1. Introduction
Cardiovascular diseases (CVDs) remain a leading cause of mortality globally, contributing to approximately 40% of all deaths [
1]. Among these, ST-elevated myocardial infarction (STEMI) poses a significant burden, with over 7 million deaths reported annually [
2]. Despite advancements in treatment modalities, the development of heart failure following STEMI remains a critical concern, increasing mortality risk by three to fourfold [
3,
4].
Patients who undergo PCI tend to have a lower risk of complications compared to those receiving medication-only therapy [
5]. In Indonesia, managing STEMI cases often involves delayed percutaneous coronary intervention (PCI), primarily due to the long distances between rural communities and specialized healthcare facilities, along with economic limitations. As a result, delayed PCI poses a significant challenge, especially for patients from lower socioeconomic backgrounds. This delay is associated with worse outcomes, including greater myocardial damage, increased fibrosis, and a higher risk of heart failure, highlighting the urgent need to address this issue in clinical practice [
6].
The pathophysiology of post-AMI complications involves complex processes of cardiac remodelling, particularly fibrosis, which significantly impacts patient outcomes [
7]. This remodelling process, characterized by structural and functional changes in the myocardium, can lead to heart failure if left uncontrolled [
8]. Recent research has highlighted the role of inflammatory mediators and fibrotic markers in this process, with Galectin-3 and Procollagen III N-terminal Propeptide (PIIINP) emerging as key biomarkers.
Colchicine, a well-established anti-inflammatory agent, has shown promise in modulating the inflammatory response following AMI. Its potential to mitigate cardiac remodeling by influencing fibrotic processes presents an intriguing therapeutic approach [
9]. Uncontrolled cardiac remodelling can culminate in heart failure, a condition where the heart is unable to pump blood effectively to meet the body's demands [
10]. Monitoring patients at high risk for this remodelling is crucial for guiding effective treatment strategies [
11]. However, the optimal timing of colchicine administration, particularly in the context of delayed PCI, and its effects on specific biomarkers of fibrosis and inflammation remain unclear. Moreover, few studies have investigated the timing of colchicine administration in relation to PCI. Colchicine administered 6 to 24 hours before PCI was found to reduce the rate of periprocedural myocardial injury (PM) injury [
12]. The use of colchicine in PCI has shown reducing major adverse cardiovascular events (MACE) [
13].
Cardiac fibrosis, a hallmark of adverse cardiac remodelling, results from the excessive deposition of extracellular matrix components, primarily collagen, in the myocardium. This process is associated with a decline in ventricular function and an increased risk of heart failure [
14]. Elevated PIIINP levels in the blood indicate increased collagen synthesis, potentially a sign of cardiac remodelling [
15]. Galectin-3 is a protein involved in diverse processes, including fibrosis, inflammation, and apoptosis [
16]. Elevated galectin-3 levels in the blood indicate increased fibrosis and inflammation, which can also contribute to cardiac remodelling and heart failure [
17].
Numerous studies have explored biomarkers like Galectin-3 and PIIINP, both of which are linked to fibrotic processes and are elevated in patients following STEMI. While the role of these biomarkers in cardiac remodelling has been extensively studied, effective therapeutic strategies to modulate these pathways remain limited. This study aims to elucidate the cardioprotective effects of colchicine on Galectin-3 and PIIINP in STEMI patients undergoing either primary or delayed PCI. Our findings suggest that colchicine's efficacy is more pronounced in delayed PCI settings, where it significantly modulates these biomarkers, offering a potential therapeutic advantage in scenarios where timely revascularization is not feasible. This research provides novel insights into the timing-dependent efficacy of colchicine in modulating post-STEMI cardiac remodelling, particularly in resource-limited settings where delayed intervention is more common.
2. Materials and Methods
2.1. Study Design and Participants
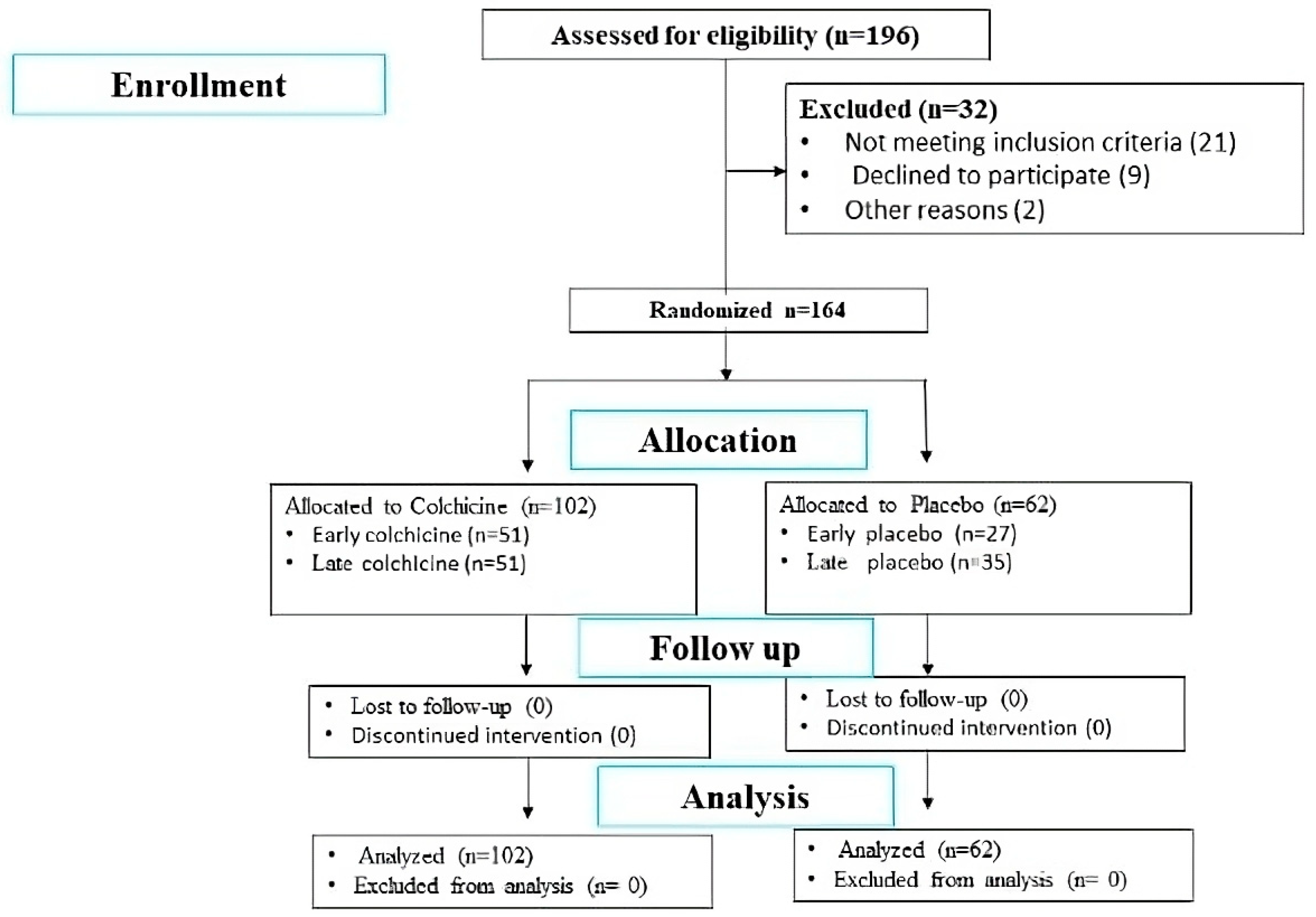
