation (5) to calculate the operational cell voltage:
where η
˅act is the activation overpotential, η
˅ohm is the ohmic overpotential, and η
˅conc is the concentration or diffusion overpotential. The activation overpotential is related to the reactions activation energy, and the catalyst and operational temperature increase can reduce this efficiency loss. The ohmic overpotential is due to ionic, electrical, and contact resistances in the electrolytic cell; the current density, cell materials and design, and temperature affect this overpotential [
13]. η
˅conc is related to mass transport, more difficult at high current densities; if H
2 and O
2 are not removed as fast as they are produced, their concentration increases decreasing the reactions kinetic [
13].
3.2. Technical Data on Electrolysis
According to the data available from the literature and market, summarized in
Table 1, it is evident that the energy consumption related to hydrogen production in commercially available devices is notably higher than the theoretical values discussed in the Subsection 3.1. Nowadays, the average specific energy consumption (ASEC) falls within the range of 55 to 60 kWh per kilogram of hydrogen produced for low-temperature electrolysers and 40-42 kWh/kg for high-temperature electrolysers.
These figures highlight that there are efficiency losses and energy requirements beyond the idealized values in practical hydrogen production systems.
Furthermore, it should be noted that the energy consumption data for hydrogen production are partially dependent on the size of the electrolyser. It is unrealistic to expect the same energy consumption value to be applicable across a wide range of systems, spanning from a few kilowatts to several megawatts. Larger-scale electrolysers may exhibit different energy efficiency levels and operational characteristics compared to smaller units. Factors such as system design, materials, and operating conditions can significantly influence the energy consumption of H2 production. Therefore, when considering the implementation of H2 production systems, it is essential to take into account the specific characteristics and scale of the electrolyser to accurately assess its energy requirements.
Technological advancements in low-temperature and high-temperature electrolysis and process optimizations can lead to improvements in energy efficiency, potentially reducing the energy consumption per unit of hydrogen produced.
Electrolysers First Law efficiency can be evaluated as in Equation (6):
that results in around 58% for low-temperature electrolysers and about 81% for high-temperature electrolysers, with the present ASECs identified in
Table 1.
For a real evaluation of electrolysers First Law efficiency, the input thermal energy should also be considered, as shown in
Figure 2.
Thus, by identifying the declared energy consumption of electrolysers as electricity consumption, it is possible to define an electrical efficiency (η
el) as the ratio of the electricity demand of the electrolytic process and the average specific energy consumption of the electrolyser, Equation (7):
evaluating the electricity demand in standard conditions, ΔG°, to have a fixed term of comparison depending only on operating the electrolysis at low or high temperatures.
Through the present ASECs identified above (
Table 1), the electrical efficiency is around 57% for low-temperature electrolysers and 77% for high-temperature electrolysers. The electrical energy demand decreases steadily as the temperature increases, and it decreases significantly above 100 °C; if the ΔG were evaluated at the operating temperature instead of in standard conditions, the electrical efficiency would surely assume lower values, especially for high-temperature electrolysers.
Table 2 summerizes the main data and results of Subsections 3.1 and 3.2.
In conventional low-temperature electrolysis, such as alkaline (ALK), proton exchange membrane (PEM), and anion exchange membrane (AEM) electrolysers, the water splitting reaction occurs at relatively low temperatures, typically around 60 to 80 °C.
Considering low-temperature real applications, their energy consumption is of the order of 55-60 kWh per kg of hydrogen produced, and this means that in the first step about 30-40% of the energy is lost.
Increasing temperature in electrolysis processes can lead to significant reductions in electrical consumption.
High-temperature electrolysis, such as by solid oxide (SO) cells, is a promising approach that utilizes elevated temperatures, typically above 600 °C often in the range of 800 to 1,000 °C, to drive the electrochemical reactions more efficiently, resulting in improved energy efficiency and reduced electricity requirements.
At elevated temperatures, the electrolysis reactions become more favourable thermodynamically. The higher operating temperature allows for faster reaction kinetics, enabling higher production rates of hydrogen. All this leads to lower overpotentials required, thus, less electrical energy is needed to drive the reactions, resulting in reduced electricity consumption.
However, it's important to note that high-temperature electrolysis comes with its challenges, such as material compatibility, thermal management, and system integration. High temperatures require appropriate materials for cell components and sealing, and efficient heat transfer mechanisms are necessary to maintain the desired operating conditions. These technical considerations need to be addressed to realize the full potential of high-temperature electrolysis.
Overall, high-temperature electrolysis holds promise for reducing electrical energy consumption, making it an area of active research and development. By leveraging the benefits of elevated temperatures, high-temperature electrolysers can contribute to the advancement of efficient and sustainable hydrogen production systems.
Continuous research on H2 production by water electrolysis is very important to increase the performance of low-temperature and high-temperature electrolysers, in various operating conditions, and during their useful life, especially to produce green H2 from renewable energy.
Electrolysis is a complex process, still being studied as regards its optimization. However, to carry out energy assessments on industrial processes, it is sufficient to refer to current literature and market data, albeit uncertain.
4. Pathways to H2 Introduction in the “Hard to Abate” Sectors
The decarbonization of the hard-to-abate sector poses significant challenges, but one potential solution lies in the electrification of industrial processes using green energy sources. While this approach holds promise, its implementation is not without difficulties, particularly in sectors like steel, cement, and glass that rely heavily on high-temperature processes and large-scale equipment. Substituting thermal energy with electricity in these processes is often impractical due to technical limitations and the sheer size of the components involved.
However, green hydrogen emerges as a viable pathway towards decarbonization in these sectors. Its unique ability to store energy, for long periods too, generated from renewable sources presents a valuable opportunity. Green hydrogen can serve as an intermediary energy carrier, enabling the efficient utilization of renewable energy across different applications. By producing hydrogen through electrolysis powered by green electricity, excess renewable energy can be stored and converted into hydrogen for subsequent use.
The integration of green hydrogen offers several advantages. Firstly, it addresses the intermittency of renewable energy sources by providing a means of energy storage that can be utilized as needed. This flexibility enhances the stability and reliability of energy supply, particularly in industries that require continuous and high-energy operations. Secondly, green hydrogen can be effectively utilized in processes that cannot be easily electrified, such as those involving high temperatures or specialized equipment. By leveraging hydrogen as a clean and versatile fuel or as a chemical reducing agent, the hard-to-abate sectors can significantly reduce their carbon emissions.
