1. Introduction
Jalapeño pepper (
Capsicum annuum L.) is a vegetable of great economic importance worldwide [
1]. In 2022, China and Mexico were the main producers with 31 and 5.8%, respectively, of a total of 53 810 million tons [
2,
3]. In Mexico, this crop is not only key to the food, economic and cultural industries, but has also seen an increase in demand since 2012 due to its antioxidant, anti-inflammatory, anticancer and antidiabetic properties [
4,
5]. As a result, Mexico exports a third of its production, mainly to the United States [
6].
Given the increasing demand and strategic value of jalapeño peppers, obtaining healthy and vigorous seedlings represents a growing problem in agriculture. In this context, seedling performance against biotic and abiotic factors is critical. Adverse abiotic conditions, such as extreme temperature variations, drought and poor soils, require seedlings emerging from seed to possess superior tolerance in order to survive and thrive. In addition, biotic challenges, including pest infestations and diseases, demand that these seedlings not only emerge quickly but also exhibit biological resistance [
7]. These factors are linked and are determinants of production success, directly affecting the sustainability and profitability of modern agriculture [
8,
9]. Despite technological advances, the efficiency with which seeds can germinate and seedlings can establish in such adverse environmental conditions remains a field under constant study, therefore, there is a growing need to strengthen seed quality to ensure a resilient agricultural future.
Faced with these challenges, researchers have sought innovative solutions to improve the germination and transplanting processes. One alternative is the
“nanopriming
” technique, an emerging and novel technology, which consists of conditioning the seeds prior to sowing. Priming consists of the imbibition of seeds for a determined time, which depends on the species, size and application, using mineral nanoparticles or mineral-based nanofertilizers. It is used with the purpose of increasing the speed and uniformity of seed germination in many economically important plant species, since this influences the quality and yield of crops [
10]. This process takes place in a specific environment, up to a point where germination related metabolic activity begins, without radicle emergence. It acts on the seeds at the molecular level, facilitating the absorption and utilization of nutrients, which results in faster and more robust growth of the roots and aerial part of the seedlings [
11,
12]. The use of nanopriming transcends agronomic benefits by offering significant environmental advantages. This technique reduces reliance on conventional chemical inputs, such as high dose fertilizers and agrochemicals, thereby reducing the potential for nitrogen pollution and other contaminants from runoff and leaching in nurseries [
13]. Furthermore, by improving resource use efficiency, nanopriming contributes to a more sustainable and environmentally friendly agriculture [
14].
Previous studies have shown that nanopriming not only increases germination rates, but also strengthens plants to better withstand transplanting stress, favoring a more uniform and healthier establishment in the field [
15]. The addition of essential micronutrients in seeds using nanopriming have shown interesting and favorable results [
16]. Zinc oxide nanoparticles in jalapeño peppers had a positive influence on early growth parameters [
17]. In addition, it alleviated zinc deficiency and improved agronomic parameters of corn seedlings [
18]. As for nanopriming with molybdenum, studies on chickpea showed that, under adverse environmental conditions, redox enzymes were activated and increased seed germination and survival [
19]. Also, molybdenum trioxide nanoparticles favored the vigor of
Vigna radiata L. seedlings, as well as production [
20]. Therefore, the adoption of innovative techniques such as nanopriming is essential to face the current challenges in the production of jalapeño peppers. This strategy not only strengthens seedlings and improves post-transplant survival rates, but also promotes more sustainable and profitable farming practices. As the industry continues to evolve, the integration of advanced technologies into agricultural production will become increasingly crucial to maintain competitiveness in the global marketplace and ensure the long-term sustainability of the jalapeño pepper crop.
In general, there is scarce literature on zinc and molybdenum nanopriming applied to the imbibition and germination of seeds, as well as to the production of vigorous jalapeño pepper seedlings. Therefore, the objective of the present study was to evaluate the effect of nanopriming of jalapeño pepper seeds, using different concentrations of a commercial Zn-Mo nanofertilizer, on imbibition curves, seed germination parameters and early-stage growth through vigorous seedling indexes of jalapeño pepper.
2. Materials and Methods
The research was divided into 2 experiments, using seeds and seedlings, respectively. The first experiment was the imbibition of seeds using nanopriming. The second experiment consisted of measuring the effect of nanopriming on germination and early growth parameters in seedlings.
2.1. Experimental Site, Plant Material and Nanofertilizer
Both experiments were carried out at the facilities of the Centro de Investigación en Alimentación y Desarrollo A.C. (CIAD) in Delicias, Chihuahua, Mexico (28°11′ N, 105°28′ W, altitude 1171 m), from 12 to 19 March 2024. Seeds of jalapeño pepper cv. M (Capsicum annuum L.), supplied by Kristen Seed S.A. de C.V. of Guadalajara, Jalisco, Mexico, were used. The nanofertilizer was the commercial product BROADACRE® ZnMo from agrichem fluagri of Guadalajara, Jalisco, Mexico. Its composition was Zn 62%, Mo 5% and 33% of an algae-based chelating agent.
2.2. Experiment 1: Seed Imbibition
2.2.1. Experimental Design and Treatments
A completely randomized design was used, where 4 treatments of the BRODACRE® ZnMo nanofertilizer and 4 replicates for each treatment were evaluated. The treatments were: Control T1 (0-0), T2 (62-5), T3 (124-10), and T4 (248-20) mg L-1 of zinc and molybdenum, respectively. To dissolve the nanofertilizer, a VWR brand magnetic mechanical stirring plate was used at a speed of 700 rpm for 40 min, followed by sonication in a Vevor Ultrasoniccleaner at 40 kHz frequency for 1 h.
2.2.2. Nanopriming of Seeds
The seed priming procedure with BRODACRE
® nanofertilizer was carried out according to Pompelli et al. [
21], with some modifications.
