Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. Methods
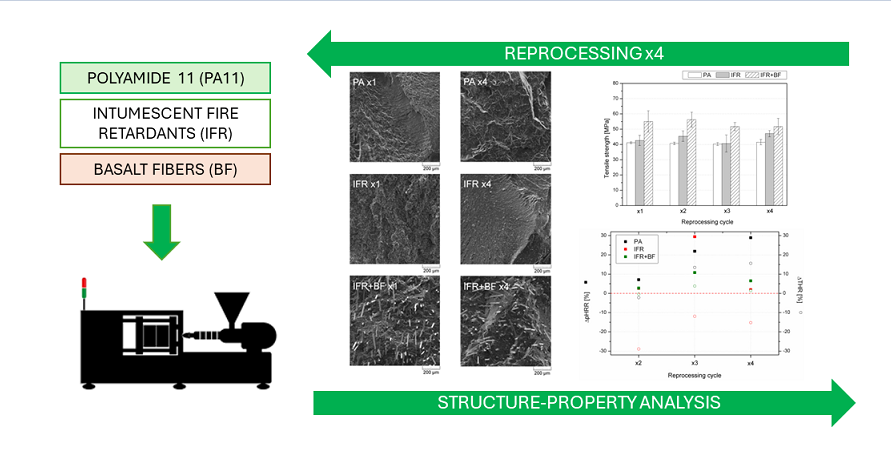
3. Results and Discussions
3.1. Structure Analysis
3.2. Mechanical Properties
3.3. Thermomechanical Properties
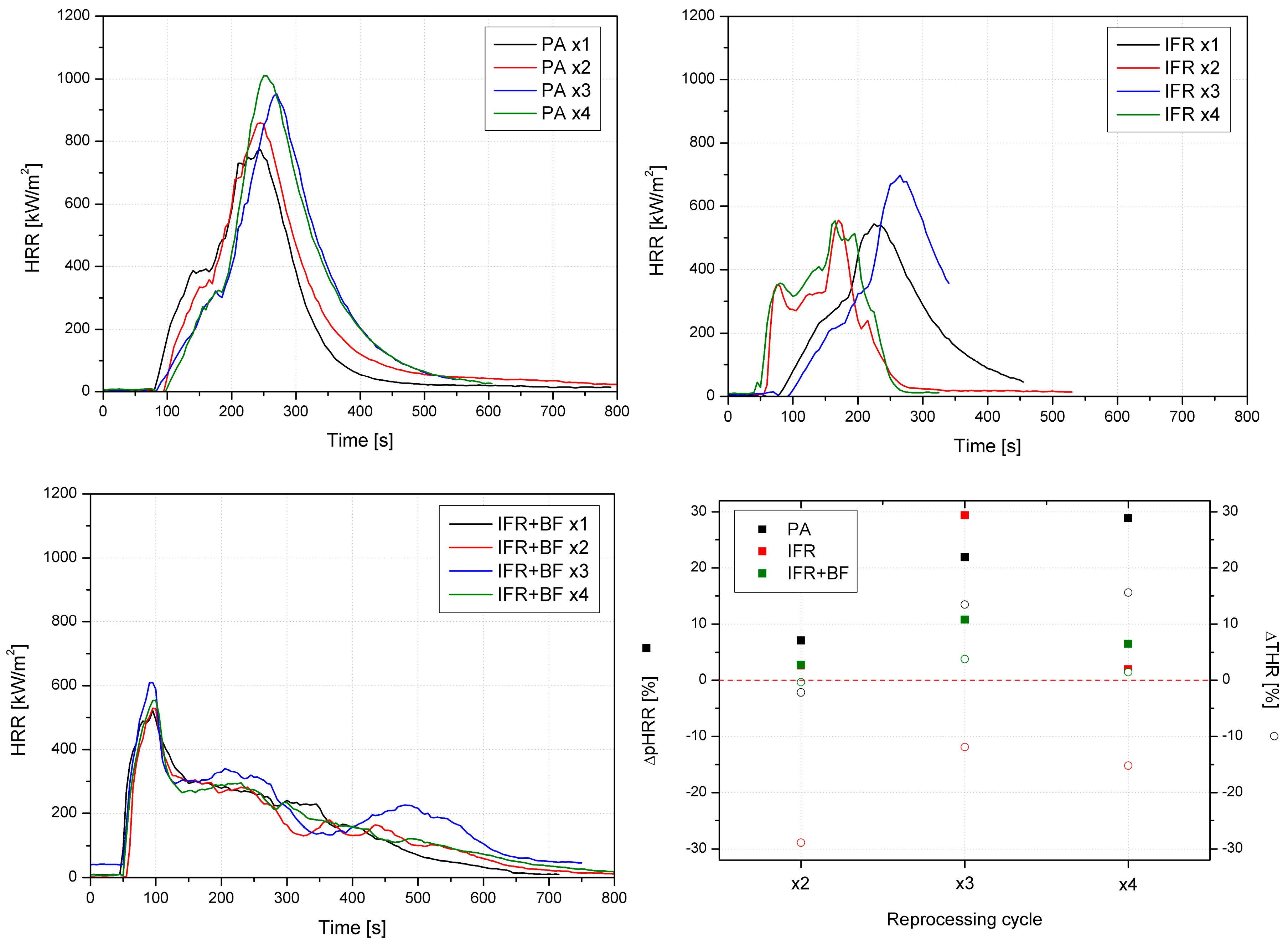
3.4. Fire Behavior
3.5. Thermal Stability and Analysis of Evolved Gaseous Products
| Material | T5% | T10% | T50% | Tonset | Tfinal | DTG peaks | Residue | ||||
| [°C] | [°C; %/min] | [%] | |||||||||
| PA x1 | 397 | 419 | 457 | 447 | - | 466 | - | 407; -0.046 |
458; -0.609 |
0.41 | |
| PA x4 | 397 | 421 | 459 | 441 | - | 475 | - | 407; -0.032 |
460; -0.307 |
0.34 | |
| IFR x1 | 300 | 323 | 395 | 309 | 353 | 412 | 184; -0.002 |
322; -0.057 |
406.; -0.437 |
3.49 | |
| IFR x4 | 301 | 323 | 392 | 306 | 346 | 408 | 186; -0.007 |
321; -0.107 |
392; -0.232 |
3.28 | |
| IFR BF x1 | 314 | 340 | 401 | 310 | 366 | 418 | - | 322; -0.044 |
397; -0.178 |
467.6; -0.015 |
21.81 |
| IFR+BF x4 | 310 | 336 | 402 | 297 | 354 | 415 | - | 325; -0.026 |
399; -0.176 |
464.8; -0.012 |
21.05 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capuano, R.; Bonadies, I.; Castaldo, R.; Cocca, M.; Gentile, G.; Protopapa, A.; Avolio, R.; Errico, M.E. Valorization and Mechanical Recycling of Heterogeneous Post-Consumer Polymer Waste through a Mechano-Chemical Process. Polymers (Basel). 2021, 13, 2783. [CrossRef]
- Kulkarni, A.; Quintens, G.; Pitet, L.M. Trends in Polyester Upcycling for Diversifying a Problematic Waste Stream. Macromolecules 2023, 56, 1747–1758. [CrossRef]
- Czarnecka-Komorowska, D.; Wiszumirska, K. Sustainability Design of Plastic Packaging for the Circular Economy. Polimery 2020, 65, 8–17. [CrossRef]
- Deopura, B.L.; Alagirusamy, R.; Joshi, M.; Gupta, B. Polyesters and Polyamides; Sawston;
- Gilbert, M. Aliphatic Polyamides. In Brydson’s Plastics Materials; Elsevier, 2017; pp. 487–511.
