Submitted:
18 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Plant Growth-Promoting Rhizobacteria (PGPR)
2.1. Biofertilization: A Sustainable Approach to Enhance Soil Fertility
2.2. Protection Against Oxidative Stress in Adverse Environmental Conditions
2.3. Production of Phytohormones by Rhizobacteria
2.4. Production of ACC Deaminase
2.5. Chemical Signals: Volatile Organic Compounds (VOCs)
2.6. Production of Siderophores
3. Plant Growth-Promoting Fungi (PGPF)
3.1. Trichoderma spp. as Biocontrol Agents and Fertilizers
3.2. Arbuscular Mycorrhizal Fungi: Extensions of Roots in Soil
4. Co-Inoculation of Beneficial Microorganisms
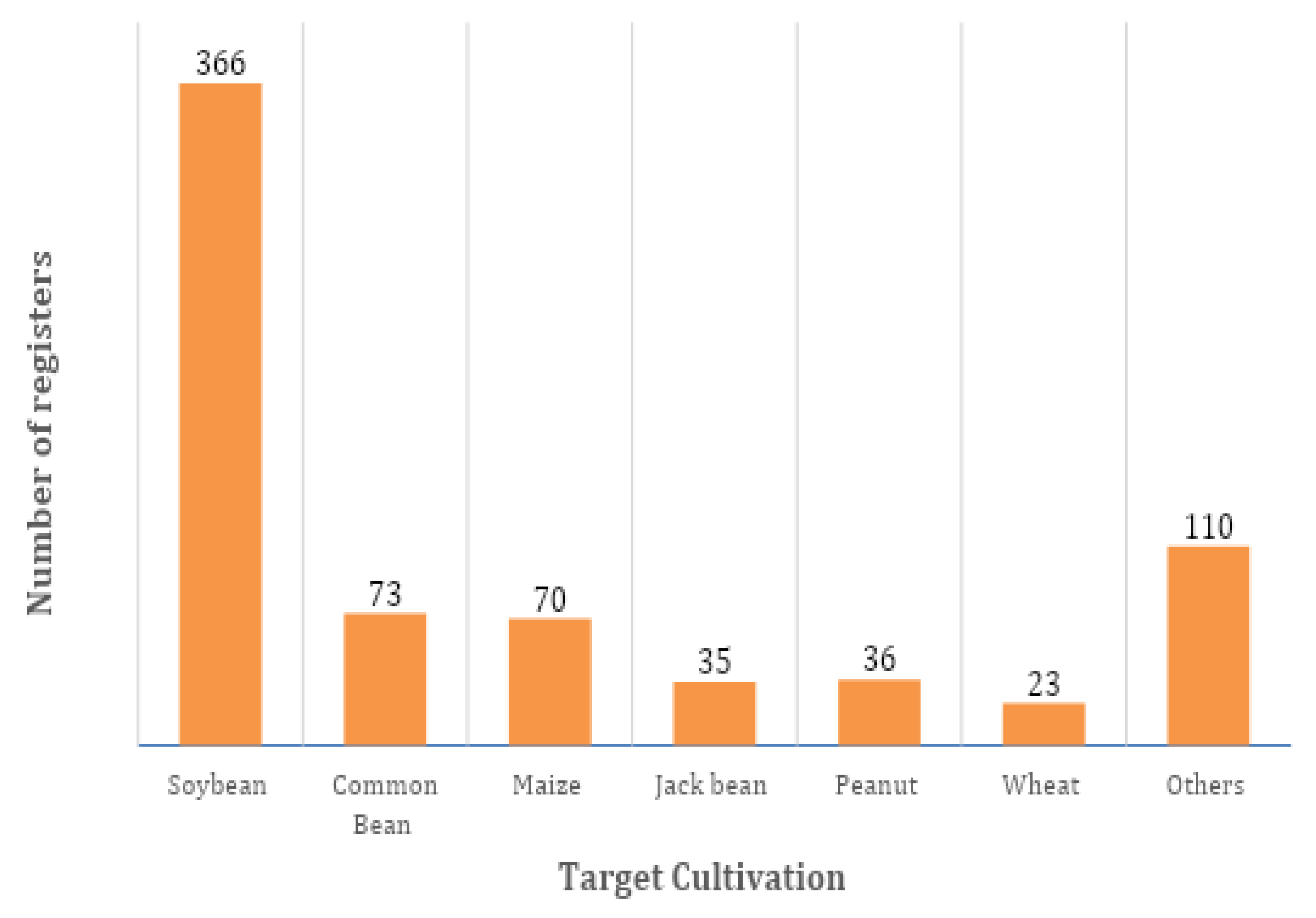
5. Biological Inoculants Registered in Brazil
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baweja P, Kumar S, Kumar G. Fertilizers and pesticides: Their impact on soil health and environment. Soil Biology, Cham: Springer International Publishing; 2020, p. 265–85.