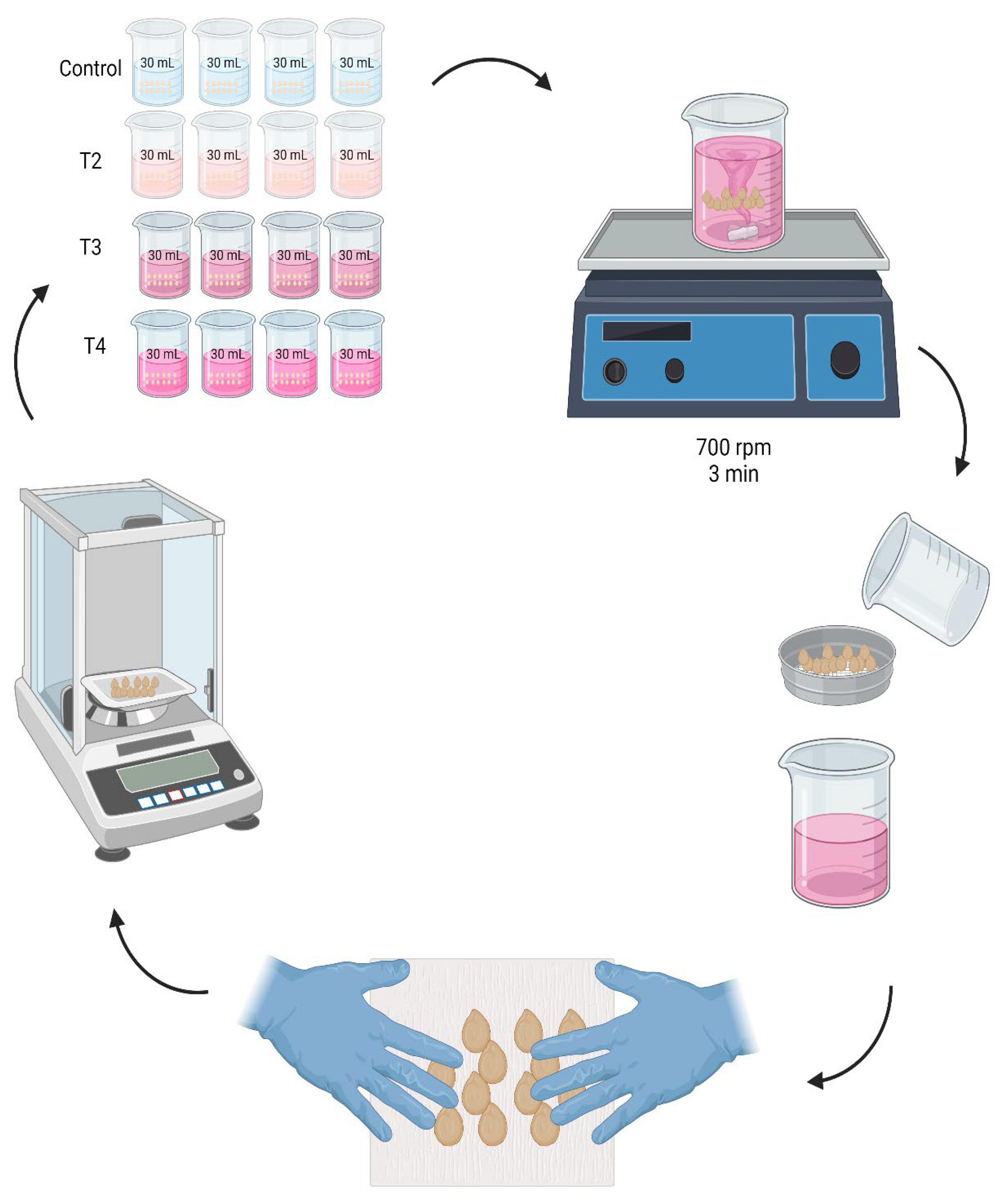
In a beaker with 30 mL of solution 100 seeds were immersed. They were shaken in a VWR brand magnetic mechanical shaker plate at a speed of 700 rpm for 3 min. Then, at 2, 4, 6, 8, 8, 10, 10, 12, 24, 36 and 48 h respectively, the seeds were removed from the beaker where they were imbibed, using a 4 mm Labalpha brand sieve to separate the seeds from the solution.
Subsequently, Fapsa C180 paper was used to absorb the excess of humidity, with the help of the hands without pressing, in a soft and superficial way the seeds were rubbed against the paper. This step was carried out until the paper had no moisture from the seeds, verifying it visually. Immediately, the seeds were weighed using an analytical balance Hr-120-C (A&D Weighin®, Japan) and the weight gained with respect to time 0 was recorded. The seeds were then immersed again in the previously separated solution (Figure 1). To standardize the imbibition, it was carried out under controlled temperature conditions at 25 ± 0.5 ° C, with a 12-h photoperiod. To avoid water loss by circulating air, the beakers were covered with Parafilm PM996 paper (Merck KGaA, Darmstadt, Germany), during each time interval between measurements.
After the imbibition time, the seeds were rinsed with tridistilled water and dried at 25 ± 0.5 ° C for 24 h. Finally, the seeds were stored in 3.8 cm x 3.8 cm Ziploc® type bags.
Figure 1.
Nanopriming of jalapeño pepper cv. M (Capsicum annuum L.) seeds, using 4 treatments: Control T1 (0-0), T2 (62-5), T3 (124-10), and T4 (248-20) mg L-1 of zinc and molybdenum, respectively, during the intervals of 2, 4, 6, 8, 8, 10, 10, 12, 24, 36, and 48 h, respectively.
Figure 1.
Nanopriming of jalapeño pepper cv. M (Capsicum annuum L.) seeds, using 4 treatments: Control T1 (0-0), T2 (62-5), T3 (124-10), and T4 (248-20) mg L-1 of zinc and molybdenum, respectively, during the intervals of 2, 4, 6, 8, 8, 10, 10, 12, 24, 36, and 48 h, respectively.
2.3. Experiment 2: Germination and Early Seedling Growth
2.3.1. Experimental Design and Treatments
A completely randomized design was used, where 4 treatments of nanopriming with ZnMo and 3 replicates with 100 seeds each for each treatment were evaluated. A control with seeds without any nanopriming and without any imbibition medium was used, and the treatments used to obtain the imbibition curves were distributed as follows: T0 is the control, T1 (0-0), T2 (62-5), T3 (124-10), and T4 (248-20) mg L-1 of zinc and molybdenum, respectively.
2.3.2. Sowing and Germination
After 48 h of the imbibition process, the seeds were sown in 338-cavity styrofoam trays. A substrate mixture of vermiculite and perlite (2:1 v/v) moistened with distilled water was used. The sowing depth was 3 mm. Then, they were taken to a germination chamber of 1 x 2 x 0.8 m (patent pending), under controlled and precise conditions automatically, which were monitored for 8 days through a visual interface with the support of the technology called Internet of Things. The temperature ranges were (20 °C–28 °C) and relative humidity (60%–80%).
2.3.3. Crop Management
On days 3, 5, and 7 after sowing, sprinkler irrigation was applied homogeneously to each tray, using a 2 L Truper sprinkler. A standard Hoagland nutrient solution modified by Sánchez et al. [
22], pH 6 ± 0.1, composed of 6 mM NH
4NO
3, 1.6 mM K
2HPO
4, 0.3 mM K
2SO
4, 4 mM CaCl
2, 1.4 mM MgSO
4, 5 µM Fe-EDDHA, 2 µM MnSO
4, 0.25 µM CuSO
4 and 0.5 µM H
3BO
3 was used. Every 48h, 500 mL per tray of nutrient solution was applied.
2.3.4. Measurements of Germination Parameters, Vigor and Morphological Indexes
The germination percentage and number of germinated seeds were counted daily during the first 8 days, and the seedling count was 5 days after sowing (DAS). Neither germination nor seedlings were detected visually before 3 DAS, considering the criterion for the beginning of radicle emergence that implies a radicle length of 1 to 2 mm, so the beginning of germination was considered at 4 DAS.
The GerminaR package [
23] was used to calculate the germination indices. The following notation is used to describe each of these variables, it is necessary to show the symbology of the equations: n
i, the number of seeds germinated in time i
n time; N, the total number of seeds in each experimental unit; k, the last day of germination evaluation; t
i, the time from the beginning of the experiment to the observation i
n; and f
i, the relative frequency of germination. These data allow the following germination indexes to be calculated:
- a)
Number of germinated seeds (ni), is the number of seeds that germinated in time k.
- b)
The percentage of seeds that complete the germination process, which is calculated with equation (1).
- c)
The mean germination time (MGT) is calculated with equation (2), this denotes the number of germinated seeds with respect to the number of germinated seeds at the time of evaluation.
- d)
The mean germination rate (MGR) is expressed as the reciprocal of MGT.
- e)
The uncertainty of germination (INC), an adaptation of the Shannon index, evaluates the uncertainty associated with the relative distribution of the germination frequency, where the germination frequency is calculated with equation (3). When uncertainty values are low, the germination frequency should be higher, since this index evaluates the degree of dispersion of germination using equation (4):
Vigor index was calculated as a function of mass with seedling fresh weight (Equation (5)) and as a function of size with seedling length (Equation (6)), where germination was expressed in decimal to simplify integer, and seedling length was the sum of radicle and plumule lengths.