To unlock the full potential of green hydrogen, concerted efforts are needed in advancing electrolysis technologies, expanding renewable energy infrastructure, and establishing supportive policies and regulations. Collaboration between industry stakeholders, research institutions, and policymakers is crucial to drive innovation and overcoming the existing challenges. By embracing green hydrogen as a valuable tool for decarbonization, the hard-to-abate sectors can move closer to achieving their sustainability goals while benefitting from a reliable and clean energy source. The study aims to address several key aspects related to the use of hydrogen in hard-to-abate industrial sectors, such as steel, glass, paper, and others, for decarbonization purposes. The following objectives are outlined:
Identification of Industrial Processes
The first objective is to identify the main industrial processes within each hard-to-abate sector that have the potential to incorporate hydrogen for decarbonization. This involves a comprehensive analysis of the different steps and processes involved in these sectors, assessing where hydrogen can be effectively utilized as a clean energy source.
Analysis of Possible Hydrogen Routes in the Process
The study will analyze and evaluate the various routes available for the use of hydrogen in the hard-to-abate sectors as a fuel. This includes examining different technologies and assessing the state-of-the-art solutions in terms of their feasibility, efficiency, and environmental impact. The goal is to identify the most suitable hydrogen-based solutions for each sector, considering factors such as process requirements, energy efficiency, and emissions reduction potential.
Use of Hydrogen produced as fuel
Another aspect to be explored is the broader role of hydrogen in the hard-to-abate sectors. The first opportunity is to use hydrogen as a fuel, in particular, the use of hydrogen as fuel in blended combustion: blended combustion refers to the practice of combining hydrogen with another fuel, such as natural gas, for thermal power production in industrial processes. Another benefit is the versatility of blended combustion. The mixture of hydrogen and natural gas, with H2 percentages up to 50%, can be utilized in existing combustion systems without requiring major modifications. This enables a smoother transition towards decarbonization, as industries can leverage their existing infrastructure and equipment while gradually incorporating higher proportions of hydrogen into the fuel blend.
Natural gas has a lower heating value typically in the range of 45-48 MJ/kg, considering a rather high methane content; assimilating natural gas with methane, a lower heating value of 50 MJ/kg ≈ 36 MJ/Nm3 can be assumed, while hydrogen has a lower heating value of 120 MJ/kg ≈ 11 MJ/Nm3.
Thus, the lower heating value of the blend can be defined as:
that, by a reference value of 1 MW of thermal energy transferred to the treated material, allows to evaluate the blend flow rate required, for example in weight:
thus, the flow rates of H
2 and methane by Equations (11) and (12):
Table 3 and
Table 4 show the lower heating value of the combustible blend, flow rates of blend, hydrogen, and methane varying the percentage of H
2 in the combustible blend, respectively in volume and in weight. Volumetric flow rates appear much bigger than mass ones due to the very low density of hydrogen (about 0.09 kg/Nm
3).
Assuming stoichiometric combustion, the reduction of carbon dioxide emissions is also estimated as the percentage of H
2 varies (
Table 3 and
Table 4).
The burner efficiency also depends on the percentage of hydrogen used; an average efficiency is assumed, for all hydrogen percentages evaluated, for a burner with air preheating: η
burner = 85-86% [
14].
To supply by hydrogen 1 MW of heat requirement for material treatment, around 35 kg/h of H
2 must be employed (
Table 4), nowadays producible by about 2 MW of low-temperature electrolysers or around 1.4 MW of high-temperature ones (
Table 1, neglecting degradation and system energy losses).
Blended combustion also offers flexibility in terms of varying hydrogen concentrations. The proportion of hydrogen in the blend can be adjusted based on specific requirements and process conditions. This adaptability allows industries to optimize combustion performance, energy efficiency, and emissions reduction according to their unique needs.
Moreover, blending hydrogen with natural gas can enhance the combustion process itself. Hydrogen has a high flame speed and wide flammability range, which can improve the stability and efficiency of combustion. The addition of hydrogen to the fuel mixture can lead to faster and more complete combustion, resulting in higher combustion efficiency and reduced pollutant emissions.
In analogy to Franco et al. [
15], energy indicators of H
2 industrial use have to be enucleated.
The heat requirement for material treatment in the industrial process is assumed equal to 1 MW, as a reference value.
In the planning stage, targets common examples of the percentage of heat requirement coverage (HRC) by hydrogen can be 10%, 15% and 20%, as shown in
Table 5. The HRC by hydrogen equals the non-renewable primary energy saving (NRPE), by replacement of fossil energy, as in Equations (13) and (14):
With 1 MW of thermal power requirement for material treatments as the reference value, in
Table 5 it can be noticed that to cover by hydrogen the 20% of the heat requirement (thus, to reduce CO
2 emissions by 20%, assuming stoichiometric combustion, Equations (13) and (14)), about 7 kg/h of H
2 are necessary in the combustible blend. This hydrogen flow rate could be produced as green H
2 by around 400 kW low-temperature electrolysers or 300 kW high-temperature ones, with present average nominal efficiencies (
Table 1) and neglecting system energy losses.
Other relevant aspects include assessing the potential use of hydrogen as a chemical element within the principal processes of these industries, such as in refining, manufacturing, and other key operations. Additionally, the study will investigate the application of hydrogen as an energy storage system, enabling the utilization of renewable energy sources by storing excess energy in the form of hydrogen for later use.
By addressing these objectives, it is possible to provide valuable insights into the integration of hydrogen in hard-to-abate industrial sectors. It will contribute to the understanding of how hydrogen can be effectively employed to decarbonize these sectors, both as a fuel and as an energy storage solution, ultimately supporting the transition to more sustainable and low-carbon industrial processes.
4.1. Steel Sector
The steel sector is characterized by its high energy consumption and is the industrial sector with the highest CO
2 emissions (
Figure 1), thus, it presents unique challenges and opportunities for decarbonization. Steel production has a fossil energy demand hovering around 3.3 TWh per million tonnes of crude steel [
2].
The steel industry is known for its energy-intensive processes, primarily in the form of high-temperature heat required for iron ore reduction and steel production. This poses challenges for electrification due to the difficulty of replacing thermal energy with electricity. Steel production is a major contributor to global carbon dioxide emissions, accounting for a significant portion of industrial CO2 output. To achieve deep decarbonization, the sector requires transformative measures to reduce its reliance on fossil fuels.
Hydrogen, produced from renewable sources, offers a carbon-neutral alternative to traditional fossil fuels used in steelmaking, such as coal and natural gas, and can play a crucial role by serving as a clean and high-temperature energy carrier.
One possible application of hydrogen is surely hydrogen use as a reducing agent in Direct Reduction of Iron (DRI) technology. In this process, hydrogen reacts with iron oxide to produce direct reduced iron, which can be further processed into steel. By replacing carbon-based reducing agents with hydrogen, the carbon emissions associated with iron and steel production can be significantly reduced [
16].
Another way hydrogen can contribute to decarbonization in the steel sector is through blended combustion. By blending hydrogen with natural gas, the carbon intensity of the combustion process can be lowered. This approach allows for a gradual transition by leveraging existing infrastructure while reducing carbon emissions in the steelmaking process [17-19].