- Olaetxea M, De Hita D, Garcia CA, Fuentes M, Baigorri R, Mora V, et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl Soil Ecol 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Etesami H, Adl SM. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. Environmental and Microbial Biotechnology, Singapore: Springer Singapore; 2020, p. 147–203.
- ANPII. Painel Interno ANPII; 2022. [cited 2024 may 20]. Available from: https://www.anpii.org.br/estatisticas/.
- Zeffa DM, Perini LJ, Silva MB, de Sousa NV, Scapim CA, Oliveira ALM de, et al. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS One 2019, 14, e0215332. [Google Scholar] [CrossRef]
- Zilli JÉ, Pacheco RS, Gianluppi V, Smiderle OJ, Urquiaga S, Hungria M. Biological N2 fixation and yield performance of soybean inoculated with Bradyrhizobium. Nutr Cycling Agroecosyst 2021, 119, 323–336. [Google Scholar] [CrossRef]
- Oteino, N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Frontiers in microbiology. 2015. [CrossRef]
- afza MR, Aliasgharzad N, Khoshru B. P solubilizing potential of some plant growth promoting bacteria used as ingredient in phosphatic biofertilizers with emphasis on growth promotion of Zea mays L. Geomicrobiol J 2020, 37, 327–335. [Google Scholar] [CrossRef]
- Sun Y, Wu J, Shang X, Xue L, Ji G, Chang S, et al. Screening of siderophore-producing bacteria and their effects on promoting the growth of plants. Curr Microbiol 2022, 79, 150. [Google Scholar] [CrossRef]
- Uzma M, Iqbal A, Hasnain S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiate. PLoS ONE n.d.; 2022.
- Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Afzal, A. Rock phosphate solubilization by plant growth-promoting Bacillus velezensis and its impact on wheat growth and yield. Geomicrobiology Journal 2023, 40, 131–142. [Google Scholar] [CrossRef]
- Mosela M, Andrade G, Massucato LR, de Araújo Almeida SR, Nogueira AF, de Lima Filho RB, et al. Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Sci Rep 2022, 12, 15284. [Google Scholar] [CrossRef]
- Rath M, Mitchell TR, Gold SE. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol Res 2018, 208, 76–84. [CrossRef]
- Gowtham, HG. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiological Research 2020, 234. [Google Scholar]
- 16. Carlos M-HJ, Stefani P-VY, Janette A-M, Melani M-SS, Gabriela P-O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol Res, 2016; 188–189, 53–61. [CrossRef]
- Zeng Q, Ding X, Wang J, Han X, Iqbal HMN, Bilal M. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environ Sci Pollut Res Int 2022, 29, 45089–45106. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, A. Future outlook of transferring biological nitrogen fixation (BNF) to cereals and challenges to retard achieving this dream. Curr Microbiol 2022, 79. [Google Scholar] [CrossRef]
- Maitra S, Praharaj S, Brestic M, Sahoo RK, Sagar L, Shankar T, et al. Rhizobium as biotechnological tools for green solutions: An environment-friendly approach for sustainable crop production in the modern era of climate change. Curr Microbiol 2023, 80, 219. [Google Scholar] [CrossRef]
- 20. Zhang W, Chen Y, Huang K, Wang F, Mei Z. Molecular mechanism and agricultural application of the NifA-NifL system for nitrogen fixation. Int J Mol Sci. [CrossRef]
- Aasfar A, Bargaz A, Yaakoubi K, Hilali A, Bennis I, Zeroual Y, et al. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front Microbiol 2021, 12, 628379. [Google Scholar] [CrossRef]
- Timofeeva A, Galyamova M, Sedykh S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Sarmah R, Sarma AK. Phosphate solubilizing microorganisms: A review. Commun Soil Sci Plant Anal 2023, 54, 1306–1315. [Google Scholar] [CrossRef]
- Pandey D, Kehri HK, Zoomi I, Singh U, Chaudhri KL, Akhtar O. Potassium solubilizing microbes: Diversity, ecological significances and biotechnological applications. Sustainable Development and Biodiversity, Cham: Springer International Publishing; 2020, p. 263–86.