This multicenter, randomized, double-blind, placebo-controlled trial was conducted from September 2022 to February 2023 across five healthcare centers in East Java, Indonesia. We enrolled 164 patients with first-time ST-segment Elevation Myocardial Infarction (STEMI). Inclusion criteria for this study were first-time STEMI patients between the ages of 30-65, presenting with chest pain lasting more than 30 minutes and ST-segment elevation ≥1 mm in at least two contiguous leads on a 12-lead electrocardiogram. Exclusion criteria included patients with previous myocardial infarction, severe renal or hepatic dysfunction, and those with contraindications to colchicine. Patients were stratified into early PCI (≤12 hours) and delayed PCI (>12 hours) groups based on their time to intervention. The study adhered to the Declaration of Helsinki and was approved by the Ethical Committee of Saiful Anwar Hospital (Approval No. 400/235/K.3/302/2020). The trial was duly registered with the International Study Registry ISRCTN (Registration Number: ISRCTN12958502, registered on 28/12/2023).
2.2. Randomization and Blinding
Participants were randomized using a computer-generated sequence to receive either colchicine or placebo, stratified by time to PCI (early: ≤12 hours; delayed: >12 hours). An independent research team managed the randomization process. Both medications were identically packaged and labelled by an external pharmacy to maintain blinding of participants, healthcare providers, and outcome assessors.
2.3. Intervention
Patients received either oral colchicine (0.5 mg daily) or a matching placebo for 12 weeks, starting on the day of admission. A loading dose of 1 mg colchicine was administered 1-2 hours pre-PCI, followed by a 0.5 mg maintenance dose starting one hour post-PCI, continued daily for one month.
2.4. Sample Size Determination
The sample size was calculated based on the primary outcomes of changes in Galectin-3 and Procollagen III N-terminal Propeptide (PIIINP) levels. The sample size was calculated to detect a medium effect size (d=0.5) with 80% power at a 0.05 alpha level, requiring 64 participants per group. To accommodate potential dropouts and non-adherence, the total sample size was increased to 164 participants, distributed as 51 in each early PCI group and 62 in the delayed intervention groups (35 colchicine, 27 placebo) (
Figure 1).
2.5. Monitoring, Safety, and Adverse Events
A systematic record was maintained for all adverse events encountered during or subsequent to medication administration. Notable adverse effects deemed related to the study were promptly reported following standardized safety reporting protocols.
2.6. Data Collection and Outcome Measures
Baseline clinical and demographic characteristics were recorded at enrolment. Primary outcomes were changes in serum Galectin-3 and PIIINP levels at 24 hours and 5 days post-PCI. Secondary outcomes included changes in left ventricular end-diastolic volume (LVEDV) and major adverse cardiac events (MACE).
2.7. Research Variables
Independent Variables: Administration of colchicine, including both loading and maintenance doses.
Dependent Variables: Changes in cardiac remodelling were assessed using echocardiographic measurements of left ventricular end-diastolic volume (LVEDV) and ejection fraction (EF).
Intermediate Variables: Levels of Galectin-3, and the collagen I to III ratio, assessed via Procollagen III N-terminal Propeptide (PIIINP).
2.8. Biomarker Quantification
Venous blood samples were collected at 24 hours and 5 days post-PCI. Serum Galectin-3 and PIIINP levels were quantified using specific enzyme-linked immunosorbent assay (ELISA) kits (Human Galectin-3 ELISA Kit, BT Lab, Cat No: E1951Hu; Human Procollagen Type III N-Terminal Propeptide ELISA Kit, Cat No: abx152740).
2.9. PIIINP Measurement Protocol
The ELISA for PIIINP involved the preparation of standard, blank, and sample wells. These were incubated with designated reagents at 37°C, followed by a series of washes. Detection reagents A and B were then applied. A colorimetric substrate solution was added to develop the colour, which turned blue, and a stop solution was subsequently used to change the colour to yellow, signalling readiness for optical density measurement at 450 nm.
2.10. Galectin-3 Measurement Protocol
The ELISA for Galectin-3, all reagents and samples were first brought to room temperature. Samples were added to labelled 8-well strips and incubated with gentle shaking for 2.5 hours at room temperature. Following this, the wells were washed, and a biotinylated Galectin-3 antibody was added. After further incubation and washing, a streptavidin solution was applied. The reaction was developed using a TMB substrate and stopped with a stop solution before measuring the absorbance at 450 nm.
