Submitted:
21 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
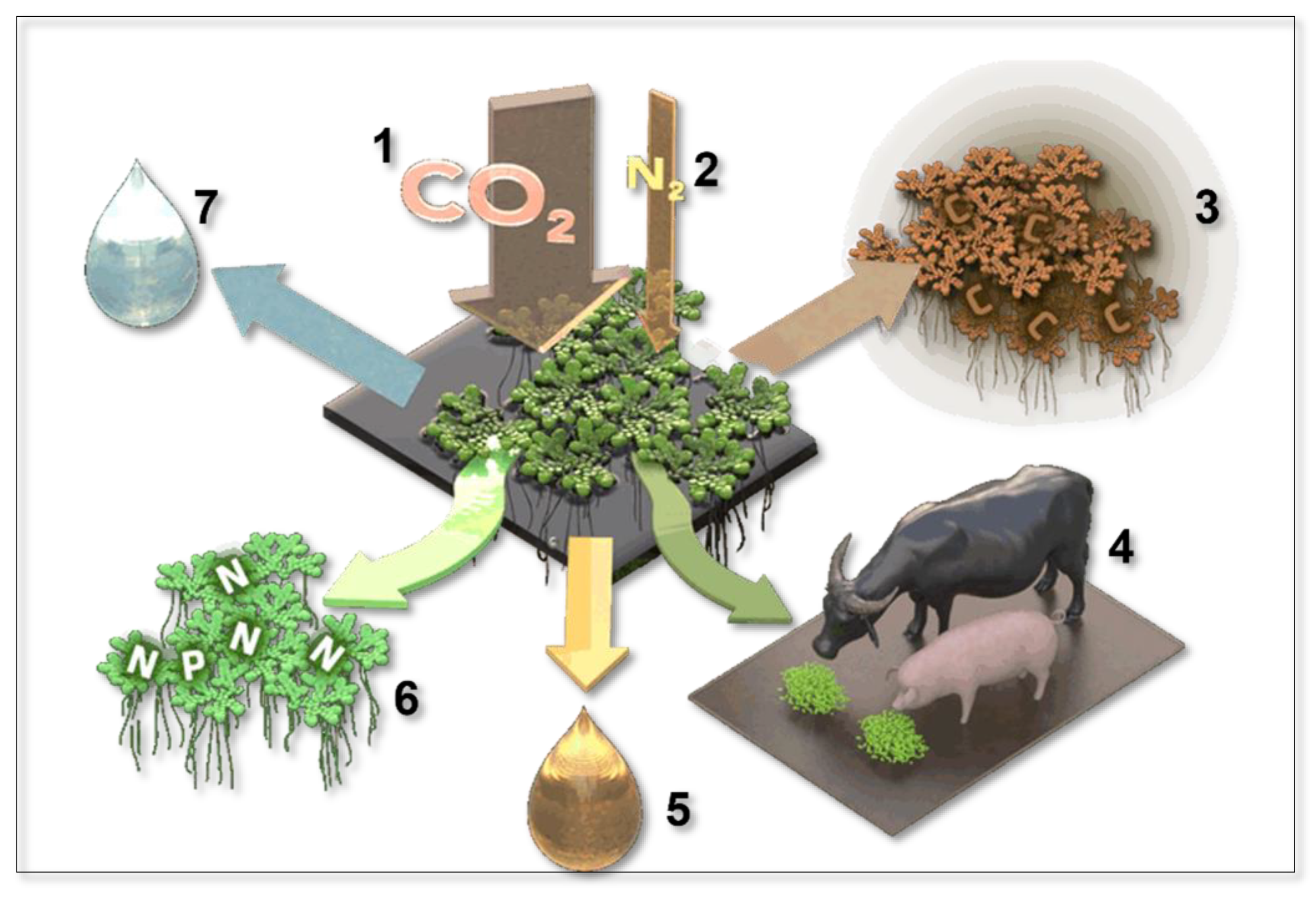
1.1. Nitrogen
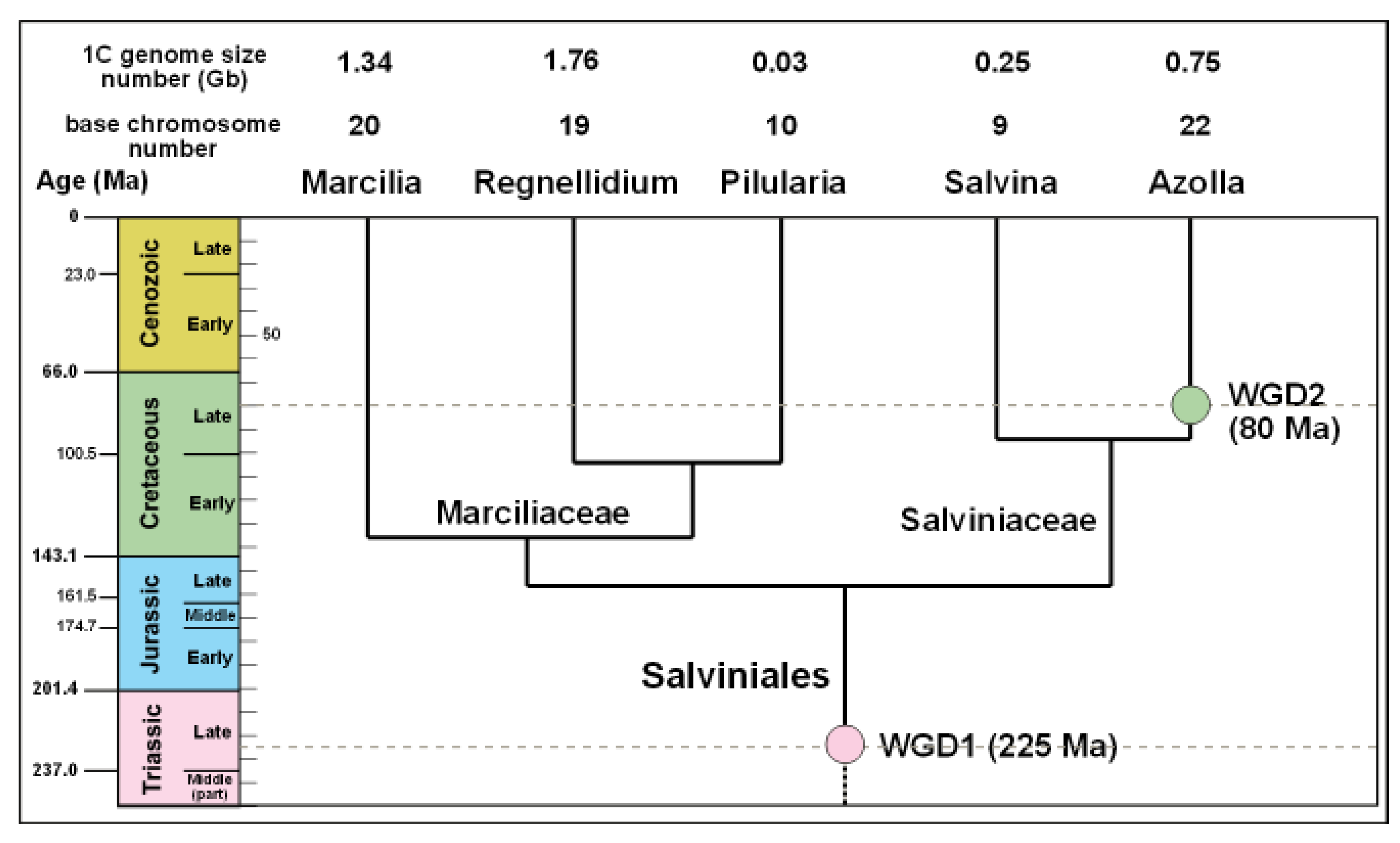
1.2. Azolla’s Suprageneric Classification
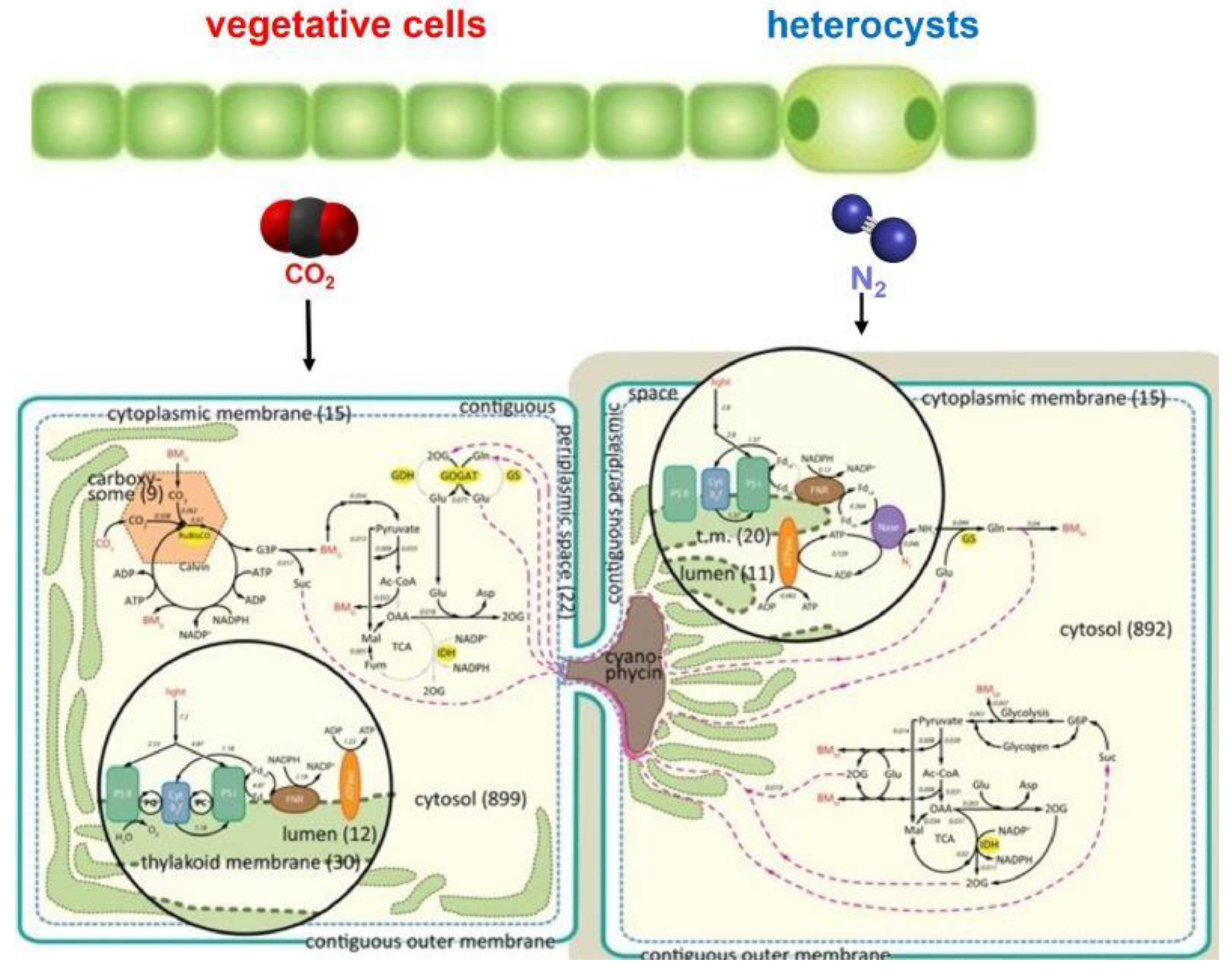
1.3. The Cyanobacterial Symbiont
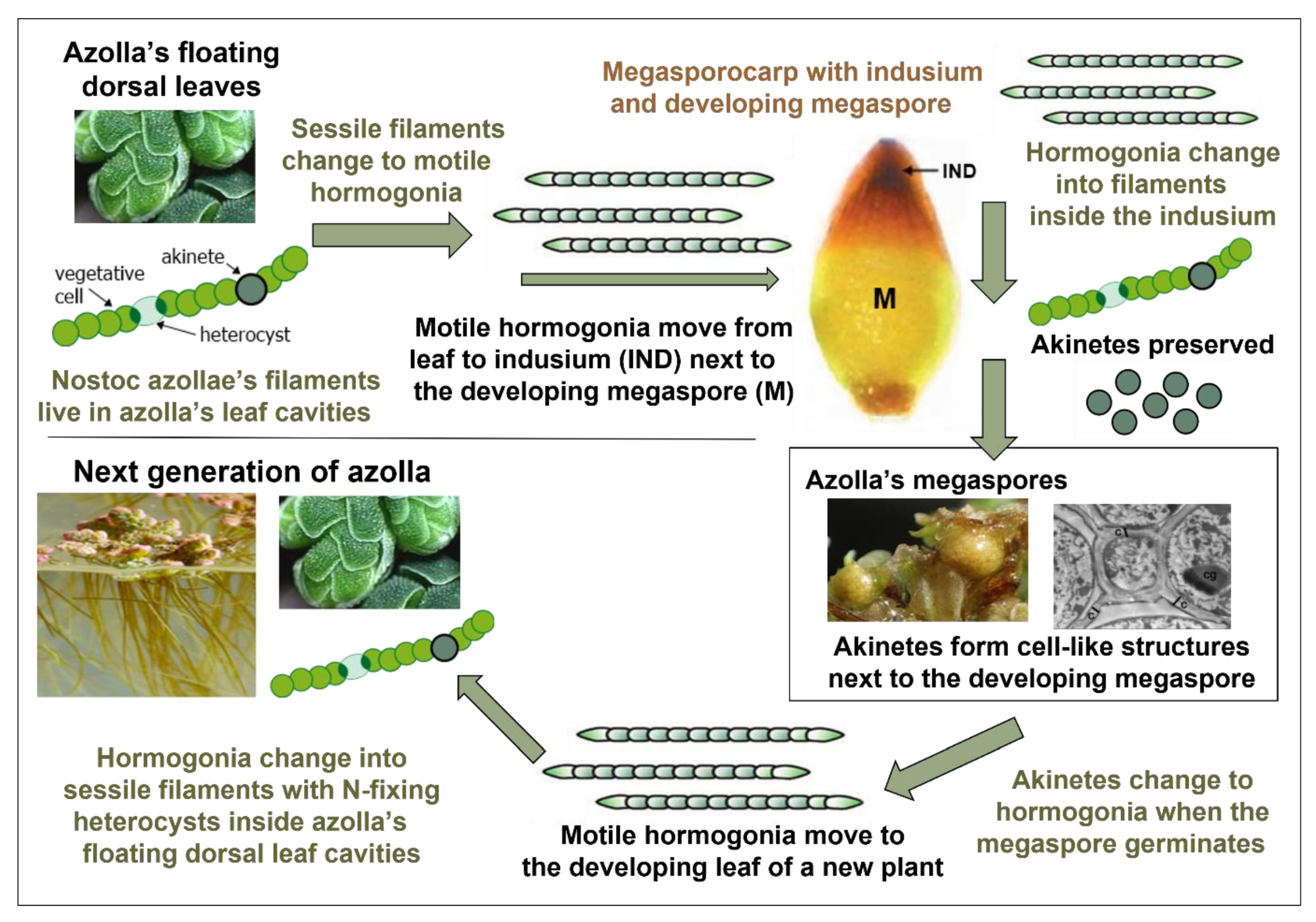
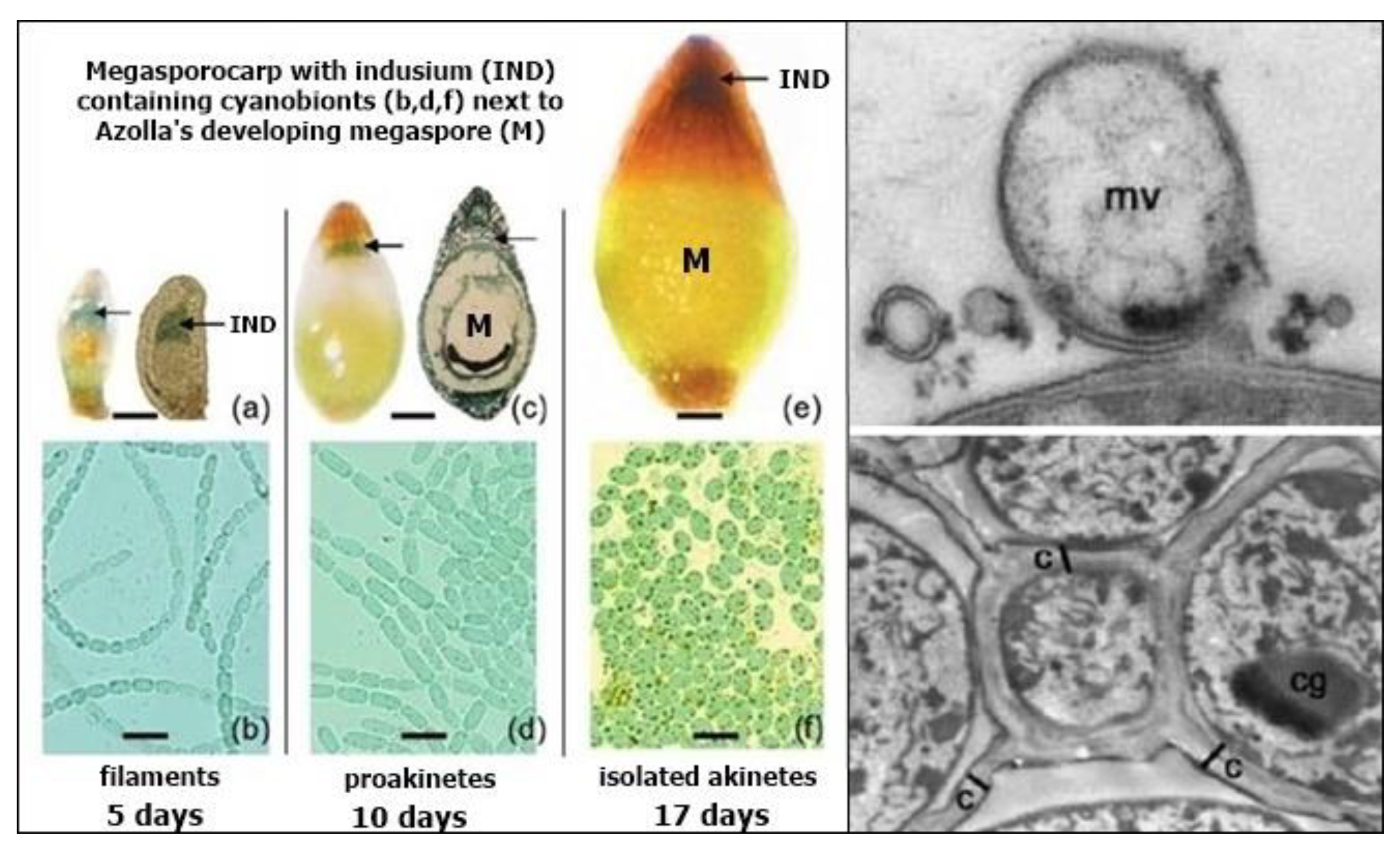
1.4. Azolla’s Transmission of N. azollae via Its Megaspores
- Sessile filaments have vegetative cells that sequester carbon dioxide and heterocysts that fix atmospheric nitrogen. Akinetes may also be present.
- Motile hormogonia only have vegetative cells, so they cannot sequester nitrogen. Hormogonia are produced by free-living species of Anabaena and Nostoc including those that have temporary symbioses with plants like Gunnera manicata. The plants emit hormogonium-inducing factor (HIF) to induce Nostoc in the soil to change into hormogonia that infect specialised stem glands in the plant, after which they change into sessile filaments including heterocysts that provide nitrogen-based compounds to the plant.
- Akinetes, or resting spores, are produced by free-living Anabaena and Nostoc, enabling them to survive adverse conditions.