- Di Lorenzo, M.L.; Longo, A.; Androsch, R. Polyamide 11/Poly(Butylene Succinate) Bio-Based Polymer Blends. Materials (Basel). 2019, 12, 2833. [CrossRef]
- Jin, X.; Cui, S.; Sun, S.; Sun, J.; Zhang, S.; Tang, W.; Bourbigot, S. The Preparation of Polyamide 11 Composites with Extremely Long Ignition Time. Polym. Adv. Technol. 2022, 33, 1202–1210. [CrossRef]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of the Fire-Retardant, Ammonium Polyphosphate, on the Thermal Decomposition of Aliphatic Polyamides. I. Polyamides 11 and 12. Polym. Degrad. STable 1992, 36, 31–41. [CrossRef]
- Sahnoune, M.; Taguet, A.; Otazaghine, B.; Kaci, M.; Lopez-Cuesta, J. Fire Retardancy Effect of Phosphorus-modified Halloysite on Polyamide-11 Nanocomposites. Polym. Eng. Sci. 2019, 59, 526–534. [CrossRef]
- Correia, C.; Gomes, T.E.P.; Gonçalves, I.; Neto, V. Reprocessability of PLA through Chain Extension for Fused Filament Fabrication. J. Manuf. Mater. Process. 2022, 6, 26. [CrossRef]
- Sikorska, W.; Richert, J.; Rydz, J.; Musioł, M.; Adamus, G.; Janeczek, H.; Kowalczuk, M. Degradability Studies of Poly(l-Lactide) after Multi-Reprocessing Experiments in Extruder. Polym. Degrad. STable 2012, 97, 1891–1897. [CrossRef]
- Ben Amor, I.; Klinkova, O.; Baklouti, M.; Elleuch, R.; Tawfiq, I. Mechanical Recycling and Its Effects on the Physical and Mechanical Properties of Polyamides. Polymers (Basel). 2023, 15, 4561. [CrossRef]
- Paszkiewicz, S.; Walkowiak, K.; Irska, I.; Mechowska, S.; Stankiewicz, K.; Zubkiewicz, A.; Piesowicz, E.; Miadlicki, P. Influence of the Multiple Injection Moulding and Composting Time on the Properties of Selected Packaging and Furan-Based Polyesters. J. Polym. Environ. 2023, 31, 722–742. [CrossRef]
- Evens, T.; Bex, G.-J.; Yigit, M.; De Keyzer, J.; Desplentere, F.; Van Bael, A. The Influence of Mechanical Recycling on Properties in Injection Molding of Fiber-Reinforced Polypropylene. Int. Polym. Process. 2019, 34, 398–407. [CrossRef]
- MohammadKarimi, S.; Neitzel, B.; Lang, M.; Puch, F. Investigation of the Fiber Length and the Mechanical Properties of Waste Recycled from Continuous Glass Fiber-Reinforced Polypropylene. Recycling 2023, 8, 82. [CrossRef]