- Wang J, Li R, Zhang H, Wei G, Li Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol 2020, 20, 38. [Google Scholar] [CrossRef]
- 26. Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front Plant Sci, 2021; 11. [CrossRef]
- Devireddy AR, Zandalinas SI, Fichman Y, Mittler R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Gowtham HG, Singh SB, Shilpa N, Aiyaz M, Nataraj K, Udayashankar AC, et al. Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants (Basel) 2022, 11, 1763. [Google Scholar] [CrossRef]
- Batool T, Ali S, Seleiman MF, Naveed NH, Ali A, Ahmed K, et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep 2020, 10, 16975. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda MDC, Santoyo G, Glick BR. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Andrade LA, Santos CHB, Frezarin ET, Sales LR, Rigobelo EC. Plant Growth-Promoting Rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Hedden P, Sponsel V. A century of gibberellin research. J Plant Growth Regul 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Urban L, Lauri F, Ben Hdech D, Aarrouf J. Prospects for increasing the efficacy of plant resistance inducers stimulating salicylic acid. Agronomy (Basel) 2022, 12, 3151. [Google Scholar] [CrossRef]
- Jasrotia S, Jasrotia R. Role of ethylene in combating biotic stress. Ethylene in Plant Biology, 2022; 388–397. [CrossRef]
- Chen H, Bullock DA Jr, Alonso JM, Stepanova AN. To fight or to grow: The balancing role of ethylene in plant abiotic stress responses. Plants 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Choudhury AR, Trivedi P, Madhaiyan M, Choi J, Choi W, Park J-H, et al. ACC deaminase producing endophytic bacteria enhances cell viability of rice (Oryza sativa L.) under salt stress by regulating ethylene emission pathway. Environ Exp Bot 2023, 213, 105411. [Google Scholar] [CrossRef]
- Andy AK, Rajput VD, Burachevskaya M, Gour VS. Exploring the identity and properties of two bacilli strains and their potential to alleviate drought and heavy metal stress. Horticulturae 2023, 9, 46. [Google Scholar] [CrossRef]
- 38. Ojuederie OB, Babalola OO. Growth enhancement and extenuation of drought stress in maize inoculated with multifaceted ACC deaminase producing rhizobacteria. Front Sustain Food Syst. [CrossRef]
- Brilli F, Loreto F, Baccelli I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front Plant Sci 2019, 10, 264. [Google Scholar] [CrossRef]
- Russo A, Pollastri S, Ruocco M, Monti MM, Loreto F. Volatile organic compounds in the interaction between plants and beneficial microorganisms. J Plant Interact 2022, 17, 840–852. [Google Scholar] [CrossRef]
- Hyder S, Rizvi ZF, los Santos-Villalobos S de, Santoyo G, Gondal A, Khalid N, et al. Applications of plant growth-promoting rhizobacteria for increasing crop production and resilience. J Plant Nutr 2023, 46, 2551–2580. [Google Scholar] [CrossRef]
- Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Ahmed E, Holmström SJM. Siderophores in environmental research: roles and applications: Siderophores in environmental research. Microb Biotechnol 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gowtham HG, Singh SB, Shilpa N, Aiyaz M, Nataraj K, Udayashankar AC, et al. Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants (Basel) 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hossain MM, Sultana F, Islam S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. Plant-Microbe Interactions in Agro-Ecological Perspectives, Singapore: Springer Singapore; 2017, p. 135–91.
- Igiehon NO, Babalola OO. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Appl Microbiol Biotechnol 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Bononi L, Chiaramonte JB, Pansa CC, Moitinho MA, Melo IS. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci Rep 2020, 10, 2858. [Google Scholar]
- Nieto-Jacobo, MF. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Frontiers in plant science. 2017. [Google Scholar]
- You J, Li G, Li C, Zhu L, Yang H, Song R, et al. Biological control and plant growth promotion by volatile organic compounds of Trichoderma koningiopsis T-51. J Fungi (Basel), 2022; 8. [CrossRef]
- Lee S, Yap M, Behringer G, Hung R, Bennett JW. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol Biotechnol 2016, 3, 7. [Google Scholar] [CrossRef]
- Lei ZHAO, Zhang Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. Journal of Integrative Agriculture 2015, 1588–1597. [Google Scholar]
- Cely MVT, de Oliveira AG, de Freitas VF, de Luca MB, Barazetti AR, Dos Santos IMO, et al. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front Microbiol 2016, 7, 720. [Google Scholar] [CrossRef]
- 53. Fayaz F, Zahedi M. Beneficial effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) nutritional status and tolerance indices under soil salinity stress. Journal of Plant Nutrition, 2021; 185–201.