2. Evidence for Azolla’s Origin
2.1. Genetic Evidence for Azolla’s Origin
2.2. Paleontological Evidence for Azolla’s Origin
2.3. Age of the Oldest Azolla Fossils
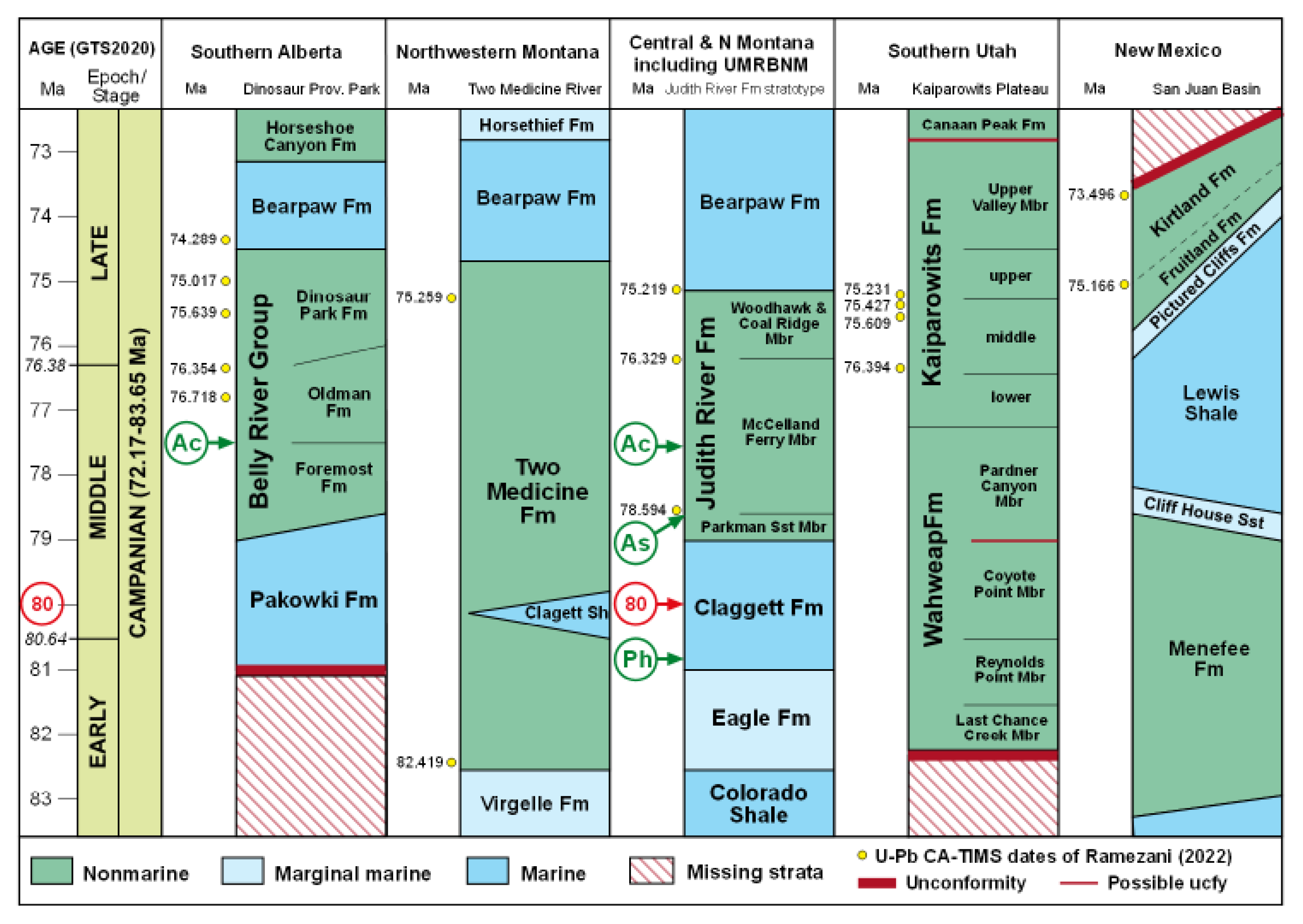
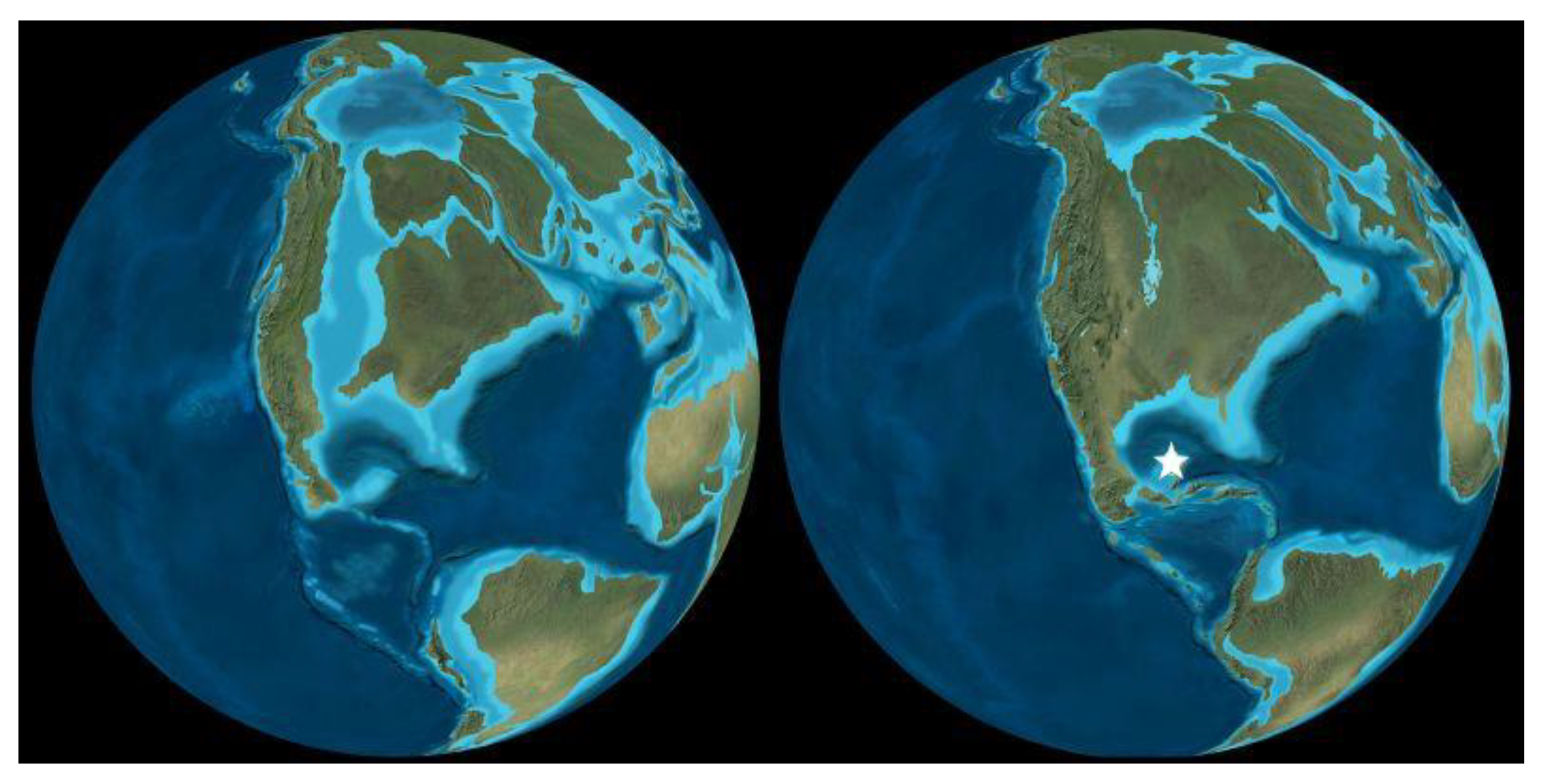
2.4. The Geological Evidence
3. Evolution of the Earliest Azolla
3.1. Temporary Symbiosis in Azolla’s Ancestor
3.2. Whole Genome Duplication (WGD)
- Chemical compounds were secreted inside the plant to induce changes in cyanobacterial mode involving motile hormogonia, sessile filaments and resting akinetes, facilitating their movement, retention and viability during the plant’s sexual reproduction. Similar chemical and environmental triggers induce the same changes in today’s free-living Anabaena and Nostoc.