2.11. Statistical Analysis
Data were analysed using GraphPad Prism software (version 8.40). Quantitative results were expressed as means ± SEM. Prior to conducting parametric tests, data were assessed for normality and variance homogeneity. The effects of colchicine compared to placebo at different time points were analysed using two-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. Significance was established at a p-value of <0.05. Additionally, adjustments for potential confounders such as age, gender, and baseline severity of MI were incorporated into the statistical models to enhance the validity and reliability of the findings.
3. Results
3.1. Baseline Characteristics
Of 196 patients assessed for eligibility, 164 patients met the inclusion criteria and were randomized (
Figure 1). Baseline characteristics were well-balanced between groups (
Table 1). The mean age was 56.93 ± 10.32 years, with a predominance of male participants (82.21%). No significant differences were observed in cardiovascular risk factors or infarct-related artery location between groups.
3.2. Effects of Colchicine on Procollagen Type III N-terminal Propeptide (PIIINP) Levels
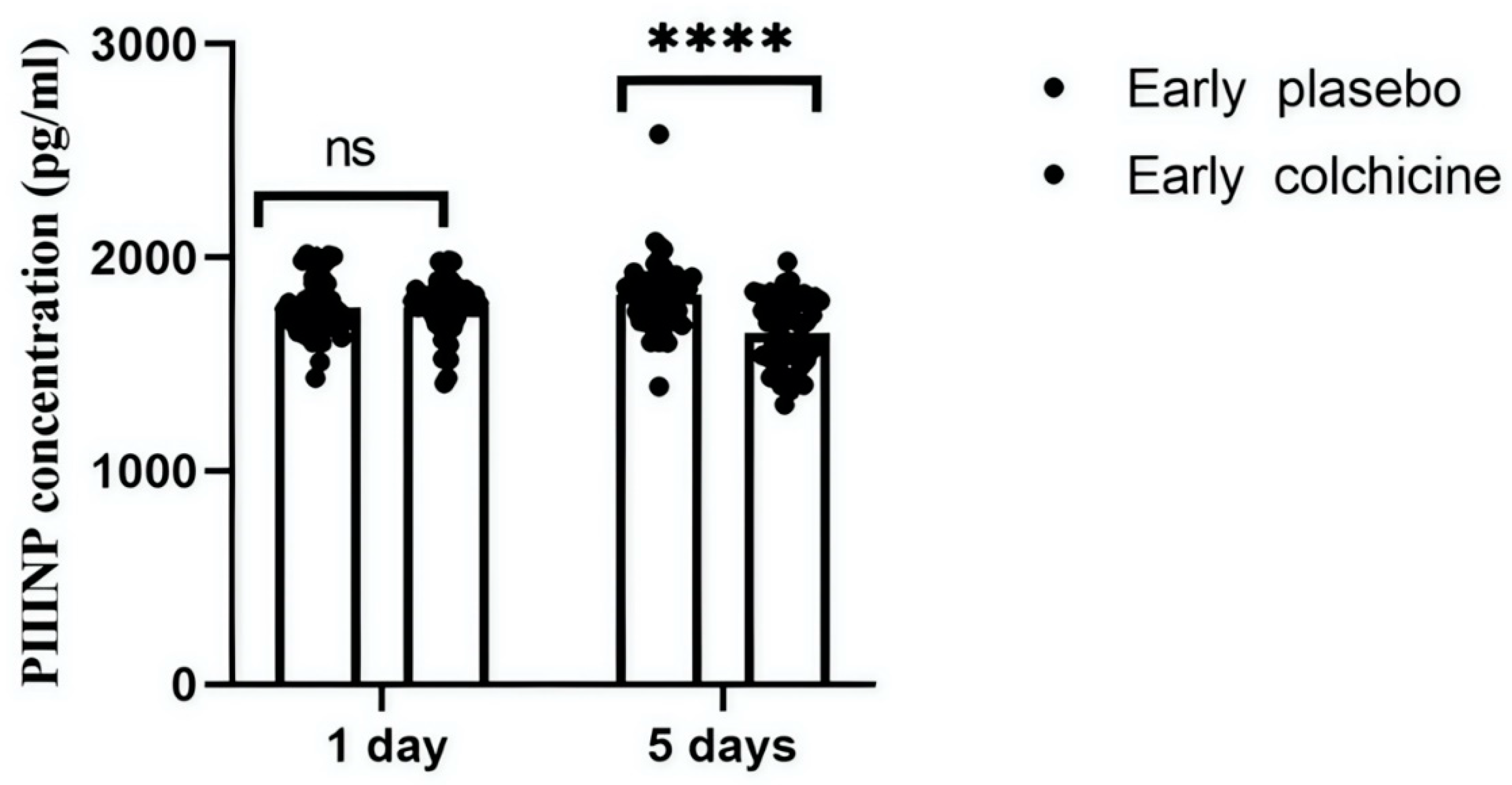
This study quantitatively analysed serum concentrations of PIIINP to evaluate the antifibrotic effects of colchicine in AMI patients following early PCI . At the initial 24-hour mark post-treatment, PIIINP levels showed no statistically significant difference between the groups, with colchicine-treated patients presenting a mean PIIINP level of 1768.41±132.08 pg/mL and the placebo group showing 1769.06±135.61 pg/mL (p>0.9999). However, a significant reduction in PIIINP levels was observed by day 5 in the colchicine group (1649.94±162.25 pg/mL) compared to the placebo group (1828.54±167.65 pg/mL, p<0.0001). These results suggest a pronounced antifibrotic effect of colchicine, particularly evident in patients undergoing early PCI (
Figure 2).
This figure illustrates the effects of colchicine on serum PIIINP concentrations in patients receiving early PCI for STEMI. The graph compares PIIINP levels between the colchicine-treated and placebo groups at two time points: 24 hours and 5 days post-MI. Initial measurements show no significant (ns) difference in PIIINP levels at 24 hours; however, by day 5, colchicine treatment significantly reduces PIIINP levels, suggesting a potential therapeutic benefit in reducing cardiac fibrosis (****p<0.0001).
