1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world, including both sexes, and the third most common cause of cancer death according to the GLOBOCAN data [
1]. The number of patients with this cancer is constantly increasing, especially in the population younger than 50 years [
2]. At the time of diagnosis, the median age of patients with colon cancer is 68 years in men and 72 years in women, and the median age in patients with rectal cancer is 63 years for both sexes. According to the Surveillance, Epidemiology and End Results Program (SEER) database in the United States, about 5% of all cases of CRC are diagnosed in patients under 45 years of age 2. A concerning increase in CRC incidence among younger individuals has resulted in altered recommendations for screening, and contemporary guidelines indicate 45 years as age-cutoff for early onset disease [3-5].
Sporadic early-onset CRC is much more common than hereditary subtype, and recent reports suggest that its molecular properties are different from late-onset tumors [
6,
7]. Early-onset disease is more often characterized by aggressive tumor histology, more distal tumor localization (descending, sigmoid colon and rectum), mucinous histology or signet ring cell histology, advanced and metastatic stage of the disease, higher tumor grade, higher rate of perineural invasion, and a positive resection margin [8-10].
Mucinous histology of the tumor observed in 10-20% of CRC patients is characterized by an abundance of extracellular mucin component that accounts for at least 50% of the tumor volume [
11,
12]. In terms of clinical pathology, this CRC subtype is significantly more common in the proximal colon than in the rectum or distal colon, shows an increased lymph node infiltration and peritoneal implants, is larger at the time of diagnosis, and is more commonly diagnosed in younger women 11. It is most often diagnosed at an advanced stage, has worse prognosis and often shows resistance to available therapies, subsequently leading to lower overall survival compared to non-mucinous CRC [
11,
12]. However, the prognostic value of mucinous histology still remains uncertain.
The process of epithelial-mesenchymal transition (EMT) is essential for embryonal development, but it also occurs during the development of epithelial tumors causing cellular transmigration that eventually leads to metastasis. In general, EMT is characterized by the decrease in the function of epithelial markers such as E-cadherin, which plays an important role in epithelial cell adhesion and tissue architecture, and increase in the expression
of mesenchymal markers such as Vimentin, which is important for maintaining cell shape and integrity [
13]. Loss of E-cadherin leads to loss of epithelial differentiation and acquisition of motility and invasiveness phenotype, and its loss is associated with progression and poor prognosis of CRC, tumor invasiveness, metastases and increased resistance to apoptosis. The EMT process is mediated and orchestrated by various transcription modulators and a number of signaling intermediates, including mucins that play a significant role in the process of cell differentiation and are associated with the aggressive behavior of metastatic tumor cells.
The members of the mucin family of glycoproteins are secreted by epithelial cells and form extracellular mucinous gel in human tissues. Mucin 1 (MUC1) is aberrantly overexpressed in CRC, participates in the regulation of the metabolic program, activates antiapoptotic proteins and induces drug resistance. These observations identify MUC1 as an attractive marker for CRC diagnosis, immunotherapy, and prognosis. Since CRC patients with high MUC1 expression in tumor tissue have a higher risk of metastasis, the level of MUC1 expression is important in guiding treatment plans, so determining MUC1 expression in CRC by immunohistochemical methods may be important for determining treatment strategies in clinical setting [
14,
15]. MUC1 can induce the expression of multiple growth factors in the survival and proliferation of tumor cells and the production of angiogenesis factors that promote the formation of new blood vessels in tumor tissues. Overexpression of MUC1 was associated with EMT and cell invasion [
13,
14,
16].
The aim of this study was to examine the diagnostic and prognostic significance of mucinous histology and epithelial-mesenchymal transition (EMT) markers in patients with early-onset CRC, as well as their association with disease severity and tumor characteristics.
2. Material and Methods
2.1. Study Subjects
The retrospective study included 106 patients who underwent surgical resection of the primary CRC at the Clinic for Digestive Surgery, University Clinical Center of Serbia in the period from 2006 to 2020. Patients aged 18-45 years were included in the study and divided into two groups according to tumor types determined based on extracellular mucin production: 53 patients with mucinous adenocarcinoma (mucinous component comprises more than 50% of tumor volume) and 53 patients with non-mucinous adenocarcinoma (no mucinous component in the tumor). Patients with partial mucin production (below 50%) were excluded from the study. Exclusion criteria were the presence of any type of hereditary polypoid or non-polypoid syndrome, chronic inflammatory bowel disease (ulcerative colitis and Crohn's disease), any type of neoadjuvant chemoradiotherapy, and the presence of other histological, both primary and metastatic types of carcinomas. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of the University Clinical Center of Serbia (Ref. No.: 175/1, Date: April 27th 2021). The informed consent was obtained from all study participants.
