Submitted:
19 April 2023
Posted:
19 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
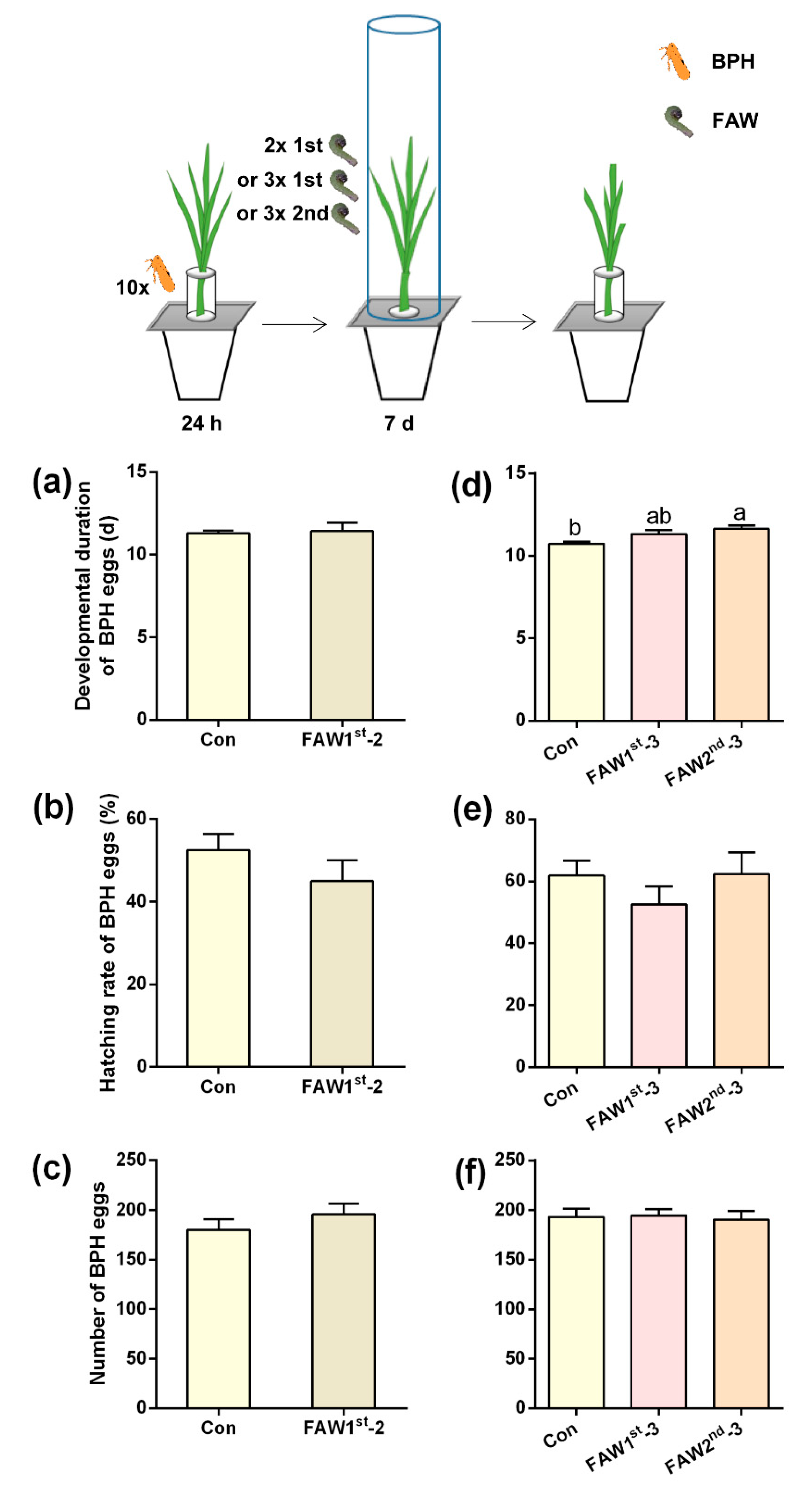
2.1. Effects of FAW larvae-infested rice plants on BPH performance
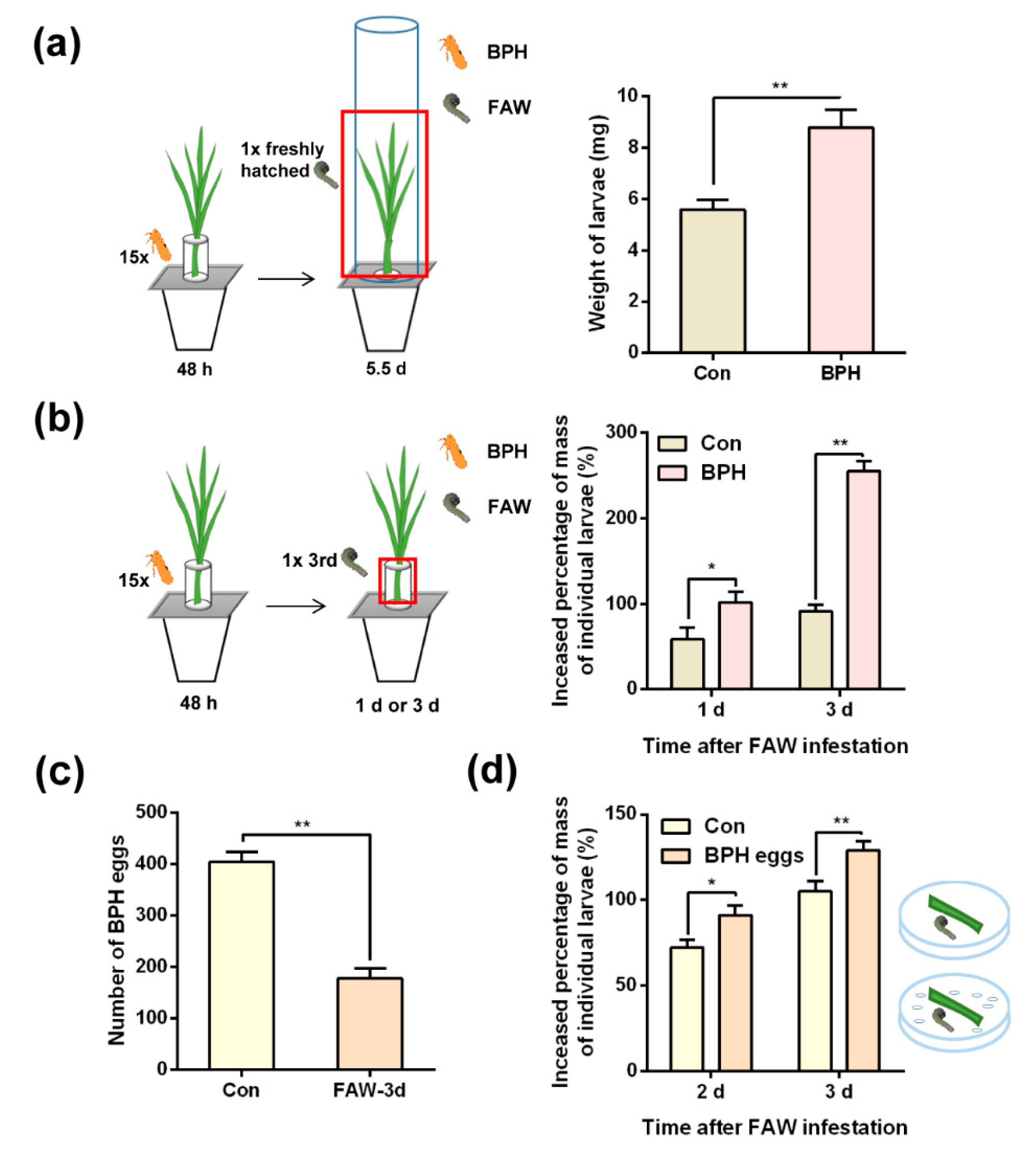
2.2. Effects of BPH-infested rice plants on performance of FAW larvae
2.3. FAW larvae infestation does not influence the attractiveness of BPH-infested plants to the parasitoid A. nilaparvatae
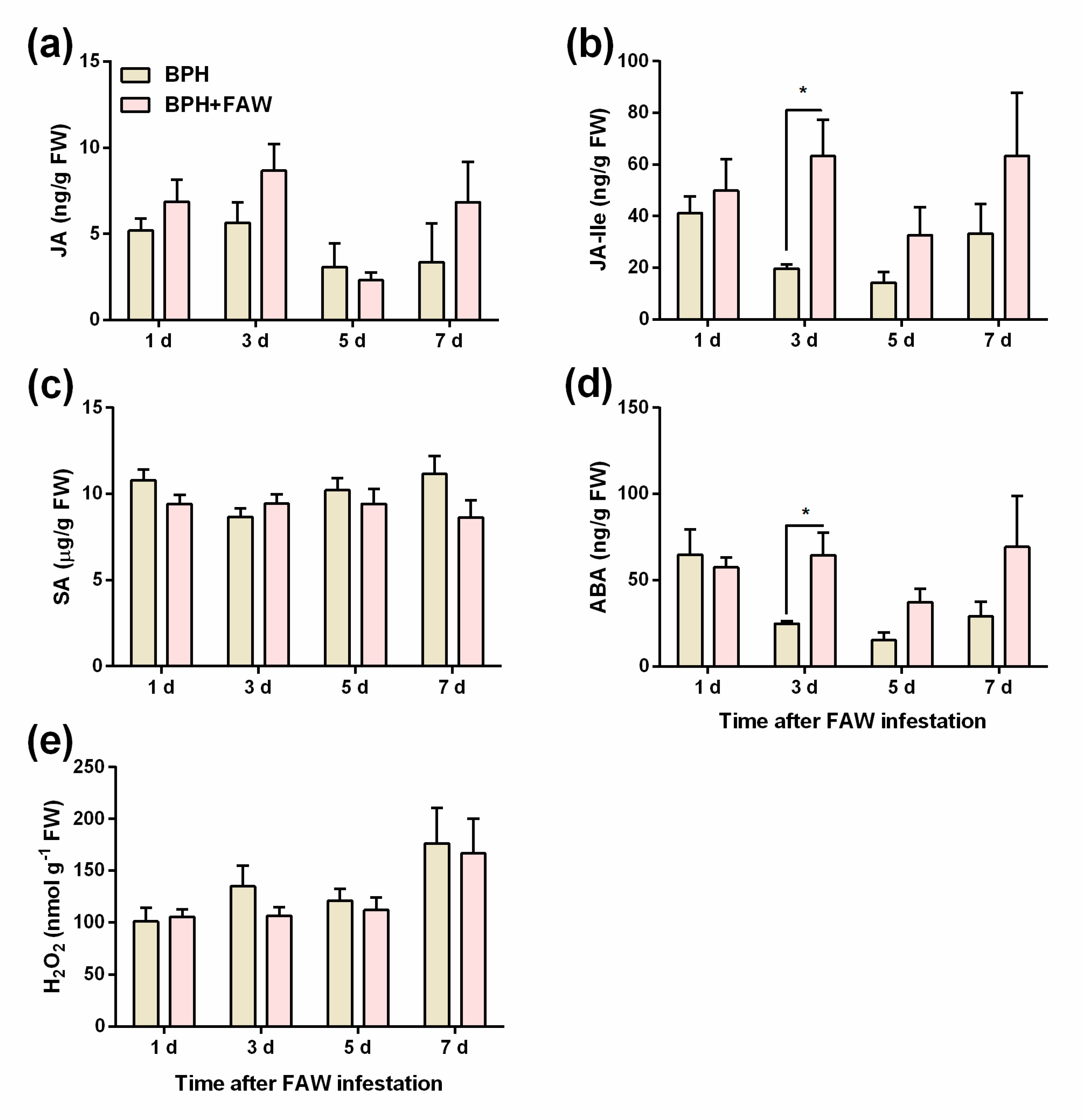
2.4. FAW larvae infestation enhances BPH-induced levels of JA-Ile and ABA but not levels of JA, SA and H2O2 in rice
2.5. FAW infestation influences BPH-induced levels of phenolamides and flavonoids in rice
3. Discussion
4. Materials and Methods
4.1. Plants and insects
4.2. Plant treatment
4.3. Bioassays
4.3.1. Effects of FAW larvae-infested rice plants on BPH performance
4.3.2. Effects of BPH-infested rice plants on the performance of FAW larvae
4.3.3. Effect of FAW larvae infestation on the attractiveness of BPH-infested plants to the parasitoid A. nilaparvatae
4.4. Analysis of JA, JA-Ile, ABA, and SA Levels
4.5. Hydrogen peroxide analysis
4.6. Analysis of phenolamides and flavonoids
4.7. Data analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Seo, P. J. Ca2+talyzing initial responses to environmental stresses. Trends Plant Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S. I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Bio. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Howe, G. A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, T.; Vandam, N. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 2005, 20, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Erb, M.; Kaplan, I. Long distance root–shoot signalling in plant–insect community interactions. Trends Plant Sci. 2013, 18, 149–156. [Google Scholar] [CrossRef]
- Papadopoulou, G. V.; van Dam, N. M. Mechanisms and ecological implications of plant-mediated interactions between belowground and aboveground insect herbivores. Ecol. Res. 2017, 32, 13–26. [Google Scholar] [CrossRef]