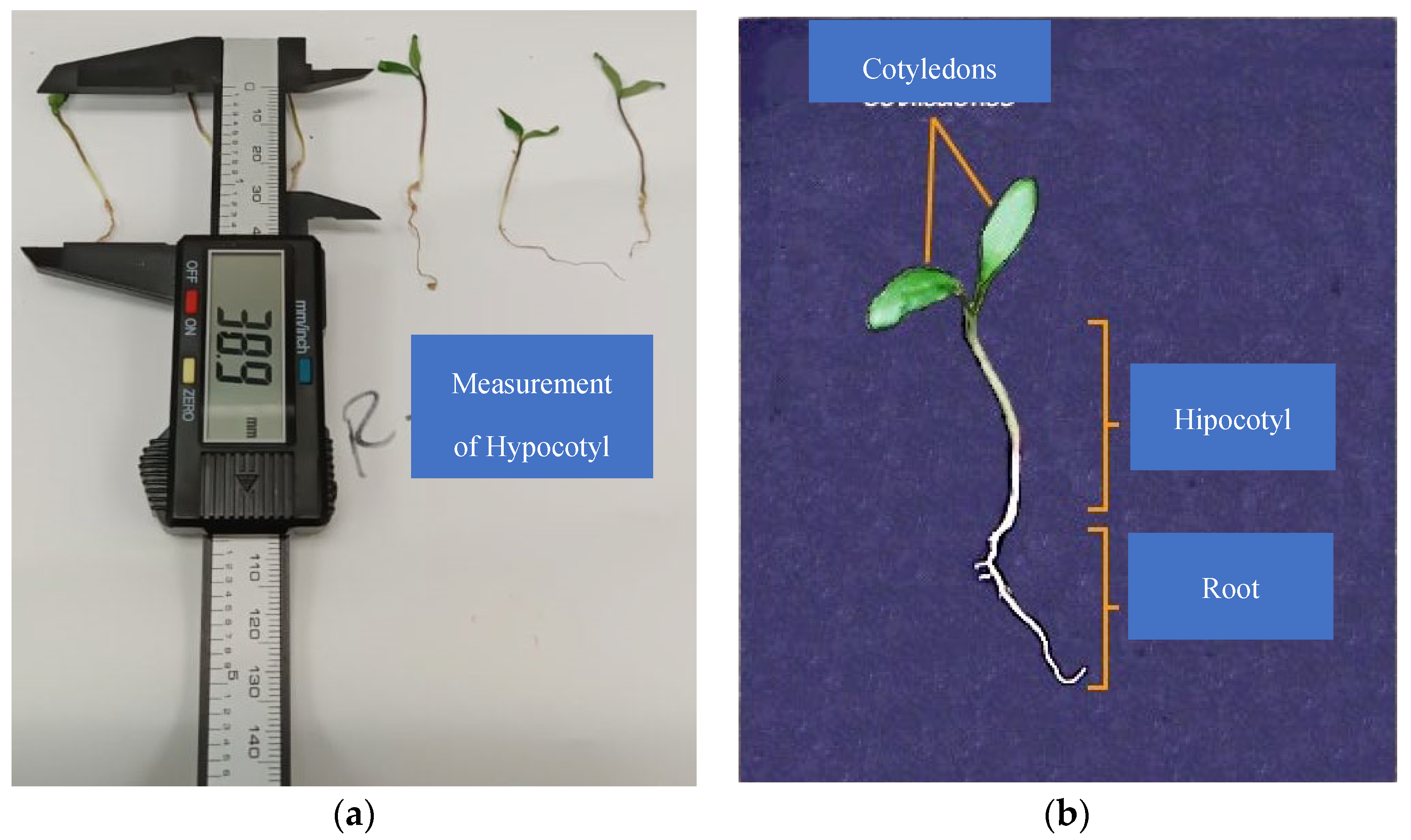
Root and plumule lengths and stem thickness were measured using a 153 mm HER-411 digital caliper (Steren®, Mexico), radicule length from the base of the hypocotyl to the apex of the radicle and plumule length from the radicle-hypocotyl intersection to the base of the cotyledons (Figure 2). Seedling fresh weight was measured using an Analytical Balance Hr-120-C (A&D Weighin®, Japan). Seedling size and biomass were measured on the 9th day after planting.
Figure 2.
(a) Plumule length measurement; (b) seedling parts to be measured.
Figure 2.
(a) Plumule length measurement; (b) seedling parts to be measured.
2.4. Statistical Analysis
Analysis of variance and mean tests were performed using Fisher’s least significant difference (LSD) (p ≤ 0.05) in SAS® version 9.0 statistical software.
3. Results and Discussion
3.1. Percentage of Imbibition
The imbibition process in seeds is essential to ensure successful germination [
24]. In this study, the nanopriming technique with zinc and molybdenum proved to be particularly effective in terms of imbibition. Specifically, the T3 treatment (124-10 mg L
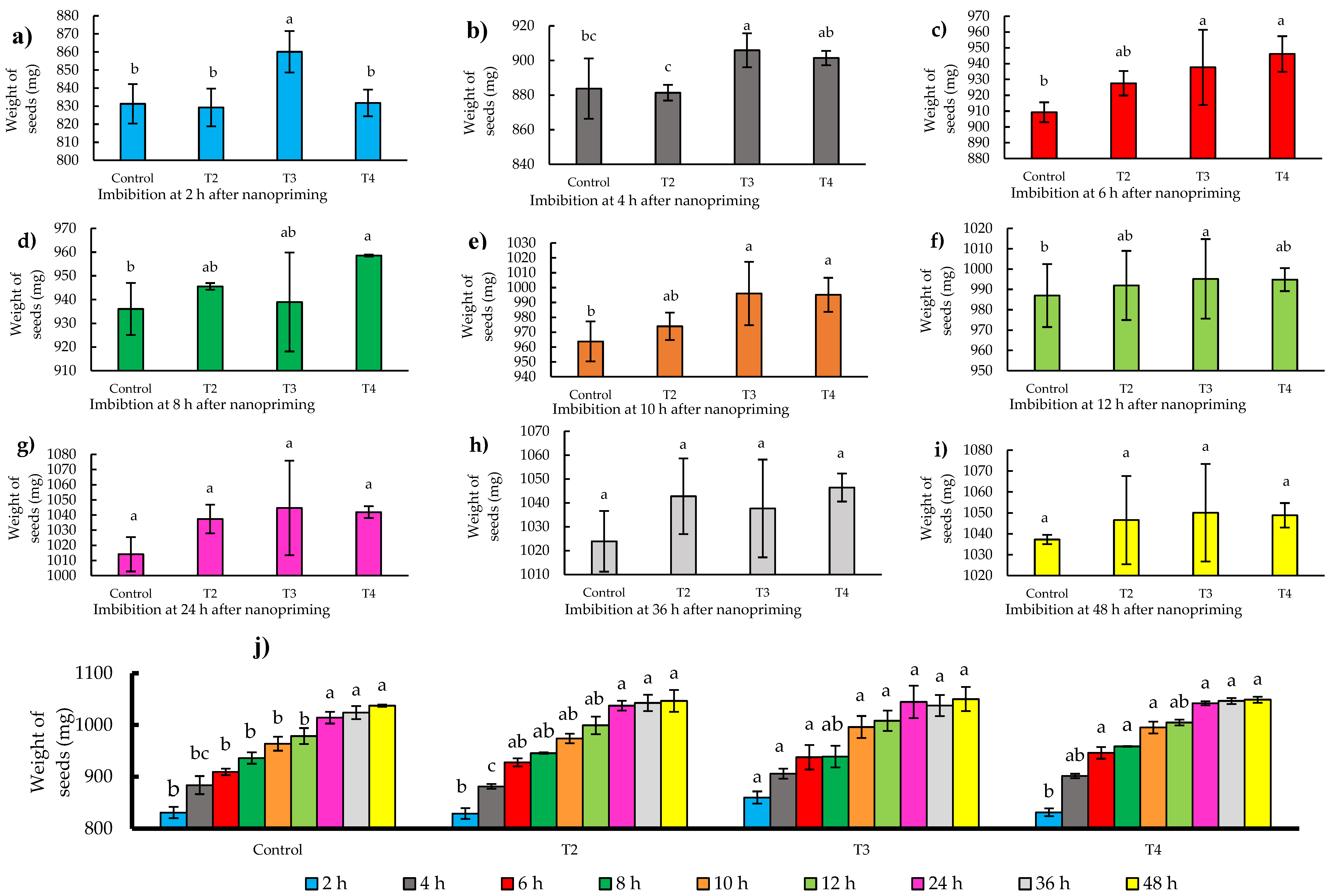
-1) proved to be the most effective (p ≤ 0.05), standing out for a significant increase in seed weight during the first 2 h of imbibition, reaching a 3% increase with respect to the initial weight.