- 54. Hu Y, Xie W, Chen B. Arbuscular mycorrhiza improved drought tolerance of maize seedlings by altering photosystem II efficiency and the levels of key metabolites. Chem Biol Technol Agric, 2020; 7. [CrossRef]
- Al-Karaki GN, Williams M. Mycorrhizal mixtures affect the growth, nutrition, and physiological responses of soybean to water deficit. Acta Physiologiae Plantarum, 2021.
- Jumrani K, Bhatia VS, Kataria S, Alamri SA, Siddiqui MH, Rastogi A. Inoculation with arbuscular mycorrhizal fungi alleviates the adverse effects of high temperature in soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef]
- Guzmán-Guzmán P, Kumar A, de Los Santos-Villalobos S, Parra-Cota FI, Orozco-Mosqueda MDC, Fadiji AE, et al. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases-A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Naher, L. , Yusuf, U. K., Ismail, A., & Hossain, K. Trichoderma spp.: a biocontrol agent for sustainable management of plant diseases. Pak J Bot 2014, 46, 1489–1493. 46,.
- Asad, SA. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases - A review. Ecol Complex 2022, 49, 100978. [Google Scholar] [CrossRef]
- Cumagun CJR. Advances in Formulation of Trichoderma for Biocontrol. Biotechnology and Biology of Trichoderma, Elsevier; 2014, p. 527–31.
- Mukhopadhyay R, Kumar D. Trichoderma: a beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt J Biol Pest Contr 2020, 30. [Google Scholar] [CrossRef]
- Hermosa R, Cardoza RE, Rubio MB, Gutiérrez S, Monte E. Secondary metabolism and antimicrobial metabolites of Trichoderma. Biotechnology and Biology of Trichoderma, Elsevier; 2014, p. 125–37.
- Stewart A, Hill R. Applications of Trichoderma in Plant Growth Promotion. Biotechnology and Biology of Trichoderma, Elsevier; 2014, p. 415–28.
- Zeilinger, S. , Gruber, S. , Bansal, R., & Mukherjee, P. K. Secondary metabolism in Trichoderma–chemistry meets genomics. Fungal biology reviews 2016, 30, 74–90. [Google Scholar]
- Junior AFC, Chagas LFB, Colonia BSO, Miller LDO, De Oliveira JC. Trichoderma asperellum (UFT201) functions as a growth promoter for soybean plant. African Journal of Agricultural Research 2019, 14, 1772–1777. [Google Scholar] [CrossRef]
- Junior AFC, Chagas LFB, Colonia BSO, Miller LDO, De Oliveira JC. Efficiency of Trichoderma asperellum UFT 201 as plant growth promoter in soybean. Afr J Agric Res 2019, 14, 263–271. [Google Scholar] [CrossRef]
- Bader AN, Salerno GL, Covacevich F, Consolo VF. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J King Saud Univ Sci 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J Hazard Mater 2021, 402, 123919. [Google Scholar] [CrossRef] [PubMed]
- Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front Plant Sci 2019, 10, 1068. [Google Scholar] [CrossRef]
- Etesami H, Jeong BR, Glick BR. Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front Plant Sci 2021, 12, 699618. [Google Scholar] [CrossRef]
- Chitarra W, Maserti B, Gambino G, Guerrieri E, Balestrini R. Arbuscular mycorrhizal symbiosis-mediated tomato tolerance to drought. Plant Signal Behav 2016, 11, e1197468. [Google Scholar] [CrossRef] [PubMed]
- Ouledali S, Ennajeh M, Ferrandino A, Khemira H, Schubert A, Secchi F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. S Afr J Bot 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Wang Y, Qiu Q, Yang Z, Hu Z, Tam NF-Y, Xin G. Arbuscular mycorrhizal fungi in two mangroves in South China. Plant Soil 2010, 331, 181–191. [Google Scholar] [CrossRef]
- Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity (Basel) 2020, 12, 370. [Google Scholar] [CrossRef]
- Djighaly PI, Diagne N, Ngom M, Ngom D, Hocher V, Fall D, et al. Selection of arbuscular mycorrhizal fungal strains to improve Casuarina equisetifolia L. and Casuarina glauca Sieb. tolerance to salinity. Ann For Sci 2018, 75. [CrossRef]
- Qin Y, Zhang W, Feng Z, Feng G, Zhu H, Yao Q. Arbuscular mycorrhizal fungus differentially regulates P mobilizing bacterial community and abundance in rhizosphere and hyphosphere. Appl Soil Ecol 2022, 170, 104294. [Google Scholar] [CrossRef]
- Kobae, Y. Dynamic phosphate uptake in arbuscular mycorrhizal roots under field conditions. Front Environ Sci, 2019; 6. [Google Scholar] [CrossRef]
- Wang F, Zhang L, Zhou J, Rengel Z, George TS, Feng G. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant Soil 2022, 481, 1–22. [Google Scholar] [CrossRef]
