Submitted:
07 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. In-Silico Study
2.1.1. Prediction of Colchicine Potential Using a Structure-Activity Relationship (SAR) Approach
2.1.2. Prediction of Target and Gene Disease Association with Cardiac Fibrosis (CF) and Acute Myocardial Infarction (AMI)
2.1.3. Pharmacology Network Analysis
2.2. In-Vitro Study
2.2.1. Induction of Hypoxia in 3T3 Cell Line
2.2.2. Measurement of HIF-1α and IL-10 Expression
2.3. Clinical Trial Study
2.3.1. Study Design and Subject Recruitment
2.3.2. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.3.3. Data Analysis
3. Results
3.1. In-Silico Study
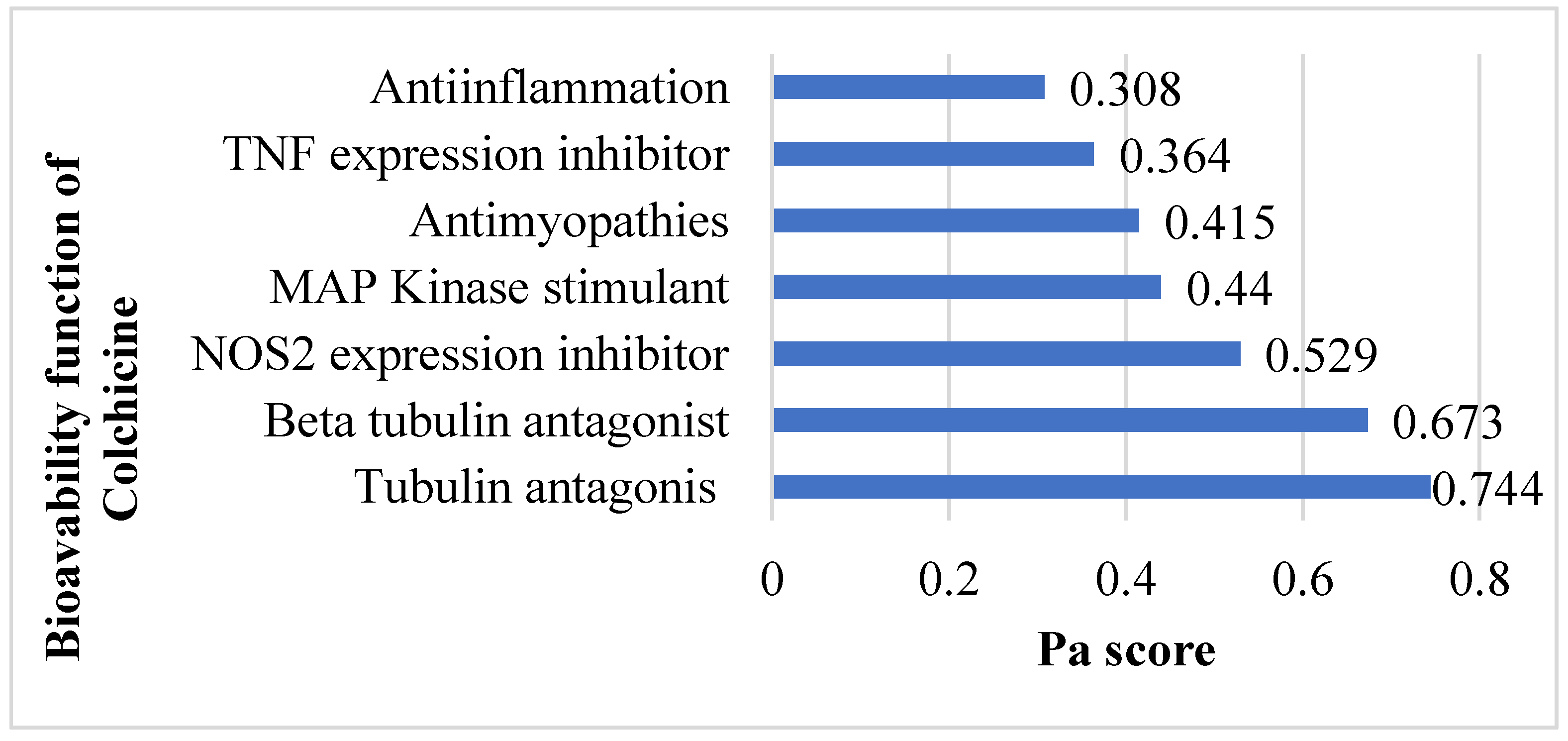
3.1.1. Prediction of Colchicine Potential Based on SAR
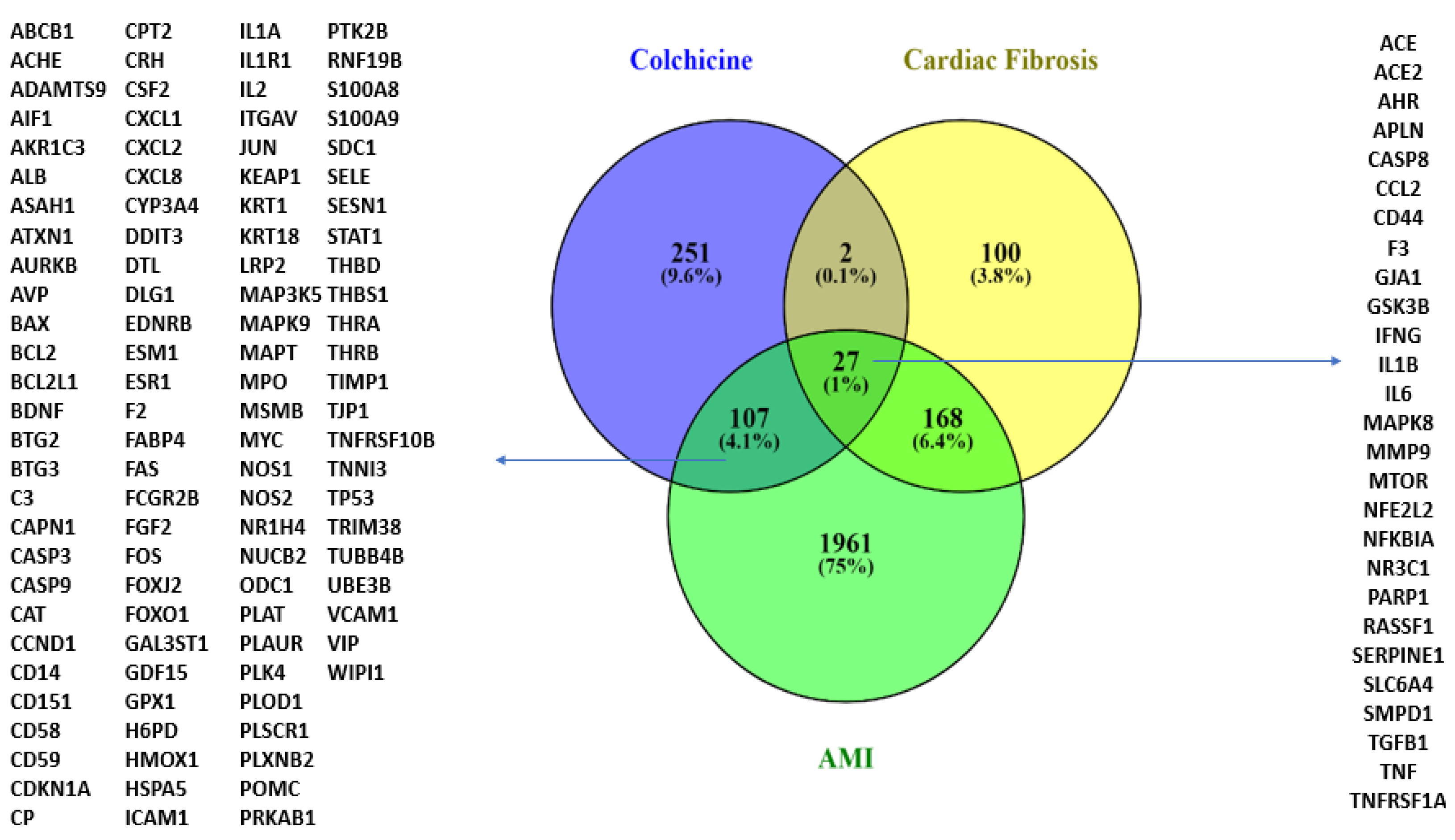