The following data were collected for all subjects: age, sex, tumor localization, degree of tumor differentiation, percentage of extracellular mucin tumor production, tumor classification using histopathological criteria based on the fifth edition of the World Health Organization (WHO) classification, and tumor disease stage using criteria of the TNM classification of the eighth edition of the American Joint Committee on Cancer from 2017. Stages of tumor disease were also determined using Dukes and Astler-Coller systems. Also, data on lymphovascular and perineural invasion, as well as tumor residual status, were collected for all patients.
2.2. Immunohistochemical Analysis
The immunohistochemical (IHC) analysis of E-cadherin, Vimentin, MUC1, and pancytokeratin was performed on five tissue sections from a single paraffin block for each subject included in the study. Pancytokeratin was used for better visualization of cancer cells and more precise determination of the tumor budding degree. Tissue sections for IHC staining were cut successively to a thickness of 4µm on Superfrost Plus plates (Thermo Scientific). The process of deparaffinization and rehydration of tissue sections was performed in accordance with standard procedures. Pre-treatment for vimentin and pancytokeratin was performed in Target Retrieval Solution, High Ph (50x), while for E-cadherin and MUC1 it was performed in Target Retrieval Solution, Low Ph (50x), brand EnVision FLEX in PTLink (DAKO). After pretreatment, automatic staining was performed for Vimentin (monoclonal mouse antibody NCL-L-VIMENTIN; dilution 1:400), and pancytokeratin (monoclonal mouse antibody AE1/AE3; DAKO; dilution 1:100) in AUTOSTAINER LINK48 (Dako), while for MUC1 (monoclonal mouse antibody MRQ-17, Cell Marque; dilution 1:100) automatic staining was performed in AUTOSTEINER 360 (Thermo Scientific). Automatic staining was performed in accordance with the manufacturer's instructions. After staining, contrast staining was performed on all sections with the help of Mayer's hematoxylin. The detection system for vimentin and pancytokeratin was performed using EnVision FLEX, and for MUC1 using Mouse / Rabbit PolyDetector Plus; Bio SB. After pretreatment, staining with E-cadherin was done manually using a monoclonal mouse antibody (SPM471; SANTA CRUZ; sc56527; dilution 1:50). The detection was performed using Mouse/Rabbit PolyDetector Plus; Bio SB. Evaluation of IHC staining was performed using a standard Leica DM1000 light microscope. After selection, the representative fields were photographed using a Leica ICC50E camera, and the QuPat free software was used for counting. The status of the EMT process was determined based on the combined analysis of E-cadherin and Vimentin expression. Tumors positive for E-cadherin and/or negative for Vimentin were considered epithelial, while tumors negative for E-cadherin and/or positive for Vimentin were classified as mesenchymal.
2.3. Evaluation of E-Cadherin Expression
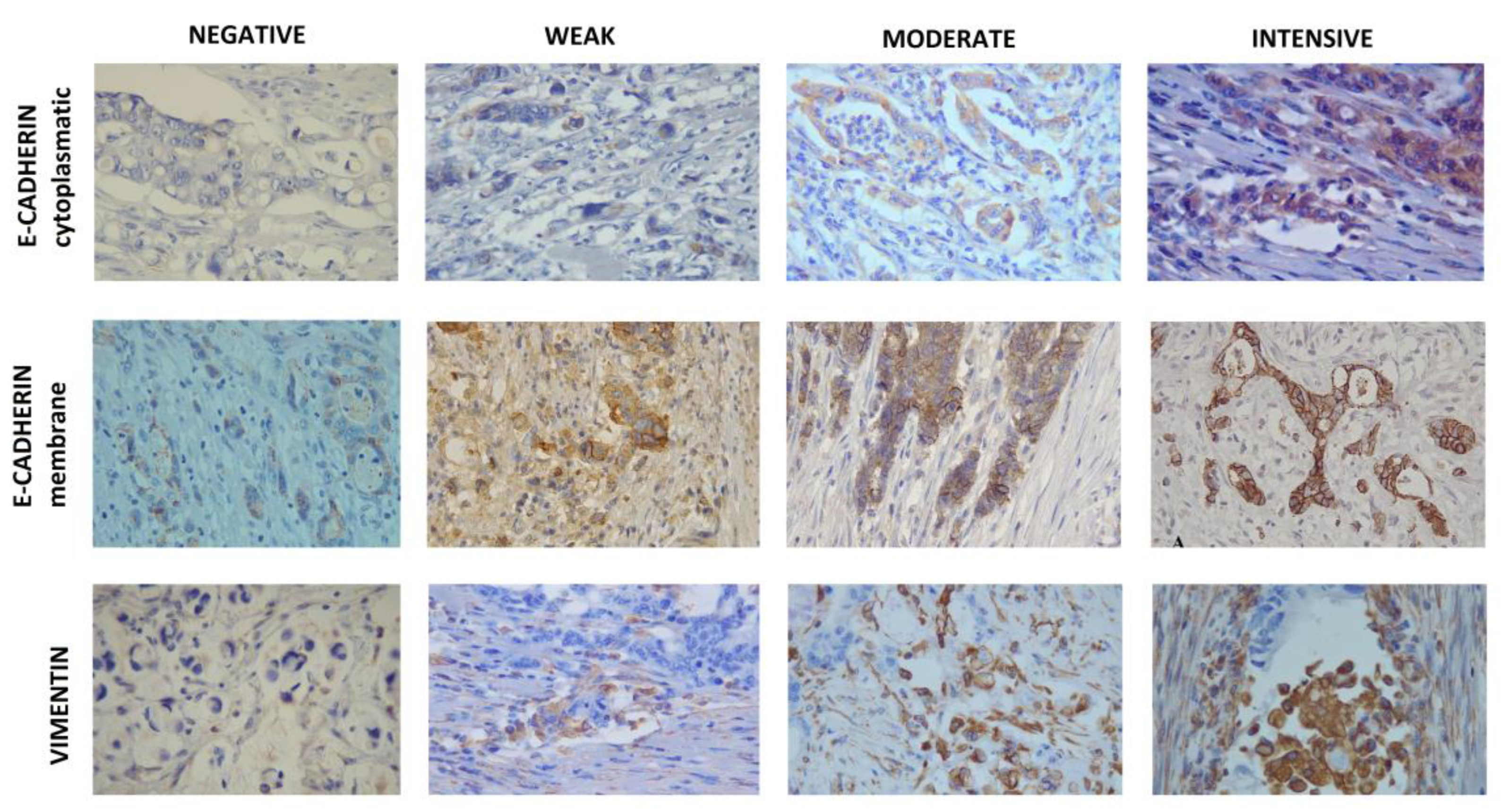