- He J-D, Chi G-G, Zou Y-N, Shu B, Wu Q-S, Srivastava AK, et al. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl Soil Ecol 2020, 154, 103592. [Google Scholar] [CrossRef]
- Wang X-Q, Wang Y-H, Song Y-B, Dong M. Formation and functions of arbuscular mycorrhizae in coastal wetland ecosystems: A review. Ecosyst Health Sustain 2022, 8. [Google Scholar] [CrossRef]
- Haq IU, Zwahlen RD, Yang P, van Elsas JD. The response of Paraburkholderia terrae strains to two soil fungi and the potential role of oxalate. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Jansa J, Hodge A. Swimming, gliding, or hyphal riding? On microbial migration along the arbuscular mycorrhizal hyphal highway and functional consequences thereof. New Phytol 2021, 230, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Bakker PAHM, Berendsen RL, Doornbos RF, Wintermans PCA, Pieterse CMJ. The rhizosphere revisited: root microbiomics. Front Plant Sci 2013, 4, 165. [Google Scholar] [CrossRef]
- Verbruggen E, Sheldrake M, Bainard LD, Chen B, Ceulemans T, De Gruyter J, et al. Mycorrhizal fungi show regular community compositions in natural ecosystems. ISME J 2018, 12, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Faghihinia M, Jansa J, Halverson LJ, Staddon PL. Hyphosphere microbiome of arbuscular mycorrhizal fungi: a realm of unknowns. Biol Fertil Soils 2023, 59, 17–34. [Google Scholar] [CrossRef]
- Spescha A, Weibel J, Wyser L, Brunner M, Hess Hermida M, Moix A, et al. Combining entomopathogenic Pseudomonas bacteria, nematodes and fungi for biological control of a below-ground insect pest. Agric Ecosyst Environ 2023, 348, 108414. [Google Scholar] [CrossRef]
- El-Sharkawy EES, Abdelrazik E. Biocontrol of Fusarium root rot in squash using mycorrhizal fungi and antagonistic microorganisms. Egypt J Biol Pest Contr 2022, 32. [Google Scholar] [CrossRef]
- Meena RS, Vijayakumar V, Yadav GS, Mitran T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Anuar MSK, Hashim AM, Ho CL, Wong M-Y, Sundram S, Saidi NB, et al. Synergism: biocontrol agents and biostimulants in reducing abiotic and biotic stresses in crop. World J Microbiol Biotechnol 2023, 39, 123. [Google Scholar] [CrossRef]
- Musyoka DM, Njeru EM, Nyamwange MM, Maingi JM. Arbuscular mycorrhizal fungi and Bradyrhizobium co-inoculation enhances nitrogen fixation and growth of green grams (Vigna radiata L.) under water stress. J Plant Nutr 2020, 43, 1036–1047. [CrossRef]
- Kavadia A, Omirou M, Fasoula DA, Louka F, Ehaliotis C, Ioannides IM. Co-inoculations with rhizobia and arbuscular mycorrhizal fungi alters mycorrhizal composition and lead to synergistic growth effects in cowpea that are fungal combination-dependent. Appl Soil Ecol 2021, 167, 104013. [Google Scholar] [CrossRef]
- De Almeida Leite R, Martins LC, Ferreira LV dos SF, Barbosa ES, Alves BJR, Zilli JE, et al. Co-inoculation of Rhizobium and Bradyrhizobium promotes growth and yield of common beans. Appl Soil Ecol 2022, 172, 104356. [Google Scholar] [CrossRef]
- Queiroz Rego, CH. Co-inoculation with Bradyrhizobium and Azospirillum increases yield and quality of soybean seeds. Agronomy Journal 2018, 110, 2302–2309. [Google Scholar] [CrossRef]
- Pérez-Rodriguez MM, Pontin M, Lipinski V, Bottini R, Piccoli P, Cohen AC. Pseudomonas fluorescens and Azospirillum brasilense Increase Yield and Fruit Quality of Tomato Under Field Conditions. J Soil Sci Plant Nutr 2020, 20, 1614–1624. [Google Scholar] [CrossRef]
- Mendes JBS, da Costa Neto VP, de Sousa CDA, de Carvalho Filho MR, Rodrigues AC, Bonifacio A. Trichoderma and bradyrhizobia act synergistically and enhance the growth rate, biomass and photosynthetic pigments of cowpea (Vigna unguiculata) grown in controlled conditions. Symbiosis 2020, 80, 133–143. [Google Scholar] [CrossRef]
- Qi R, Lin W, Gong K, Han Z, Ma H, Zhang M, et al. Bacillus co-inoculation alleviated salt stress in seedlings cucumber. Agronomy (Basel) 2021, 11, 966. [Google Scholar] [CrossRef]
- 97. Leite R da C, Pereira YC, Oliveira-Paiva CA de, Moraes AJG de, Silva GB da. Increase in yield, leaf nutrient, and profitability of soybean co-inoculated with Bacillus strains and Arbuscular mycorrhizal fungi. Rev Bras Cienc Solo, 2022; 46. [CrossRef]
- Brasil. Inoculantes; 2024. [cited 2024 April 27]. Available from: https://www.atermaisdigital.cnptia.embrapa.br/web/inoculantes.