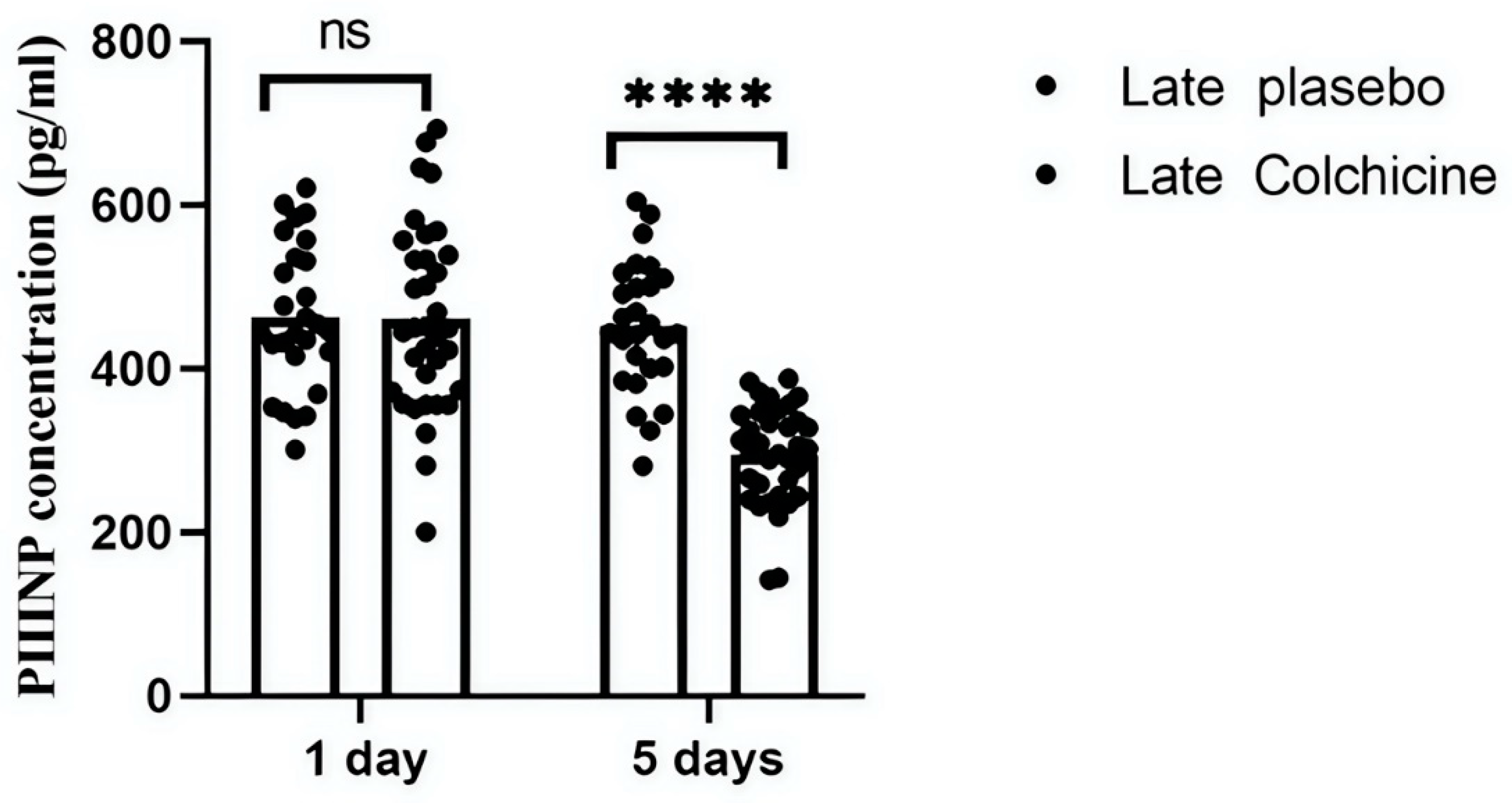
Further analysis evaluated colchicine's efficacy in AMI-STEMI patients who received late revascularization. Contrary to early PCI outcomes, colchicine significantly altered PIIINP levels at both 24 hours and day 5 in these settings. At 24 hours, the colchicine-treated group's PIIINP levels (461.26±113.77 pg/mL) were similar to those in the placebo group (463.19±89.29 pg/mL, p>0.9999). However, by day 5, a highly significant difference was observed between the colchicine (451.48±93.00 pg/mL) and placebo (494.91±60.76 pg/mL) groups (p<0.0001) (p<0.0001) (
Figure 3).
This figure depicts the longitudinal effects of colchicine on PIIINP levels in patients with STEMI undergoing delayed PCI. Serum levels of PIIINP were measured at 24 hours and 5 days post-intervention. The results indicate that colchicine does not significantly affect PIIINP levels at 24 hours in this later intervention group; however, a highly significant difference is observed between the colchicine and placebo groups at day 5.
These findings suggest that the timing of colchicine administration may be crucial for its effectiveness in modulating fibrotic biomarkers and highlight the importance of early PCI in guiding therapeutic strategies in clinical settings. ns: not significant; ****: p<0.0001 revealed a highly significant reduction in PIIINP levels, confirming colchicine’s antifibrotic effect.