- Andrzejewski, J.; Barczewski, M.; Czarnecka-Komorowska, D.; Rydzkowski, T.; Gawdzińska, K.; Thakur, V.K. Manufacturing and Characterization of Sustainable and Recyclable Wood-Polypropylene Biocomposites: Multiprocessing-Properties-Structure Relationships. Ind. Crops Prod. 2024, 207, 117710. [CrossRef]
- Delva, L.; Hubo, S.; Cardon, L.; Ragaert, K. On the Role of Flame Retardants in Mechanical Recycling of Solid Plastic Waste. Waste Manag. 2018, 82, 198–206. [CrossRef]
- Zhang, S.; Wang, P.; Tan, L.; Huang, H.; Jiang, G. Relationship between Screw Structure and Properties of Recycled Glass Fiber Reinforced Flame Retardant Nylon 46. RSC Adv. 2015, 5, 13296–13306. [CrossRef]
- Davis, R.D.; Gilman, J.W.; VanderHart, D.L. Processing Degradation of Polyamide 6/Montmorillonite Clay Nanocomposites and Clay Organic Modifier. Polym. Degrad. STable 2003, 79, 111–121. [CrossRef]
- Deng, C.-L.; Du, S.-L.; Zhao, J.; Shen, Z.-Q.; Deng, C.; Wang, Y.-Z. An Intumescent Flame Retardant Polypropylene System with Simultaneously Improved Flame Retardancy and Water Resistance. Polym. Degrad. STable 2014, 108, 97–107. [CrossRef]
- Yan, H.; Zhao, Z.; Wang, Y.; Jin, Q.; Zhang, X. Structural Modification of Ammonium Polyphosphate by DOPO to Achieve High Water Resistance and Hydrophobicity. Powder Technol. 2017, 320, 14–21. [CrossRef]
- Sałasińska, K.; Celiński, M.; Mizera, K.; Barczewski, M.; Kozikowski, P.; Leszczyński, M.K.; Domańska, A. Moisture Resistance, Thermal Stability and Fire Behavior of Unsaturated Polyester Resin Modified with L-Histidinium Dihydrogen Phosphate-Phosphoric Acid. Molecules 2021, 26, 932. [CrossRef]
- Pandian, A.; Vairavan, M.; Jebbas Thangaiah, W.J.; Uthayakumar, M. Effect of Moisture Absorption Behavior on Mechanical Properties of Basalt Fibre Reinforced Polymer Matrix Composites. J. Compos. 2014, 2014, 1–8. [CrossRef]
- Davies, P.; Pomies, F.; Carlsson, L.A. Influence of Water Absorption on Transverse Tensile Properties and Shear Fracture Toughness of Glass/Polypropylene. J. Compos. Mater. 1996, 30, 1004–1019. [CrossRef]