This result is remarkable, as T3-treated seeds reached a weight of 860 mg, in contrast to the control that recorded a weight of 828 mg, as shown in Figure 2(a). During the first 12 h, T3 showed a superior response in solution uptake compared to the control, reflected in Figures 2(a) to 2(f). Furthermore, no significant differences were observed at time intervals after 12 h, specifically at 24, 36, and 48 h, respectively, nor were there significant percentage increases in weight exceeding 1%, as detailed in Figures 2(g) through 2(i). This finding highlights the critical importance of the early imbibition stages in the T3 treatment for successful seed germination.
Several studies have documented similar effects on the imbibition of priming-treated seeds. Portuguez et al. [
25] observed in
I. rugosum Salisb that, by applying 0.25% KNO
3 for 16 to 24 h, seed dormancy was broken with minimal absorption, identifying this treatment as the optimum in their study. On the other hand, Da Silva et al. [
26] reported that in
E. stipitata McVaught, the highest water uptake occurs between 12 and 24 h using priming with distilled water. Furthermore, Rai-Kalala et al. [
27] found in
Triticum aestivum L. an increased tolerance to drought stress when nanopriming with ZnSO
4 at 10 mg L
-1 for 18 h of imbibition.
3.2. Imbibition Curves
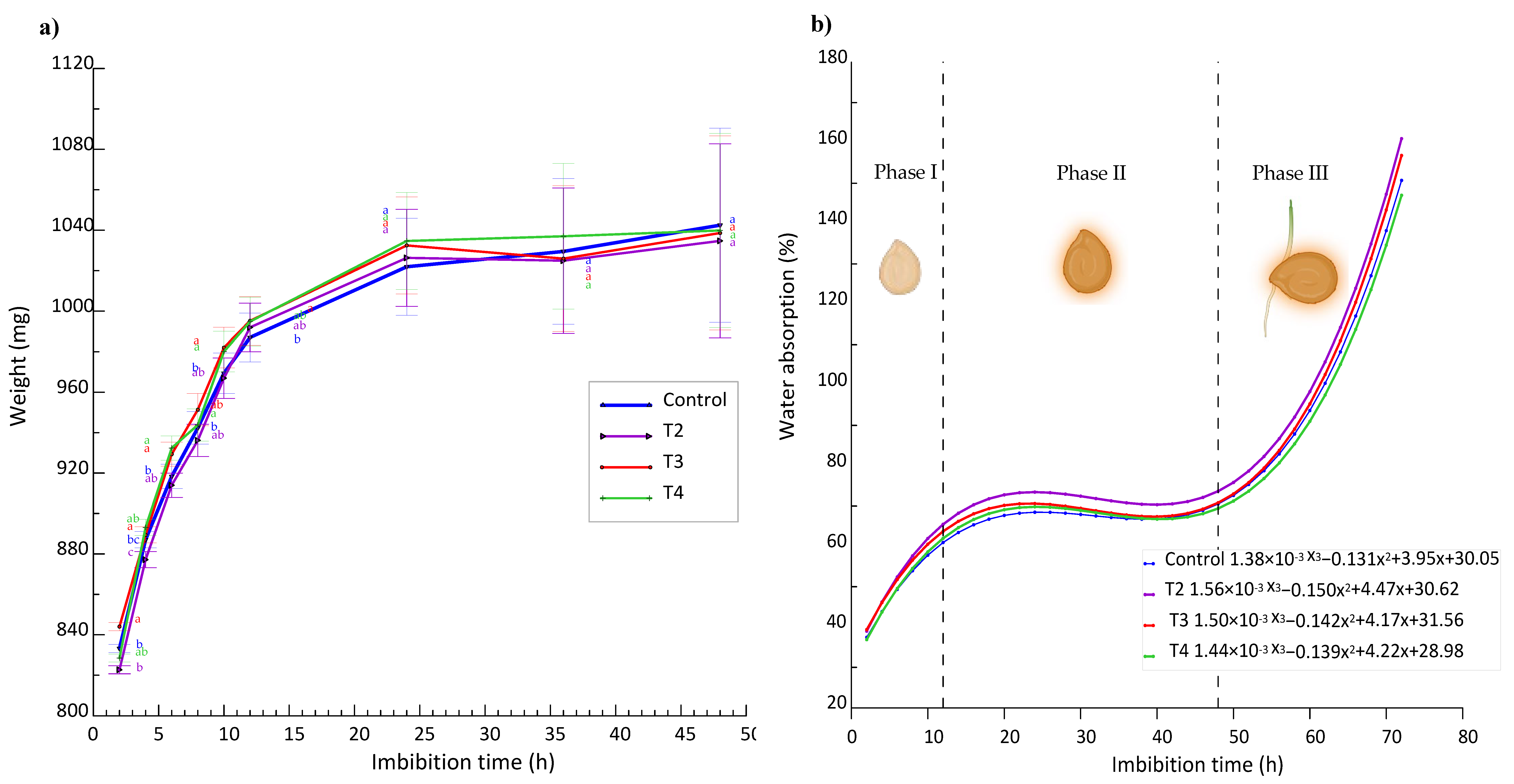
In the present study, the imbibition curves show a gradual increase in seed weight for each of the nanopriming treatments over all time intervals, as illustrated in Figure 2(j). This observation is consistent with findings in the scientific literature reporting rapid water uptake in the early stages followed by a slowdown in later stages.
For example, Finch-Savage and Leubner-Metzger [
28] explain that, during the initial imbibition phase, seeds undergo rapid water uptake, essential to reactivate metabolic processes that were dormant. This rapid increase in seed weight, as observed in Figure 3(b) of our study, where the weight increases up to 65% in the first 12 h, reflects the critical rehydration of internal tissues necessary for germination. This pattern has been corroborated by studies such as that of Bewley et al. [
29], who emphasize the importance of this phase for the activation of essential biochemical and physiological mechanisms in the seed.
However, after these first 12 h, our study observes that the increase in weight is reduced to less than 1%, being almost imperceptible, as indicated in Figure 3 (a). This phenomenon is in line with that reported by Kaya et al. [
30], who suggest that, after the rapid absorption phase, seeds enter a plateau phase where water absorption stabilizes while more complex metabolic processes are initiated.
Finally, Phase III of our experiment, which begins after 48 h, shows an exponential increase in weight due to seed protrusion and the onset of root emergence. This phase is crucial as it marks the beginning of embryo development protruding from the seed, a finding that is supported by the work of Nonogaki et al. [
31], who emphasize that the transition to the germination phase proper is critical for the reproductive success of the plant.
Figure 2.
(a-i) Imbibition of jalapeño pepper (Capsicum annuum L.) seeds during different time intervals (2-48 h); (j) Histogram of imbibition times by treatments. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤0.05). Mean values ± standard error.