3.3. Effects of Colchicine on Galectin-3 Levels
In the early PCI group, colchicine significantly reduced Galectin-3 levels at 24 hours (461.94 ± 125.03 pg/mL vs. 531.61 ± 141.59 pg/mL, p=0.0040) and 5 days (237.06 ± 63.18 pg/mL vs. 565.53 ± 103.41 pg/mL, p<0.0001) compared to placebo (
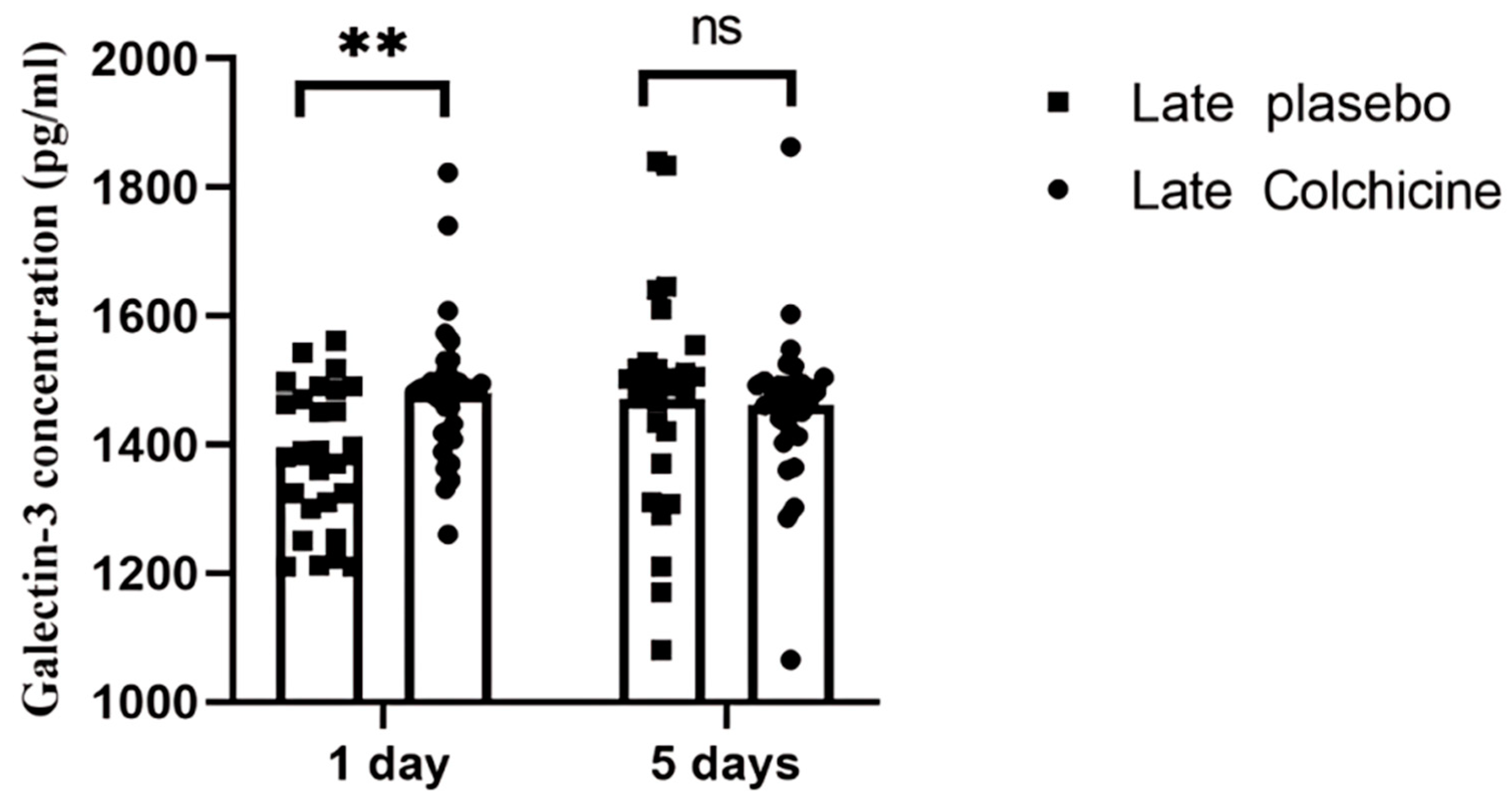
Figure 4). In the delayed PCI group, colchicine initially increased Galectin-3 levels at 24 hours (1479.5 ± 104.25 pg/mL vs. 1382.45 ± 109.91 pg/mL, p=0.0066) but showed a non-significant reduction by day 5 (1461.04 ± 114.94 pg/mL vs. 1470.75 ± 172.90 pg/mL, p>0.9999) (
Figure 5).
This figure illustrates the significant reduction in serum galectin-3 levels at 24 hours and 5 days post-treatment in the colchicine-treated group, compared to the placebo group, highlighting its effectiveness in early PCI scenarios. These results underscore colchicine's potential to influence cardiac fibrosis outcomes through galectin-3 modulation. ****: p<0.0001, highly statistically significant; **: p<0.01, statistically significant.
This figure highlights the significant increase in serum galectin-3 levels at 24 hours post-treatment in the colchicine group compared to the placebo group. By day 5, a non-significant reduction in galectin-3 levels was observed in the colchicine-treated patients compared to the placebo group. The data suggest a potential delayed anti-inflammatory effect of colchicine in modulating inflammatory pathways linked to cardiac remodelling in STEMI patients undergoing delayed PCI. ns: not significant; **: p<0.01, statistically significant.
Our analysis revealed that colchicine administration significantly impacted Galectin-3 and PIIINP levels depending on the timing of PCI. In the delayed PCI group, Galectin-3 levels showed an initial increase at 24 hours (p < 0.01), indicating heightened fibrotic and inflammatory activity. However, by day 5, colchicine significantly reduced PIIINP levels (p < 0.0001), suggesting its potential in modulating the later stages of fibrosis, even when intervention is delayed.
4. Discussion
The baseline characteristics of the 164 participants were uniformly distributed across all groups. This included a balanced demographic of males and females, aged 30-65 years, all presenting with first-time STEMI. Key clinical parameters such as duration of chest pain, ST-segment elevation, and cardiac enzyme levels were recorded to ensure comparability at the outset. The demographic and clinical profiles confirm that the study groups were well-matched, supporting the integrity of the trial's outcomes. Our study meticulously profiled the baseline characteristics of AMI- STEMI patients, capturing a demographic primarily in their late fifties, with a majority being male. Notably, smoking prevalence was markedly high, especially in the early PCI-placebo group, though not significantly different when compared with the late colchicine group. The incidence of conventional cardiovascular risk factors such as hypertension, diabetes mellitus, and dyslipidaemia showed no statistically significant differences across groups, reinforcing the comparability of the cohorts. The involvement of the infarct-related artery, specifically the left anterior descending (LAD) artery, was variably noted across the groups, yet without significant differences, ensuring a balanced baseline for evaluating the effects of colchicine therapy (unpublished data).