Green hydrogen can also serve as an energy storage medium for the steel sector, enabling the integration of renewable energy sources. Excess renewable electricity can be used to produce hydrogen through electrolysis during periods of low electrical demand or high renewable power generation. The stored hydrogen can then be utilized during peak demand or when renewable energy supply is limited, providing a reliable and flexible energy source for steel production.
4.2. Cement Sector
The cement production is the second industrial sector for the highest carbon dioxide emissions, as shown in
Figure 1. Cement manufacturing requires about 0.5 TWh of fossil energy per Mtonnes of clinker [
2].
Cement production involves high-temperature processes, such as limestone calcination and clinker production, which require substantial amounts of thermal energy. These processes typically rely on fossil fuels like coal and natural gas, leading to significant CO
2 emissions. Hydrogen can play a crucial role in decarbonizing the cement sector by serving as a clean and sustainable alternative to fossil fuels [
20].
Hydrogen can be utilized as an alternative fuel in cement kilns, replacing or supplementing traditional fossil fuels.
Clinker, a key ingredient in cement production, is produced through the heating of limestone and other materials in a kiln. This process generates substantial CO2 emissions due to the calcination of limestone. Hydrogen, when used as a reducing agent, can potentially replace the traditional carbon-intensive calcination process.
The cement sector can also benefit from the use of hydrogen as an energy storage solution and for integrating renewable energy sources.
4.3. Glass Sector
The glass sector encompasses various processes involved in the production of glass products. Manufacturing requires about 2.5 TWh of fossil energy per Mtonnes of flat glass, and around 1.6 per container glass [
2]. These processes typically involve high-temperature operations, such as glass melting, refining, and forming.
One possibility is the use of hydrogen as a direct fuel in glass furnaces, replacing fossil fuels in the form of blended combustion. Hydrogen combustion produces only water vapour as a byproduct, eliminating greenhouse gas emissions. This substitution can significantly reduce the carbon footprint of glass production [18, 21].
Another potential application of hydrogen is as a reducing agent in glass manufacturing. Hydrogen can act as an alternative to carbon-based reducing agents, such as coke or coal, in the production of specific types of glass. By using hydrogen, the industry can avoid carbon emissions associated with traditional reduction processes.
Furthermore, hydrogen can contribute to energy storage and management.
4.4. Pulp and Paper Sector
The pulp and paper sector is the fifth industrial sector for the highest CO
2 emissions (
Figure 1). Its fossil energy demand varies from about 1.4 TWh per Mtonnes of board and packaging paper, to around 2 for tissue and graphic paper, to about 3.4 TWh per million tonnes of chemical pulp [
2].
The paper sector encompasses various processes involved in the production of paper and related products. These processes typically include wood pulping, papermaking, and paper coating. The industry relies heavily on thermal energy, primarily obtained from fossil fuels, for tasks such as drying, heating, and steam generation.
As in the other sectors, one possible application is the use of hydrogen for thermal processes in place of fossil fuels; but hydrogen can be utilized in the pulping process as a chemical agent for delignification, a crucial step in paper production. Hydrogen peroxide, derived from hydrogen, can be employed as an eco-friendly bleaching agent, replacing chlorine-based chemicals.
5. Case Study: Implementing Hydrogen in the Steel Sector
Steel has been a central component of industrial society since its inception, symbolizing the advent of the industrial era. The economic significance of steel is exemplified by its substantial production growth over the years. In 1950, at the onset of post-war recovery, global steel production stood at almost 190 million tonnes; this figure skyrocketed to 850 Mtonnes in 2000 and reached the staggering maximum of 1,962 Mtonnes in 2021 [
22].
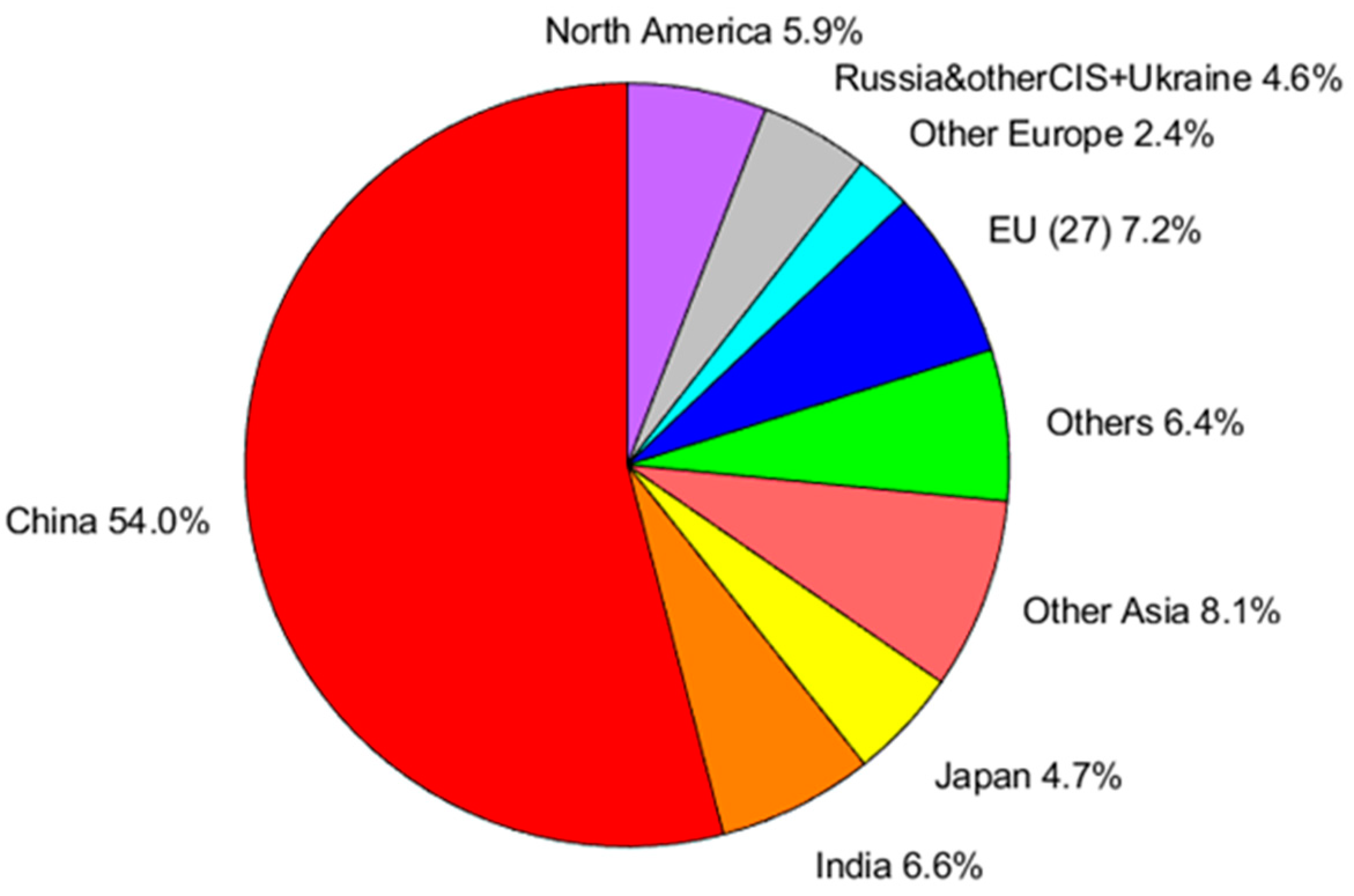
Crude steel production for the 64 countries reporting to the World Steel Association was 1885 Mtonnes in 2022 [22, 23], as shown in
Figure 3 and
Table 6.