3.1.2. Prediction of Targets and Genes Related to Colchicine, AMI, and CF
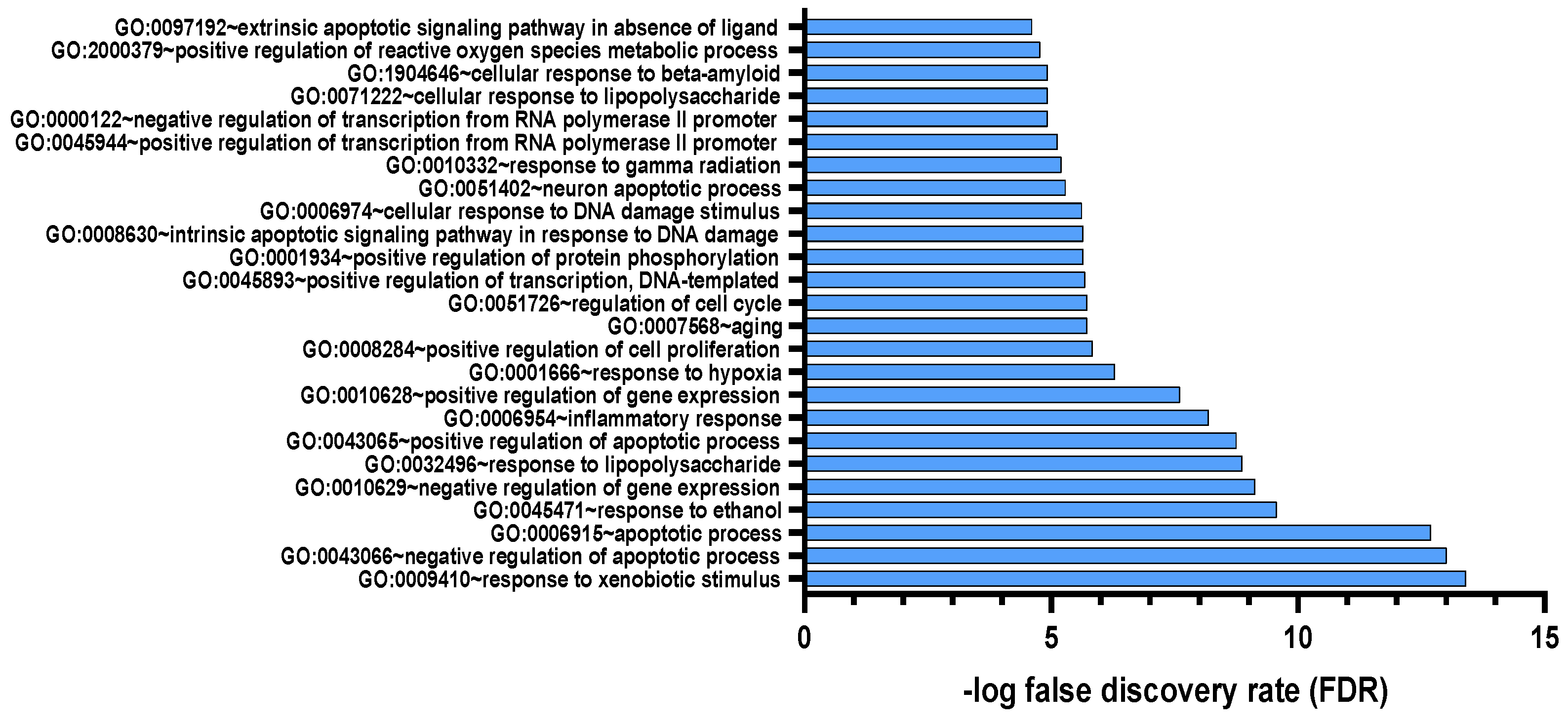
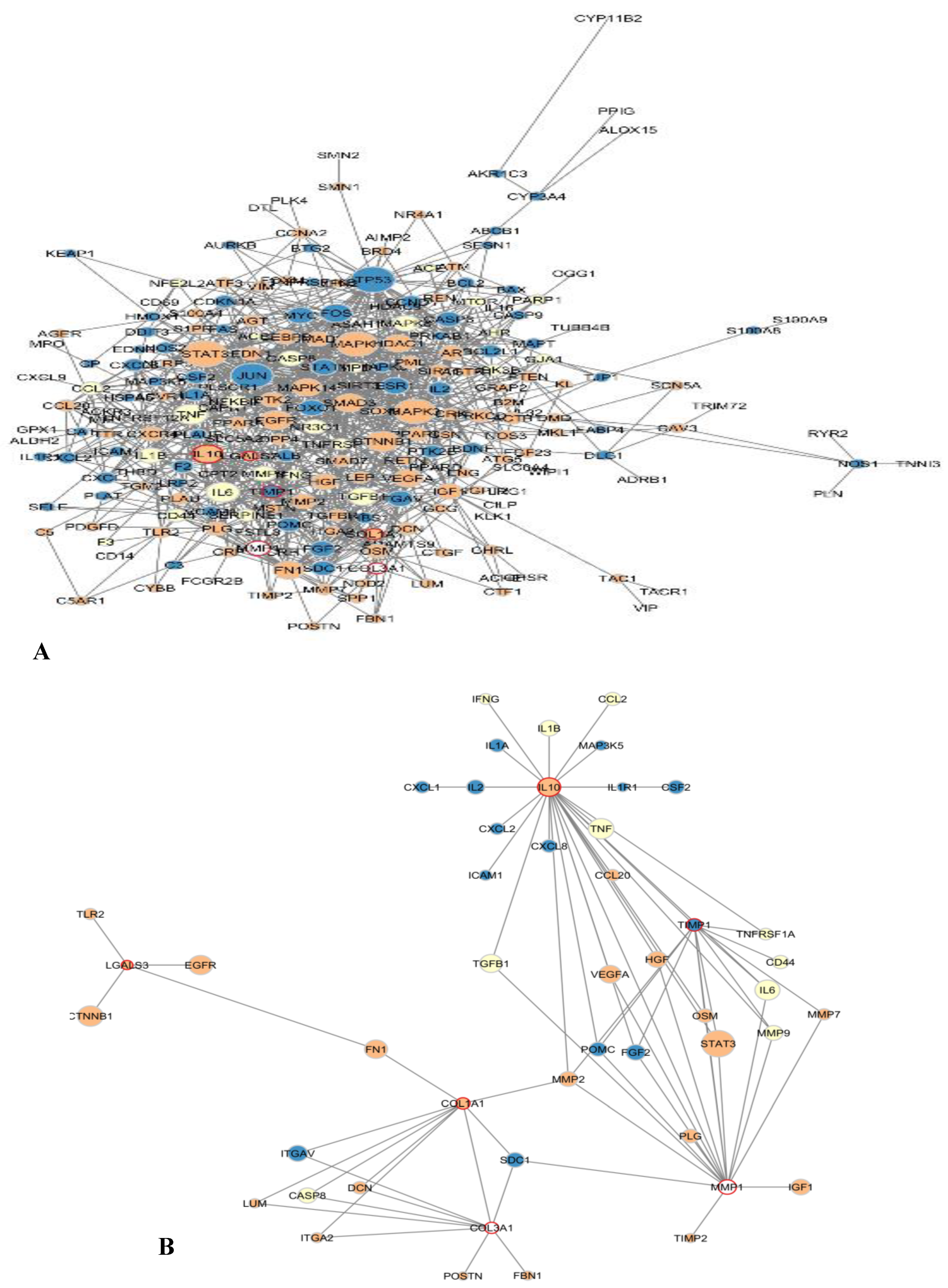
3.1.3. Pharmacology Network Analysis
3.2. In-Vitro Study
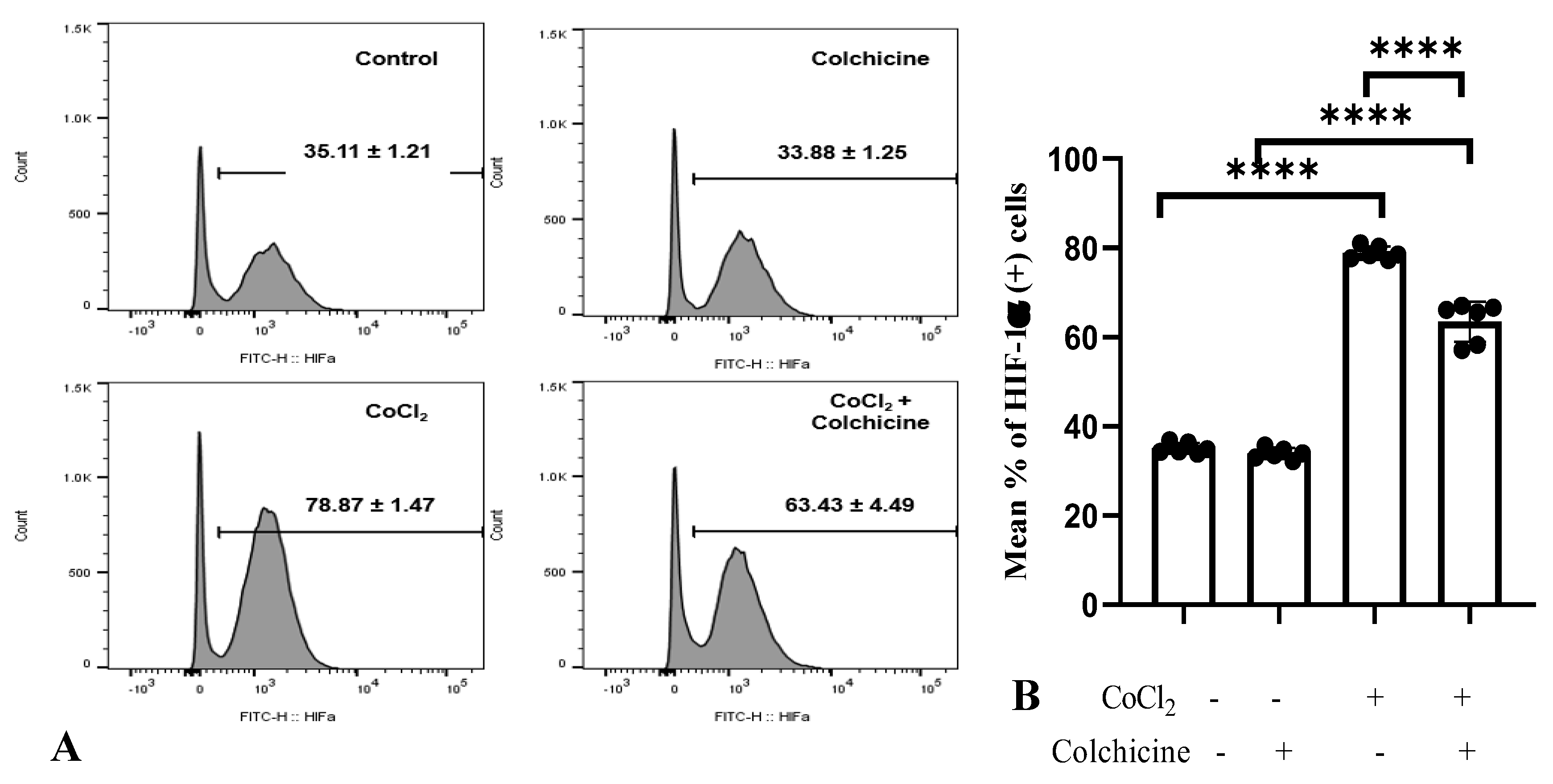
3.2.1. HIF-1α Expression
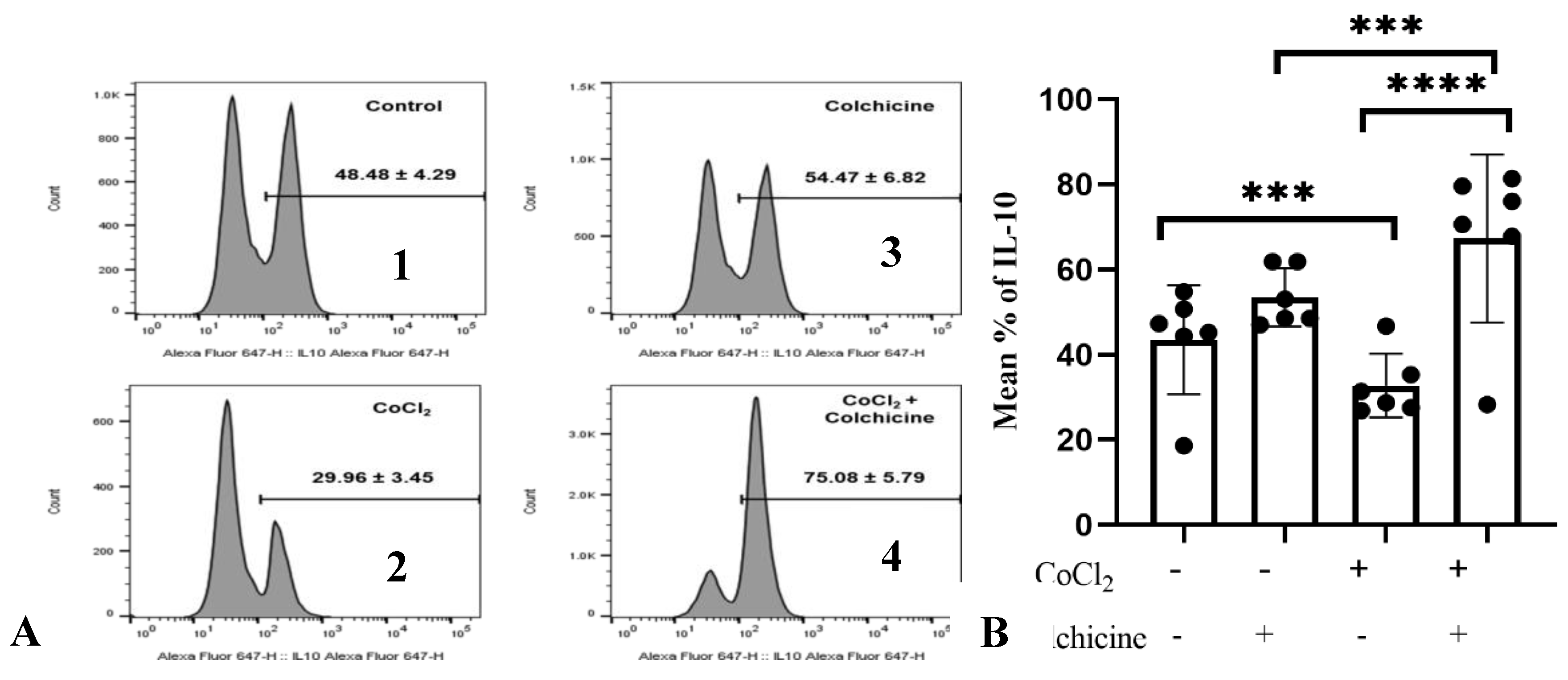
3.2.2. IL-10 Expression
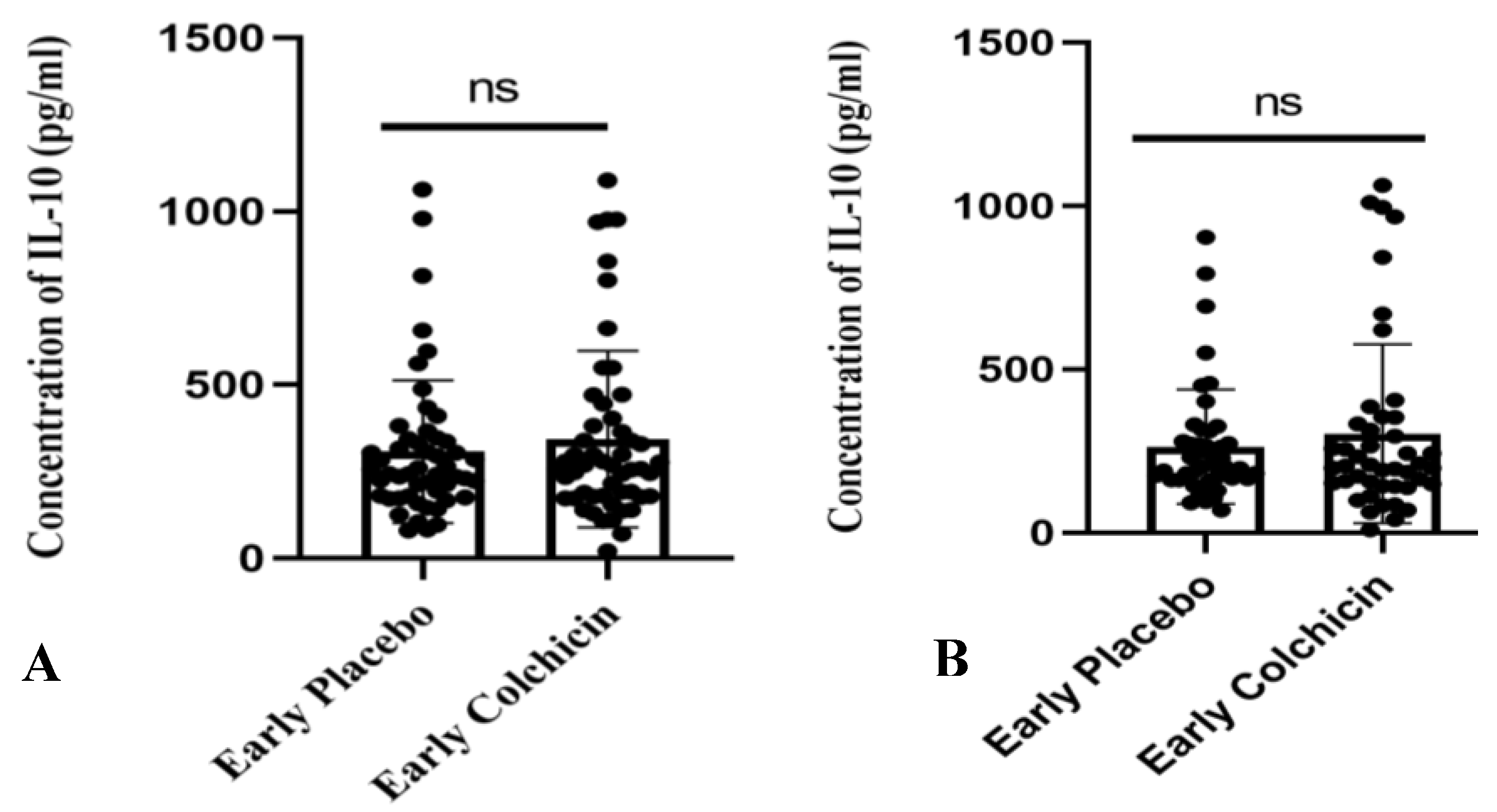
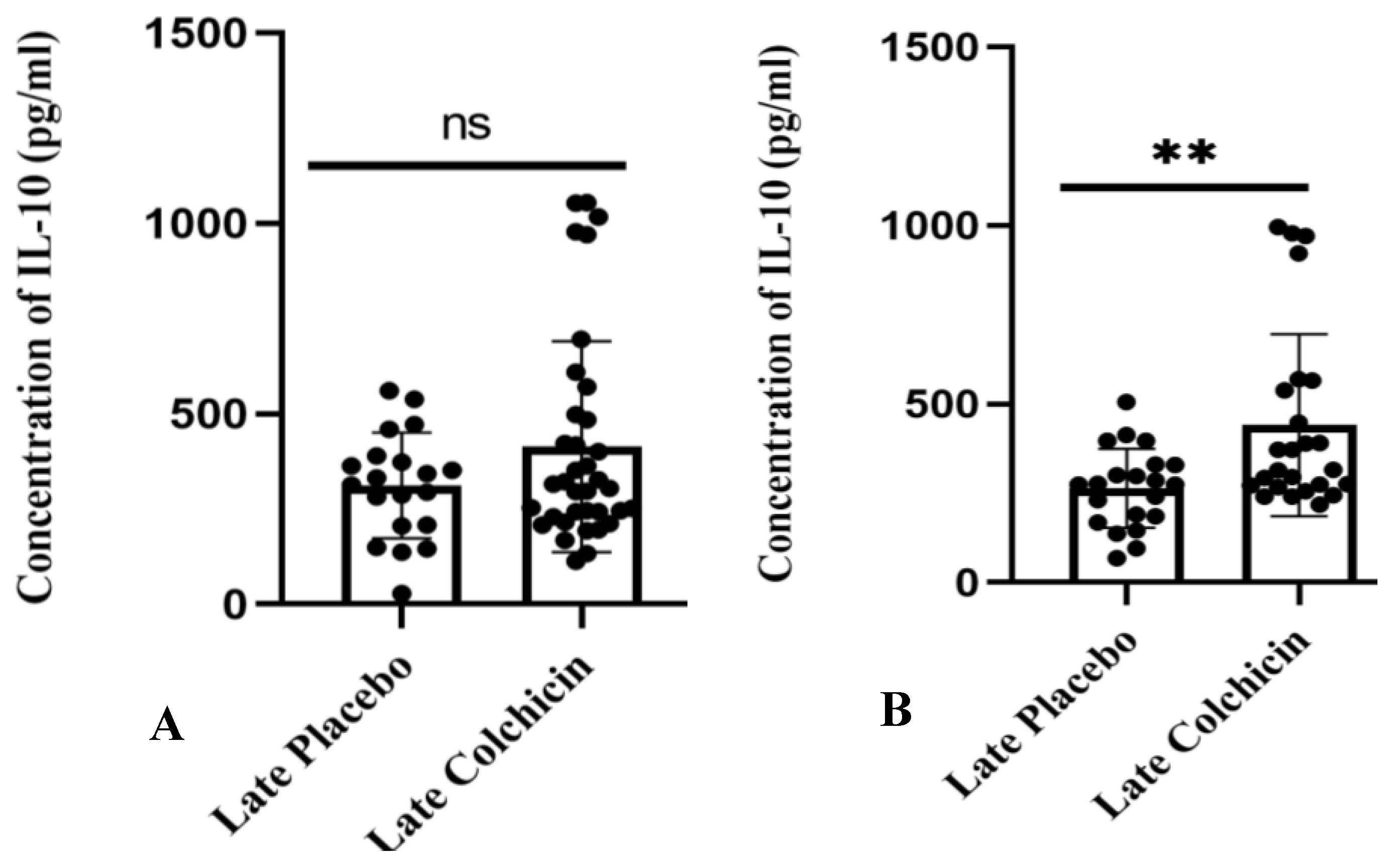