For evaluation of E-cadherin expression, the entire cross-section at x4 and x10 lens magnification was first examined to find the infiltrative zone of tumor spread that was the only one to be assessed at x40 lens magnification by observing at least 100 cancer cells. Both membrane and cytoplasmic staining were evaluated. For the cell membrane a four level scale was used: 1) +++ for continuous staining of the membrane with the creation of a honeycomb-shaped pattern; 2) ++ for continuous staining present in 40–90% of membranes; 3) + for continuous staining present in 10–39% of membranes; 4) - for staining in <10% membranes. Cytoplasmic staining was also classified into four categories: 1) 0 for no noticeable staining, 2) 1 for weak but still noticeable staining, 3) 2 for moderate, obviously positive, but still weak staining, 4) 3 for strong, intense staining. The membrane (MI) and cytoplasmis (CI) staining indexes were calculated based on the intensity of membrane or cytoplasmic staining and the proportion of positively stained cancer cells taken into account, using the following formula: I = 0 * f0 + 1 * f1 + 2 * f2 + 3 * f3, where I is the staining index, and f0-f3 cell fractions showing a defined level of staining intensity (0 to 3). Theoretically, the staining index is in the range between 0 and 3 [
17,
18]. Values of staining index greater than 0.5 were considered positive.
2.4. Evaluation of Vimentin Expression
Vimentin expression in cancer cells was first assessed by examining the cross section as a whole under low power magnification (lens x4), and then confirmed by high power magnification (lens x20 and x40). At least ten visual fields were observed, predominantly in the region of the tumor invasive front, in parallel with the same visual fields used for E-catherine staining. The immunoreactivity scoring system was applied according to the following two criteria: (1) the proportion of positively stained cells: 0 for 0%, 1 for ≤ 1%, 2 for 1-10%, 3 for 11–33%, 4 for 34–66 % and 5 for 67-100%; and (2) color intensity: 0 for colorless, 1 for pale, 2 for yellow, 3 for brown. The overall Vimentin score was calculated according to the modified Allerd scoring system by summing the two criteria into a single score: 0-1 negative, 2-3 weak positivity, 4-6 moderate positivity, 7-8 strong positivity [
13,
19].
2.5. Evaluation of MUC1 Expression
For evaluation of MUC1 expression the selected sections were first examined in full at x4 and x10 lens magnification, and since very heterogeneous antibody expression occurs at individual sections, 10 HPF visual fields were and counting was performed using x40 lens magnification [
20]. The immunostaining intensity of individual cells was evaluated on a scale from 0 (no staining) to +4 (strongest intensity). In addition, the percentage of stained cells for each of the intensities was determined. The percentage of cells at each intensity was multiplied by the appropriate intensity value to obtain an immunohistochemical score ranging from 0 to 4. The value of the score ≥0.5 or at least 25% of tumor cells was considered positive expression [
21]. For the samples with positive expression, the degree of positivity was determined: 0 for no positive cells, 1 for less than 5%, 2 for 5-29%, 3 for 30-59%, and 4 for more than 60% of positive cells [
22].