- A series of passages inside the plant enabled the cyanobacteria to travel from the plant’s leaf cavities to a chamber, called the indusium, next to its developing megaspore. After germination of the megaspore, a channel opened in the embryonic leaf (cotyledon), enabling the cyanobacteria to travel from the indusium to dorsal leaf cavities as they developed in the new plant.
- The cyanobionts’ movement through the passages was directed by chemicals secreted inside the plant, augmented by a negative nitrogen gradient, similar to the attraction of today’s Nostoc in the soil to Gunnera’s stem nodules.
3.3. Germination and Propagation
4. Identifying Azolla’s Ancestor
5. Discussion
Appendix A. Questionable pre-Campanian records of Azolla and Azollopsis
References
- Pi, H.-W.; Lin, J.-J.; Chen, C.-A.; Wang, P.-H.; Chiang, Y.-R.; Huang, C.-C.; Young, C.-C.; Li, W.-H. Origin and Evolution of Nitrogen Fixation in Prokaryotes. Mol. Biol. Evol. 2022, 39, msac181. [Google Scholar] [CrossRef] [PubMed]
- Stüeken, E.E.; Buick, R.; Guy, B.M.; Koehler, M.C. Isotopic Evidence for Biological Nitrogen Fixation by Molybdenum-Nitrogenase from 3.2 Gyr. Nature 2015, 520, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Berja, N.S. The Growth of Four Species of Azolla as Affected by Temperature. Aquat. Bot. 1983, 15, 175–185. [Google Scholar] [CrossRef]
- Bujak, A.; Bujak, J. Azolla’s Use as a Biofertilizer and Livestock Feed. In Ferns; Marimuthu, J., Fernández, H., Kumar, A., Thangaiah, S., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 671–695. ISBN 9789811661693. [Google Scholar]
- Ansari, M.A.; Sharma, V.P. Role of Azolla in Controlling Mosquito Breeding in Ghaziabad District Villages (U.P.). Indian J. Malariol. 1991, 28, 51–54. [Google Scholar] [PubMed]
- Mwingira, V.; Mayala, B.; Senkoro, K.; Rumisha, S.; Shayo, H., Elizabeth; Mlozi, P.; Mboera, L. Mosquito Larval Productivity in Rice-Fields Infested with Azolla in Mvomero District, Tanzania. Tanzan. J. Health Res. 2009, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Reuben, R. Evaluation of the Water Fern Azolla Microphylla for Mosquito Population Management in the Rice-Land Agro-Ecosystem of South India. Med. Vet. Entomol. 1991, 5, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Bharati, K. Influence of Incorporation or Dual Cropping of Azolla on Methane Emission from a Flooded Alluvial Soil Planted to Rice in Eastern India. Agric. Ecosyst. Amp Environ. 2000. [Google Scholar] [CrossRef]
- Mujiyo, Sunarminto, B.; Hanudin, E.; Widada, J.; Syamsiyah, J. Methane Emission on Organic Rice Experiment Using Azolla. Int. J. Appl. Environ. Sci. 2016, 11, 295–308.
- Xu, H.; Zhu, B.; Liu, J.; Li, D.; Yang, Y.; Zhang, K.; Jiang, Y.; Hu, Y.; Zeng, Z. Azolla Planting Reduces Methane Emission and Nitrogen Fertilizer Application in Double Rice Cropping System in Southern China. Agron. Sustain. Dev. 2017, 37, 29. [Google Scholar] [CrossRef]
- Bujak, J.; Bujak, A. The Azolla Story: A Message from the Future.; The Azolla Foundation, 2020; ISBN 1-5272-8335-6.
- Winstead, D.; Di Gioia, F.; Jauregui, M.; Jacobson, M. Nutritional Properties of Raw and Cooked Azolla Caroliniana Willd., an Aquatic Wild Edible Plant. Food Sci. Nutr. 2024, 12, 2050–2060. [Google Scholar] [CrossRef]
- Carrapiço, F. Azolla as a Superorganism. Its Implication in Symbiotic Studies. In Symbioses and Stress: Joint Ventures in Biology; Seckbach, J., Grube, M., Eds.; Springer Netherlands: Dordrecht, 2010; pp. 225–241. ISBN 978-90-481-9449-0. [Google Scholar]
- Carrapico, F. Azolla and Bougainville’s Voyage Around the World. In Current Advances in Fern Research; 2018; pp. 251–267 ISBN 978-3-319-75102-3.
- Saunders, R.M.K.; Fowler, K. The Supraspecific Taxonomy and Evolution of the Fern Genus Azolla (Azollaceae). 1993. [CrossRef]
- De Benedetti, F.; Zamaloa, M.D.C.; Gandolfo, M.A.; Cúneo, N.R. Reinterpretation of Paleoazolla: A Heterosporous Water Fern from the Late Cretaceous of Patagonia, Argentina. Am. J. Bot. 2020, 107, 1054–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-W.; Brouwer, P.; Carretero-Paulet, L.; Cheng, S.; de Vries, J.; Delaux, P.-M.; Eily, A.; Koppers, N.; Kuo, L.-Y.; Li, Z.; et al. Fern Genomes Elucidate Land Plant Evolution and Cyanobacterial Symbioses. Nat. Plants 2018, 4, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Metzgar, J.S.; Schneider, H.; Pryer, K.M. Phylogeny and Divergence Time Estimates for the Fern Genus Azolla (Salviniaceae). Int. J. Plant Sci. 2007, 168, 1045–1053. [Google Scholar] [CrossRef]
- Singh, P.; Khan, A.; Srivastava, A. Chapter 16 - Heterocyst and Akinete Differentiation in Cyanobacteria: A View toward Cyanobacterial Symbiosis. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press, 2020; pp. 235–248 ISBN 978-0-12-819311-2.
- Malatinszky, D.; Steuer, R.; Jones, P.R. A Comprehensively Curated Genome-Scale Two-Cell Model for the Heterocystous Cyanobacterium Anabaena Sp. PCC 7120. Plant Physiol. 2017, 173, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Becking, J.H. Endophyte Transmission and Activity in the Anabaena-Azolla Association. Plant Soil 1987, 100, 183–212. [Google Scholar] [CrossRef]
- Watzer, B.; Forchhammer, K.; Watzer, B.; Forchhammer, K. Cyanophycin: A Nitrogen-Rich Reserve Polymer. In Cyanobacteria; IntechOpen, 2018 ISBN 978-1-78923-705-4.