Asia emerges as the dominant region, with major producers including China, India, and Japan, closely followed by Europe and North America,
Figure 3. In
Table 6, it can be noticed that the top ten steel producing countries in 2022 together cover about 85% of the global production. These statistics highlight the vital role that steel plays in various sectors and its contribution to economic development and infrastructure worldwide.
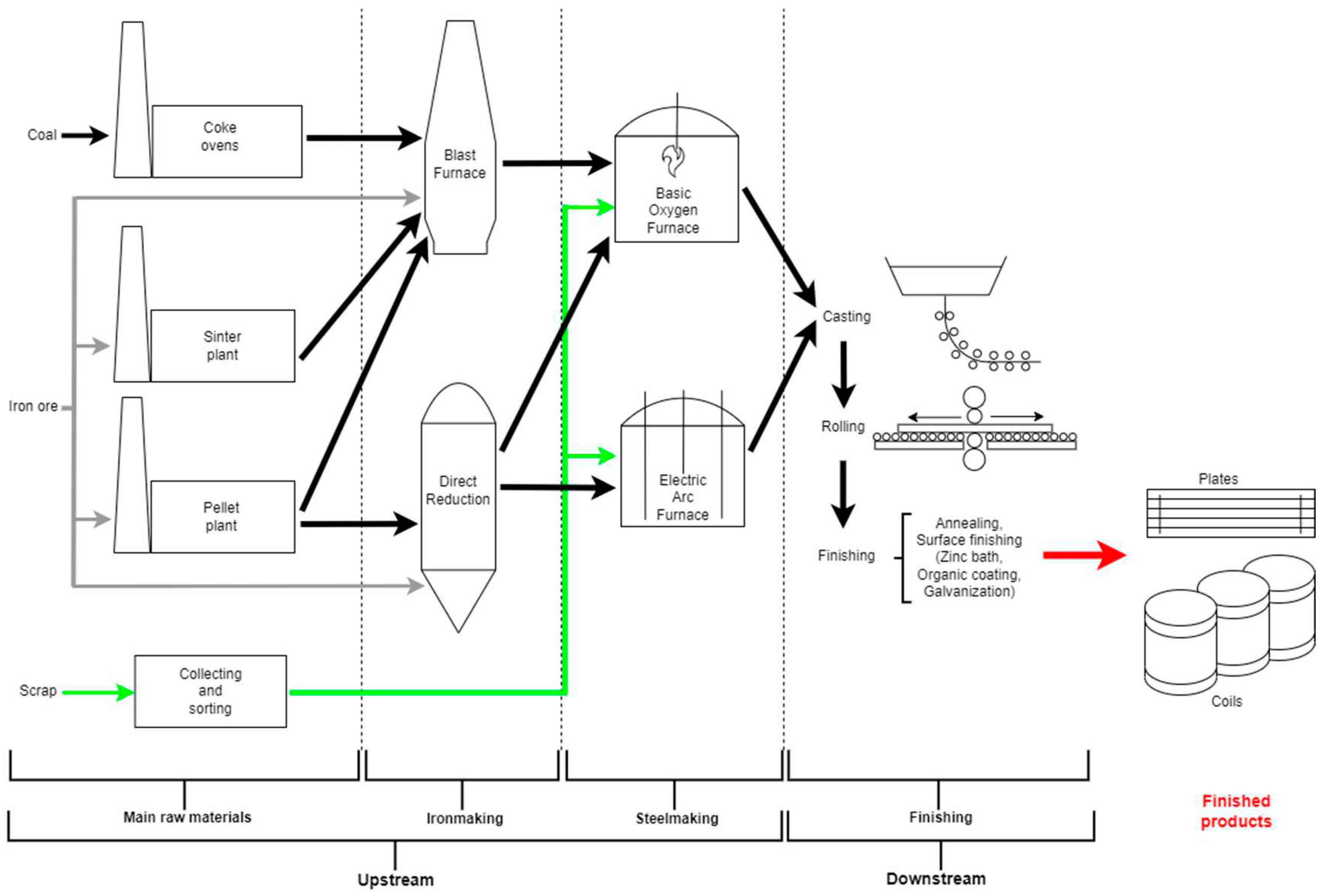
Steel production is a continuous process that demands significant energy input and results in high emissions. The steel production cycle can be divided into two primary phases: the upstream phase and the downstream phase. The upstream phase involves the transformation of raw materials into semi-finished products. This phase includes processes such as iron ore mining, coke production, and iron and steelmaking in blast furnaces or electric arc furnaces. During this phase, the raw materials are converted into steel billets, slabs, or other semi-finished forms. The downstream phase involves the further processing of these semi-finished products to obtain the final steel products. This phase encompasses various operations, such as the hot or cold working of sheets (plates), sheets and rolls (sheets and coils), as well as additional processing of semi-finished products like slabs and billets. The downstream phase also includes several treatments and applications to enhance the quality and characteristics of the steel. These treatments may involve chemical processes like pickling to remove surface oxide, mechanical processing such as cold rolling to refine the shape and thickness, heat treatment to adjust mechanical properties, and surface treatments like galvanizing to prevent oxidation and impart specific properties to the steel.
Figure 4 provides an integral overview of the complete process.