2.6. Pancytokeratin Staining and Tumor Budding Evaluation
Pancytokeratin immunostaining was performed to facilitate identification of cancer cells and tumor budding evaluation. Pancytokeratin staining was scored as follows: 0 for no staining; 1 for less than 5% of tumor cells; 2 for 5-25% of tumor cells; 3 for 25-50% of tumor cells; 4 for more than 50% of tumor cells [
23]. To determine the degree of tumor budding, the H&E stained section with the largest degree of buds was selected, then a hot spot was identified on the x10 lens. The buds were counted in the selected hot spot at x20 lens and tumor budding was scored using a three-step system proposed by the International Consensus Conference on Tumor Budding (ITBCC) as used by the Japan Colon Cancer Association and Rectum: 0–4 buds — low tumor budding (Bd1); 5–9 buds — moderate tumor budding (Bd 2); 10 or more buds — high tumor budding (Bd3) [
24].
2.7. Statistical Analysis
Statistical analyses were performed using Statistical Package for Social Sciences 21.0 (SPSS Inc., Chicago, Illinois, USA). Data were expressed as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. The normality of continuous data and homogeneity of variance were tested by one-sample Kolmogorov-Smirnov test and Levene’s test, respectively. Differences between groups for categorical data were analyzed by Fisher’s Exact test and Pearson’s Chi-squared test, while for continuous data Independent Samples Mann-Whitney U test was used. Logistic regression was performed to analyze the impact of each independent variable on the likelihood of an event of interest. Curves of probabilities for overall survival (OS) were constructed using the Kaplan-Meier product-limit method; the median of survival analysis with a corresponding 95% confidence interval (CI) was used for description, and the log-rank test was utilized for testing differences between curves. P value less than 0.05 was considered statistically significant.
3. Results
The expression of epithelial marker E-cadherin and mesenchymal marker Vimentin were analyzed in tumor tissue of 106 patients with colorectal cancer diagnosed before the age of 45 years. The patients were recruited for two groups according to the tumor histology - mucinous (53 patients) and non-mucinous (53 patients). The tissue sections were selected to contain the invasive tumor front and E-cadherin and Vimentin score were determined (
Figure 1). In addition to total E-cadherin score, membrane and cytoplasmic scores were also analyzed. The clinical and pathological characteristics of patients and scores for analyzed EMT markers are shown in
Table 1.
Mucinous tumors were significantly less differentiated than non-mucinous tumors (P=0.007), and they were characterized by significantly more advanced stage (P=0.027). Also, mucinous tumors affected right colon significantly more frequently than other tumor locations (P=0.039). Lower average expression of E-cadherin and higher average expression of Vimentin were detected in non-mucinous vs. mucinous tumors. In comparison to non-mucinous, mucinous tumors had a significantly higher cytoplasmic E-cadherin score (P=0.043). Total and membrane E-cadherin scores were also higher in patients with mucinous tumors, although without statistical significance. More non-mucinous tumors were vimentin-positive tumors than mucinous (22,6% vs.16,9%), and the average Vimentin score was higher in non-mucinous vs. mucinous tumors (0.85±1.73 vs. 0.62±1.47).
When patients with epithelial and mesenchymal tumors were compared regardless of the mucinous histology, patients with mesenchymal tumors were 1.6 years younger and there were 9.4% more women among them (
Table 2). No significant difference in MUC1 score was observed between epithelial and mesenchymal tumors. Epithelial tumors were significantly better differentiated than mesenchymal tumors (
P=0.034). Patients with mesenchymal tumors had significantly more prominent tumor budding (
P<0.001).
Binomial and ordinal logistic regression (adjusted for age and sex) were performed to evaluate the impact of Mucin 1, E-cadherin and Vimentin on mucinous histology and disease severity. The results indicated Mucin 1 as an independent predictor of tumor differentiation (odds ratio of 3.312, 95% CI [1.418-7.737]; p=0.006). Tumors with increased cytoplasmatic E-cadherin expression are 2.982 times more likely to have mucinous histology (95% CI [1.130-7.868]; p=0.027), while the increase in cytoplasmatic E-cadherin score was negatively associated with tumor grade (odds ratio of 0.228, 95% CI [0.072-0.722]; p=0.012). Vimentin was identified as an independent predictor of tumor budding (odds ratio of 2.738, 95% CI [1.519-4.934]; p=0.001).