Figure 2.
(a-i) Imbibition of jalapeño pepper (Capsicum annuum L.) seeds during different time intervals (2-48 h); (j) Histogram of imbibition times by treatments. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤0.05). Mean values ± standard error.
Figure 3.
(a) Imbibition curves vs. weight gain by effect of treatments. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤0.05). Mean values ± standard error; (b) Trend curves of % solution uptake vs. imbibition hours and different germination stages.These results highlight the relevance of a detailed understanding of imbibition stages and their impacts on seed germination. The implications of these findings are fundamental for the optimization of pregerminative treatments and for improving seed germination rates and seed vigor in agricultural and species conservation applications.
Figure 3.
(a) Imbibition curves vs. weight gain by effect of treatments. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤0.05). Mean values ± standard error; (b) Trend curves of % solution uptake vs. imbibition hours and different germination stages.These results highlight the relevance of a detailed understanding of imbibition stages and their impacts on seed germination. The implications of these findings are fundamental for the optimization of pregerminative treatments and for improving seed germination rates and seed vigor in agricultural and species conservation applications.
3.2. Germination Parameters
3.2.1. Number of Seeds Germinated
The number of germinating seeds is a vital indicator of reproductive success and survival of plants in various environments [
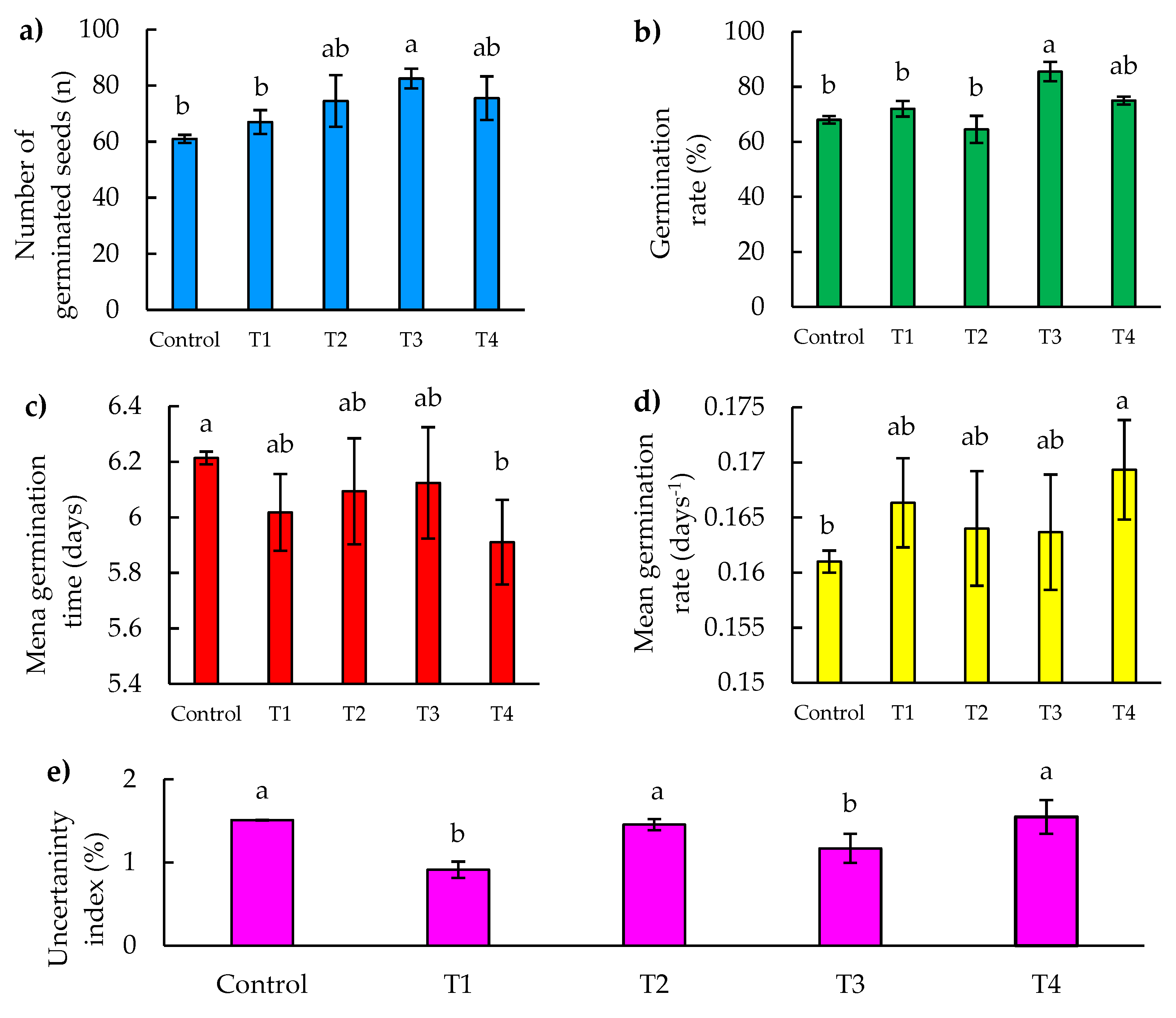
32]. In our study, the T3 treatment showed a significant increase in the number of germinated seeds, reaching 20% more than the control at 8 days after sowing (DAS), as shown in Figure 4 (a).
Figure 4.
Seed germination parameters as a function of time (8 DAS) of jalapeño pepper (Capsicum annuum L.), under Zn-Mo nanopriming treatments. (a) Number of germinated seeds, (b) Percentage of seed germination, (c) Mean germination time, (d) Mean germination rate, (e) Uncertainty index. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤ 0.05). Mean values ± standard error.
Figure 4.
Seed germination parameters as a function of time (8 DAS) of jalapeño pepper (Capsicum annuum L.), under Zn-Mo nanopriming treatments. (a) Number of germinated seeds, (b) Percentage of seed germination, (c) Mean germination time, (d) Mean germination rate, (e) Uncertainty index. Different letters indicate significant differences between treatments (Fisher’s LSD test p ≤ 0.05). Mean values ± standard error.
This result is in agreement with the research of Finch-Savage and Bassel [
33], who demonstrated that success in the early stages of germination can dramatically influence plant population dynamics, especially in competitive and stressful environments. Although treatments T2 and T4 showed no significant statistical differences compared to T3, the superior performance of T3 suggests that the specific composition of this treatment may be better aligned with the physiological needs of seeds during imbibition and the early stages of germination. This finding is in line with the work of Baskin and Baskin [
34], who identified that fine tuning of priming treatments can result in significant improvements in germination rate and consistency.
As for treatments T2 and T4, there were statistically no significant differences with respect to T3; however, T3 was more effective.
3.2.2. Germination Percentage
The germination percentage is a crucial indicator that measures the viability of seeds and their ability to develop into healthy and robust seedlings, which is essential for the survival and successful establishment of plants in any ecological or agricultural environment [
35]. In terms of germination percentage, there was a significant difference found between the T3 treatment and the control treatments, T1 and T2, highlighting the efficacy of T3 in improving seed germination. In particular, T3 showed a 25% increase in the percentage of germinated seeds compared to the control and 32% more than T2 at 8 days after sowing (DAS), as illustrated in Figure 4 (b). These results indicate that T3 provides optimal conditions that favor germination.
Previous studies have shown that proper management of environmental and chemical conditions during seed treatment can significantly influence germination rate. According to Kildisheva et al
. [
36], seed priming is a technique that can optimize the metabolic processes necessary to break dormancy and trigger germination more rapidly and uniformly.