- Mysiukiewicz, O.; Sałasińska, K.; Barczewski, M.; Celiński, M.; Skórczewska, K. Effect of Intumescent Flame Retardants on Non-isothermal Crystallization Behavior of High-density Polyethylene. Polym. Eng. Sci. 2022, 62, 2230–2242. [CrossRef]
- Song, J.; Liu, J.; Zhang, Y.; Chen, L.; Zhong, Y.; Yang, W. Basalt Fibre-Reinforced PA1012 Composites: Morphology, Mechanical Properties, Crystallization Behaviours, Structure and Water Contact Angle. J. Compos. Mater. 2015, 49, 415–424. [CrossRef]
- Patti, A.; Acierno, S.; Nele, L.; Graziosi, L.; Acierno, D. Sustainable Basalt Fibers vs. Traditional Glass Fibers: Comparative Study on Thermal Properties and Flow Behavior of Polyamide 66-Based Composites. ChemEngineering 2022, 6, 86. [CrossRef]
- Ichazo, M..; Albano, C.; González, J.; Perera, R.; Candal, M.. Polypropylene/Wood Flour Composites: Treatments and Properties. Compos. Struct. 2001, 54, 207–214. [CrossRef]
- Mrozek, K.; Chen, S. Selective Induction Heating to Eliminate the Fundamental Defects of Thin-walled Moldings Used in Electrical Industry. J. Appl. Polym. Sci. 2017, 134. [CrossRef]
- Zhang, Q.; Mo, Z.; Liu, S.; Zhang, H. Influence of Annealing on Structure of Nylon 11. Macromolecules 2000, 33, 5999–6005. [CrossRef]
- Park, S.D.; Todo, M.; Arakawa, K.; Koganemaru, M. Effect of Crystallinity and Loading-Rate on Mode I Fracture Behavior of Poly(Lactic Acid). Polymer (Guildf). 2006, 47, 1357–1363. [CrossRef]
- Fidecka, K.; Giacoboni, J.; Picconi, P.; Vago, R.; Licandro, E. Quantification of Amino Groups on Halloysite Surfaces Using the Fmoc-Method. RSC Adv. 2020, 10, 13944–13948. [CrossRef]
- Iorio, M.; Santarelli, M.L.; González-Gaitano, G.; González-Benito, J. Surface Modification and Characterization of Basalt Fibers as Potential Reinforcement of Concretes. Appl. Surf. Sci. 2018, 427, 1248–1256. [CrossRef]
- Gupta, V.B.; Mittal, R.K.; Sharma, P.K.; Mennig, G.; Wolters, J. Some Studies on Glass Fiber-reinforced Polypropylene. Part II: Mechanical Properties and Their Dependence on Fiber Length, Interfacial Adhesion, and Fiber Dispersion. Polym. Compos. 1989, 10, 16–27. [CrossRef]