- Conab. Boletim de safra de grãos - 7o Levantamento - Safra 2023/24 2024. Available from: URL: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos.
- Jones, F. Os primeiros inoculantes. revistapesquisa.fapesp.br. 2019. [cited 2023 September 24]. https://revistapesquisa.fapesp.br/os-primeiros-inoculantes/.
- Telles TS, Nogueira MA, Hungria M. Economic value of biological nitrogen fixation in soybean crops in Brazil. Environ Technol Innov 2023, 31, 103158. [Google Scholar] [CrossRef]
- Paulitsch F, dos Reis FB Jr, Hungria M. Twenty years of paradigm-breaking studies of taxonomy and symbiotic nitrogen fixation by beta-rhizobia, and indication of Brazil as a hotspot of Paraburkholderia diversity. Arch Microbiol 2021, 203, 4785–4803. [Google Scholar] [CrossRef]
- Pavinato PS, Cherubin MR, Soltangheisi A, Rocha GC, Chadwick DR, Jones DL. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci Rep 2020, 10, 15615. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int J Agron 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Sipert S, Cohim E, do Nascimento FRA. Identification and quantification of main anthropogenic stocks and flows of potassium in Brazil. Environ Sci Pollut Res Int 2020, 27, 32579–32593. [Google Scholar] [CrossRef]
- Schueler TA, Dourado ML, Videira SS, da Cunha CD, Rizzo ACL. Biosolubilization of verdete: An alternative potassium source for agriculture fertilizer. Biocatal Agric Biotechnol 2021, 34, 102031. [Google Scholar] [CrossRef]
- 107. Costa AD, Santos SR dos, Pereira GL, Santos WO. Glauconite as a potential source of potassium in Brazilian agriculture - a review. Cienc Agron, 2024; 55. [CrossRef]
- Brasil. Plano Nacional de Fertilizantes 2050; 2022. [cited 2024 April 27]. Available from: https://www.gov.br/planalto/pt-br/assuntos-estrategicos/documentos/planos/plano-nacional-fertilizantes/view.