- Bottrell, D. G.; Schoenly, K. G. Resurrecting the ghost of green revolutions past: the brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Hogenhout, S. A.; Ammar, E.; Whitfield, A. E.; Redinbaugh, M. G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Whitfield, A. E.; Falk, B. W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479-480, 278–289. [Google Scholar] [CrossRef]
- Hattori, M.; Sogawa, K. Oviposition behavior of the rice brown planthopper, Nilaparvata lugens (Stål), and its electronic monitoring. J. Insect Behav. 2002, 15, 283–293. [Google Scholar] [CrossRef]
- Sogawa, K. The rice brown planthopper - feeding physiology and host plant interactions. Annu. Rev. Entomol. 1982, 27, 49–73. [Google Scholar] [CrossRef]
- Muduli, L.; Pradhan, S. K.; Mishra, A.; Bastia, D. N.; Samal, K. C.; Agrawal, P. K.; Dash, M. Understanding brown planthopper resistance in rice: genetics, biochemical and molecular breeding approaches. Rice Science 2021, 28, 532–546. [Google Scholar] [CrossRef]
- Mishra, A.; Barik, S. R.; Pandit, E.; Yadav, S. S.; Das, S. R.; Pradhan, S. K. Genetics, mechanisms and deployment of brown planthopper resistance genes in rice. Crit. Rev. Plant Sci. 2022, 41, 91–127. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I. A.; Pieterse, C. M. J.; Van Wees, S. C. M. Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Z.; Meng, J.; Zhou, P.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, B.; Cheng, J. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]
- Montezano, D. G.; Specht, A.; Sosa-Gómez, D. R.; Roque-Specht, V. F.; Sousa-Silva, J. C.; Paula-Moraes, S. V.; Peterson, J. A.; Hunt, T. E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P. A.; Day, R.; Desneux, N.; Harrison, R. D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022. [Google Scholar] [CrossRef]
- Kumar, R. M.; Gadratagi, B.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Sparks, A. N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P. L.; Sankung, S. B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. Plos One 2016, 11, e165632. [Google Scholar] [CrossRef] [PubMed]
- Erik, S. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef]