Clinical outcomes following colchicine administration were assessed, focusing on changes in LVEDV and the incidence of MACE. The increase in LVEDV, an indicator of cardiac function deterioration, was observed in nearly half of the patients in the early placebo group, with similar proportions noted in the early colchicine group. The late treatment groups had lower LVEDV increase percentages, but these differences did not achieve statistical significance. MACE, a critical clinical endpoint, was notably absent in both early PCI groups. The late placebo group documented a singular MACE occurrence, whereas the late colchicine group reported none, underscoring colchicine's safety profile. Overall, statistical analysis confirmed that colchicine's effect on both LVEDV increase and MACE prevention was not significantly different from placebo, indicating its potential non-inferiority in managing post-infarction outcomes (unpublished data).
This investigation centres on patients with STEMI, defined as an ST elevation greater than 1 mm (0.1 mV) in two or more contiguous leads [
18]. The process of cardiac remodelling post-STEMI is influenced by various risk factors, including age, obesity, diabetes, hypertension, dyslipidaemia, and chronic ischemia resulting from coronary artery disease, alongside lifestyle factors such as smoking [
19]. Post-STEMI inflammation can persist due to ongoing parietal stress on the myocardial wall [
20]. This stress activates major neurohormonal pathways, specifically the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system, exacerbating fibrosis and accelerating apoptotic cellular changes [
21]. These physiological changes contribute to adverse ventricular remodelling, characterized by alterations in heart structure and function, significantly elevating the risk of heart failure and subsequent mortality [
22].
Research by Tardif et al. (2021), has shown that a low dose of colchicine (0.5 mg daily) administered over 22.6 months post-MI can significantly lower the risk of ischemic cardiac events such as death, resuscitated cardiac arrest, and recurrent myocardial infarction, compared to a placebo. This finding supports the initiation of colchicine treatment within three days post-MI to mitigate the risk of subsequent cardiovascular events [
23].
Our study reveals ground-breaking insights into the role of colchicine in modulating cardiac fibrosis and inflammation, especially in the context of delayed PCI for STEMI patients. While early PCI demonstrated reductions in Galectin-3 and PIIINP, signifying controlled fibrotic and inflammatory responses, the delayed PCI group presented a remarkable finding. Despite the heightened fibrotic activity marked by the significant increase in Galectin-3 within the first 24 hours post-PCI, colchicine administration successfully reversed this trajectory by significantly lowering PIIINP by day five. This indicates colchicine's potential to intervene in the late-stage fibrotic process, offering protection even when revascularization is delayed—a critical advantage for regions where timely access to PCI is a challenge.
Elevated levels of PIIINP may signify not only the normal fibrogenesis associated with healing but also excessive collagen deposition and disorganized healing, which are indicative of an amplified pro-inflammatory response and extensive tissue damage within the myocardium. Such pathological remodelling can lead to contractile dysfunction and disturbances in cardiac electrophysiology, consequently reducing myocardial pump efficiency and increasing the risk of arrhythmias [
24]. Furthermore, elevated PIIINP levels post-MI have been correlated with adverse structural changes in the heart, particularly poor left ventricular function within the first year following the infarction, irrespective of the initial size of the infarct [
25]. This biomarker is critical in regulating cellular interactions and healing processes within the cardiac tissue [
26].
Elevated galectin-3 levels, indicative of myocardial damage, can trigger an inflammatory cascade that promotes macrophage infiltration, fibroblast activation, and excessive collagen deposition, leading to interstitial [
27,
28]. Our findings suggest that colchicine effectively modulates these processes, highlighting its potential in managing cardiac remodelling and fibrosis post-STEMI. This study underscores the pivotal role of galectin-3 following cardiac injury, where macrophages release this lectin to facilitate the fibrotic process by promoting collagen deposition and scar tissue formation [
29]. Galectin-3 further drives the differentiation of fibroblasts into myofibroblasts, central to scar formation and extracellular matrix remodelling, resulting in increased myocardial stiffness and thickness. Such structural changes contribute to diastolic dysfunction, often manifesting as a consequence of extensive myocardial scarring [
30,
31].
Furthermore, data from a preceding study indicated that colchicine substantially lowered NT-proBNP levels compared to placebo, suggesting a beneficial effect on cardiac hypertrophy and remodelling processes (unpublished data). NT-proBNP is a marker secreted by cardiomyocytes under stress conditions such as myocardial infarction, and it possesses vasodilatory, diuretic, and natriuretic properties that aid in reducing cardiac preload and wall stress [
32].
These findings suggest that colchicine could be a powerful therapeutic tool ultimately mitigating the adverse remodelling often seen with delayed intervention. By targeting key fibrotic markers like Galectin-3 and PIIINP, colchicine offers a novel pathway to improve long-term outcomes in STEMI patients undergoing delayed PCI, making it a potential game-changer in the management of acute cardiac events where timely intervention is not feasible. Our findings further support the role of colchicine in modulating not only fibrotic biomarkers like Galectin-3 and PIIINP but also key anti-inflammatory markers such as IL-10, as previously reported in our earlier studies. The significant increase in IL-10 levels following colchicine administration in a hypoxia-induced inflammation model reinforces its potential to reduce pro-inflammatory cytokine activity and promote myocardial healing in AMI-STEMI patients [
33].
The lack of significant differences in LVEDV changes and MACE incidence between colchicine and placebo groups in our study may be due to the relatively short follow-up period. Longer-term studies are needed to determine whether the observed biomarker changes translate into clinically meaningful outcomes. Several limitations of our study warrant consideration. First, we focused on two key biomarkers, a more comprehensive panel including additional inflammatory and fibrotic markers could provide a more complete picture of colchicine's effects. Lastly, longer follow-up periods would be valuable in assessing the long-term impact of colchicine on cardiac remodelling and clinical outcomes.
5. Conclusions
Our study demonstrates that colchicine significantly modulates fibrotic and inflammatory markers in AMI-STEMI patients, especially those undergoing delayed PCI. Despite the initial increase in Galectin-3, indicating heightened fibrosis, colchicine reduced PIIINP by day five, suggesting its ability to mitigate late-stage fibrosis. These findings highlight colchicine’s potential as a therapeutic strategy to improve outcomes in delayed PCI, offering new hope for patients in regions with limited access to timely interventions.