The operational costs of steel production are divided into various components, with raw materials accounting for 40-45%, energy for 35-40%, and the remaining portion allocated to labour, depreciation, and maintenance. Given its energy-intensive nature, the steel industry relies on both thermal and electrical energy, with a minimum requirement of 18 gigajoules per tonne of steel. Moreover, the environmental impact of steel production is substantial, primarily attributed to CO
2 emissions during coke processing and blast furnace reduction, resulting in an approximate production of 2 tonnes of CO
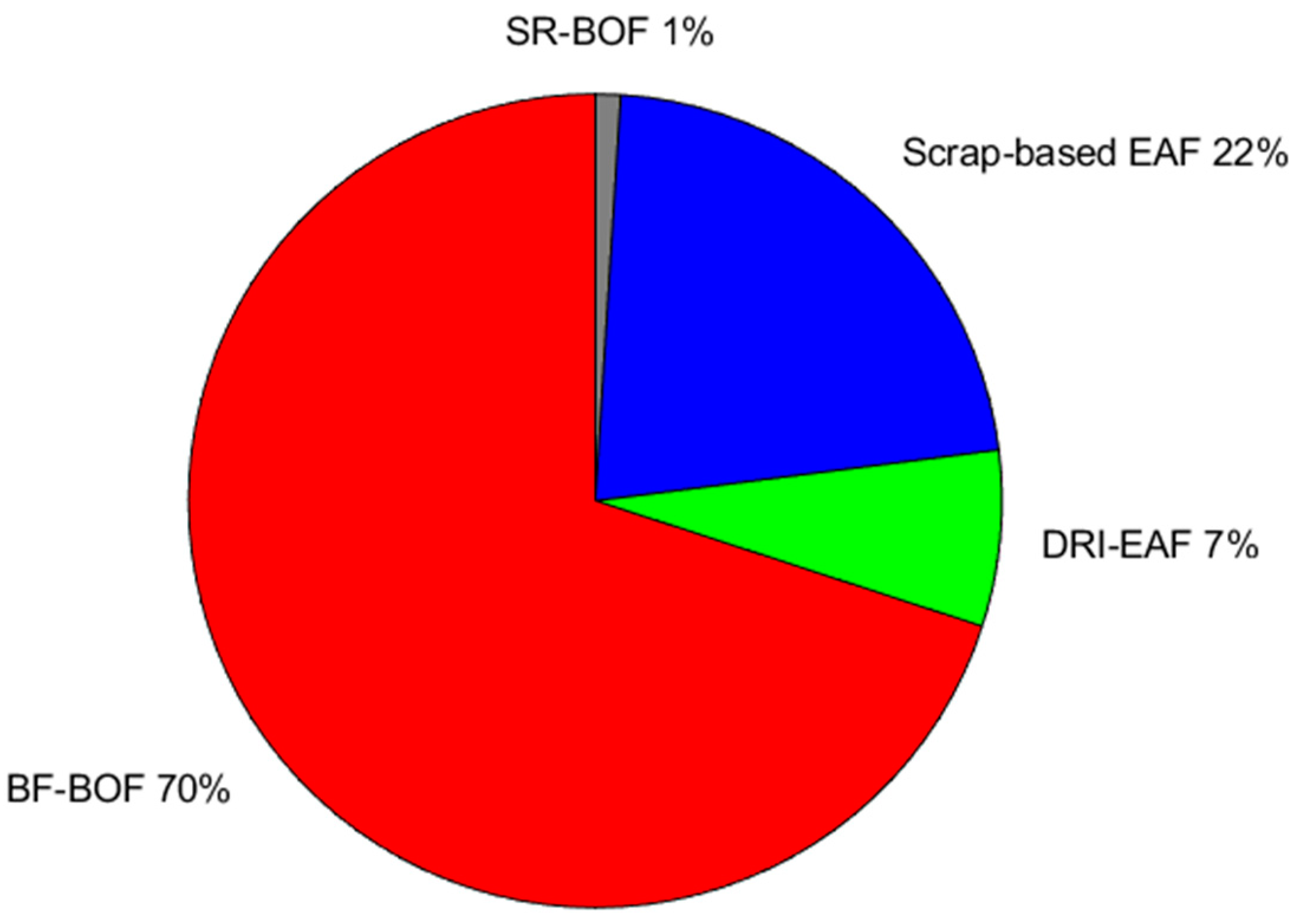
2 per tonne of steel. Currently, the majority of primary steel production (70%) utilizes the conventional blast furnace and converter system with oxygen injection,
Figure 5 [
24]; the remaining portion is primarily derived from recycled scrap in electric arc furnaces (EAF), while a small fraction is produced using older generation melting furnaces. This distribution highlights the prevailing dominance of traditional steelmaking methods and the growing significance of recycling processes in mitigating environmental impacts.
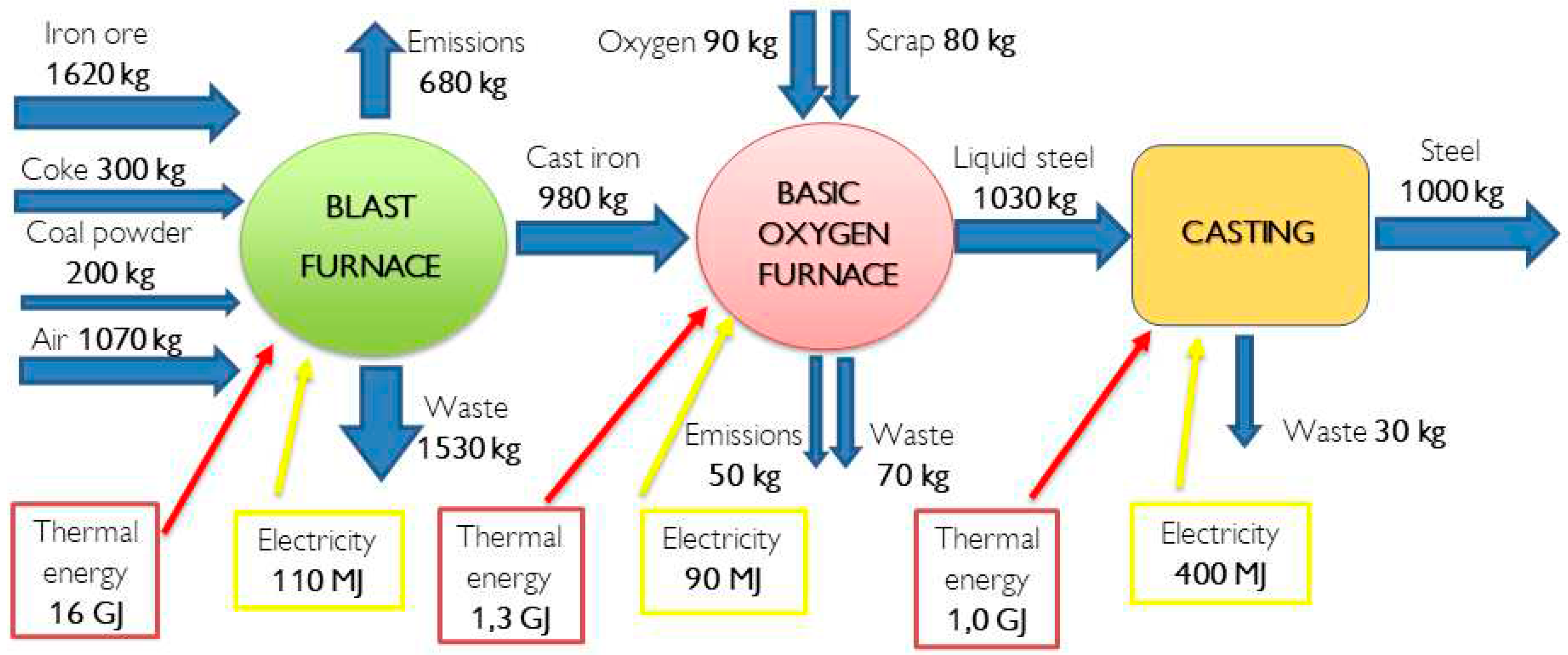
To fully grasp the potential role of hydrogen, it is essential to understand the energy and mass balance of the process, particularly in technologies like the Basic Oxygen Furnace (BOF). The authors have developed a representation, depicted in the accompanying figure, to shed light on this aspect. It is important to note that the data presented are derived from a model and may not reflect precise real-world values. However, they offer a representative insight into the technology and its characteristics.
