Submitted:
17 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
TPS Biology
TPS Monitoring
2. Materials and Methods
2017. Field Trial: Efficacy of Gambusia Affinis and Predatory Beetles at Controlling TPS
2018. Field Trial: Comparing Different Application Rates against Previous Successful Rate
Assessing Monitoring Practices
Statistical Analysis
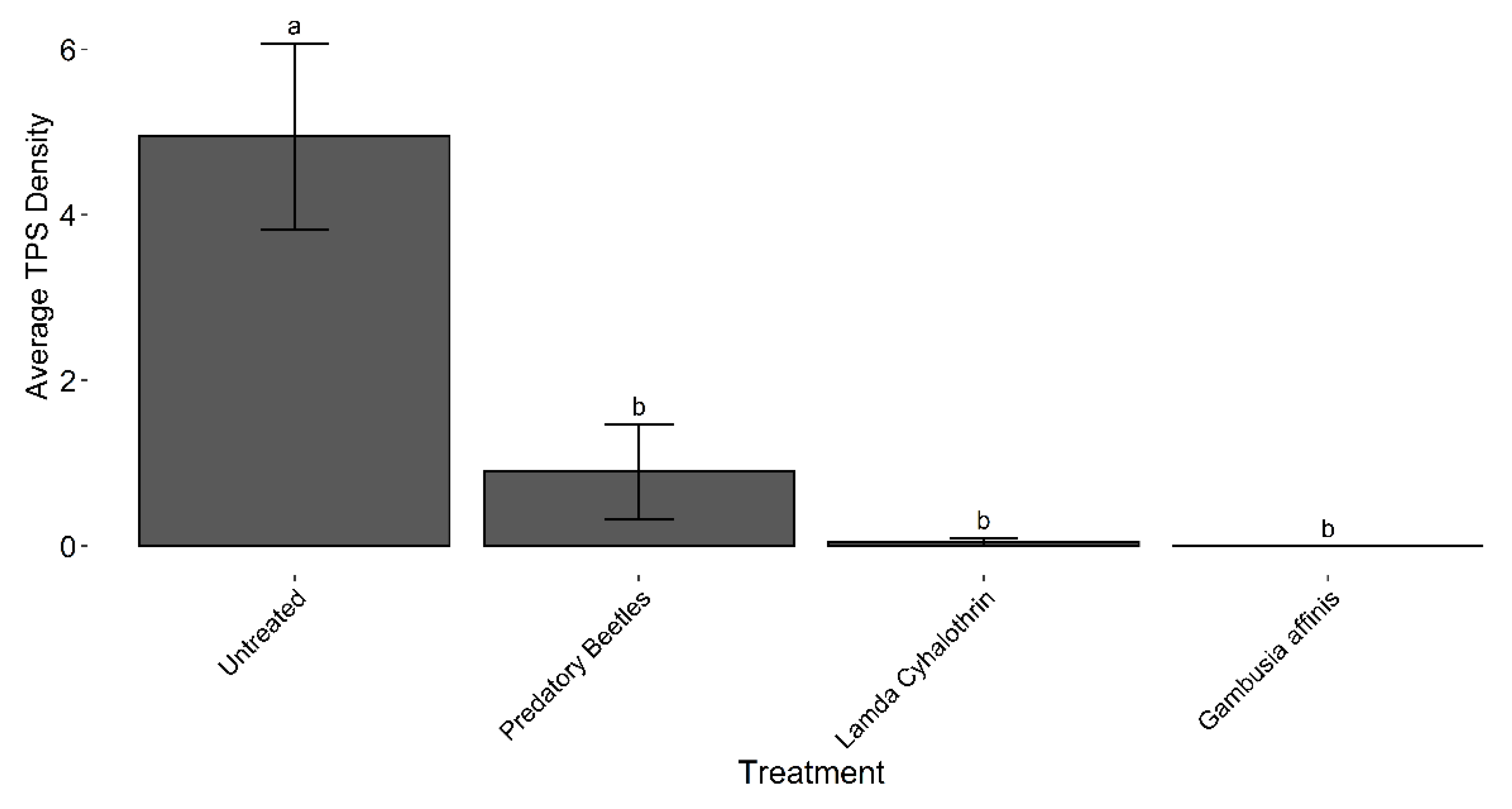
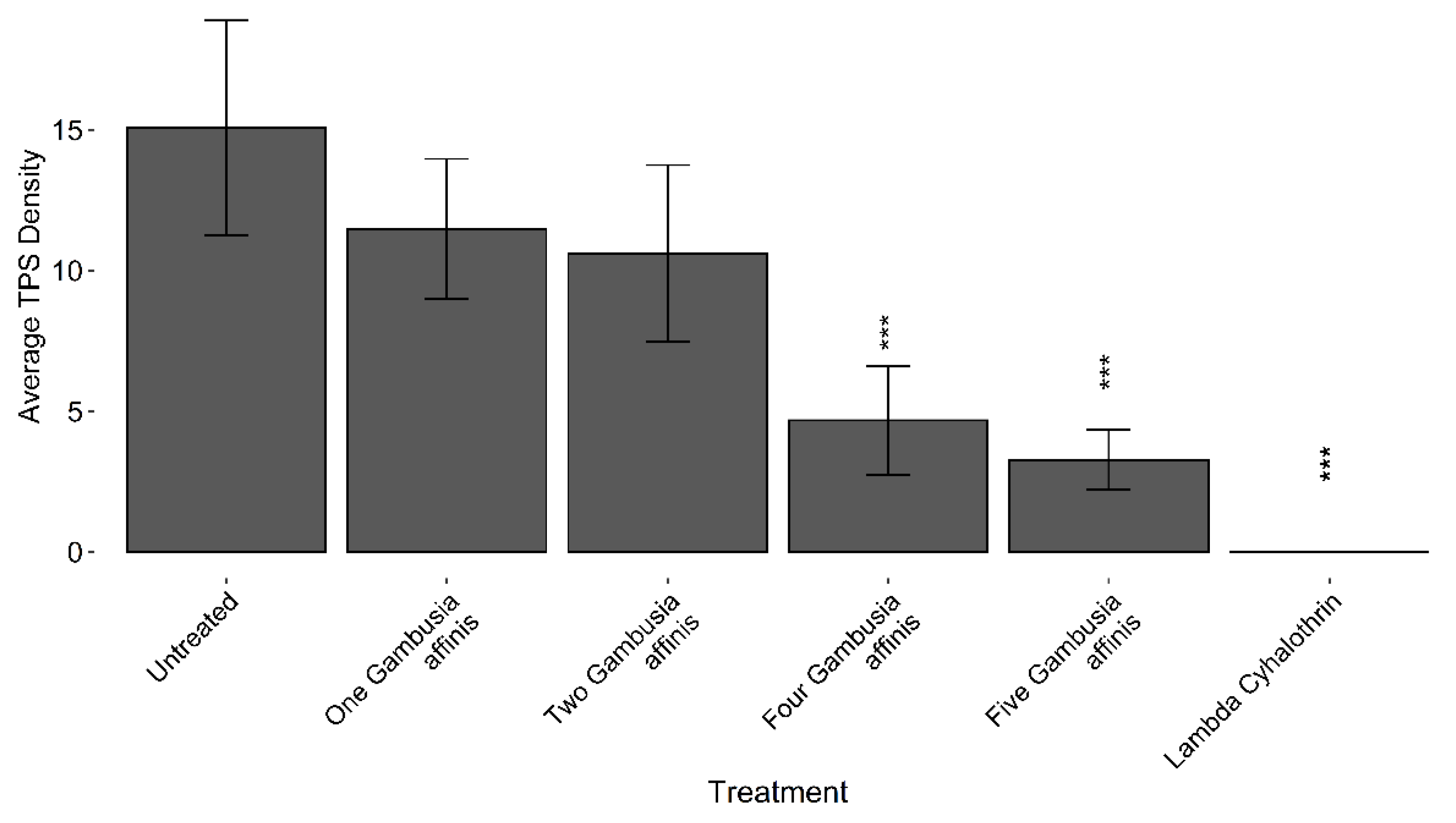