Although no significant differences were found between the control and the other treatments except T3, this may indicate that while T3 is formulated in a way that maximizes efficacy, the other treatments may not be sufficiently differentiating from the control in terms of impact on germination. As suggested by Halmer [
37], the selection of priming agents and their concentrations should be specific and carefully adjusted for each seed type and growing conditions, which could explain the variability in germination response among the observed treatments.
3.2.3. Mean Germination Time and Mean Germination Rate
The importance of mean germination time (MGT) and mean germination rate (MGR) is highlighted in studies evaluating seed germination capacity and vigor [
38]. In our study, a significant difference in mean germination time was identified between the T4 treatment and the control. The T4 treatment recorded a mean germination time of 5.91 days, significantly faster than the control, which presented the longest time with 6.21 days (Figure 4 (c)). This indicates that T4 improves germination efficiency. No significant differences were observed between the control and the other treatments, with the exception of T4. Furthermore, when considering the mean germination rate, which is the inverse of the mean germination time, T4 stands out notably for its efficiency, accelerating germination 5% faster than the control, representing a reduction of approximately 0.3 days or 7 h (Figure 4 (d)).
According to Farooq et al. [
39], rapid germination is essential to optimize the use of favorable environmental conditions, which is critical in agricultural environments where optimal windows for germination are limited. On the other hand, Kaya et al. [
30] suggest that the efficacy of priming treatments, as observed with T4, can significantly modify the rate of water uptake and dormancy breakdown, facilitating a more rapid and uniform onset of seed growth. These findings highlight the importance of selecting and optimizing seed treatment techniques to improve germination. In agricultural contexts, this can translate into more effective crop establishment, which is crucial for maximizing yields and resource efficiency [
40].
3.2.4. Uncertainty Index of Germination
Uncertainty in seed germination is a crucial aspect in agriculture and plant biology, as it directly affects plant productivity, seedling survival, and ultimately yields and overall crop quality. This uncertainty can be influenced by a variety of environmental and genetic factors [
41].
In our study, in the uncertainty index, significant differences were found between treatments T1 and T3 compared to the control, T2 and T4. Specifically, treatment T1 showed the lowest uncertainty index in germination at 8 (DAS), being 42% lower compared to T4, which presented the highest value (Figure 4 (e)).
A study by Xu et al. [
42] showed that factors such as light, temperature, salinity and osmotic stress have a significant impact on germination and seedling emergence in
Cucumis melo L. var. Agrestis Naud. marking the importance of controlling these factors to reduce uncertainty in germination. On the other hand, Hayashi et al. [
41] highlighted the influence of microenvironmental and management factors on the variability of germination time, which reinforces the need to analyze these aspects to improve predictability in plant production. In addition, Soltani et al. [
43] reviewed how environmental cues affect germination of non-dormant seeds, concluding that a better understanding of these cues can improve the efficiency of seedling establishment and reduce uncertainty in germination.
3.3. Morphological and Seedling Vigor Parameters
Table 1 shows the results of morphological parameters and vigor of jalapeño pepper (
Capsicum annuum L.) seedlings under different doses of nanopriming. The analysis of variance for plumule length showed statistically significant differences (p ≤ 0.05) between the T3 treatment and the other treatments, highlighting T3 with a mean value of 36.1 mm, 25% higher than T1, which presented the lowest mean value of 28.5 mm.
For the root length variable, no statistically significant differences (p ≤ 0.05) were observed among treatments. However, T3 had the highest average value with 35.9 mm, while T1 had the lowest average value with 30.6 mm.
Regarding stem diameter, statistically significant differences (p ≤ 0.001) were found among all treatments with respect to the control. The T4 treatment presented the highest value, being 112% thicker than the control.
In the seedling fresh weight parameter, there were statistically significant differences (p ≤ 0.05) between T3 and T4 compared to the control. The T4 treatment had the highest value with 33.1 mg, while the control presented the lowest value with 20.9 mg, showing a difference of 58% between the two.
Analysis of the seedling vigor I index revealed statistically highly significant differences (p ≤ 0.001) between treatments T3 and T4 with respect to the control, with T3 showing 53% more vigor I than the control. The vigor II index showed similar com-portance, with highly significant differences (p ≤ 0.001) between T3 and the control, with T3 being 57% more vigorous than the control.
The use of nanopriming to improve the morphological characteristics and vigorousness of seedlings has been studied in several investigations. For example, a study by Li et al. [
44] showed that nanoparticle treatment significantly improved germination and initial seedling growth in different plant species. This research supports the findings of our study, where nanopriming treatments showed significant improvements in several growth parameters.
In addition, Soltani et al. [
45] reviewed the impact of various environmental cues on seed germination, highlighting the importance of factors such as temperature and water availability, which are also critical in the context of nanopriming treatments.
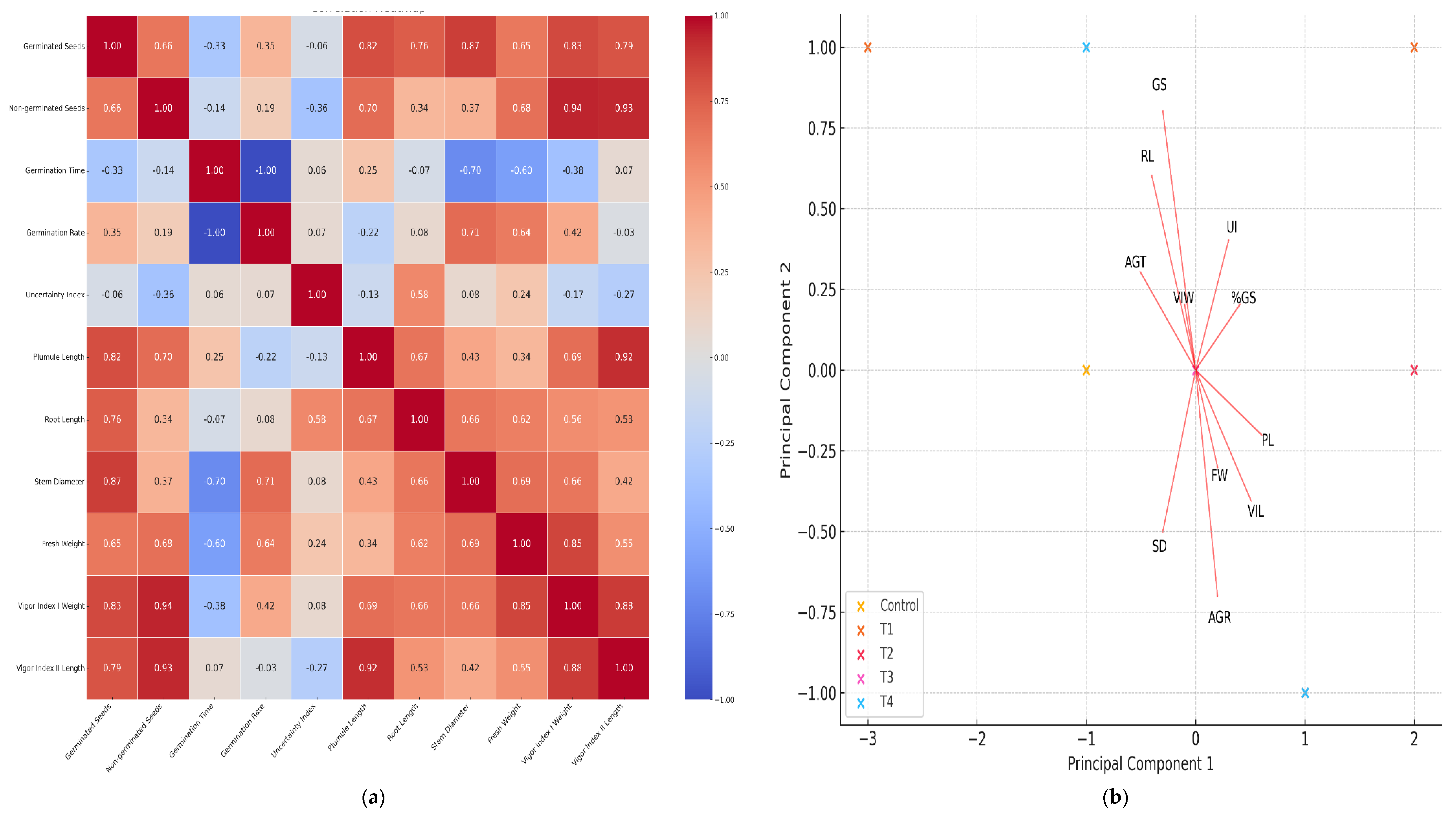
3.4. Correlation Analysis and Heat Map
The heat map was generated to find correlations between germination and early growth variables in our second experiment. Figure 5 (a) shows that vigor index I with seed weight and percentage of germinated seeds have a very high correlation (0.94), suggesting that improvement in the percentage of germinated seeds is strongly associated with an increase in the vigor index weighted by seedling weight.