- Pliquet, M.; Rapeaux, M.; Delange, F.; Bussiere, P.O.; Therias, S.; Gardette, J.L. Multiscale Analysis of the Thermal Degradation of Polyamide 6,6: Correlating Chemical Structure to Mechanical Properties. Polym. Degrad. STable 2021, 185, 109496. [CrossRef]
- Domingos, E.; Pereira, T.M.C.; Castro, E.V.R. de; Romão, W.; Sena, G.L. de; Guimarães, R.C.L. Monitorando a Degradação Da Poliamida 11 (PA-11) via Espectroscopia Na Região Do Infravermelho Médio Com Transformada de Fourier (FTIR). Polímeros 2012, 23, 37–41. [CrossRef]
- Bahrami, M.; Abenojar, J.; Martínez, M.A. Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties. Polymers (Basel). 2021, 13, 3553. [CrossRef]
- Roger, A.; Sallet, D.; Lemaire, J. Photochemistry of Aliphatic Polyamides. 4. Mechanisms of Photooxidation of Polyamides 6, 11, and 12 at Long Wavelengths. Macromolecules 1986, 19, 579–584. [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Molecular and Macromolecular Structure Changes in Polyamide 11 during Thermal Oxidation. Polym. Degrad. STable 2014, 108, 123–132. [CrossRef]
- Prabhakar, M.N.; Raghavendra, G.M.; Vijaykumar, B.V.D.; Patil, K.; Seo, J.; Jung-il, S. Synthesis of a Novel Compound Based on Chitosan and Ammonium Polyphosphate for Flame Retardancy Applications. Cellulose 2019, 26, 8801–8812. [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal Degradation Study of Intumescent Flame Retardants by TG and FTIR: Melamine Phosphate and Its Mixture with Pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [CrossRef]
- Chen, Y.; Wang, Q. Reaction of Melamine Phosphate with Pentaerythritol and Its Products for Flame Retardation of Polypropylene. Polym. Adv. Technol. 2007, 18, 587–600. [CrossRef]
- Wang, Q.; Ding, Y.; Randl, N. Investigation on the Alkali Resistance of Basalt Fiber and Its Textile in Different Alkaline Environments. Constr. Build. Mater. 2021, 272, 121670. [CrossRef]
- Najafi, S.K.; Tajvidi, M.; Chaharmahli, M. Long-term Water Uptake Behavior of Lignocellulosic-high Density Polyethylene Composites. J. Appl. Polym. Sci. 2006, 102, 3907–3911. [CrossRef]
- Fu, H.; Xu, H.; Liu, Y.; Yang, Z.; Kormakov, S.; Wu, D.; Sun, J. Overview of Injection Molding Technology for Processing Polymers and Their Composites. ES Mater. Manuf. 2020. [CrossRef]