3.3. Clinical Trial Study
3.3.1. Baseline Characteristics of AMI Patients
| Early placebo | Early colchicine | pvalue | Late placebo | Late colchicine | pvalue | |
| Age (years) (Mean±SD) | 57.34±10.75 | 58.60±9.13 | 0.479 | 56.39±10.85 | 59.08±9.41 | 0.457 |
| Sex (Male), n (%) | 84 (85.71%) | 42 (84%) | 0.810 | 41 (83.67%) | 40 (76.92%) | 0.459 |
| Smoker, n (%) | 86 (87.76%) | 43 (86%) | 0.800 | 33 (67.35%) | 26 (50%) | 0.106 |
| Hypertension, n (%) | 34 (39.80%) | 19 (38%) | 0.720 | 32 (67.35%) | 35 (67.31%) | 0.837 |
| Diabetes Melitus, n (%) | 9 (9.18%) | 5 (10%) | >1.000 | 6 (12.24%) | 12 (23.08%) | 0.197 |
| Dyslipidaemia, n (%) | 14 (14.29%) | 11 (22%) | 0.253 | 5 (10%) | 5 (9.62%) | >1.000 |
| Infarct related artery (LAD), n (%) | 29 (29.59%) | 15 (30%) | >1.000 | 29 (59.18%) | 27 (51.92%) | 0.670 |
| Infarct related artery (Non LAD), n (%) | 69 (70.41%) | 35 (70%) | >1.000 | 20 (40.82%) | 25 (48.08%) | 0.670 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaptoge, S.; Pennells, L.; De Bacquer, D. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. The Lancet Global Health 2019, 7, pp.e1332–45. [CrossRef]
- Kementrian Kesehatan RI. Riset Kesehatan Dasar (Riskesdas) 2018.