- Hodgskiss, M.S.W.; Crockford, P.W.; Peng, Y.; Wing, B.A.; Horner, T.J. A Productivity Collapse to End Earth’s Great Oxidation. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 17207–17212. [Google Scholar] [CrossRef]
- Knoll, A.H.; Nowak, M.A. The Timetable of Evolution. Sci. Adv. 2017, 3, e1603076. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, B.E.; de Vos, J.M.; Antonelli, A.; Bagheri, H.C. Evolution of Multicellularity Coincided with Increased Diversification of Cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. 2013, 110, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Christman, H.; Meeks, J.C. DNA Microarray Comparisons of Plant Factor- and Nitrogen Deprivation-Induced Hormogonia Reveal Decision-Making Transcriptional Regulation Patterns in Nostoc Punctiforme. J. Bacteriol. 2008, 190, 7382–7391. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.C. Symbiosis between Nitrogen-Fixing Cyanobacteria and Plants: The Establishment of Symbiosis Causes Dramatic Morphological and Physiological Changes in the Cyanobacterium. BioScience 1998, 48, 266–276. [Google Scholar] [CrossRef]
- Risser, D.D. Hormogonium Development and Motility in Filamentous Cyanobacteria. Appl. Environ. Microbiol. 2023, 89, e0039223. [Google Scholar] [CrossRef] [PubMed]
- Crow, K.D.; Wagner, G.P.; SMBE Tri-National Young Investigators Proceedings of the SMBE Tri-National Young Investigators’ Workshop 2005. What Is the Role of Genome Duplication in the Evolution of Complexity and Diversity? Mol. Biol. Evol. 2006, 23, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ekman, M.; Tollbäck, P.; Klint, J.; Bergman, B. Protein Expression Profiles in an Endosymbiotic Cyanobacterium Revealed by a Proteomic Approach. Mol. Plant-Microbe Interactions® 2006, 19, 1251–1261. [Google Scholar] [CrossRef]
- Ekman, M.; Tollbäck, P.; Bergman, B. Proteomic Analysis of the Cyanobacterium of the Azolla Symbiosis: Identity, Adaptation, and NifH Modification. J. Exp. Bot. 2008, 59, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Nylander, J.A.; Bergman, B. Genome Fluctuations in Cyanobacteria Reflect Evolutionary, Developmental and Adaptive Traits. BMC Evol. Biol. 2011, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Larsson, J.; Vigil-Stenman, T.; Nylander, J.A.A.; Ininbergs, K.; Zheng, W.-W.; Lapidus, A.; Lowry, S.; Haselkorn, R.; Bergman, B. Genome Erosion in a Nitrogen-Fixing Vertically Transmitted Endosymbiotic Multicellular Cyanobacterium. PLoS ONE 2010, 5, e11486. [Google Scholar] [CrossRef]
- Watanabe, I. Azolla-Symbiosis - Its Physiology and Use in Tropical Agriculture. In Microbiology of Tropical Soils and Plant Productivity; Dommergues, Y.R., Diem, H.G., Eds.; Springer Netherlands: Dordrecht, 1982; pp. 169–185. ISBN 978-94-009-7529-3. [Google Scholar]
- Bergman, B.; Johansson, C.; Söderbäck, E. The Nostoc-Gunnera Symbiosis. New Phytol. 1992, 122, 379–400. [Google Scholar] [CrossRef] [PubMed]
- White, J. Palynodata Datafile: 2006 Version. Geol. Surv. Can. Open File 2008, 5793. [Google Scholar]
- Brinkhuis, H.; Schouten, S.; Collinson, M.E.; Sluijs, A.; Damsté, J.S.S.; Dickens, G.R.; Huber, M.; Cronin, T.M.; Onodera, J.; Takahashi, K. Episodic Fresh Surface Waters in the Eocene Arctic Ocean. Nature 2006, 441, 606–609. [Google Scholar] [CrossRef]
- Collinson, M.E.; Barke, J.; van der Burgh, J.; van Konijnenburg-van Cittert, J.H.A. A New Species of the Freshwater Fern Azolla (Azollaceae) from the Eocene Arctic Ocean. Rev. Palaeobot. Palynol. 2009, 155, 1–14. [Google Scholar] [CrossRef]
- Hall, J.W. Studies on Fossil Azolla; Primitive Types of Megaspores and Massulae from the Cretaceous. Am. J. Bot. 1969, 56, 1173–1180. [Google Scholar] [CrossRef]
- Boytsova, E.P. Upper Cretaceous Spore-Pollen Complexes of the Turgay Depressions. (In: Atlas of Upper Cretaceous, Paleocene and Eocene Spore-Pollen Complexes of Various Regions of the USSR). [ Verkhne Melovye Sporovo-Pyl’tsevye Kompleksy Turgayskogo Progiba. (In: Atlas Verkhne Melovykh Paleotsenorvykh i Eotsenovykh Sporov Pyl’tsevykh Kompleksov Nekotorykh Rayonov SSSR.). Tr. VSEGEI Novaya SeriyaLeningrad 1960, 30, 28–49. [Google Scholar]
- Romanovskaya, G.M.; Stelmak, N.K. Short Description of Spores and Pollen from Upper Cretaceous Deposits of the Turgay Basin. (In: Atlas of Upper Cretaceous, Paleocene and Eocene Spore-Pollen Complexes of Various Regions of the USSR). [ Kratkoe Opisanie Spor i Pyl’tsy Iz Verkhnemelovykh Otlozheniy Turgayskog Progiba. (In: Atlas Verkhne Melovykh Paleotsenorvykh i Eotsenovykh Sporov Pyl’tsevykh Kompleksov Nekotorykh Rayonov SSSR.). Tr. VSEGEI Novaya SeriyaLeningrad 1960, 30, 200–269. [Google Scholar]
- Juyal, N.P.; Sharma, J.; Chopra, A.S.; Misra, C.M. Palynostratigraphy and Paleoenvironment Analysis of the Mesozoic - Cenozoic Succession of the Wells Bhavadevarapalli, Bobbarlanka and Peddapalem in Krishna Delta. Contrib. XV Indian Colloq. Micropaleontol. Stratigr. 1996, 607–614. [Google Scholar]
- Ramanujam, C.J.K. Remain of Azollaceae from Late Albian of Cauvery Basin and Their Evolutionary Significance. Geophytology 1996, 25, 105–111. [Google Scholar]
- Faddeeva, I.Z. Palynological Characteristics of Lower Mesozoic Coal Deposits of Kazakhstan. (In: History of Lower Mesozoic Coal Accumulation in Kazakhstan. Part 3) [Palinologicheshaya Kharacteristika Nizhnemezozoic Kikh Uglenoshykh Otlozhenii Kazakhstana (In-Istoriya Nizhnemezozoiskogo Uglenakopleniya v Kazakastane]. Tr. Lab. Geol. UglyaAkademiya Nauk SSR 1963, 20, 143–185. [Google Scholar]
- Panova, L.A. Lower Cretaceous Spore-Pollen Complexes of Central Asia. (In: Atlas of Lower Cretaceous Spore-Pollen Complexes of Some Regions of USSR. I.M.Pokyovsksys and N.K.Stel’mak, Editors) [Nizhnemelovye Sporovo-Pyl’tsevye Kompleksy Sredney Azii. (In: Atlas Nizhnemelovykh Sporovo-Pyl’tsevykh Kompleksov Nekotorykh Reyonov SSSR. I.M.Pokyovsksys and N.K.Stel’mak,Editors)]. Tr. VSEGEI Novaya Seriya 1964, 124, 40–48. [Google Scholar]
- Andreeva, E.M.; Boytsova, E.P.; Koltsova, T.T.; Komarova, N.O.; Kruchinina, N.V.; Lyuber, A.A. Palaeopalynology. (Three Volumes: Volume I: Methods. Volume II: Complexes of Palynomorphs of the Precambrian-Holocene of the USSR. Volume III: Figures and Plates. 1966, 141. [Google Scholar]
- Bondarenko, N.M.; Bocharnikova, A.D.; Kara-Murza, E.N.; Koltsova, T.T.; Kopytova, E.A. A List of Plant Spores and Pollen of Valanginian Deposits in Some Districts of the USSR. (In: Atlas of Lower Cretaceous Spore-Pollen Complexes of Some Regions of USSR. I.M.Pokyovsksys and N.K.Stel’mak, Editors) [Spisok Rastenii Spory i Pyl’tsa Kotorykh Vstrecheny v Valankhinskikh i Valankhii-Goterivski Kh Otlozheniyakh Nekotorykh Raionov SSSR. (In: Atlas Nizhnemelovykh Sporovo-Pyl’tsevykh Kompleksov Nekotorykh Reyonov SSSR. I.M.Pokyovsksys and N.K.Stel’mak,Editors)]. Tr. VSEGEI Novaya Seriya 1964, 124, 322–339. [Google Scholar]
- Faddeeva, I.Z. Part 5. Lower Mesozoic Megaspores of the Or-Ilek Region and Their Stratigraphic Significance. (In: Palynological Validation of the Stratigraphic Division of the Lower Mesozoic Coal-Bearing Deposits of Or’-Ilek Region.) [5.Nizhnemezozoiskie Megaspory Or’-Ilekskogo Raiona i Ikh Stratigraficheskoe Znachenie In(Palinologischeskoe Obosnovanie Stratigraficheskogo Raschlene Nizhnemezozoickikh Uglenosnykh Otlozhenii Or’-Ilekskogo Raiona]. Geol. InstitutOtdelenie Geol. Uglya Goryuchikh SlantsevAkademiya Nauk SSRVNIGI 1965, 1–116.