A comprehensive analysis reveals that the primary energy consumption in the steelmaking process is primarily attributed to the operation of the Furnace. Specifically, the energy requirement amounts to approximately 18 GJ of total energy per metric tonne of hot-rolled coil steel. This value aligns closely with the findings reported in existing literature, which indicate energy consumption ranging from 18 to 22 GJ per tonne of steel.
Obviously, considering the conventional BF-BOF process, represented in
Figure 6, another promising avenue lies in the application of hydrogen for blended combustion, synergistically combining it with natural gas. This approach optimizes combustion efficiency and reduces emissions, contributing to the overall sustainability of the steel sector.
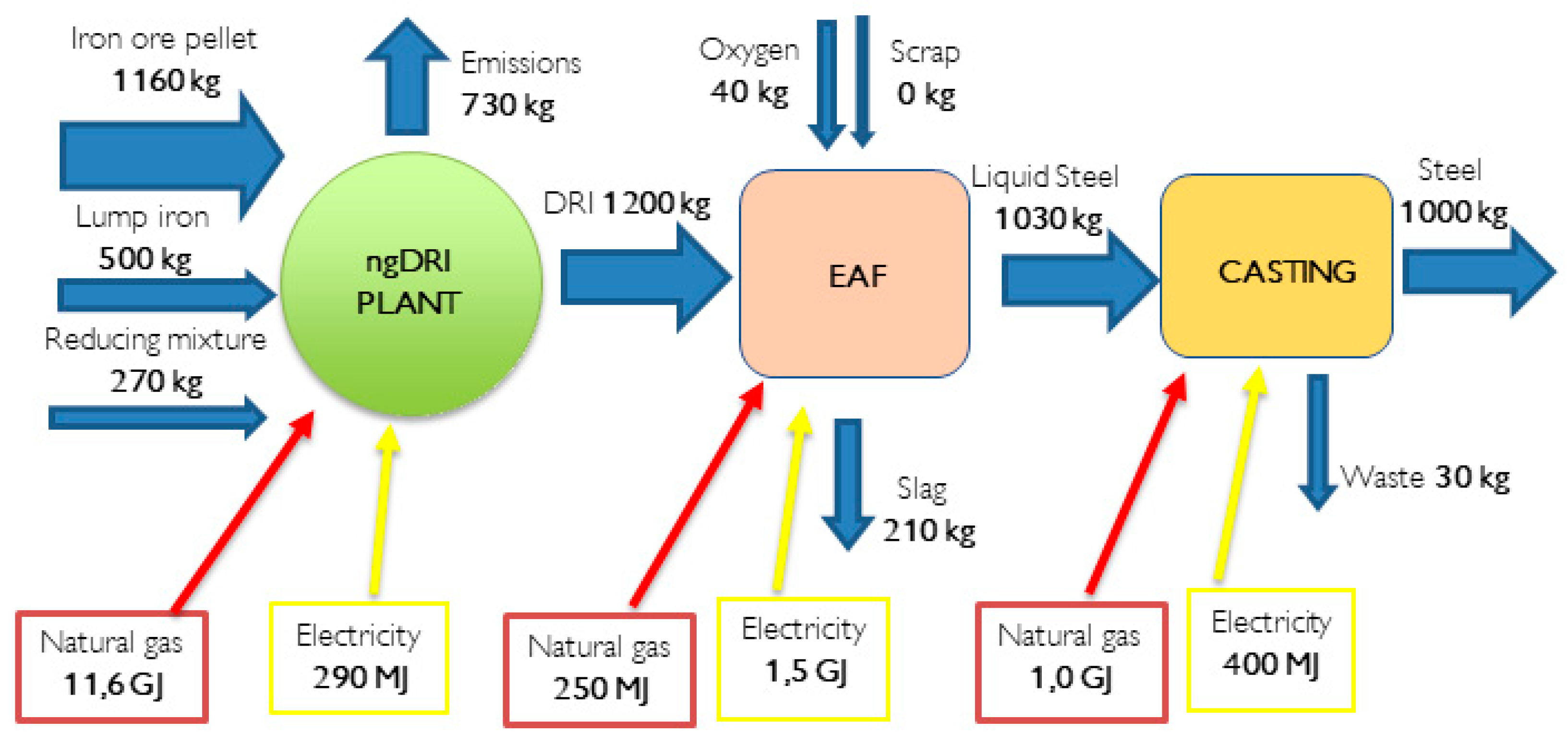
An alternative technology for steel production is the Direct Reduction Iron (DRI) process, which currently satisfies 7% of global steel demand. DRI plants typically employ a shaft furnace fuelled by methane instead of coal. The chemical process involves reforming, either internal or external to the furnace, to generate syngas consisting of CO and H2. This syngas enables the direct reduction of iron at temperatures around 800 °C, resulting in the formation of solid iron known as DRI (Direct Reduced Iron).
Hydrogen serves as an effective reducing agent in the DRI process, facilitating high productivity and iron metallization exceeding 90%, ensuring complete oxidation. However, it is important to note that hydrogen reduction reactions are endothermic, leading to increased energy requirements in these plants. In contrast, CO sustains the process through exothermic reactions. A numerical example of the DRI-EAF (Electric Arc Furnace) process demonstrates the lower demand for iron ore, which is introduced in a porous form to enhance the reduction rate. The energy consumption in the ngDRI-EAF process ranges from 14 to 18 GJ per tonne of steel.
Figure 7 provides an approximate energy and mass balance of the process. The consistency between the observed energy consumption values and those documented in the literature reinforces the validity and reliability of the data. It underscores the significance of the Furnace operation in determining the overall energy consumption in steel production. Understanding this energy-intensive aspect is crucial for identifying opportunities to optimize energy efficiency and explore potential pathways for decarbonization.
To reduce CO2 emissions in the steel industry, a transformation of the steelmaking process is underway. This transformation involves the adoption of various techniques such as direct reduced iron (DRI) with different reduction degrees, hot briquetted iron (HBI), hydrogen plasma smelting reduction (HPSR), and electric smelters for low-grade ores. To uphold their commitment to zero waste, the industry must gain a comprehensive understanding of by-products like slag.