- Reddy, H.; Hanif, A.M.; Rizkian, T.; Wahyudi. The Role of Galectin-3 in Acute Myocardial Infarction: A Narrative Literature Review. Bioscmed 2022, 6, 2858–2865. [CrossRef]
- Michiels, C. Physiological and Pathological Responses to Hypoxia. The American Journal of Pathology 2004, 164, pp.1875–82. [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E. IL-10 Inhibits Inflammation and Attenuates Left Ventricular Remodeling After Myocardial Infarction via Activation of STAT3 and Suppression of HuR. Circulation Research 2009, 104. [CrossRef]
- Hartman, M.H.T.; Groot, H.E.; Leach, I.M. Translational overview of cytokine inhibition in acute myocardial infarction and chronic heart failure. Trends in Cardiovascular Medicine 2018, 28, pp. 369–379. [CrossRef]
- Gourdie, R.G.; Dimmeler, S.; Kohl, P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 2016, 15(9), pp. 620-38. [CrossRef]
- Ma, J.; Tai, Y.; Fan, M.; Wang, Z. Cardiac Rehabilitation of Patients with Acute ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention in a Han Population in Northern China: A Prospective Cohort Study. Int J Gen Med 2021, 4, pp. 4959–65.
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; De Boer, R.A. From Inflammation to Fibrosis—Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr Heart Fail Rep 2017, 14, pp. 235–250. [CrossRef]
- Chacko, L.P.; Howard, J.; Rajkumar, C. Effects of Percutaneous Coronary Intervention on Death and Myocardial Infarction Stratified by Stable and Unstable Coronary Artery Disease: A Meta-Analysis of Randomized Controlled Trials. Circ Cardiovascular Quality and Outcomes 2020, 13, p. e006363. [CrossRef]
- Zheng, W.; Yu, C.; Liu, J. Patients with ST-segment elevation of myocardial infarction miss out on early reperfusion: When to undergo delayed revascularization. J Geriatr Cardiol 2017, 14, pp. 524–531.
- Ribeiro, S.A.; Lopes, C.; Amaral, R.; Amaral, A. The therapeutic potential of colchicine in the complications of COVID19. Could the immunometabolic properties of an old and cheap drug help? Metabolism Open 2020, 7, p. 100045. [CrossRef]
- Nawabi, A.Q.; Hassan, W.; Chen, L. Is Colchicine a New Game-Changer in Patients with Acute Coronary Syndrome? Cureus 2022. [CrossRef]
- Diaz-Arocutipa, C.; Benites-Meza, J.K.; Chambergo-Michilot, D. Efficacy and Safety of Colchicine in Post–acute Myocardial Infarction Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Cardiovasc Med 2021, 8, p. 676771. [CrossRef]
- Zhang, D.; Li, L.; Li, J. Colchicine improves severe acute pancreatitis-induced acute lung injury by suppressing inflammation, apoptosis and oxidative stress in rats. Biomedicine & Pharmacotherapy 2022, 153, p. 113461. [CrossRef]
- Liu, H.; Beck, T.N.; Golemis, E.A.; Serebriiskii, I.G. Integrating in silico resources to map a signaling network. Methods Mol Biol 2014, 1101, pp. 197-245. [CrossRef]
- Basanagouda, M.; Jadhav, V.B.; Kulkarni, M.V.; Rao, R.N. Computer Aided Prediction of Biological Activity Spectra: Study of Correlation between Predicted and Observed Activities for Coumarin-4-Acetic Acids. Indian J Pharm Sci 2015, 73, pp. 88–92.
- Mujalli, A.; Banaganapalli, B.; Alrayes, N.M. Myocardial infarction biomarker discovery with integrated gene expression, pathways and biological networks analysis. Genomics 2020, 112, pp. 5072-85. [CrossRef]
- Tao, Y.; Gao, C.; Qian, D. Regulatory mechanism of fibrosis-related genes in patients with heart failure. Front Genet 2022, 13, p. 1032572. [CrossRef]
- Shahid, M.; Azfaralariff, A.; Law, D. Comprehensive computational target fishing approach to identify Xanthorrhizol putative targets. Sci Rep 2021, 11, p. 1594. [CrossRef]
- Wu, Y.; Zhu, Y.; Xie, N. A network pharmacology approach to explore active compounds and pharmacological mechanisms of a patented Chinese herbal medicine in the treatment of endometriosis. PLoS ONE 2022, 17, p. e0263614. [CrossRef]
- Kim, T.H.; Yu, G.R.; Kim, H. Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro. CIMB 2023, 45, pp. 1314–32. [CrossRef]
- Foutch, D.; Pham, B.; Shen, T. Protein conformational switch discerned via network centrality properties. Computational and Structural Biotechnology Journal 2021, 19, pp. 3599–608. [CrossRef]
- Zhao, C.; Moreno-Nieves, U.; Di Battista, J.A. Chemical Hypoxia Brings to Light Altered Autocrine Sphingosine-1-Phosphate Signalling in Rheumatoid Arthritis Synovial Fibroblasts. Mediators of Inflammation 2015, pp. 1–12. [CrossRef]
- Liu, R.; Liu, Q.; Wang, K. Comparative analysis of gene expression profiles in normal hip human cartilage and cartilage from patients with necrosis of the femoral head. Arthritis Res Ther 2016, 18, p. 98. [CrossRef]
- Wang Y, Zhang Y, Li J; et al. (2023b) Hypoxia Induces M2 Macrophages to Express VSIG4 and Mediate Cardiac Fibrosis After Myocardial Infarction. Theranostics 2023, 13:2192–2209. [CrossRef]
- Lestari, S.R.; Rifa’i, M. Regulatory T cells and anti-inflammatory cytokine profile of mice fed a high-fat diet after single-bulb garlic (Allium sativum L.) oil treatment. Trop J Pharm Res 2019, 17, p. 2157. [CrossRef]
- Al-Masoudi, F.I.; Ali, B.H. Evaluation of Nesfatin – 1 and Other Biochemical Markers in diabetic Neuropathy Iraqi patients before and after treatment with tegretol. Annals of the Romanian Society for Cell Biology 2021, pp. 9422–29. Available online: http://annalsofrscb.ro/index.php/journal/article/view/3682.