- Wu, K.; Zhang, Y.; Hu, W.; Lian, J.; Hu, Y. Influence of Ammonium Polyphosphate Microencapsulation on Flame Retardancy, Thermal Degradation and Crystal Structure of Polypropylene Composite. Compos. Sci. Technol. 2013, 81, 17–23. [CrossRef]
- Xu, M.; Liu, H.; Ma, K.; Li, B.; Zhang, Z. New Strategy towards Flame Retardancy through Design, Synthesis, Characterization, and Fire Performance of a Chain Extender in Polyamide 6 Composites. Polym. Eng. Sci. 2019, 59. [CrossRef]
- Rusin-Żurek, K.; Kuciel, S.; Kurańska, M. The Effect of Funcionalized Ethylene-n-Octene Copolymer on Mechanical Properties of BioPET with Organic Waste Fillers. Polimery 2023, 68, 330–336. [CrossRef]
- Ortega, Z.; McCourt, M.; Romero, F.; Suárez, L.; Cunningham, E. Recent Developments in Inorganic Composites in Rotational Molding. Polymers (Basel). 2022, 14, 5260. [CrossRef]
- Czarnecka-Komorowska, D.; Bryll, K.; Kostecka, E.; Tomasik, M.; Piesowicz, E.; Gawdzińska, K. The Composting of PLA/HNT Biodegradable Composites as an Eco-Approach to the Sustainability. Bull. Polish Acad. Sci. Tech. Sci. 2021, 136720–136720. [CrossRef]
- Švehlová, V.; Polouček, E. About the Influence of Filler Particle Size on Toughness of Filled Polypropylene. Die Angew. Makromol. Chemie 1987, 153, 197–200. [CrossRef]
- Gupta, V.B.; Mittal, R.K.; Sharma, P.K.; Mennig, G.; Wolters, J. Some Studies on Glass Fiber-reinforced Polypropylene. Part I: Reduction in Fiber Length during Processing. Polym. Compos. 1989, 10, 8–15. [CrossRef]
- Prajapati, R.S.; Jain, S.; Shit, S.C. Development of Basalt Fiber-reinforced Thermoplastic Composites and Effect of PE-g-MA on Composites. Polym. Compos. 2017, 38, 2798–2805. [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Andrzejewski, J.; Matykiewicz, D.; Medycki, D.; Kloziński, A.; Skórczewska, K.; Szostak, M. Thermo-mechanical and Mechanical Behavior of Hybrid Isotactic Polypropylene Glass Fiber Reinforced Composites (GFRC) Modified with Calcium Carbonate (CaCO3). Polym. Eng. Sci. 2020, 60, 1588–1603. [CrossRef]