- Zilli M, Scarabello M, Soterroni AC, Valin H, Mosnier A, Leclère D, et al. The impact of climate change on Brazil’s agriculture. Sci Total Environ 2020, 740, 139384. [Google Scholar] [CrossRef] [PubMed]
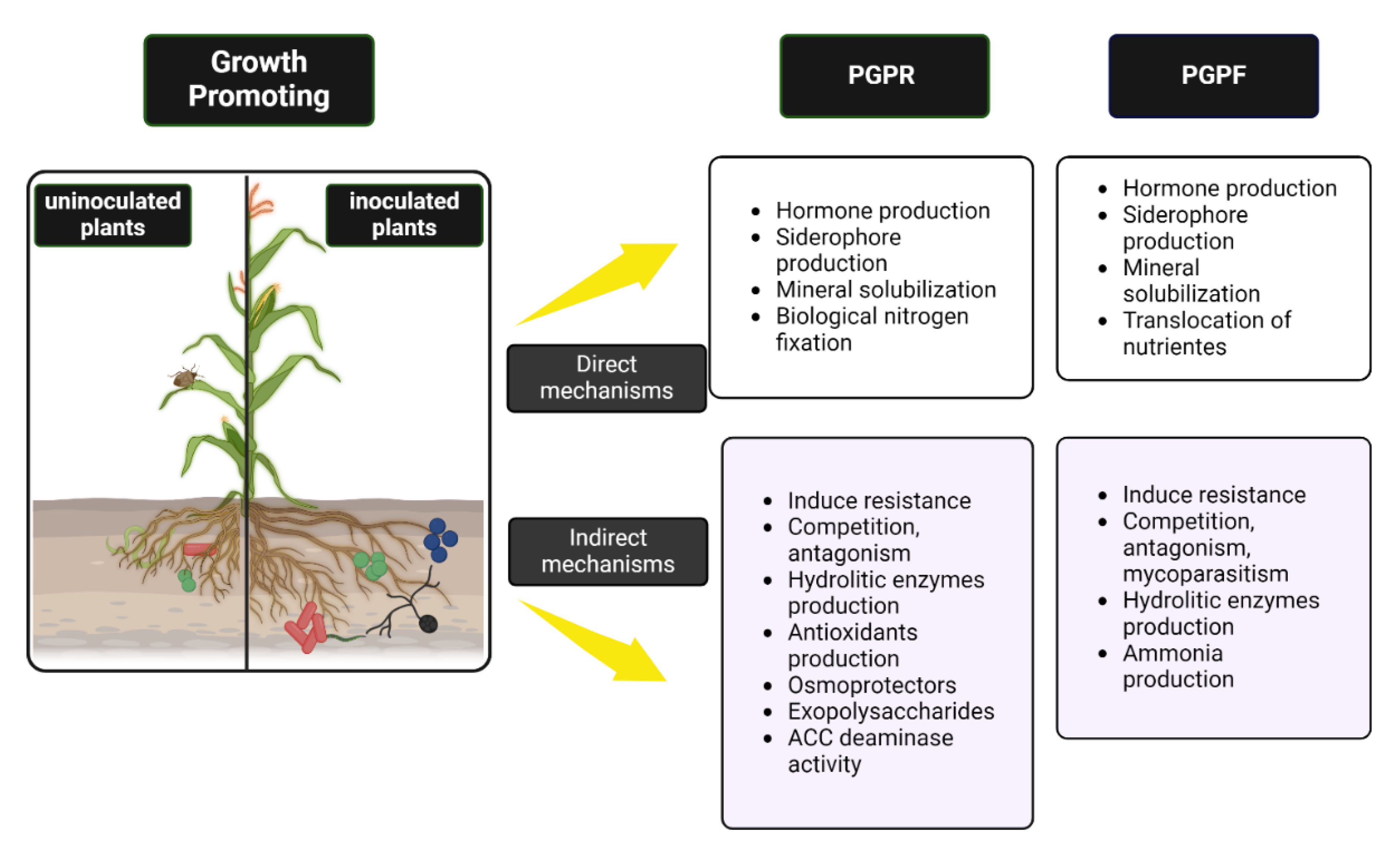
| Specie | Mechanism of growth promotion | Culture | Reference |
|---|---|---|---|
| Azospirillum brasilense | Nitrogen fixation | Maize (Zea mays) | [5] |
| Bradyrhizobium sp. | Nitrogen fixation | Soybean (Glycine max) | Zilli et al., 2021[6] |
| Pseudomonas sp. | Phosphate solubilization | Pea (Pisum sativum) | Oteino et al. 2015[7] |
| Pseudomonas sp. | Phosphate solubilization | Maize (Zea mays) | Sarikhani et al., 2020[8] |
| Pseudomonas brassicae | Siderophore production | Mung bean (Vigna radiata) | Sun et al. 2022[9] |
| Pseudomonas aeruginosa | Phytohormone production | Mung bean (Vigna radiata) | Uzma et al., 2022[10] |
| Pseudomonas putida | Antioxidant activity | Maize (Zea mays L.) | Sandhya et al., 2010[11] |
| Bacillus velezensis | Phosphate solubilization and phytohormone production | Wheat (Triticum aestivum) | Afzal et al.2023[12] |
| Bacillus velezensis | Phosphate solubilization | Soybean (Glycine max) and Maize (Zea mays) | Mosela et al. 2022[13] |
| Bacillus mojavensis | VOCs production | Arabidopsis thaliana | Rath et al., 2018[14] |
| Bacillus subtilis | ACC deaminase activity | Tomato (Solanum lycopersicum) | Gowtham et al., 2020[15] |
| Serratia sp. | ACC deaminase activity and phytohormone production | Sunflower (Helianthus annuus) | Carlos et al., 2026[16] |
| Specie | Mechanism of growth promotion | Culture | Reference |
|---|---|---|---|
| Trichoderma sp. | Phosphate solubilization | Soybean (Glycine max) | [47] |
| Trichoderma sp. | VOCs production | Arabidopsis thaliana | [48] |
| T. koningiopsis | VOCs production | Arabidopsis thaliana | [49] |
| T. viride | VOCs production | Tomato (Solanum lycopersicum) | [50] |
| T. asperellum | Phosphate solubilization and phytohormone production | Cucumber (Cucumber sativus) | [51] |
| Rhizophagus clarus | Increase of P and N content | Soybean (Glycine max) and Cotton (Gossypium hirsutum) | [52] |
| Glomus. intraradices | Salt tolerance | Wheat (Triticum aestivum) | [53] |
| Rhizophagus irregularis | Drought tolerance | Maize (Zea mays) | [54] |
| Mix of Rhizophagus clarus, R. intraradices, Septoglomus deserticola, Funneliformis mosseae | Water déficit tolerance | Soybean (Glycine max) | [55] |
| Rhizophagus irregularis , Funneliformis mosseae, and Funneliformis geosporum | High temperature tolerance | Soybean (Glycine max) | [56] |