- Yang, P.; Chang, X. The occurrence, influence, prevention and control strategies of Spodoptera frugiperda in Aisa and Africa. China Plant Protection 2019, 39, 88–90. [Google Scholar]
- Yang, X.; Liu, Y.; Luo, M.; Li, Y.; Wang, W.; Fei, W.; Hong, J. This is the first time that Spodoptera frugiperda has been found in Jiangcheng, Yunnan province. Yunnan Agriculture 2019, 72. [Google Scholar]
- Jiang, Y.; Liu, J.; Xie, M.; Li, Y. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Protection 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Jiang, Y.; Liu, J.; Wu, K.; Xiao, Y. Molecular characterization analysis of fall armyworm populations in China. Plant Protection 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Zhang, H. A preliminary report on rice seedling damage caused by Spodoptera frugiperda in Yunxiao grassland of Fujian province and biotype identification of Spodoptera frugiperda. China Plant Protection 2020, 20, 41–43. [Google Scholar]
- Yang, J.; Tao, Y.; Liu, Q.; Zheng, Z.; Zhou, H. In Wuxue, Hubei province, armyworm was found to harm rice seedlings. China Plant Protection 2020, 40, 44–45. [Google Scholar]
- Pechan, T.; Ye, L.; Chang, Y.; Mitra, A.; Lin, L.; Davis, F. M.; Williams, W. P.; Luthe, D. S. A unique 33-KD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other lepidoptera. Plant Cell 2000, 12, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. A.; Christensen, S. A.; Alborn, H. T.; Hiltpold, I. Detecting the conspecific: herbivory-induced olfactory cues in the fall armyworm (Lepidoptera: Noctuidae). Metabolites 2021, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Marti, G.; Villard, N.; Doyen, G. A.; Wolfender, J.; Turlings, T. C. J.; Erb, M. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011, 68, 901–911. [Google Scholar] [CrossRef]
- Bentivenha, J. P. F.; Baldin, E. L. L.; Montezano, D. G.; Hunt, T. E.; Paula-Moraes, S. V. Attack and defense movements involved in the interaction of Spodoptera frugiperda and Helicoverpa zea (Lepidoptera: Noctuidae). J. Pest Sci. 2017, 90, 433–445. [Google Scholar] [CrossRef]