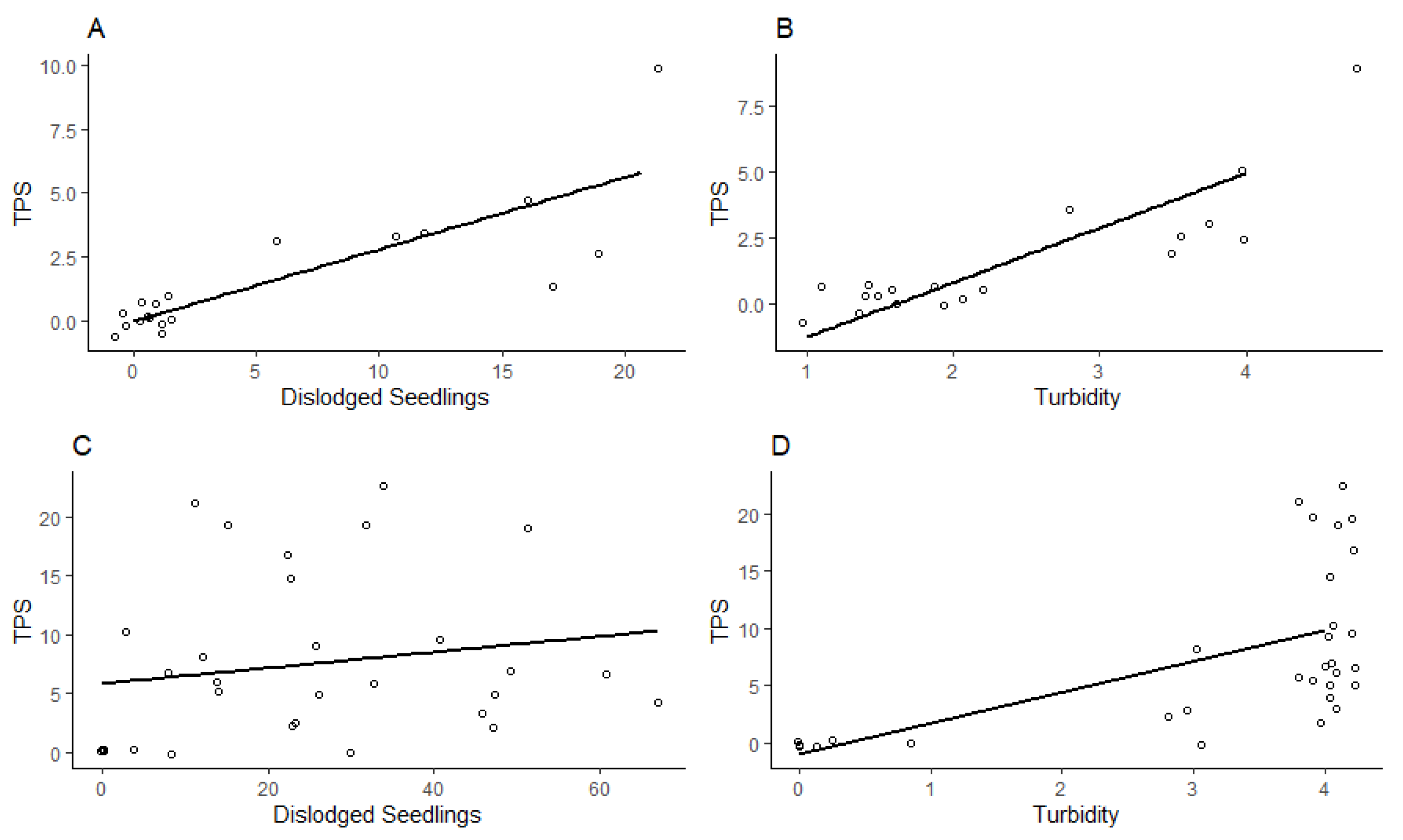
3. Results
4. Discussion
Considerations for the Use of Gambusia in Rice Paddies
Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linder, F. 1952. Contributions to the morphology and taxonomy of the Branchiopoda Notostraca with special reference to the North American species. Proceedings United States National Museum, 102:1-69.
- Longhurst, A.R. 1955. A review of the Notostraca. Bulletin of the British Museum of Zoology. 3(1):1-57.
- Scholnick, D.A. 1995. Sensitivity of metabolic rate, growth and fecundity of tadpole shrimp, Triops longicaudatus to environmental variation. Biology Bulletin, 189:22-28.
- Fry, L.L. , Mulla, M.S. 1996. Optimal conditions for rearing the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae), A biological control agent against mosquitoes. Journal of the American Mosquito Control Association, 12(3):446-453.