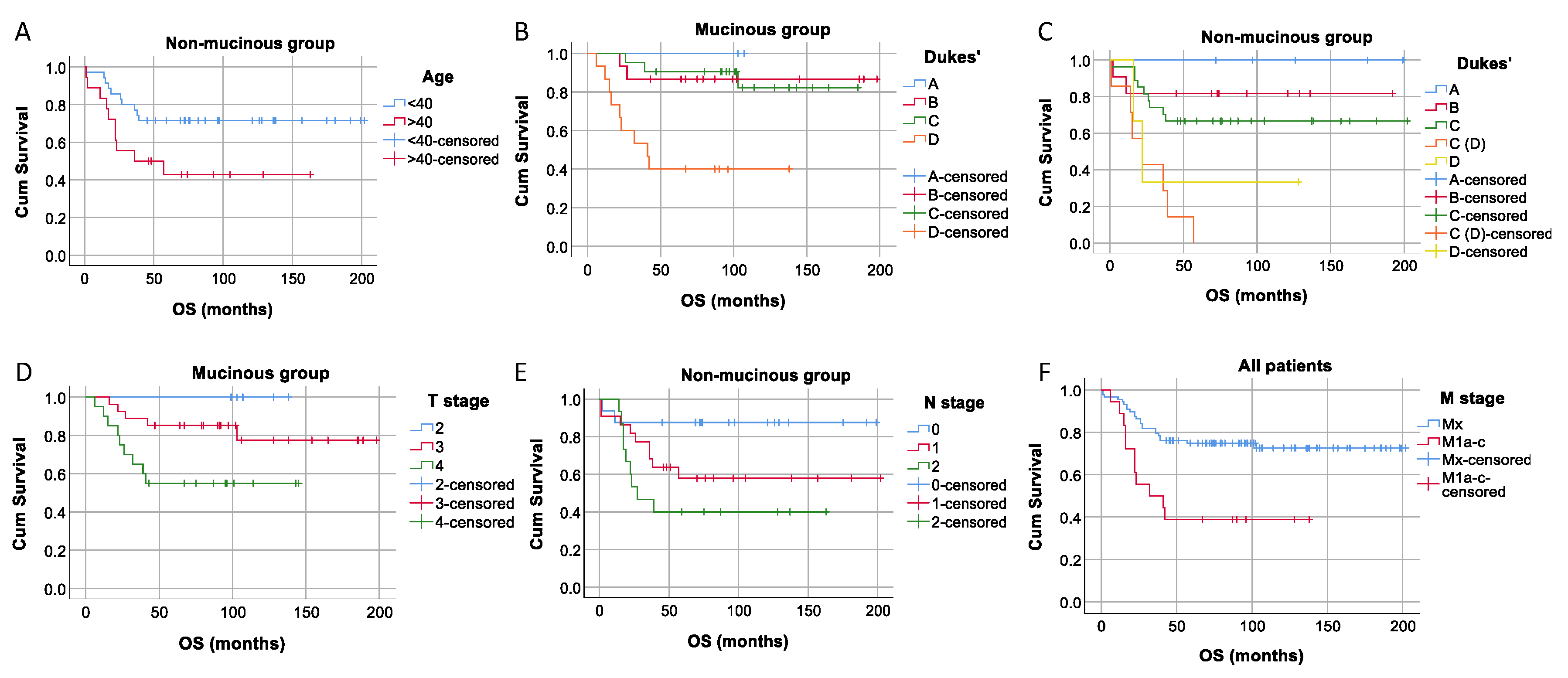
We tested if there was a significant difference in overall survival between patients with mucinous and non-mucinous tumors, epithelial and mesenchymal tumor types, as well as between positive and negative tumors for MUC1, membrane E-cadherin, cytoplasmatic E-cadherin, and Vimentin and found no statistically significant differences in survival distributions for any of investigated groups. We also performed a survival analysis for all collected patients' demographics and clinical and pathological characteristics (as seen in
Table 1) separately in the complete patients' group and mucinous and non-mucinous groups. We found significant differences in survival time between the patients younger and older than 40 years of age in the non-mucinous group (
P=0.026), based on the Dukes' classification for mucinous and non-mucinous groups (
P=0.001 for both), based on the T stage for mucinous tumors (
P =0.022), N stage for a non-mucinous group (
P=0.033), and between the Mx and M1a-c stages for all patients (
P=0.001) (
Figure 2).
4. Discussion
Expression of EMT markers, E-cadherin and Vimentin, along with expression of MUC1 were analyzed in a cohort of patients with early-onset CRC in order to explore the role of EMT in mucinous histology. Up to date, the vast majority of studies dealing with EMT in cancer focused either on its role in metastatic disease, its potential as a prognostic factor in primary tumors or its association with other relevant cellular processes, such as fibrosis [
25]. In spite of its importance for the clinical management, potential significance of EMT in specific histological subtypes of malignant disease was neglected. Although mucins have been demonstrated to be involved in the EMT process and also in the enrichment of cancer stem cell population in different cancer types, only a handful of recent studies investigated the EMT in mucinous histology [26-28].
The study was conducted in patients with early-onset disease, which represents a specific subset of CRC characterized by a predominance of mucinous tumors [
29]. This subset of patients was shown to harbor genetic and non-genetic determinants of risk for CRC and these individuals would highly benefit from preventive measures [
30,
31].The total number of patients enrolled in the study is relatively small due to the age-cutoff value and other inclusion criteria. However, the exclusion of patients older than 45 years, those with familial forms of cancer, ulcerative colitis or partial mucin production (below 50%) and those who underwent neoadjuvant chemoradiotherapy was expected to result in better patient stratification. Although our study was limited to tissue samples and did not characterize the underlying molecular mechanism of EMT
in vitro, the expression of EMT markers in tumor tissue was studied in the invasive tumor front, since EMT occurs in this region when cancer cells come in contact with stromal cells, as well as various signaling molecules and stromal cell derivatives [32-34].