- Petrosyants, M.A. A Study of Microfossils for Revealing Old Sedimentation Environment [Issledovanie Mikrofossilii Dlya Byyableniya Obstanovok Drevnego Osadkonakopleniya. Izv. Akad. Nauk SSSR Seriya Geol. 1984, 1984, 73–79. [Google Scholar]
- Sedova, M.A. Description of Spores and Pollen in Neocomian Deposits of the Vitimsk Plateau and Transbaikal. (In: Atlas of Lower Cretaceous Spore-Pollen Complexes of Some Regions of USSR. I.M.Pokyovsksys and N.K.Stel’mak, Editors) [Opisanie Spor i Pyl’tsy Iz Nedkomskikh Otlozhenii Vitimskogo Plostogor’Ya i Zabaikal’Ya. (In: Atlas Nizhnemelovykh Sporovo-Pyl’tsevykh Kompleksov Nekotorykh Reyonov SSSR. I.M.Pokyovsksys and N.K.Stel’mak,Editors)]. Tr. VSEGEI Novaya Seriya 1964, 124, 280–295. [Google Scholar]
- Freeman, M. Scouting Magazine. Available online: https://scoutingmagazine.org/2019/08/paddling-in-the-wake-of-lewis-and-clark/ (accessed on 24 May 2024).
- Gradstein, F.M.; Ogg, J.G.; Schmitz, D.; Ogg, M. Geologic Time Scale 2020 - 1st Edition; Elsevier, 2020; ISBN 978-0-12-824360-2.
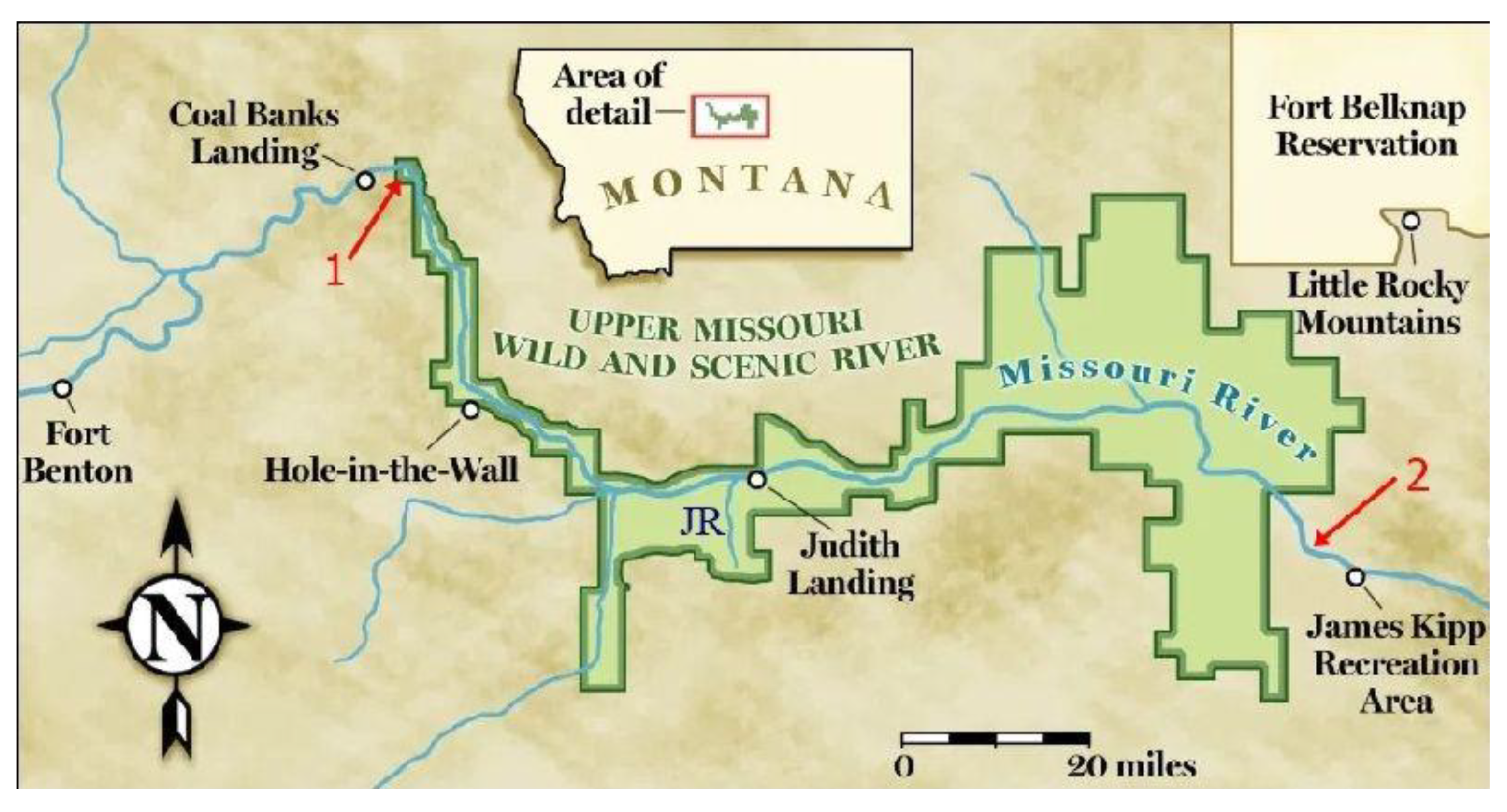
- Rogers, R.R.; Kidwell, S.M.; Deino, A.L.; Mitchell, J.P.; Nelson, K.; Thole, J.T. Age, Correlation, and Lithostratigraphic Revision of the Upper Cretaceous (Campanian) Judith River Formation in Its Type Area (North-Central Montana), with a Comparison of Low- and High-Accommodation Alluvial Records. J. Geol. 2016, 124, 99–135. [Google Scholar] [CrossRef]
- Ramezani, J.; Beveridge, T.L.; Rogers, R.R.; Eberth, D.A.; Roberts, E.M. Calibrating the Zenith of Dinosaur Diversity in the Campanian of the Western Interior Basin by CA-ID-TIMS U-Pb Geochronology. Sci. Rep. 2022, 12, 16026. [Google Scholar] [CrossRef]
- Blakey, R. Deep Time MapsTM. Available online: https://deeptimemaps.com/ (accessed on 24 May 2024).