- Maffi, M. 1962. Triops granaries (Lucas) (Crustacea) as a natural enemy of mosquito larvae. Nature, (Lond.) 195:722.
- Tietze, N.S. , Mulla, M.S. 1989. Prey size selection by Triops longicaudatus (Notostraca: Triopsidae) feeding on immature stages of Culex quinquefasciatus. Journal of the American Mosquito Control Association. 5:392-396.
- Tietze, N.S. , Mulla, M.S. 1990. Influence of the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae), stocking rate of Culex tarsalis development in experimental field microcosms. Journal of the American Mosquito Control Association, 6:265–269.
- Tietze, N.S. , Mulla, M.S. 1991. Biological control of Culex mosquitoes (Diptera: Culicidae) by the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae). Journal of Medical Entomology, 28:24–31.
- Fry, L.L. , Mulla, M.S., Adams, C.W. 1994. Field introduction and establishment of the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae), a biological control agent of mosquitoes. Biological Control. 4:113–124.
- Amazon Inc. 2019. Toyops. https://www.amazon.com/Toyops-HG24TRI-Triops-Hanging-Kit/dp/B003L2CQBK/ref=sr_1_3?keywords=Tadpole+shrimp&qid=1553536366&s=hpc&sr=8-3/ (Accessed 25 March 2019).
- Grigarick, A.A. , Lange, W.H., Finfrock, D.C. 1961. Control of the tadpole shrimp, Triops longicaudatus, in California rice fields. Journal of Economic Entomology. 54:36–40.
- Rosenberg, L.E. 1947. Apus as a pest in California rice fields. California Department of Agriculture Bulletin. 36(20): 42–48.
- Grigarick, A.A. 1963. Rice plant injury: By invertebrate pests. California Agriculture, 17(8):6-7.
- Grigarick, A.A. , Lynch, J.H., Way J.O. 1985. Controlling tadpole shrimp. California Agriculture March-April pp 12-13. http://calag.ucanr.edu/archive/?type=pdf&article=ca.v039n03p12. (Accessed 05 March 2019). 05 March.
- Linquist, B. , Fischer, A., Godfrey, L.D., Greer, C., Hill, J., Koffler, K., Moeching, M., Mutters, R., van Kessel, C. 2008. Minimum tillage could benefit California rice farmers. California Agriculture. 62(1):24–29.
- UCCE. (2015). University of California Cooperative Extension (UCCE) Rice Production Manual. http://rice.ucanr.edu/Stand_Establishment/ (accessed 23 Febuary 2019).
- UCCE. (2018). University of California Cooperative Extension (UCCE) Rice Production Manual. http://rice.ucanr.edu/Stand_Establishment/ (accessed 23 Febuary 2019).
- Takahashi, F. 1977. Pioneer life of tadpole shrimp, Triops spp. (Notostraca:Triopsidea). Applied Entomological Zoology, 12 (2): 104-117.
- Linquist, B. , Koffler, K., Hill, J., van Kessel, C. 2011. Rice field drainage affects nitrogen dynamics and management. California Agriculture. 65(2):80-84.
- Bird, J. A, Eagle, A.J., Horwath, W.R., Hair., M.W., Zilbert, E.E., van Kessel C. 2002. Long-term studies find benefits, challenges in alternative rice straw management. California Agriculture, 56(2): 69-75.
- Report to the Legislature. 2000. Progress Report of the Phase Down of Rice Straw Burning in the Sacramento Valley Air Basin. California Air Resources Board. California Department of Food and Agriculture. (Accessed 09 March 2019).
- ACC. 2012.American Commodity Company LCC. The Facts About California Rice Production, As reported in part from May, 2012, Environmental Sustainability Report prepared by the California Rice Commission. http://www.accrice.com/sustainability/the-facts-about-california-rice-production/ (accessed 10 January 2019).
- Van Soest, P.J. 2006. Rice straw, the role of silica and treatments to improve quality. Animal Feed Science and Technology. 130(3-4): 137-171.
- Blank, R. 2006. Spatial and temporal variation in the composition and biomass of algae present in selected California rice fields. Journal of Freshwater Ecology. 21, 649–656.