| Species | Culture | Benefits | References |
| Bradyrhizobium diazoefficiens and Rhizobium tropici | Commom beans (Phaseolus vulgaris) | Growth promotion and grain yield | [92] |
| Bradyrhizobium japonicum and Azospirillum brasilense | Soybean (Glycine max) | Increased yield components, grain yield and seed quality | [93] |
| Pseudomonas fluorescens and Azospirillum brasilense | Tomato (Solanum lycopersicum) | Increased yield and fruit quality | [94] |
| Bradyrhizobium sp. and Trichoderma sp. | Cowpea (Vigna unguiculata) | Increased the growth rate, biomass and photosynthetic pigments | [95] |
| B. licheniformis and B. subtilis | Cucumber (Cucumber sativus) | Alleviated Salt Stress | [96] |
| B. subitilis, B. megaterium and Rhizophagus intraradices | Soybean (Glycine max) | Increase leaf nutrient and in yield | [97] |
| Rhizophagus irregulares and Bradyrhizobium sp. | Mung bean (Vigna radiata) | Growth promotion and alleviated water stress | [90] |
| Cultura | Gênero | Single inoculation | Co-inoculation |
|---|---|---|---|
| Soybean (Glycine max) | Bradyrhizobium sp. | 246 |
B. Japonicum + B. Elkani (52) B. Japonicum + A. brasilense (4) A. brasilense + P. fluorescense (2) B. megaterium + B. subtilis (2) B. subtilis + B. elkani (3) R. intraradices + Claroideoglomus claroideum (2) B. subtilis + B. elkani +Parabhurkodelia nodosa (3) B. subtilis + B. amyloliquefacens +B. pumilus (1) P. fluorescense + B. amyloliquefacens + Priestia megaterium (1) |
| Azospirillum sp. | 28 | ||
| Bacillus sp. | 8 | ||
| Pseudomonas sp. | 6 | ||
| Trichoderma sp. | 5 | ||
| Rhizophagus sp. (Rhizoglomus) | 5 | ||
| Commom beans (Phaseolus vulgaris) | Rhizobium sp. | 65 | Bacillus megaterium + Bacillus subtillis (1) |
| Azospirilum sp. | 6 | ||
| Bacillus sp. | 1 | ||
| Maize (Zea mays) | Azospirillum sp. | 41 |
B. megaterium + B. subtilis (2) A. brasilense + P. fluorescense (2) B. megaterium + Lysinobacillus sp. (1) B. licheniformis + B. aryabhatai (2) B. Japonicum + A. brasilense (2) R. intraradices + Claroideoglomus claroideum (2) B. subtilis + B. amyloliquefacens +B. pumilus (1) |
| Bacillus sp. | 8 | ||
| Rhizophagus sp. (Rhizoglomus) | 5 | ||
| Pseudomonas sp. | 3 | ||
| Methylobacterium sp. | 1 | ||
| Peanut (Arachis hypogaea) | Bradyrhizobium sp. | 36 | - |
| Jack bean (Canavalia ensiformis) | Bradyrhizobium sp. | 35 | - |
| Wheat (Triticum aestivum) | Azospirilum sp. | 23 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