In addition, the vigor index I with length and plumule length also show a high correlation (0.92), indicating that seedling length is a determining factor in their overall vigor. Stem diameter and germinated seeds (0.87) show a positive correlation, which may indicate that seeds germinated under optimal conditions tend to develop seedlings with thicker stems. It is also seen that root length and plumule length have a correlation of (0.77), suggesting that good root development is associated with healthier aerial growth. The strong correlations between vigor indexes and germination metrics reinforce the importance of proper seed treatment to ensure success in the early stages of seedling development.
These results offer the possibility of adjusting seed conditions and treatments to optimize both germination and early seedling growth, which could result in more efficient and sustainable agricultural practices.
3.5. Principal Component Analysis
Principal component analysis (PCA) reduces the dimensionality of the data and helps to identify underlying patterns. In this case, multiple variables have been reduced to two principal components.
The analysis shows the distribution of treatments in the space defined by the first two principal components. Each point represents a specific treatment, and similar treatments tend to cluster together (Figure 5 (b)).
Principal Component 1 (PC1): Explains 54.67% of the total variation in the data.
Principal Component 2 (PC2): Explains 23.81% of the total variation in the data.
Treatments T3 and T4 appear to be separated from the control and the other treatments in the principal component space. This suggests that these treatments have distinctive characteristics compared to the others.
Treatments such as T1 and T2 are closer to the control, indicating that their effects on the measured variables are more similar to the control than to T3 and T4. The first two principal components explain approximately 78.48% of the total variation in the data. Treatments T3 and T4 show more pronounced effects on morphological and vigorous variables, while T1 and T2 are more similar to the control. This information can be crucial to optimize growing conditions and improve agricultural efficiency.
Figure 5.
(a) Pearson correlation analysis represented in a heat map; (b) Principal component analysis. Both figures are made with the variables of germination and early growth, where the effect of nanopriming on seeds of Jalapeño bell pepper (Capsicum annuum L.) can be seen.
Figure 5.
(a) Pearson correlation analysis represented in a heat map; (b) Principal component analysis. Both figures are made with the variables of germination and early growth, where the effect of nanopriming on seeds of Jalapeño bell pepper (Capsicum annuum L.) can be seen.
4. Conclusions
The use of nanopriming with a dose of 124-10 mg L-1 of ZnMo, respectively, improved the increase of vigor indexes with weight and stem length, reflecting a higher germination rate in seeds, as well as seedlings that are more resistant to various stress factors. Therefore, it is observed that the use of ZnMo nanopriming promotes the viability of producing jalapeño peppers (Capsicum annuum L.) and also contributes to the sustainability of this crop.
Based on these findings, the Zinc-Molybdenum nanopriming method could potentially transform jalapeño pepper cultivation by improving seed quality and resistance. This could lead to more robust crop yields and reduce the need for chemical inputs such as fertilizers and pesticides, thus lowering production costs and environmental impact. In addition, the improved initial performance of seedlings suggests that they could be more resistant to environmental stresses such as drought and temperature fluctuations, critical factors in the context of climate change.
These aspects highlight the potential of nanopriming to contribute to the adaptation and sustainability of agricultural practices, ensuring food security and economic stability for farmers focused on jalapeño pepper production.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: E.H.O.-C. and E.S.; Data collection: L.U.C.-E., S.P.-Á.; J.J.P.-C and C.L.F.-L.; Analysis and interpretation of results: C.C.-M.; J.C.A.-P. and E.M.-M.; draft manuscript preparation: E.H.O.-C., C.A.R.-E. and E.S. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Data Availability Statement
The authors declare that all data discussed in the study are available in the manuscript.
Acknowledgments
We would like to thank the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONHACyT— Mexico) for the support provided by the scholarship granted to Erick Humberto Ochoa Chaparro through the “Becas Nacionales CONAHCYT” program with CVU No. 843732.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vasileva, K.; Todorova, V.; Masheva, S. Evaluation of collection of pepper (Capsicum spp.) resources for resistance to Verticillium dahliae Kleb. Bulgarian Journal of Agricultural Science. 2019, 25, 1030–1038. [Google Scholar]
- Sánchez-Toledano, B.I.; Gómez, D.M.J.C.; Cuevas-Reyes, V.; Salgado-Beltrán, L. Characterization of the preferences towards jalapeño peppers from the perspective of the Sonoran consumers. Agro Productividad. 2021, 14, 55–61. [Google Scholar] [CrossRef]
- Sánchez-Toledano, B.I.; Cuevas-Reyes, V.; Kallas, Z.; Zegbe, J.A. Preferences in jalapeño pepper attributes: a choice study in Mexico. Foods. 2021, 10, 3111. [Google Scholar] [CrossRef] [PubMed]
- García-Vásquez, R.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C.; Pérez-Ochoa, M.L.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Chávez-Servia, J.L. Bioactive and nutritional compounds in fruits of pepper (Capsicum annuum L.) landraces conserved among indigenous communities from Mexico. AIMS Agriculture & Food. 2023, 8, 832–850. [Google Scholar]
- Sánchez Toledano, B.I.; Camarena Gómez, D.M.J.; López Santiago, M.A.; Cuevas Reyes, V. Consumer Preferences of Jalapeño Pepper in the Mexican Market. Horticulturae. 2023, 9, 684. [Google Scholar] [CrossRef]
- Rodríguez-Barajas, N.; Montalvo-González, E.; Villagrán, Z.; González-Torres, S.; Villaruel-López, A.; Esparza, L.M.A. Chili and Pepper Byproducts. In FOOD BYPRODRUCTS, 1st ed.; Martin-Del-Campo, S.T., Cardador-Martínez, A., Ramírez-Anaya, J.D.P., Eds.; Nova Science Publishers: New York, USA; Volume 1, pp. 75–96.