Supplementary Materials
All data underlying the results are available as part of the article and no additional source data are required. have been deposited and are publicly available. Specifically, the data can be accessed through the provided link to the Google_Spreadsheet:
https://docs.google.com/spreadsheets/d/1iqg6-u6I8I44a7lpEBeHbeVxI2r-gokd/edit#gid=1670558133. This statement will replace any previous statements within the manuscript, and it reflects our commitment to transparency and accessibility of research data. We appreciate your attention to this matter.
Author Contributions
Conceptualization, S.D.H. and M.S.R.; methodology, S.D.H.; software, D.Q.; validation, M.S.R., D.S. and A.A.; formal analysis, S.D.H.; investigation, S.D.H.; resources, S.D.H.; data curation, S.D.H.; writing—original draft preparation, S.D.H..; writing—review and editing, R.A.N.; visualization, B.L.; supervision, M.S.R., D.S. and A.A.; project administration, S.D.H.; funding acquisition, S.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Saiful Anwar Hospital (Approval No. 400/235/K.3/302/2020). The trial was duly registered with the International Study Registry ISRCTN (Registration Number: ISRCTN12958502, registered on 28/12/2023).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required. have been deposited and are publicly available. Specifically, the data can be accessed through the provided link to the Google_Spreadsheet:
https://docs.google.com/spreadsheets/d/1iqg6-u6I8I44a7lpEBeHbeVxI2r-gokd/edit#gid=1670558133. This statement will replace any previous statements within the manuscript, and it reflects our commitment to transparency and accessibility of research data. We appreciate your attention to this matter.
Acknowledgments
The authors would like to thank all staff from the Department of Cardiology and Cardiology and Vascular Medicine, Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia, and Division of Cardiovascular Sciences, University of Manchester, United Kingdom, for supporting this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aw, K. L., Koh, A., Lee, H. L., Kudzinskas, A., & De Palma, R. (2022). Colchicine for symptomatic coronary artery disease after percutaneous coronary intervention. Open Heart, 9(1), e001887. [CrossRef]
- Azevedo, P. S., Polegato, B. F., Minicucci, M. F., Paiva, S. A. R., & Zornoff, L. A. M. (2016). Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arquivos Brasileiros de Cardiologia. [CrossRef]
- Blanda, V., Bracale, U. M., Di Taranto, M. D., & Fortunato, G. (2020). Galectin-3 in Cardiovascular Diseases. International Journal of Molecular Sciences, 21(23), 9232. [CrossRef]
- Bouabdallaoui, N., & Tardif, J.-C. (2021). Colchicine in the Management of Acute and Chronic Coronary Artery Disease. Current Cardiology Reports, 23(9), 120. [CrossRef]
- Bouabdallaoui, N., Tardif, J.-C., Waters, D. D., Pinto, F. J., Maggioni, A. P., Diaz, R., Berry, C., Koenig, W., Lopez-Sendon, J., Gamra, H., Kiwan, G. S., Blondeau, L., Orfanos, A., Ibrahim, R., Grégoire, J. C., Dubé, M.-P., Samuel, M., Morel, O., Lim, P., … Roubille, F. (2020). Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). European Heart Journal, 41(42), 4092–4099. [CrossRef]
- Cao, Jia, & Zhu. (2019). BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. International Journal of Molecular Sciences, 20(8), 1820. [CrossRef]
- Cole, J., Htun, N., Lew, R., Freilich, M., Quinn, S., & Layland, J. (2021). Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention: The COPE-PCI Pilot Trial. Circulation: Cardiovascular Interventions, 14(5), e009992. [CrossRef]
- De Boer, R. A., Yu, L., & Van Veldhuisen, D. J. (2010). Galectin-3 in Cardiac Remodeling and Heart Failure. Current Heart Failure Reports, 7(1), 1–8. [CrossRef]
- Díaz-Alvarez, L., & Ortega, E. (2017). The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators of Inflammation, 2017, 1–10. [CrossRef]
- Ding, Y., Wang, Y., Zhang, W., Jia, Q., Wang, X., Li, Y., Lv, S., & Zhang, J. (2020). Roles of Biomarkers in Myocardial Fibrosis. Aging and Disease, 11(5), 1157. [CrossRef]
- Fan, D., Takawale, A., Lee, J., & Kassiri, Z. (2012). Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & Tissue Repair, 5(1), 15. [CrossRef]
- Fonseca, F. A., & Izar, M. C. (2022). Role of Inflammation in Cardiac Remodeling After Acute Myocardial Infarction. Frontiers in Physiology, 13, 927163. [CrossRef]
- Frunza, O., Russo, I., Saxena, A., Shinde, A. V., Humeres, C., Hanif, W., Rai, V., Su, Y., & Frangogiannis, N. G. (2016). Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. The American Journal of Pathology, 186(5), 1114–1127. [CrossRef]
- Fujino, M., Ishihara, M., Ogawa, H., Nakao, K., Yasuda, S., Noguchi, T., Ozaki, Y., Kimura, K., Suwa, S., Fujimoto, K., Nakama, Y., Morita, T., Shimizu, W., Saito, Y., Hirohata, A., Morita, Y., Inoue, T., Okamura, A., Uematsu, M., … Miyamoto, Y. (2017). Impact of symptom presentation on in-hospital outcomes in patients with acute myocardial infarction. Journal of Cardiology, 70(1), 29–34. [CrossRef]
- Gudowska, M., Gruszewska, E., Panasiuk, A., Cylwik, B., Swiderska, M., Flisiak, R., Szmitkowski, M., & Chrostek, L. (2017). High serum N-terminal propeptide of procollagen type III concentration is associated with liver diseases. Gastroenterology Review, 3, 203–207. [CrossRef]