- Bazan, P.; Nosal, P.; Wierzbicka-Miernik, A.; Kuciel, S. A Novel Hybrid Composites Based on Biopolyamide 10.10 with Basalt/Aramid Fibers: Mechanical and Thermal Investigation. Compos. Part B Eng. 2021, 223, 109125. [CrossRef]
- Lv, Z.; Wang, K.; Qiao, Z.; Wang, W. The Influence of Modified Zeolites as Nucleating Agents on Crystallization Behavior and Mechanical Properties of Polypropylene. Mater. Des. 2010, 31, 3804–3809. [CrossRef]
- Tomaszewska, J.; Klapiszewski, Ł.; Skórczewska, K.; Szalaty, T.J.; Jesionowski, T. Advanced Organic-Inorganic Hybrid Fillers as Functional Additives for Poly(Vinyl Chloride). Polimery 2017, 62, 19–26. [CrossRef]
- Abdel Hakim, A.E. Improving the Vicat Softening Point of Poly (Vinyl Chloride) Mixtures through Blending with Different Polymers and Inorganic Fillers. Egypt. J. Chem. 2021, 0–0. [CrossRef]
- Vasanthkumar, P.; Balasundaram, R.; Senthilkumar, N.; Palanikumar, K.; Lenin, K.; Deepanraj, B. Thermal and Thermo-Mechanical Studies on Seashell Incorporated Nylon-6 Polymer Composites. J. Mater. Res. Technol. 2022, 21, 3154–3168. [CrossRef]
- Fornes, T.D.; Paul, D.R. Modeling Properties of Nylon 6/Clay Nanocomposites Using Composite Theories. Polymer (Guildf). 2003, 44, 4993–5013. [CrossRef]
- Cui, L.; Wang, P.; Zhang, Y.; Zhang, L.; Chen, Y.; Wang, L.; Liu, L.; Guo, X. Combined Effect of α-Nucleating Agents and Glass Fiber Reinforcement on a Polypropylene Composite: A Balanced Approach. RSC Adv. 2017, 7, 42783–42791. [CrossRef]
- Schartel, B.; Hull, T.R. Development of Fire-Retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [CrossRef]
- Barczewski, M.; Sałasińska, K.; Kloziński, A.; Skórczewska, K.; Szulc, J.; Piasecki, A. Application of the Basalt Powder as a Filler for Polypropylene Composites With Improved Thermo-Mechanical Stability and Reduced Flammability. Polym. Eng. Sci. 2019, 59. [CrossRef]
- Levchik, S. V. Introduction to Flame Retardancy and Polymer Flammability. In Flame Retardant Polymer Nanocomposites; Wiley, 2007; pp. 1–29.
- Xia, Y.; Jin, F.; Mao, Z.; Guan, Y.; Zheng, A. Effects of Ammonium Polyphosphate to Pentaerythritol Ratio on Composition and Properties of Carbonaceous Foam Deriving from Intumescent Flame-Retardant Polypropylene. Polym. Degrad. STable 2014, 107, 64–73. [CrossRef]