- Khan, H.A.; Ziaf, K.; Amjad, M.; Iqbal, Q. Exogenous application of polyamines improves germination and early seedling growth of hot pepper. Chilean Journal of Agricultural Research. 2012, 72, 429–433. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Humic substances improve vegetable seedling quality and post-transplant yield performance under stress conditions. Agriculture. 2020, 10, 254. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation management and growth-promoting treatments affect tomato transplant production and plant growth after transplant. Agronomy. 2020, 10, 1504. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Sharma, R.; Rajput, P.; Singh, A.K. Nano-Priming Technology for Sustainable Agriculture. Biogeosystem Technique. 2021, 8, 79–92. [Google Scholar]
- El-Maarouf-Bouteau, H. The seed and the metabolism regulation. Biology. 2022, 11, 168. [Google Scholar]
- Zhang, H.Y.; Su, W.H. Classification, uptake, translocation, and detection methods of nanoparticles in crop plants: a review. Environmental Science: Nano. 2024, 11, 1847–1870. [Google Scholar] [CrossRef]
- Yadav, N.; Bora, S.; Devi, B.; Upadhyay, C.; Singh, P. Nanoparticle-Mediated Defense Priming: A Review of Strategies for Enhancing Plant Resilience Against Biotic and Abiotic Stresses. Plant Physiology and Biochemistry. 2024, 108796. [Google Scholar]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Gan, Y. Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability. 2022, 14, 14880. [Google Scholar]
- Oh, S.; Cave, G.; Lu, C. Vitamin B12 (Cobalamin) and micronutrient fortification in food crops using nanoparticle technology. Frontiers in Plant Science. 2021, 12, 668819. [Google Scholar] [CrossRef] [PubMed]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno-García, G.; Juárez-Maldonado, A.; Betancourt-Galindo, R.; González-Morales, S.; Sánchez-Vega, M.; Méndez-Lopez, A. Zinc oxide nanoparticle morphology modify germination and early growth of bell pepper seedlings. Biotecnia. 2023, 25, 5–15. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatalysis and Agricultural Biotechnology. 2020, 29, 101778. [Google Scholar] [CrossRef]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Andronov, E.E.; Gonchar, L.N.; Kalenskaya, S.M.; Chebotar, V.K. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis. 2017, 73, 57–69. [Google Scholar] [CrossRef]
- Nanda, J.; Dwibedi, S.K.; Samant, P.K.; Patnaik, G.P.; Paikaray, R.K.; Bal, M.; Rath, B.S. Effect of molybdenum trioxide nanoparticle-mediated seed priming on the productivity of green gram (Vigna radiata L.). Environment Conservation Journal. 2023, 24, 131–139. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodriguez-Páez, L.A. Imbibition and germination of seeds with economic and ecological interest: Physical and biochemical factors involved. Sustainability. 2023, 15, 5394. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J. M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. cv. Strike) under high NH4NO3 application rates. Scientia horticulturae. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Benites-Alfaro, O. E.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application GerminaQuant for R. Wiley Ecological Research. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Woodstock, W. L. Seed imbibition: a critical period for successful germination. Seed Technology Journal. 1988, 1, 1–15. [Google Scholar]
- Portuguez-García, M.P.; Rodríguez-Ruiz, A.M.; Porras-Martínez, C.; González-Lutz, M.I. Imbibition and temperature to rupture latency of Ischaemum rugosum Salisb. UCR. 2020, 31, 793–802. [Google Scholar]
- da Silva Moura, M.L.; Chagas, E.A.; Smiderle, O.J.; Vilaça, R.; Chagas, P.C.; de Moura, E.A.; Farias, E.E. Biometric characterization, water absorption curve and vigor on araçá-boi seeds. International Journal of Plant Biology. 2016, 7, 6265. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R. S.; Jajoo, A. H2O2 signaling regulates seed germination in ZnO nanoprimed wheat (Triticum aestivum L.) seeds for improving plant performance under drought stress. Environmental and Experimental Botany. 2021, 189, 10456. [Google Scholar] [CrossRef]
- Finch-Savage, W. E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New phytologist. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Germination. In Seeds; Springer: New York, USA, 2013. [Google Scholar]
- Kaya, M.D.; Okçu, G.; Atak, M.; Cıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). European journal of agronomy. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—still a mystery. Plant Science. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Nonogaki, H.; Chen, F.; Bradford, K.J. Mechanisms and genes involved in germination sensu stricto. Seed Development, Dormancy and Germination. Annual Plant Reviews. 2007, 27, 264–304. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: extending performance beyond adaptation. Journal of experimental botany 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J. M.; Baskin, C.C. What kind of seed dormancy might palms have? Seed Science Research. 2014, 24, 17–22. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Mattana, E.; Acosta, A.T.; Bacchetta, G. Seed germination responses to varying environmental conditions and provenances in Crucianella maritima L., a threatened coastal species. Comptes Rendus. Biologies, 2012, 335, 26–31. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Cross, A.T. Dormancy and germination: making every seed count in restoration. Restoration Ecology. 2020, 28, 256–265. [Google Scholar] [CrossRef]
- Halmer, P. Methods to improve seed performance in the field. Handbook of seed physiology. 2004, 125–165. [Google Scholar]
- Yılmaz, T.; Yılmaz, M.; Doğu, A.; Bölgesindeki, R.; Kizilağac. Alnus glutinosa L. Gaertn. Popülasyonlarinin Tohum Özellikleri. Turkish Journal of Forest Science. 2021, 5, 150–164. [Google Scholar] [CrossRef]
- Farooq, S.; Onen, H.; Tad, S.; Ozaslan, C.; Mahmoud, S. F.; Brestic, M.; El-Shehawi, A. M. The influence of environmental factors on seed germination of Polygonum perfoliatum L.: Implications for management. Agronomy. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Falleri, E. Dormancy breaking in Cornus sanguinea seeds. Seed Science and Technology. 2004, 32, 1–4. [Google Scholar] [CrossRef]
- Hayashi, E.; Amagai, Y.; Maruo, T.; Kozai, T. Phenotypic analysis of germination time of individual seeds affected by microenvironment and management factors for cohort research in plant factory. Agronomy. 2020, 10, 1680. [Google Scholar] [CrossRef]
- Xu, H.; Su, W.; Zhang, D.; Sun, L.; Wang, H.; Xue, F.; Wu, R. Influence of environmental factors on Cucumis melo L. var. agrestis Naud. Seed germination and seedling emergence. PLoS one. 2017, 12, e0178638. [Google Scholar]
- Soltani, E.; Baskin, C.C.; Gonzalez-Andujar, J.L. An overview of environmental cues that affect germination of nondormant seeds. Seeds. 2022, 1, 146–151. [Google Scholar] [CrossRef]
- Donglin, L.; Yaquin, J.; Chengjing, Y.; Yuan, X. Effects of cold stratification on the endogenous hormone, dormancy and germination of Cornus walteri Wanger seeds. Bangladesh Journal of Botany. 2020, 49, 507–514. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Gonzalez-Andujar, J.L. An overview of environmental cues that affect germination of nondormant seeds. Seeds. 2022, 1, 146–151. [Google Scholar] [CrossRef]
Table 1.
Plumule length, Root length, Stem diameter, Fresh weight, Vigor index I, Vigor index II, of jalapeño pepper (Capsicum annum L.) seedlings under nanopriming treatments at different doses of Zn-Mo nanofertilizer.
Table 1.
Plumule length, Root length, Stem diameter, Fresh weight, Vigor index I, Vigor index II, of jalapeño pepper (Capsicum annum L.) seedlings under nanopriming treatments at different doses of Zn-Mo nanofertilizer.
| Treatments |
Plumule length (mm) |
Root length (mm) |
Steam diameter (mm) |
Fresh weight (mg) |
Vigor index I (weight) |
Vigor index II (length) |
T0
(Control) |
28.9 |
33.0 |
0.26 |
20.9 |
18.43 |
19.64 |
T1
(0-0) mg L−1
|
28.5 |
30.6 |
0.43*** |
21.2 |
20.98 |
20.51 |
T2
(62-5) mg L−1
|
31.4 |
35.3 |
0.55*** |
20.9 |
19.46 |
20.26 |
T3
(124-10) mg L−1
|
36.1* |
35.9 |
0.55*** |
28.9* |
28.31*** |
30.9*** |
T4
(248-20) mg L−1
|
29.5 |
35.5 |
0.63*** |
33.1* |
25.42*** |
22.11 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).