The contribution of hydrogen in the steel sector encompasses various dimensions that offer transformative benefits. Primarily, hydrogen serves as a crucial facilitator for the integration of green energy sources into steel production. By functioning as a storage medium, hydrogen enables the effective utilization of renewable energy, addressing the challenges associated with its direct application. Moreover, hydrogen demonstrates its potential as a versatile chemical reducing agent within the steelmaking process, particularly in technologies like Direct Reduced Iron (DRI).
This direct utilization of hydrogen, H
2DRI, enhances process efficiency and promotes decarbonization.
Figure 8 provides a possible mass and energy balance of the process H
2DRI-EAF.
In the case of H2DRI, the energy requirement experiences a significant increase, estimated to be in the range of 15-18 GJ per tonne of steel. However, this increase is primarily attributed to a larger share of electricity in the process. If the electricity used is generated from renewable sources, the overall energy consumption would be lower compared to the conventional BF-BOF process. Additionally, the electric share in the shaft furnace (SF) can be further reduced through heat recovery from waste fumes.
When considering the feedstock of hydrogen, there are two options: either producing hydrogen on-site through electrolysis or sourcing it from an external supply chain. In both cases, the emissions are significantly reduced by up to 80% compared to the BF-BOF process. This reduction in emissions highlights the environmental benefits of utilizing hydrogen in the DRI process.
Integrating renewable energy sources for electricity production and implementing hydrogen as a reducing agent in the H
2DRI process present opportunities for reducing carbon emissions and achieving greater sustainability in steel production. The potential for emissions reduction and the use of renewable energy sources make H
2DRI a promising pathway for decarbonizing the steel industry and transitioning towards more environmentally friendly steelmaking processes. Affirming all energy intensity in terms of final energy in
Table 7, the H
2DRI-EAF energy requirement appears smaller than BOF-BF one, and comparable and potentially smaller than ngDRI-EAF one, with a much larger share of electricity.
The potential of hydrogen to revolutionize green energy integration and process optimization in the steel industry is undeniably significant. However, accurately assessing the scale of this transformation is paramount, given the formidable power requirements involved. The high energy demands inherent to steelmaking processes present challenges in evaluating feasible pathways for hydrogen implementation. Careful consideration of the scale and magnitude of the problem is essential to ensure realistic and effective solutions. This assessment delves into the intricate task of evaluating the real-world implementation of hydrogen in the steel industry, taking into account the substantial power levels required. It emphasizes the need for a comprehensive understanding of the energy landscape, process requirements, and technological capabilities to accurately gauge the viability and scalability of hydrogen-based solutions.
The complex interplay between hydrogen, green energy integration, and process optimization in the steel industry requires careful analysis.
6. Conclusions
This paper has explored the potential applications of hydrogen in the hard-to-abate sectors, highlighting its versatility and significance in decarbonization efforts. The initial examination provided an overview of hydrogen production methods, emphasizing the importance of sustainable and green hydrogen production.
Current efficiencies prove to be around 60% for low-temperature electrolysers, and 80% for high-temperature electrolysers, by a literature and market analysis of their nominal powers. The electrical efficiency assumes lower values for the high-temperature technology evaluating the electrical energy demand at the operating temperatures, instead of in standard conditions.
Research, experimentation, and experience on H2 production are underway, and more and more necessary to increase electrolysers performance in the various operating conditions, both at the beginning of their life and over time, especially with a view to producing green H2 from renewable energy.
The subsequent analysis delved into the diverse possibilities for hydrogen utilization in the different hard-to-abate sectors.
Overall, this paper has provided insights into the multiple ways in which hydrogen can contribute to decarbonization efforts in the hard-to-abate sectors. The findings underscore the importance of considering hydrogen as a versatile solution, offering alternative fuel options, chemical integration, and energy storage capabilities. As the world continues its transition towards a low-carbon future, harnessing the potential of hydrogen in these sectors will be crucial in achieving sustainable and environmentally friendly industrial practices in most of the considered “hard to abate” sectors.
One prominent strategy is the use of hydrogen as an alternative fuel in blended combustion, where it can be combined with existing fuels like natural gas to reduce carbon emissions. This approach offers a practical and incremental pathway towards decarbonization, leveraging the existing infrastructure while achieving significant emission reductions.
By our analysis of blended combustion, about 40 Nm3/h of H2 are necessary in the combustible blend to cover by hydrogen the 10% of 1 MW (reference value) of heat requirement for material treatment. This hydrogen flow rate could be produced as green H2 by around 200 kW low-temperature electrolysers or 150 kW high-temperature ones, with present average nominal efficiencies and neglecting system energy losses.
The second relevant use is referred to the use of hydrogen as a chemical element within the industrial processes themselves. In sectors such as steel, glass, and paper, hydrogen can act as a valuable reducing agent, enabling cleaner and more sustainable production methods. By replacing carbon-intensive inputs with hydrogen, these industries can significantly reduce their environmental impact. Finally, hydrogen demonstrates potential as an energy storage system, enabling the integration of renewable energy sources in the hard-to-abate sectors. By storing excess renewable energy in the form of hydrogen, it can be utilized during periods of high demand or when renewable energy generation is low, ensuring a more reliable and sustainable energy supply.
In all the cases considered, maintaining a careful balance of energy is crucial. The paper has also conducted practical tests on various routes for introducing hydrogen into the steel sector, specifically focusing on the analysis of potential contributions in the BOF and DRI processes. The implementation of hydrogen in the DRI process has shown promise and garnered interest. . The H2DRI-EAF technology energy requirement can be smaller than BOF-BF one, and comparable with ngDRI-EAF one, with a much bigger share of electricity, thus, potentially more renewable energy use. Typically, the energy requirement per tonne of steel produced decreases from 19 to 16.5 gigajoules (GJ). However, it is essential to evaluate each specific case thoroughly, considering the potential benefits and drawbacks associated with the introduction of hydrogen.
Author Contributions
Conceptualization, A.F. and C.G.; formal analysis, A.F. and C.G.; methodology, A.F. and C.G.; data curation, C.G.; supervision, A.F.; writing—original draft preparation, A.F.; writing—review and editing, A.F. and C.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors really thanks for the financial support the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 - Call for tender No. 1561 of 11.10.2022 of Ministero dell’Università e della Ricerca (MUR); Project funded by the European Union – NextGenerationEU. Award Number: Project code PE0000021, Concession Decree No. 1561 of 11.10.2022 adopted by Ministero dell’Università e della Ricerca (MUR), according to attachment E of Decree No. 1561/2022, Project title “Network 4 Energy Sustainable Transition – NEST”.