Considering the observed heterotopic expression of E-cadherin, we determined both the expression of membrane (MI) and cytoplasmic (CI) expression of E-cadherin. Patients with mucinous type early-onset CRC had a statistically significantly higher E-cadherin CI, compared to patients with non-mucinous tumor histology. Total and MI scores for E-cadherin were also higher in mucinous compared to non-mucinous tumor, but without statistical significance. Previous studies have shown that aberrant expression of cytoplasmic E-cadherin may reflect an increased likelihood of metastasis [
18]. Cytoplasmic E-cadherin expression in primary tumors was significantly higher in patients with recurrence during their follow-up, compared to those who did not have relapses, while it was lower in those who did not have primary metastases and in those who did not develop disease recurrences over time 18. These findings suggest that cytoplasmic (aberrant) expression of E-cadherin reflects the unfavorable biological behavior of CRC [
18]. Our study showed that in the group of mucinous CRC a higher cytoplasmic (aberrant) expression of E-cadherin coincided with later tumor stage (28.2% vs. 5.7% stage IV), which also correlates with previous research [
18].
The group of non-mucinous early-onset CRC patients had more Vimentin positive patients and higher vimentin score than the group with mucinous histology. Many studies showed that as cancer progresses, Vimentin expression increases, while E-cadherin expression decreases [13,35-37]. Colon epithelial cells normally show strong membrane expression of E-cadherin, which reflects the normal localization of this intercellular adhesive molecule [
18]. When the expression of epithelial markers decreases and expression of mesenchymal markers increases, the cells tend to separate from their place of origin. The process of EMT is associated with primary tumor growth, regional lymph node infiltration, vascular invasion, cancer grade and stage progression, tumor invasiveness, cancer progression to metastatic stage, and overall poor prognosis [
13,
38,
39]. Although our study did not show a statistically significant difference in the overall survival in patients with increased cytoplasmic expression of E-cadherin, previous research showed an association between this aberrant expression in the invasive margin and adverse overall survival time in CRC [
18,
40]. Similarly, the Vimentin score was not significantly associated with overall survival in the present study, while previous research showed that elevated expression of Vimentin can serve as a novel biomarker for worse prognosis and poor overall survival in CRC [
16].
As expected, MUC1 expression was statistically higher in mucinous compared to non-mucinous tumors. Increased MUC1 expression was previously found to be a predictor of poor prognosis and overall survival in CRC, as it correlated with higher TNM stage, depth of invasion, lymph node and distant metastases [
14,
41]. These findings suggest that MUC1 expression is a promising prognostic factor for CRC and may serve as a valuable biomarker for identifying metastatic potential of the disease, but it may also be a promising target for future immunotherapy with the idea of reducing the risk of metastasis and increasing survival in patients with CRC [
42,
43].
Analysis of clinical and pathological data in our study indicates that early-onset disease, regardless of histology, is overrepresented in men, which correlates with other studies [
10,
44,
45]. As expected, non-mucinous tumors had a lower grade than mucinous (94.3% vs. 73.6%) [
46,
47]. In mucinous tumors group, there were more tumors of right colon, while non-mucinous were predominantly located in left colon and rectum, as confirmed by other studies [
8,
48,
49]. Additionally, hereditary mucinous forms are more frequently localized in right colon in comparison to sporadic mucinous forms [
50]. Lymphatic and vascular invasion were somewhat more pronounced in mesenchymal vs. epithelial tumors, and these tumors were of a higher Dukes stage at the time of diagnosis, but without statistical significance.
Epithelial tumors were significantly better differentiated compared to mesenchymal (
P= 0.034), which correlates with the finding that the preservation of E-cadherin correlates with better tumor differentiation [
51]. Patients with mesenchymal tumors were 1.6 years younger and there were 9.4% more women in this group in comparison to mesenchymal tumors. Patients with mesenchymal tumors had a statistically significantly more prominent tumor budding (P<0.001), which correlates with recent studies that reveal the connection of tumor budding and EMT [
52]. Loss or decreased expression of E-cadherin seen in tumor buds was also observed in our samples, which is consistent with previous reports [
53,
54]. Reduced expression or partial presence of E-cadherin on the membrane and heterotopic expression (alteration of membrane to cytoplasmic expression) was also observed in tumor buds, as in other studies [
52,
55].
5. Conclusion
Mucinous tumors had a significantly higher cytoplasmic E-cadherin score, they were significantly less differentiated, more advanced and affected right colon more frequently than other tumor locations in comparison to non-mucinous tumors. Epithelial tumors were significantly better differentiated and with less prominent tumor budding than mesenchymal tumors. The process of EMT appears to be more prominent in tumors of younger patients regardless of mucinous histology. EMT status and especially cytoplasmic E-cadherin expression may represent useful tools for patient stratification and choice of therapy in early-onset CRC. This pilot study indicates some peculiarities in the status of EMT markers in younger CRC patients, and further studies are needed to reveal underlying molecular mechanism of EMT in early-onset CRC.