- Emon, N.U.; Alam, S.; Rudra, S. Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum : In vivo, in vitro, and in silico approaches. Food Science & Nutrition 2021, 9, pp. 833–46. [CrossRef]
- Liu, Y.; Xu, S.; Zu, T. Reversal of TET-mediated 5-hmC loss in hypoxic fibroblasts by ascorbic acid. Laboratory Investigation 2019, 99, pp. 1193–202. [CrossRef]
- Qiu, L.; Wang, W.; Sa, R.; Liu, F. Prevalence and Risk Factors of Hypertension, Diabetes, and Dyslipidemia among Adults in Northwest China. International Journal of Hypertension 2021, pp. 1–10. [CrossRef]
- Boden, W.E.; Miller, M.G.; McBride, R. Testosterone concentrations and risk of cardiovascular events in androgen-deficient men with atherosclerotic cardiovascular disease. American Heart Journal 2020, 224, pp. 65–76. [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, pp. 524–33. [CrossRef]
- Ambreen, S.; Fatima, S.; Elwakiel, A. Hypercoagulability Impairs Plaque Stability in Diabetes-Induced Atherosclerosis. Nutrients 2022, 14, 1991. [CrossRef]
- Logeart D.; Taille, Y.; Derumeaux, G. Patterns of left ventricular remodeling post-myocardial infarction, determinants, and outcome. Clin Res Cardiol 2024. [CrossRef]
- Lok, D.J.; Lok, S.I.; Bruggink-André, De La Porte, P.W. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol 2013, 102, pp. 103–10. [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med Res Methodol 2021, 21, 241. [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals (Basel) 2020, 13(1), p. 8. doi: 10.3390/ph13010008. Erratum in: Pharmaceuticals (Basel). 2020 Apr 20;13(4):E72. [CrossRef]
- Puurand, M.; Tepp, K.; Timohhina, N. Tubulin βII and βIII Isoforms as the Regulators of VDAC Channel Permeability in Health and Disease. Cells 2019, 8, p. 239. [CrossRef]
- Leung, Y.Y.; Hui, L.L.Y.; Kraus, V.B. Colchicine --- update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015, 45, pp. 341–50. [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016, 119, pp. 91–112. [CrossRef]
- Stafford, N.; Assrafally, F.; Prehar, S. Signaling via the Interleukin-10 Receptor Attenuates Cardiac Hypertrophy in Mice During Pressure Overload, but not Isoproterenol Infusion. Front Pharmacol 2020, 11, p. 559220. [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J of Applied Toxicology 2019, 39, pp. 556–70. [CrossRef]
- Michiels, C. Physiological and Pathological Responses to Hypoxia. The American Journal of Pathology 2004, 164, pp. 1875–82. [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 2017, 112, 33. [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014, 11, pp. 255–65. [CrossRef]
- Kandilarov, I.; Gardjeva, P.; Georgieva-Kotetarova, M.; Zlatanova, H.; Vilmosh, N.; Kostadinova, I.; Katsarova, M.; Atliev, K.; Dimitrova, S. Effect of Plant Extracts Combinations on TNF-α, IL-6 and IL-10 Levels in Serum of Rats Exposed to Acute and Chronic Stress. Plants 2023, 12, 3049. [CrossRef]
- Cabral-Santos, C.; De Lima Jr, E.A.; Fernandes, I.M.D.C. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. Journal Cellular Physiology 2019, 234, pp. 9956-65. [CrossRef]
- Shoemaker, J.; Saraiva, M.; O’Garra, A. GATA-3 Directly Remodels the IL-10 Locus Independently of IL-4 in CD4+ T Cells. The Journal of Immunology 2006, 176, pp. 3470–79. [CrossRef]
- Stumpf, C.; Petzi, S.; Seybold, K. Atorvastatin enhances interleukin-10 levels and improves cardiac function in rats after acute myocardial infarction. Clinical Science 2009, 116, pp. 45–52. [CrossRef]
- Hofmann, U.; Frantz, S. Role of T-cells in myocardial infarction. Eur Heart J 2016, 37, pp. 873–9. [CrossRef]
- Meng, X.; Li, J.; Yu, M. Transplantation of mesenchymal stem cells overexpressing IL10 attenuates cardiac impairments in rats with myocardial infarction. Journal Cellular Physiology 2018, 233, pp. 587-95. [CrossRef]
- Lakoski, S.G.; Liu, Y.; Brosnihan, K.B.; Herrington, D.M. Interleukin-10 concentration, and coronary heart disease (CHD) event risk in the estrogen replacement and atherosclerosis (ERA) study. Atherosclerosis 2008, 197, pp. 443–7. [CrossRef]
- Kaur, K.; Sharma, A.K.; Singal, P.K. Significance of changes in TNF-α and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. American Journal of Physiology-Heart and Circulatory Physiology 2006, 291, pp. H106–13. [CrossRef]
- Hulsmans, M.; Sager, H.B.; Roh, J.D. Cardiac macrophages promote diastolic dysfunction. Journal of Experimental Medicine 2018, 215, pp. 423–40. [CrossRef]
- Frieler, R.A.; Mortensen, R.M. Immune Cell and Other Noncardiomyocyte Regulation of Cardiac Hypertrophy and Remodeling. Circulation 2015, 131, pp. 1019–30. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