The authors would like to express their sincere appreciation for the support and guidance provided by Dr. Eng. Isabella Biasci throughout the development of the analysis presented in Section 5. Dr. Biasci's insights have significantly contributed to the comprehensive understanding of the role of hydrogen in decarbonizing the steel industry.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ASEC |
average specific energy consumption, kWh/kgH2 |
| ∆G |
Gibbs free-energy change, kJ/molreac or kWh/kgH2 |
| ∆H |
enthalpy change, kJ/molreac or kWh/kgH2 |
| ∆S |
entropy change, kJ/molreac/K |
| Ecell
|
cell voltage, V |
| η |
efficiency, % |
| η˅
|
overpotential, V |
| F |
Faraday constant, C/mole−
|
| HRC |
heat requirement coverage, % |
| LHV |
lower heating value, MJ/kg or MJ/Nm3
|
| ṁ |
mass flow rate, kg/h |
| NRPES |
non-renewable primary energy saving, % |
| q̇ |
volumetric flow rate, Nm3/h |
| T |
temperature, °C or K |
| U |
cell voltage, V |
| Subscripts, superscripts, acronyms and abbreviations |
| AEM |
anion exchange membrane |
| ALK |
alkaline |
| BF |
blast furnace |
| BOF |
basic oxygen furnace |
| DRI |
direct reduced iron |
| e− |
electron |
| EAF |
electric arc furnace |
| el |
electrical |
| (g) |
gaseous state |
| (l) |
liquid state |
| MC |
molten carbonate |
| n |
number of moles of electrons |
| ng |
natural gas |
| PCC |
proton conductive cell |
| PEM |
proton exchange membrane |
| reac |
reaction |
| rev |
reversible |
| SO |
solid oxide |
| SMR |
steam methane reforming |
| ° |
in standard conditions (1 atm and 25 °C) |
References
- Mäkitie, T.; Steen, M. The energy sector: an industrial perspective on energy transitions. In Handbook of Industrial Development; Bianchi, P., Labory, S., Tomlinson, P.R., Eds.; Edward Elgar Publishing: Glos, UK, 2023. [Google Scholar]
- Neuwirth, M.; Fleiter, T.; Manz, P.; Hofmann, R. The future potential hydrogen demand in energy-intensive industries - a site-specific approach applied to Germany. Energy Convers. Manag. 2022, 252, 115052. [Google Scholar] [CrossRef]
- Zaiter, I.; Ramadan, M.; Bouabid, A.; El-Fadel, M.; Mezher, T. Potential utilization of hydrogen in the UAE's industrial sector. Energy 2023, 128108. [Google Scholar] [CrossRef]
- Azadnia, A.H.; McDaid, C.; Andwari, A.M.; Hosseini, S.E. Green hydrogen supply chain risk analysis: A european hard-to-abate sectors perspective. Renew. Sust. Energ. Rev. 2023, 182, 113371. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Sabathier, J.; Guedes, F.; Reigstad, G.A.; Straus, J.; Wolfgang, O.; Ouassou, J.A.; Askeland, M.; Hjorth, I.; Skjelbred, H.I.; Andersson, L.E.; Douguet, S.; Villavicencio, M.; Trüby, J.; Brauer, J.; Cabot, C. Hydrogen and the decarbonization of the energy system in europe in 2050: A detailed model-based analysis. Renew. Sust. Energ. Rev. 2022, 167, 112779. [Google Scholar] [CrossRef]
- van der Spek, M.; Banet, C.; Bauer, C.; Gabrielli, P.; Goldthorpe, W.; Mazzotti, M.; Munkejord, S.T.; Røkke, N.A.; Shah, N.; Sunny, N.; Sutter, D.; Trusler, J.M.; Gazzani, M. (2022). Perspective on the hydrogen economy as a pathway to reach net-zero CO2 emissions in Europe. Energy Environ. Sci. 2022, 15(3), 1034–1077. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Xue, J.; Wang, X.; Li, Q. Hydrogen as a carrier of renewable energies toward carbon neutrality: State-of-the-art and challenging issues. Int. J. Miner. Metall. Mater. 2022, 29(5), 1073–1089. [Google Scholar] [CrossRef]
- Abad, A.V.; Dodds, P.E. Green hydrogen characterisation initiatives: Definitions, standards, guarantees of origin, and challenges. Energy Policy 2020, 138, 111300. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2020, 13(1), 298. [Google Scholar] [CrossRef]
- Industry – CO2 emissions. Available online: https://www.iea.org/energy-system/industry (accessed on 17 07 2023).
- Chang, R.; Overby, J. Chemistry, 13th Edition; McGraw-Hill Education: New York, United States of America, 2019; pp. 806–841. [Google Scholar]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43(24), 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Amores, E.; Sánchez, M.; Rojas, N.; Sánchez-Molina, M. Renewable hydrogen production by water electrolysis. In Sustainable fuel technologies handbook; Academic Press, 2021; pp. 271–313. [Google Scholar] [CrossRef]
- Wünning, J.G. Hydrogen for process heat generation. Student research project, RWTH Aachen University, Aachen, 1989.
- Franco, A.; Miserocchi, L.; Testi, D. Energy Indicators for Enabling Energy Transition in Industry. Energies 2023, 16, 581. [Google Scholar] [CrossRef]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of hydrogen direct reduction for fossil-free steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Cokain, K; Cochran, M.; Schalles, D. CO2 Reduction Options for High Temperature Industrial Combustion. AFRC 2021 Symposium, Houston, Texas, 11 10 2021.
- von Schéele, J. Decarbonizing and Use of Hydrogen in Reheat Furnaces. Aachener Ofenbau- und Thermoprozess-Kolloquium, Aachen, Germany, 07 10 2021.
- Sankowski, L.; Kaiser, F.; Schmitz, N.; Schwotzer, C.; Pfeifer, H. CO2-neutral Process Heating for Carburizing Furnaces–an Ecological Analysis. J. Heat Treatm. Mat. 2023, 78(1), 3–16. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Cezeliano, A.S.; Darmanto, P.S.; Aziz, M. Thermodynamic analysis of hydrogen utilization as alternative fuel in cement production. S. Afr. J. Chem. Eng. 2022, 42, 23–31. [Google Scholar] [CrossRef]
- Gärtner, S. , Rank, D.; Heberl, M.; Gaderer, M.; Dawoud, B.; Haumer, A.; Sterner, M. Simulation and techno-economic analysis of a power-to-hydrogen process for oxyfuel glass melting. Energies 2021, 14(24), 8603. [Google Scholar] [CrossRef]
- World steel in figures 2023 now available. Available online: https://worldsteel.org/media-centre/press-releases/2023/world-steel-in-figures-2023-now-available/ (accessed on 17 07 2023).
- 2023 Press releases. Available online: https://worldsteel.org/media-centre/press-releases/2023/ (accessed on 17 07 2023).
- Global crude steel production by process route and scenario, 2019-2050. Available online: https://www.iea.org/data-and-statistics/charts/global-crude-steel-production-by-process-route-and-scenario-2019-2050 (accessed on 17 07 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).