- Dalman, S.; Lucas, S. Tyrannosaurid Teeth from the Claggett Formation of the Elk Basin, Late Cretaceous of Western North America. N. M. Mus. Nat. Hist. Sci. Bull. 2016, 71, 83–89. [Google Scholar]
- Paerl, H.W.; Gardner, W.S.; McCarthy, M.J.; Peierls, B.L.; Wilhelm, S.W. Algal Blooms: Noteworthy Nitrogen. Science 2014, 346, 175–175. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.G.; Bergman, B.; Nierzwicki-Bauer, S.A.; Rai, A.N.; Schüßler, A. Cyanobacterial-Plant Symbioses. In The Prokaryotes: Volume 1: Symbiotic associations, Biotechnology, Applied Microbiology; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, 2006; pp. 331–363. ISBN 978-0-387-30741-1. [Google Scholar]
- Adams, D.G.; Duggan, P.S.; Jackson, O. Cyanobacterial Symbioses. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Netherlands: Dordrecht, 2012; pp. 593–647. ISBN 978-94-007-3855-3. [Google Scholar]
- Adams, D.G.; Duggan, P.S. Cyanobacteria–Bryophyte Symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. Chapter 10 - The Nutraceutical Potential of Cyanobacteria. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press, 2022; pp. 287–330 ISBN 978-0-12-821491-6.
- Chiu, W.-L.; Peters, G.A.; Levieille, G.; Still, P.C.; Cousins, S.; Osborne, B.; Elhai, J. Nitrogen Deprivation Stimulates Symbiotic Gland Development in Gunnera Manicata. Plant Physiol. 2005, 139, 224–230. [Google Scholar] [CrossRef]
- Conradi, F.D.; Mullineaux, C.W.; Wilde, A. The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment. Life Basel Switz. 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Faluweki, M.K.; Goehring, L. Structural Mechanics of Filamentous Cyanobacteria. J. R. Soc. Interface 2022, 19, 20220268. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, B.; Meeks, J.C.; Risser, D.D. Evidence That a Modified Type IV Pilus-like System Powers Gliding Motility and Polysaccharide Secretion in Filamentous Cyanobacteria. Mol. Microbiol. 2015, 98, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Mullineaux, C.W. Motility in Cyanobacteria: Polysaccharide Tracks and Type IV Pilus Motors. Mol. Microbiol. 2015, 98, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Wong, F.C.Y.; Meeks, J.C. DNA Binding Properties of the HrmR Protein of Nostoc Punctiforme Responsible for Transcriptional Regulation of Genes Involved in the Differentiation of Hormogonia. Mol. Microbiol. 2003, 47, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Splitt, S. Sucrose Analog Sucralose Is Potent Inhibitor of Hormogonium Differentiation in Nostoc Punctiforme. Pac. Undergrad. Res. Creat. Conf. PURCC 2015. [Google Scholar]
- Cohen, M.F.; Sakihama, Y.; Takagi, Y.C.; Ichiba, T.; Yamasaki, H. Synergistic Effect of Deoxyanthocyanins from Symbiotic Fern Azolla Spp. on hrmA Gene Induction in the Cyanobacterium Nostoc Punctiforme. Mol. Plant-Microbe Interact. MPMI 2002, 15, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Bergman, B.; Chen, B.; Zheng, S.; Xiang, G.; Rasmussen, U. Cellular Responses in the Cyanobacterial Symbiont during Its Vertical Transfer between Plant Generations in the Azolla Microphylla Symbiosis. New Phytol. 2009, 181, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Stough, J.B. Palynomorphs from South America | KU Biodiversity Institute and Natural History Museum. Univ. Kans. Publ. 1968, 32, 1–12. [Google Scholar]
- Hoshino, Y.; Nettersheim, B.J.; Gold, D.A.; Hallmann, C.; Vinnichenko, G.; van Maldegem, L.M.; Bishop, C.; Brocks, J.J.; Gaucher, E.A. Genetics Re-Establish the Utility of 2-Methylhopanes as Cyanobacterial Biomarkers before 750 Million Years Ago. Nat. Ecol. Evol. 2023, 7, 2045–2054. [Google Scholar] [CrossRef]
- Sweet, A.R.; Hills, L.V. A Detailed Study of the Genus Azollopsis. Can. J. Bot. 1974, 52, 1625–1642. [Google Scholar] [CrossRef]
- Paulina-Carabajal, A.; Barrios, F.T.; Méndez, A.H.; Cerda, I.A.; Lee, Y.-N. A Late Cretaceous Dinosaur and Crocodyliform Faunal Association–Based on Isolate Teeth and Osteoderms–at Cerro Fortaleza Formation (Campanian-Maastrichtian) Type Locality, Santa Cruz, Argentina. PLoS ONE 2021, 16, e0256233. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K. Azolla from the Upper Cretaceous Edmonton Formation, Alberta, Canada. Can. J. Earth Sci. 1968, 5, 915–919. [Google Scholar] [CrossRef]
- Hall, J.W. A New Genus of Salviniaceae and a New Species of Azolla from the Late Cretaceous. Am. Fern J. 1968, 58, 77–88. [Google Scholar] [CrossRef]
- Boytsova, E.P. Lower Cretaceous Spore-Pollen Complexes of the Central Asia Trans-Urals. >(In: Atlas of Lower Cretaceous Spore-Pollen Complexes of Some Regions of USSR. I.M.Pokyovsksys and N.K.Stel’mak, Editors) [Nizhnemelovye Sporovo-Pyl’tsevye Kompleksy Sredney Azii. (In- Atlas Nizhnemelovykh Sporovo-Pyl’tsevykh Kompleksov Nekotorykh Reyonov SSSR. I.M.Pokyovsksys and N.K.Stel’mak,Editors)]. 1964, 124, 71–81.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