- Guo, Q., Zhao, Y., Li, J., Huang, C., Wang, H., Zhao, X., Wang, M., & Zhu, W. (2021). Galectin-3 Derived from HucMSC Exosomes Promoted Myocardial Fibroblast-to-Myofibroblast Differentiation Associated with β -catenin Upregulation. International Journal of Stem Cells, 14(3), 320–330. [CrossRef]
- Handari, S. D., Rohman, M. S., Sargowo, D., Aulanni’am, Nugraha, R. A., Lestari, B., & Oceandy, D. (2024). Novel Impact of Colchicine on Interleukin-10 Expression in Acute Myocardial Infarction: An Integrative Approach. Journal of Clinical Medicine, 13(16), 4619. [CrossRef]
- Hara, A., Niwa, M., Noguchi, K., Kanayama, T., Niwa, A., Matsuo, M., Hatano, Y., & Tomita, H. (2020). Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules, 10(3), 389. [CrossRef]
- Hoeker, G. S., James, C. C., Tegge, A. N., Gourdie, R. G., Smyth, J. W., & Poelzing, S. (2020). Attenuating loss of cardiac conduction during no-flow ischemia through changes in perfusate sodium and calcium. American Journal of Physiology-Heart and Circulatory Physiology, 319(2), H396–H409. [CrossRef]
- Jenča, D., Melenovský, V., Stehlik, J., Staněk, V., Kettner, J., Kautzner, J., Adámková, V., & Wohlfahrt, P. (2021). Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Failure, 8(1), 222–237. [CrossRef]
- Khanna, A., Zamani, M., & Huang, N. F. (2021). Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. Journal of Cardiovascular Development and Disease, 8(11), 137. [CrossRef]
- Kochan, A., Lee, T., Moghaddam, N., Milley, G., Singer, J., Cairns, J. A., Wong, G. C., Jentzer, J. C., Van Diepen, S., Alviar, C., & Fordyce, C. B. (2023). Reperfusion Delays and Outcomes Among Patients With ST-Segment–Elevation Myocardial Infarction With and Without Cardiogenic Shock. Circulation: Cardiovascular Interventions, 16(6). [CrossRef]
- Leancă, S. A., Crișu, D., Petriș, A. O., Afrăsânie, I., Genes, A., Costache, A. D., Tesloianu, D. N., & Costache, I. I. (2022). Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life, 12(8), 1111. [CrossRef]
- Leung, Y. Y., Yao Hui, L. L., & Kraus, V. B. (2015). Colchicine—Update on mechanisms of action and therapeutic uses. Seminars in Arthritis and Rheumatism, 45(3), 341–350. [CrossRef]
- Li, L., Zhang, Q., Lei, X., Huang, Y., & Hu, J. (2020). MAP4 as a New Candidate in Cardiovascular Disease. Frontiers in Physiology, 11, 1044. [CrossRef]
- López, B., González, A., Ravassa, S., Beaumont, J., Moreno, M. U., San José, G., Querejeta, R., & Díez, J. (2015). Circulating Biomarkers of Myocardial Fibrosis. Journal of the American College of Cardiology, 65(22), 2449–2456. [CrossRef]
- Ma, K., Gao, W., Xu, H., Liang, W., & Ma, G. (2022). Role and Mechanism of the Renin-Angiotensin-Aldosterone System in the Onset and Development of Cardiorenal Syndrome. Journal of the Renin-Angiotensin-Aldosterone System, 2022, 1–8. [CrossRef]
- Nikolov, A., & Popovski, N. (2022). Extracellular Matrix in Heart Disease: Focus on Circulating Collagen Type I and III Derived Peptides as Biomarkers of Myocardial Fibrosis and Their Potential in the Prognosis of Heart Failure: A Concise Review. Metabolites, 12(4), 297. [CrossRef]
- Petrie, J. R., Guzik, T. J., & Touyz, R. M. (2018). Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Canadian Journal of Cardiology, 34(5), 575–584. [CrossRef]
- Plata-Mosquera, C. A., Bernal-Tórres, W., Herrera-Escandón, Á. A., Uribe-Posso, L. P., Rodríguez-Casanova, Á. M., Casanova-Valderrama, M. E., Vivas-Mayor, M., Puerta-Mesa, A. C., & Martínez-Aristizabal, J. (2021). Sacubitril/valsartan reduces levels of procollagen types I and III and correlates with reverse cardiac remodeling. REC: CardioClinics, 56(1), 14–21. [CrossRef]
- Richter, B., Gwechenberger, M., Socas, A., Zorn, G., Albinni, S., Marx, M., Wolf, F., Bergler-Klein, J., Loewe, C., Bieglmayer, C., Binder, T., Wojta, J., & Gössinger, H. D. (2011). Time course of markers of tissue repair after ablation of atrial fibrillation and their relation to left atrial structural changes and clinical ablation outcome. International Journal of Cardiology, 152(2), 231–236. [CrossRef]
- Salari, N., Morddarvanjoghi, F., Abdolmaleki, A., Rasoulpoor, S., Khaleghi, A. A., Hezarkhani, L. A., Shohaimi, S., & Mohammadi, M. (2023). The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovascular Disorders, 23(1), 206. [CrossRef]
- Seropian, I. M., Cassaglia, P., Miksztowicz, V., & González, G. E. (2023). Unraveling the role of galectin-3 in cardiac pathology and physiology. Frontiers in Physiology, 14, 1304735. [CrossRef]
- Singh, A., Dwivedi, S., Pradhan, A., Narain, V. S., Sethi, R., Chandra, S., Vishwakarma, P., Chaudhary, G., Bhandari, M., & Sharma, A. (2021). Isolated ST-Elevation Myocardial Infarction Involving Leads I and aVL: Angiographic and Electrocardiographic Correlations from a Tertiary Care Center. Cardiology Research and Practice, 2021, 1–7. [CrossRef]
- Sygitowicz, G., Maciejak-Jastrzębska, A., & Sitkiewicz, D. (2021). The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules, 12(1), 46. [CrossRef]
- Xiu, W.-J., Yang, H.-T., Zheng, Y.-Y., Ma, Y.-T., & Xie, X. (2019). Delayed PCI 12 Hours after the Onset of Symptoms Is Associated with Improved Outcomes for Patients with ST-Segment Elevation Myocardial Infarction: A Real-World Study. Journal of Interventional Cardiology, 2019, 1–11. [CrossRef]
- Zhao, W., Zhao, J., & Rong, J. (2020). Pharmacological Modulation of Cardiac Remodeling after Myocardial Infarction. Oxidative Medicine and Cellular Longevity, 2020, 1–11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).