Author Contributions
Conceptualization, G.B. and A.N..; methodology, A.D.R., R.J., P.S., V.M., and G.B.; formal analysis, A.D.R., S.D., R.J., S.R.S., J.R., and A.N.; investigation, A.D.R, S.D., R.J and S.R.S.; data curation, A.D.R.; writing—original draft preparation, A.D.R., S.D. and A.N.; writing—review and editing, A.D.R., S.D., R.J., S.R.S., P.S., V.M., J.R., G.B. and A.N.; visualization, A.D.R., J. R.; supervision, P.S., V.M., G.B. and A.N.; funding acquisition, P.S., V.M., G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Strategic Project of the Serbian Academy of Sciences and Arts - Molecular basis of response to chemoradiotherapy in rectal cancer (MOHERATEKA), F-69 and the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant number 451-03-47/2023-01/200110.
Sample collection
The retrospective study included 106 patients who underwent surgical resection of the primary CRC at the Clinic for Digestive Surgery, University Clinical Center of Serbia in the period from 2006 to 2020.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of the University Clinical Center of Serbia (Ref. No.: 175/1, Date: April 27th 2021).
Informed Consent Statement
The informed consent was obtained from all study participants.
Data Availability Statement
Data supporting reported results can be obtained from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Lancet. GLOBOCAN 2018: counting the toll of cancer. Lancet. 09 22 2018;392(10152):985. [CrossRef]
- Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 02 2019;13(2):109-131. [CrossRef]
- Haghighat S, Sussman DA, Deshpande A. US Preventive Services Task Force Recommendation Statement on Screening for Colorectal Cancer. JAMA. Oct 05 2021;326(13):1328. [CrossRef]
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. Jul 2018;68(4):250-281. [CrossRef]
- Gupta S, Bharti B, Ahnen DJ, et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer. Jul 01 2020;126(13):3013-3020. [CrossRef]
- Stigliano V, Sanchez-Mete L, Martayan A, Anti M. Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol. Sep 21 2014;20(35):12420-30. [CrossRef]
- Cavestro GM, Mannucci A, Zuppardo RA, Di Leo M, Stoffel E, Tonon G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig Liver Dis. 06 2018;50(6):521-532. [CrossRef]
- Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 06 15 2019;125(12):2002-2010. [CrossRef]
- Weinberg BA, Marshall JL. Colon Cancer in Young Adults: Trends and Their Implications. Curr Oncol Rep. 01 18 2019;21(1):3. [CrossRef]
- Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin Colorectal Cancer. 12 2017;16(4):293-299.e6. [CrossRef]
- Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 03 29 2019;39(1):13. [CrossRef]
- Dai D, Zhou B, Zhong Y, Jin H, Wang X. Survival of patients with resected primary colorectal mucinous adenocarcinoma: A competing risk nomogram analysis. Oncol Lett. Dec 2019;18(6):6594-6604. [CrossRef]
- Niknami Z, Muhammadnejad A, Ebrahimi A, Harsani Z, Shirkoohi R. Significance of E-cadherin and Vimentin as epithelial-mesenchymal transition markers in colorectal carcinoma prognosis. EXCLI J. 2020;19:917-926. [CrossRef]
- Li C, Liu T, Yin L, Zuo D, Lin Y, Wang L. Prognostic and clinicopathological value of MUC1 expression in colorectal cancer: A meta-analysis. Medicine (Baltimore). Mar 2019;98(9):e14659. [CrossRef]
- Guo M, You C, Dou J. Role of transmembrane glycoprotein mucin 1 (MUC1) in various types of colorectal cancer and therapies: Current research status and updates. Biomed Pharmacother. Nov 2018;107:1318-1325. [CrossRef]
- Ponnusamy MP, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, Batra SK. Emerging role of mucins in epithelial to mesenchymal transition. Curr Cancer Drug Targets. Nov 2013;13(9):945-56. [CrossRef]
- Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. Aug 2002;41(2):127-33. [CrossRef]
- Elzagheid A, Algars A, Bendardaf R, et al. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol. Jul 21 2006;12(27):4304-9. [CrossRef]
- Toiyama Y, Yasuda H, Saigusa S, et al. Increased expression of Slug and Vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis. Nov 2013;34(11):2548-57. [CrossRef]
- Kasprzak A, Siodła E, Andrzejewska M, et al. Differential expression of mucin 1 and mucin 2 in colorectal cancer. World J Gastroenterol. Sep 28 2018;24(36):4164-4177. [CrossRef]
- Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. Oct 2000;6(10):4017-25.
- Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. Mar 09 2007;5:31. [CrossRef]
- Yamagishi H, Imai Y, Okamura T, et al. Aberrant cytokeratin expression as a possible prognostic predictor in poorly differentiated colorectal carcinoma. J Gastroenterol Hepatol. Dec 2013;28(12):1815-22. [CrossRef]
- Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 09 2017;30(9):1299-1311. [CrossRef]
- Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. Jun 2020;21(6):341-352. [CrossRef]
- Gurzu S, Bara T, Molnar C, et al. The epithelial-mesenchymal transition induces aggressivity of mucinous cystic neoplasm of the pancreas with neuroendocrine component: An immunohistochemistry study. Pathol Res Pract. Jan 2019;215(1):82-89. [CrossRef]
- Sugimoto R, Uesugi N, Yamada N, et al. Sarcomatoid change associated with epithelial-mesenchymal transition in mucinous tubular and spindle cell carcinoma of the kidney: a case report. Int J Clin Exp Pathol. 2019;12(7):2767-2771.
- Bhuyan G, Arora R, Ahluwalia C, Sharma P. Epithelial-mesenchymal transition in serous and mucinous epithelial tumors of the ovary. J Cancer Res Ther. 2019;15(6):1309-1315. [CrossRef]
- Silla IO, Rueda D, Rodríguez Y, García JL, de la Cruz Vigo F, Perea J. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol. Dec 14 2014;20(46):17288-96. [CrossRef]
- Archambault AN, Su YR, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. 04 2020;158(5):1274-1286.e12. [CrossRef]
- Archambault AN, Lin Y, Jeon J, et al. Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr. Jun 2021;5(3):pkab029. [CrossRef]
- Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. Aug 28 2001;98(18):10356-61. [CrossRef]
- Rosivatz E, Becker I, Bamba M, et al. Neoexpression of N-cadherin in E-cadherin positive colon cancers. Int J Cancer. Sep 20 2004;111(5):711-9. [CrossRef]
- Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. Nov 2015;25(11):675-686. [CrossRef]
- Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 2008 Nov-Dec 2008;28(6A):3815-26.
- Bruun J, Kolberg M, Nesland JM, Svindland A, Nesbakken A, Lothe RA. Prognostic Significance of β-Catenin, E-Cadherin, and SOX9 in Colorectal Cancer: Results from a Large Population-Representative Series. Front Oncol. 2014;4:118. [CrossRef]
- Hugo HJ, Gunasinghe NPAD, Hollier BG, et al. Epithelial requirement for in vitro proliferation and xenograft growth and metastasis of MDA-MB-468 human breast cancer cells: oncogenic rather than tumor-suppressive role of E-cadherin. Breast Cancer Res. Jul 27 2017;19(1):86. [CrossRef]
- Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. Sep 2011;68(18):3033-46. [CrossRef]
- Kim SA, Inamura K, Yamauchi M, et al. Loss of CDH1 (E-cadherin) expression is associated with infiltrative tumour growth and lymph node metastasis. Br J Cancer. Jan 19 2016;114(2):199-206. [CrossRef]
- Lee SJ, Choi SY, Kim WJ, et al. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. Jun 19 2013;8:99. [CrossRef]
- Zeng Y, Zhang Q, Zhang Y, et al. MUC1 Predicts Colorectal Cancer Metastasis: A Systematic Review and Meta-Analysis of Case Controlled Studies. PLoS One. 2015;10(9):e0138049. [CrossRef]
- Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. Feb 19 2007;25(9):1607-18. [CrossRef]
- Gao T, Cen Q, Lei H. A review on development of MUC1-based cancer vaccine. Biomed Pharmacother. Dec 2020;132:110888. [CrossRef]
- Abualkhair WH, Zhou M, Ahnen D, Yu Q, Wu XC, Karlitz JJ. Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age. JAMA Netw Open. 01 03 2020;3(1):e1920407. [CrossRef]
- Gausman V, Dornblaser D, Anand S, et al. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. 11 2020;18(12):2752-2759.e2. [CrossRef]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 01 2020;76(2):182-188. [CrossRef]
- Hu X, Li YQ, Li QG, Ma YL, Peng JJ, Cai S. Mucinous Adenocarcinomas Histotype Can Also be a High-Risk Factor for Stage II Colorectal Cancer Patients. Cell Physiol Biochem. 2018;47(2):630-640. [CrossRef]
- Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg. Mar 2016;20(3):648-55. [CrossRef]
- Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. Aug 2018;11(4):264-273. [CrossRef]
- Chiang JM, Yeh CY, Changchien CR, Chen JS, Tang R, Chen JR. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int J Colorectal Dis. Aug 2010;25(8):941-7. [CrossRef]
- He X, Chen Z, Jia M, Zhao X. Downregulated E-cadherin expression indicates worse prognosis in Asian patients with colorectal cancer: evidence from meta-analysis. PLoS One. 2013;8(7):e70858. [CrossRef]
- Yamada N, Sugai T, Eizuka M, et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum Pathol. 02 2017;60:151-159. [CrossRef]
- Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. Nov 2010;1(7):651-61. [CrossRef]
- Galván JA, Helbling M, Koelzer VH, et al. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget. Jan 20 2015;6(2):874-85. [CrossRef]
- Bronsert P, Enderle-Ammour K, Bader M, et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol. Nov 2014;234(3):410-22. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).