- Mutua, J. M.; Mutyambai, D. M.; Asudi, G. O.; Khamis, F.; Niassy, S.; Jalloh, A. A.; Salifu, D.; Magara, H. J. O.; Calatayud, P.; Subramanian, S. Competitive plant-mediated and intraguild predation interactions of the invasive Spodoptera frugiperda and resident stemborers Busseola fusca and Chilo partellus in maize cropping systems in Kenya. Insects 2022, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Sokame, B. M.; Musyoka, B.; Mohammed, S. A.; Tamiru, A.; Bruce, A.; Anderson, P.; Karlsson Green, K.; Calatayud, P. Cannibalism and intraguild predation involved in the intra- and inter-specific interactions of the invasive fall armyworm, Spodoptera frugiperda, and lepidopteran maize stemborers. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Polis, G. A.; Holt, R. D. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Polis, G. A.; Myers, C. A.; Holt, R. D. The ecology and evolution of intraguild predation - potential competitors that eat each other. Annu. Rev. Ecol. Evol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Hua, H.; Ma, W. Comparative transcriptome analysis of defense response of rice to Nilaparvata lugens and Chilo suppressalis infestation. Int. J. Biol. Macromol. 2020, 163, 2270–2285. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Gobel, C.; Francis, F.; Haubruge, E.; Wathelet, J. P.; du Jardin, P.; Feussner, I.; Fauconnier, M. L. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef]
- Leitner, M.; Boland, W.; Mithöfer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Moran, P. J. P. J.; Thompson, G. A. G. A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Influence of infestation by herbivores with different feeding habits or treatment by β-glucosidase on levels of main defense-related signals in rice plants. Master, Zhejiang University, Hang Zhou, 2006; p. 31. [Google Scholar]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E. E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 2000, 12, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhu, L.; He, G. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Fox, L. R. Cannibalism in natural populations. Annu. Rev. Ecol. Evol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Polis, G. A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Evol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Bose, A. P. H. Parent–offspring cannibalism throughout the animal kingdom: a review of adaptive hypotheses. Biol. Rev. 2022, 97, 1868–1885. [Google Scholar] [CrossRef]