- Pagacz, J.; Leszczyńska, A.; Modesti, M.; Boaretti, C.; Roso, M.; Malka, I.; Pielichowski, K. Thermal Decomposition Studies of Bio-Resourced Polyamides by Thermogravimetry and Evolved Gas Analysis. Thermochim. Acta 2015, 612, 40–48. [CrossRef]
- Ferry, L.; Sonnier, R.; Lopez-Cuesta, J.-M.; Petigny, S.; Bert, C. Thermal Degradation and Flammability of Polyamide 11 Filled with Nanoboehmite. J. Therm. Anal. Calorim. 2017, 129, 1029–1037. [CrossRef]
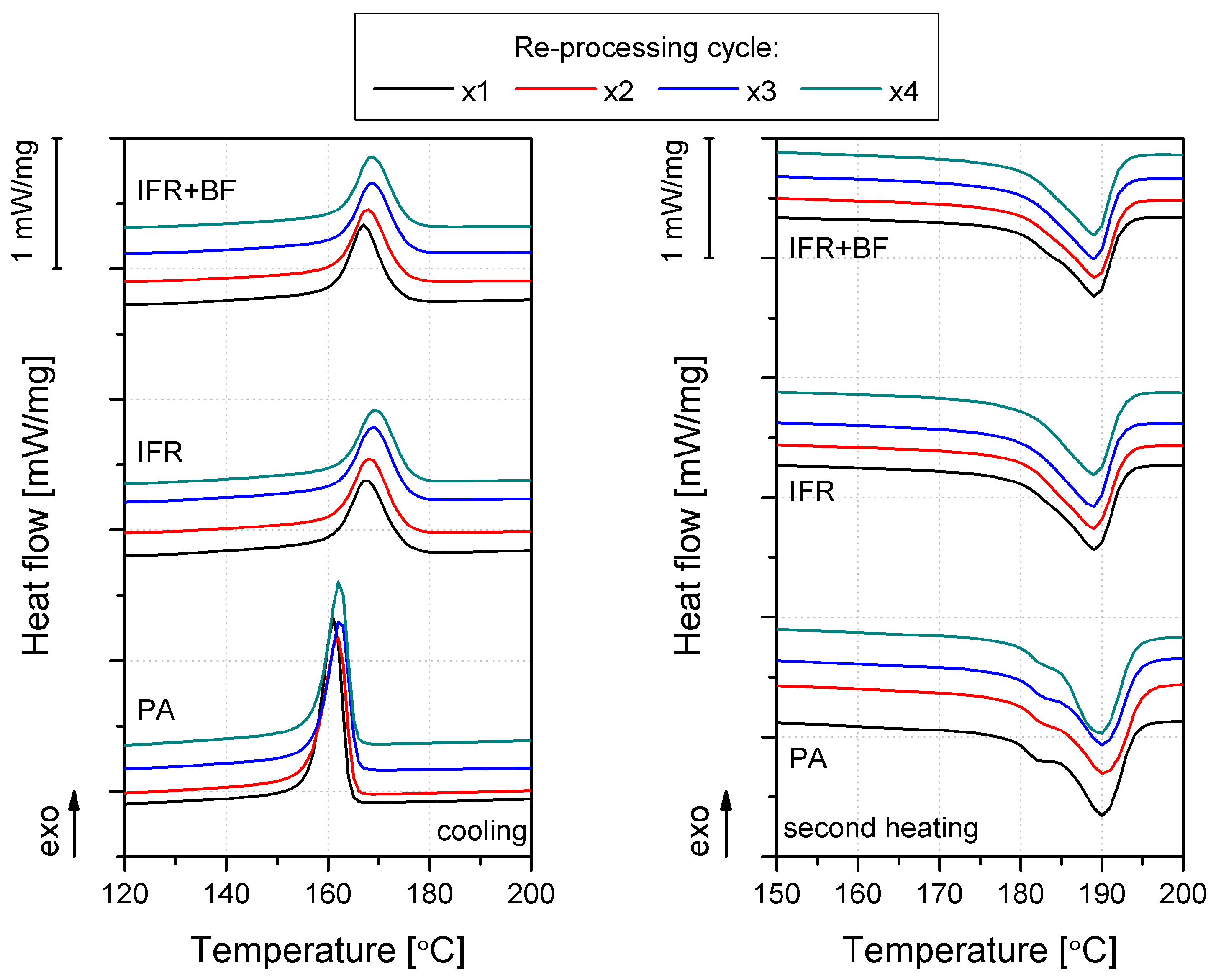
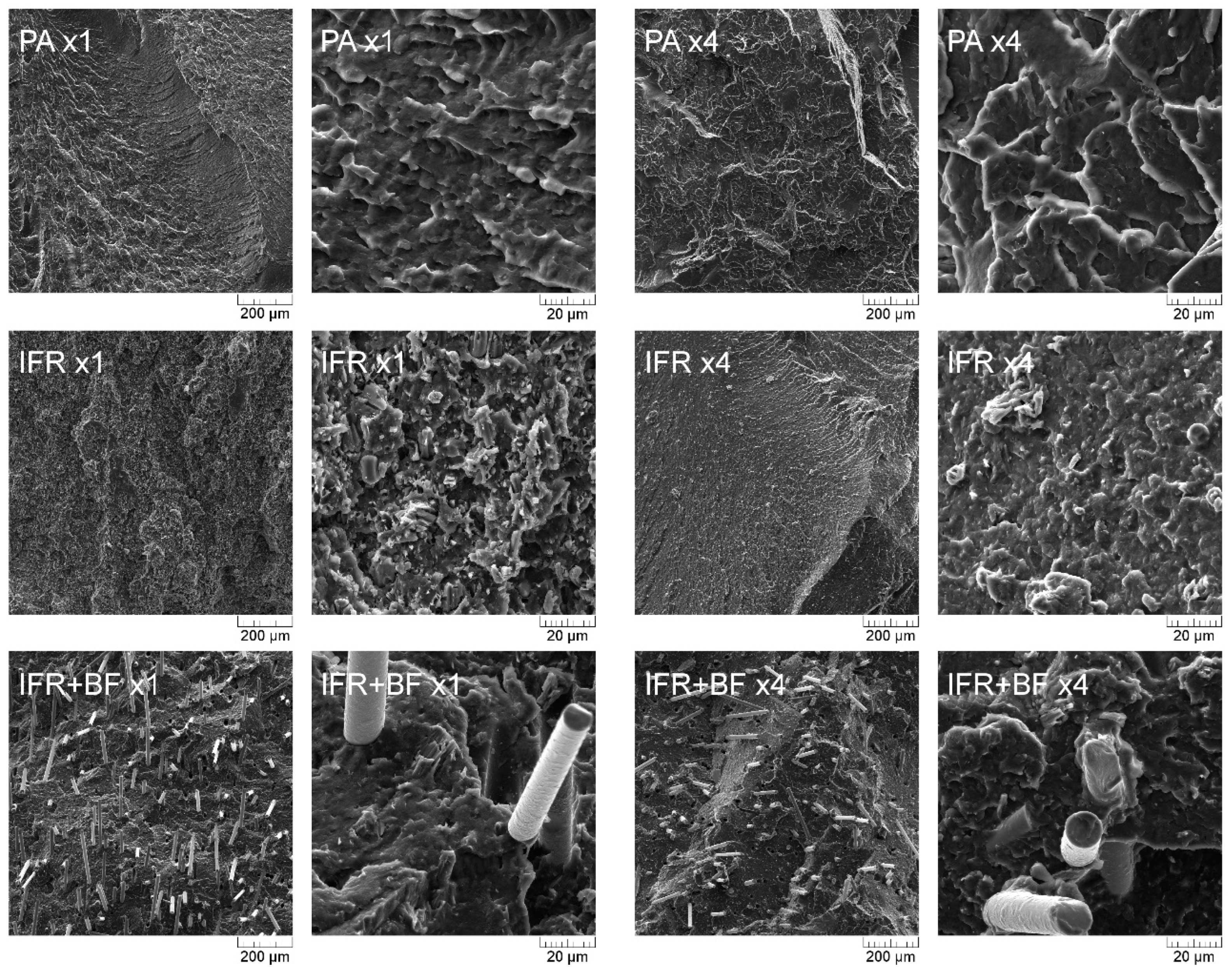

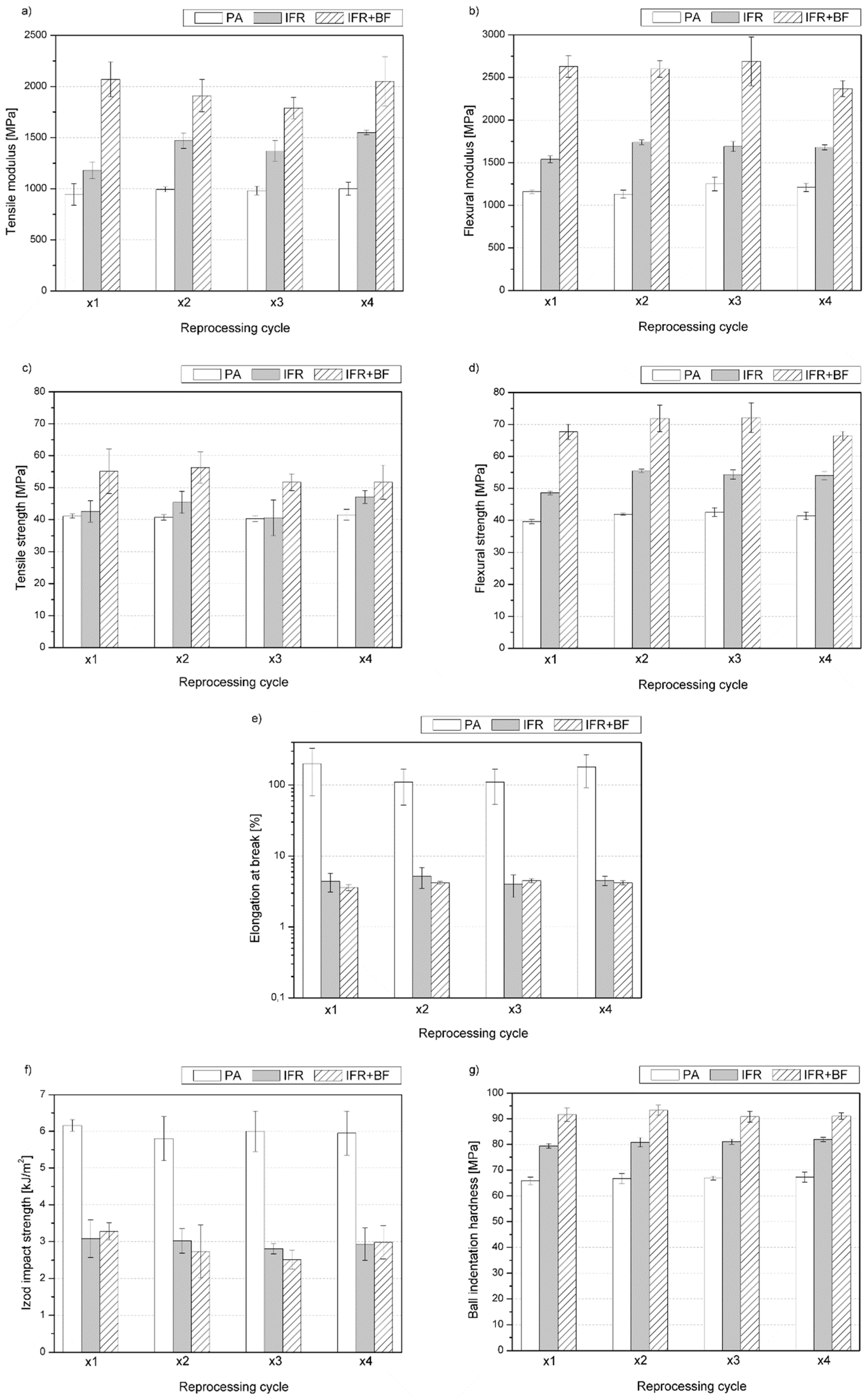
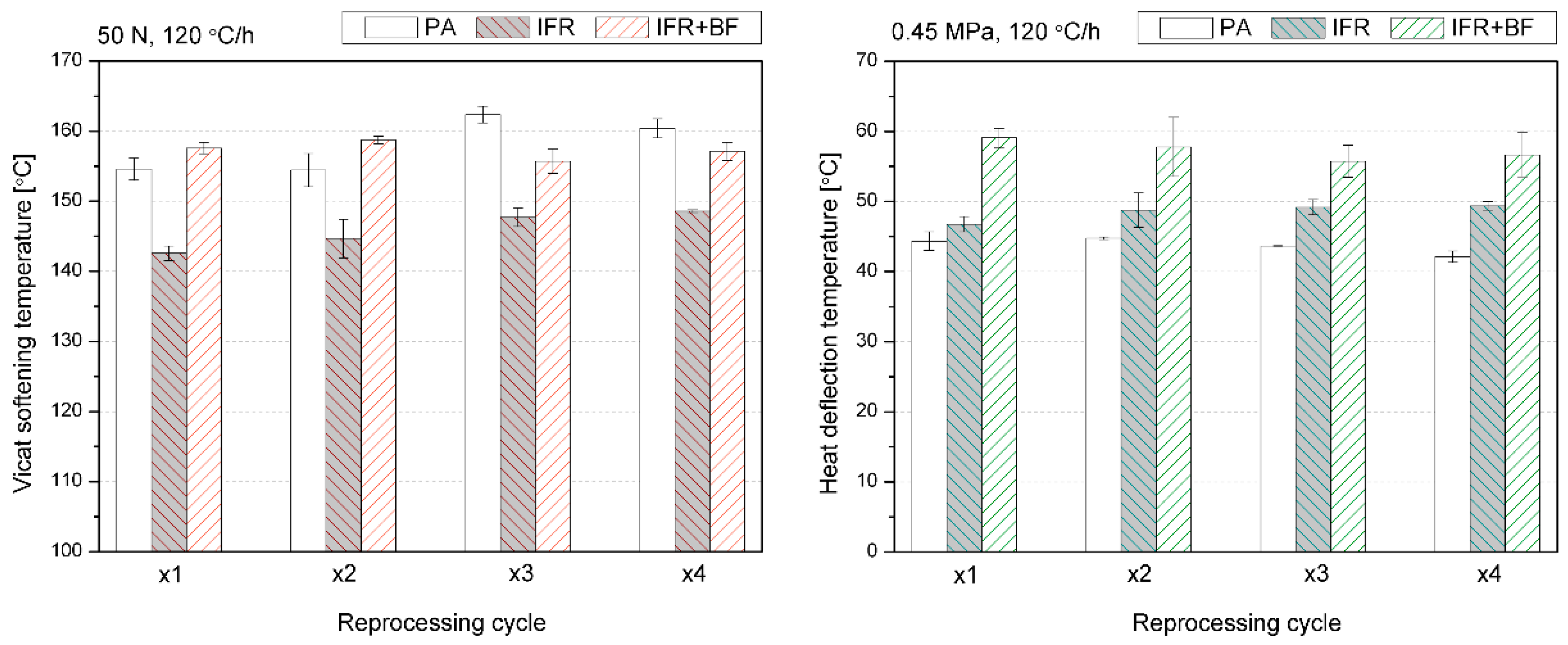
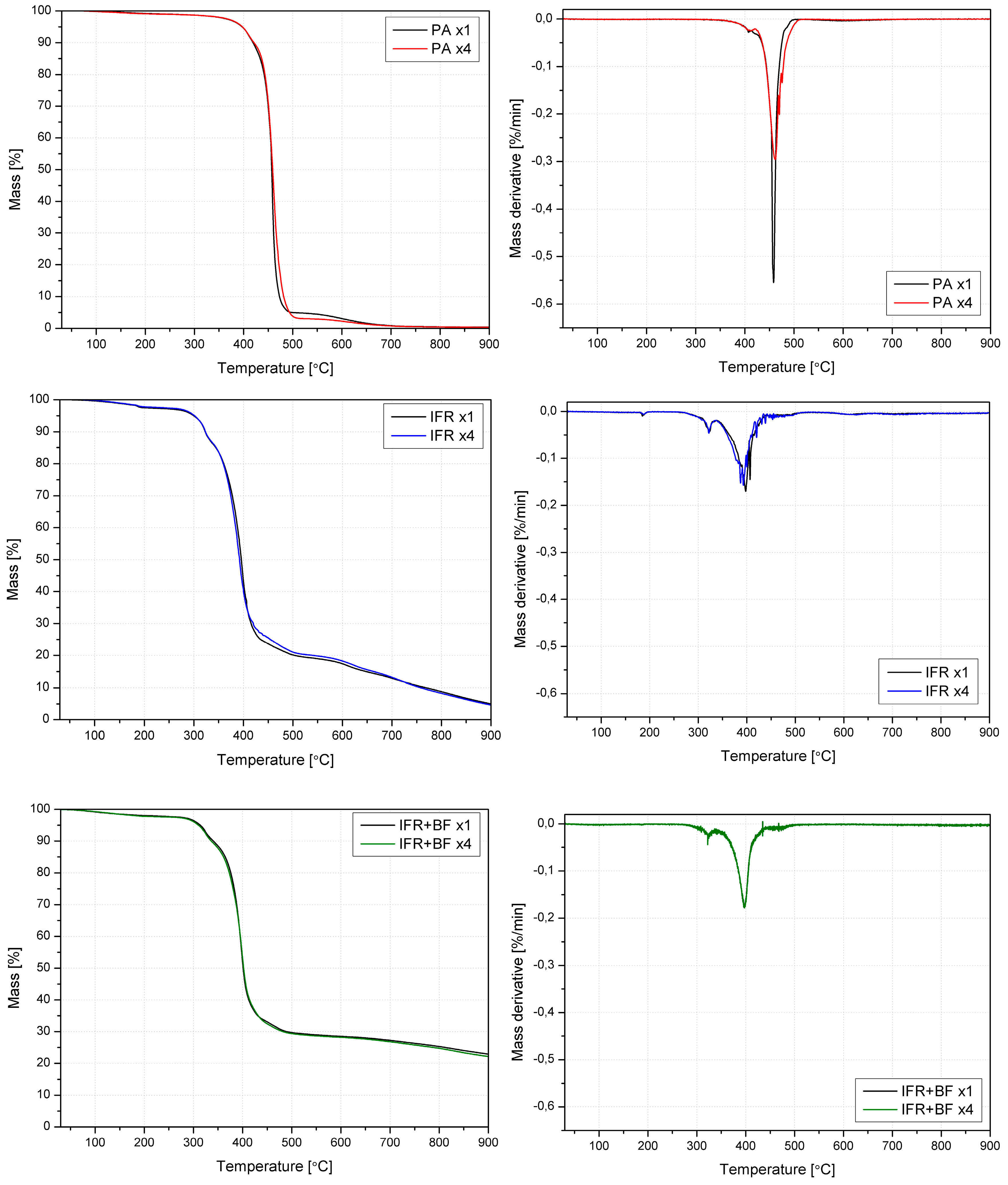
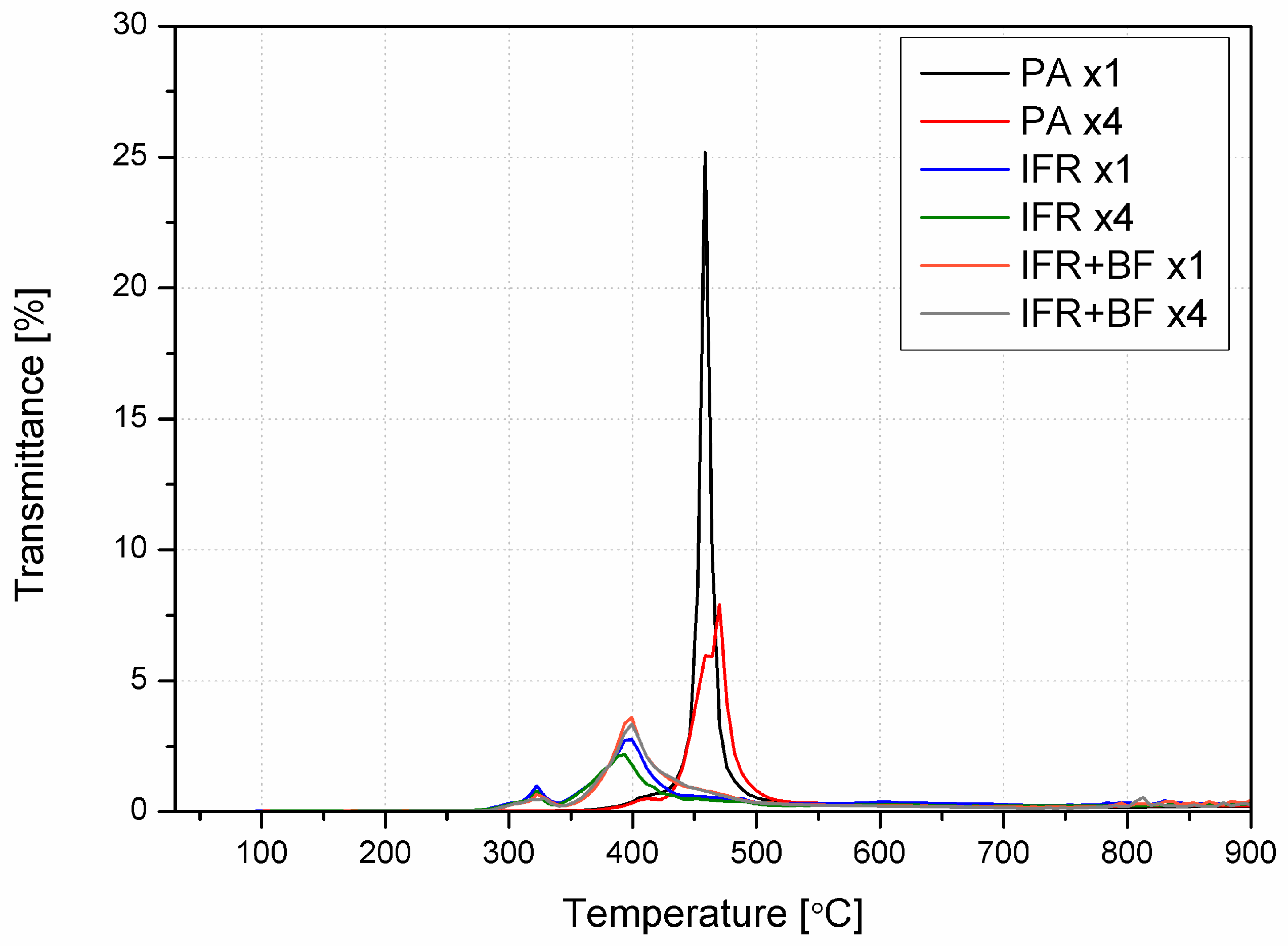
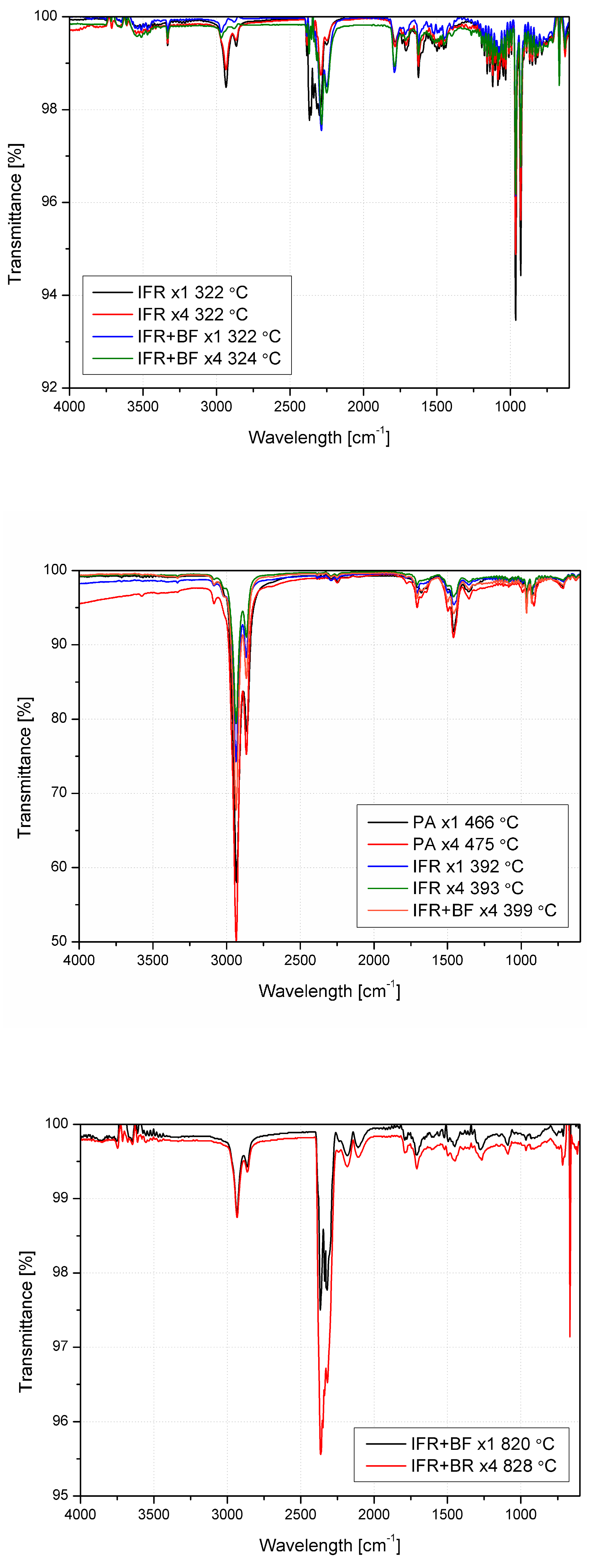
| Material | PA | IFR | IFR+BF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Processing cycle | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| TC [°C] | 161.1 | 161.8 | 162.3 | 162.5 | 167.5 | 168.1 | 168.8 | 169.5 | 166.9 | 167.8 | 168.6 | 169.7 |
| TM2 [°C] | 190.0 182.1 |
190.2 182.4 |
190.1 182.6 |
189.7 182.4 |
189.1 | 189.0 | 188.9 | 189.1 | 189.2 | 189.2 | 189.1 | 189.2 |
| Xc* [%] | 21.9 | 23.1 | 21.4 | 20.5 | 26.3 | 26.2 | 26.1 | 26.1 | 24.9 | 25.4 | 25.7 | 25.5 |
| * Xc =ΔHM2/(ΔHM100%*(1-Φ)), ΔHM2 – melting enthalpy; ΔHM100% for PA11 = 200 J/g [30], Φ – mass fraction of the filler. | ||||||||||||
| Material | TTI | pHRR | MARHE | THR | EHC | TSR |
| s | kW/m2 | kW/m2 | MJ/m2 | MJ/kg | m2/m2 | |
| PA x1 | 76 | 774 | 351 | 89 | 36 | 1247 |
| PA x2 | 74 | 829 | 327 | 130 | 29 | 1228 |
| PA x3 | 65 | 944 | 354 | 127 | 35 | 1443 |
| PA x4 | 70 | 998 | 362 | 147 | 37 | 1346 |
| IFR x1 | 46 | 529 | 224 | 150 | 20 | 669 |
| IFR x2 | 67 | 543 | 234 | 79 | 15 | 676 |
| IFR x3 | 67 | 685 | 232 | 56 | 25 | 676 |
| IFR x4 | 57 | 539 | 284 | 70 | 18 | 846 |
| IFR+BF x1 | 58 | 507 | 261 | 67 | 28 | 726 |
| IFR+BF x2 | 74 | 521 | 244 | 108 | 25 | 1709 |
| IFR+BF x3 | 58 | 562 | 245 | 112 | 28 | 1565 |
| IFR+BF x4 | 73 | 540 | 245 | 109 | 28 | 1699 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