- DPR, Department of Pesticide Regulation. 2015. Summary of Pesticide Use Report Data. https://www.cdpr.ca.gov/docs/pur/pur15rep/15sum.htm#trendscom (accessed 05 November 2016).
- Espino, L. 2016. TPS Control Issues. University of California, Agriculture and Natural Resources (UCANR) UC Rice Blog, California Rice Production. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=21130/ (accessed 14 February 2019).
- Fischer, D. C. 1990. Rate of evolution in living fossils. In D.E.G. Briggs & P.R. Krauther (Eds) Paleobiology. London: Blackwell Scientific, pp. 152-159.
- Mantovani, B. , Cesar, M., Scanabissi, F. 2004. Molecular taxonomy and phylogeny of the ‘living fossil’ lineages Triops and Lepidurus (Branchiopoda: Notostraca) Academy of Science and Letter. Zoological scripta. 33: 367-374.
- Boix, D. , Sala, J., Gascón S., Brucet, S. 2006. Predation in a Temporary pond with special attention to the trophic role of Triops cancriformis (Crustacea: Branchiopoda: Notostraca) Hydrobiologia. 571: 341-353.
- Hengherr, S. , Heyer, A.G., Brümmer, F., Schill, R.O. 2011. Trehalose and Vitreous States: Desiccation Tolerance of Dormant Stages of the Crustaceans Triops and Daphnia. Journal of Physiological and biochemical zoology. 84(2): 147-153.
- Igarishi, K. 1970. Ecological studies on Triops longicaudatus (Notostraca) inhabiting Shonai District, Japan. Journal of Yamagata Agriculture. For Soc., 23:33-39.
- Scott, S. R, Grigarick, A.A. 1979. Laboratory studies of factors affecting egg hatch of Triops longicaudatus (LeConte) (Notostraca: Triopsidae). Hydrobiologia, 63(2):145-152.
- Fry, L.L. , Mulla, M.S. 1992. Effect of drying period and soil moisture on egg hatch of the tadpole shrimp (Notostraca: Triopsidae). Journal of Economic Entomology. 85(1):65-69.
- Davis, J.M. , Madison, D. 2000. The Ontogeny of Light-Dark Response in Triops longicaudatus as a Response to Changing Selective Pressures. Crustaceana, 73(3): 283-288.
- California Mosquito & Vector Control. 1991. Controlling Mosquitoes with Mosquito Fish. Clarkcountynv.gov. Chromeextension://efaidnbmnnnibpcajpcglclefindmkaj/https://webfiles.clarkcountynv.gov//Public%20Works/Vector/Controlling%20Mosquitoes%20with%20Mosquito%20Fish.pdf, (Accessed March 2, 2021).
- Pyke, Graham H. 2005. A review of the biology of Gambusia affinis and G. holbrooki. Reviews in Fish Biology and Fisheries, 15, 339–365.
- R.G. Otto.1973. Temperature tolerance of the mosquitofish, Gambusia affinis (Baird and Girard) J. Fish Biol. J. Fish Biol. 5, 575–585.
- S.C. Stearns R.D. Sage. 1980. Maladaption in a marginal population of the mosquito fish. Gambusia affinis Evolution, 34 65–75.
- Miura, T. , Takahashi, R.M., Wilder, W.H. 1984. Impact of the mosquito fish (Gambusia affinis) on a rice field ecosystem when used as a mosquito control agent. Mosquito News, 44(4): 510-517.
- Hoy, J. B. , Reed, D. E. 1970. Biological control of Culex tarsalis in a California rice field. Mosquito News. 30:222-230.
- Hoy, J. B. , Reed, D. E. 1971. The efficacy of mosquitofish for control of Culex tarsalis in California rice fields. Mosquito News, 31:567-572.
- Farley, D. G. , Younce, L.C. 1977. Stocking date versus efficacy of Gambusia affinis in Fresno County rice fields. Proc. California Mosquito & Vector Control Association. 45:83-86.
- Bence, J. R. , Murdoch, W.W. 1986. Prey size selection by the mosquitofish Gambusia affinis relation to optimal diet theory. Ecology, 67(2):324-336.
- Wurtsbaugh, W. , Cech J.J., Compton, J. 1980. Effect of fish size on prey size selection in Gambusia affinis. Proceedings from California Mosquito & Vector Control Association, 48:48-51.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