- Wise, D. H. Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu. Rev. Entomol. 2006, 51, 441–465. [Google Scholar] [CrossRef]
- Awmack, C. S.; Leather, S. R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Jimenez-Perez, A.; Wang, Q. Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J. Insect Behav. 2004, 17, 511–522. [Google Scholar] [CrossRef]
- Rhainds, M. Size-dependent realized fecundity in two lepidopteran capital breeders. Environ. Entomol. 2015, 44, 1193–1200. [Google Scholar] [CrossRef]
- Lou, Y.; Cheng, J. Simulation analysis on coordinated effects of rice varieties and Anagrus nilaparvatae Pang et Wang on brown planthopper. Nilaparvata lugens (Stål). Journal of Biomathematics 1999, 14, 470–478. [Google Scholar]
- Wu, J. Agricultural Entomology (Northern Version); China Agriculture Press: Beijing, 2003; p. 146. [Google Scholar]
- Xu, J.; Wang, X.; Zu, H.; Zeng, X.; Baldwin, I. T.; Lou, Y.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Wang, S.; Xie, P.; Liu, J. A key ABA hydrolase gene, OsABA8OX3 is involved in rice resistance to Nilaparvata lugens by affecting callose deposition. J. Asia-Pac. Entomol. 2019, 22, 625–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhou, S.; Lou, Y.; Lu, J. Silencing an E3 ubiquitin ligase gene OsJMJ715 enhances the resistance of rice to a piercing-sucking herbivore by activating ABA and JA signaling pathways. Int. J. Mol. Sci. 2021, 22, 13020. [Google Scholar] [CrossRef]
- Stevenson, P. C.; Kimmins, F. M.; Grayer, R. J.; Raveendranath, S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lugens. Entomol. Exp. Appl. 1996, 80, 246–249. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: a review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Alamgir, K. M.; Hojo, Y.; Christeller, J. T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I. T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. W.; Zhou, P. Y.; Mo, X. C.; Hu, L. F.; Jin, N.; Chen, X.; Yu, Z. X.; Meng, J. P.; Erb, M.; Shang, Z. C.; et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. P. Natl. Acad. Sci. Usa. 2020, 117, 12017–12028. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R. J.; Harborne, J. B.; Kimmins, F. M.; Stevenson, P. C.; Wijayagunasekera, H. N. P. Phenolics in rice phloem sap as sucking deterrents to the brown planthopper, Nilaparvata lugens. Acta Hortic. 1994, 381, 691–694. [Google Scholar] [CrossRef]
- Hao, P.; Feng, Y.; Zhou, Y.; Song, X.; Li, H.; Ma, Y.; Ye, C.; Yu, X. Schaftoside interacts with NlCDK1 protein: a mechanism of rice resistance to brown planthopper, Nilaparvata lugens. Front. Plant Sci. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Chalmers, J. A.; Raj, S.; Thaler, J. S. Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 2005, 143, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. J.; Zheng, S. J.; van Loon, J. J.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proc Natl Acad Sci U S A 2009, 106, 21202–21207. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Su, S.; Liu, Q.; Jiao, Y.; Peng, Y.; Li, Y.; Turlings, T. C. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. Elife 2020, 9, e55421. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J. S.; Köllner, T. G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef]
- Erb, M.; Foresti, N.; Turlings, T. C. A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Biol. 2010, 10, 247. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, PH, USA, 1976. [Google Scholar]
- Vattikuti, J.; Sailaja, V.; Prasad, Y. G.; Katti, G. R.; Chirutkar, P. M.; Rao, G. R.; Padmakumari, A. P.; Padmavathi, C.; Prabhakar, M. Temperature driven development of the rice brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). J. Agrometeorol. 2019, 21, 131–140. [Google Scholar] [CrossRef]
- Lu, J.; Robert, C. A. M.; Riemann, M.; Cosme, M.; Mène-Saffrané, L.; Massana, J.; Stout, M. J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef]
- De Ascensao, A. R. F. D.; Dubery, I. A. Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 2003, 63, 679–686. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




