Introduction
Generally, ticks are divided into two large families, Ixodidae (hard ticks) and Argasidae (soft ticks) with various species and genera, are among the most critical obligate ectoparasites of animals, especially livestock and poultry [
1]. These blood-sucking ectoparasites as pathogenic vectors transmit bacteria, viruses, and protists to hosts, including animals and humans. These pathogens cause various diseases (e.g., bacterial diseases (Q fever, Lyme disease, borreliosis, relapsing fever, and borreliosis), fungal diseases (dermatophilosis), protozoal diseases (babesiosis and theileriosis), and rickettsial diseases (ehrlichiosis, Brazilian spotted fever, anaplasmosis, and Rocky Mountain spotted fever) [
2,
3]. Since sex ratio is a critical parameter determining the status and dynamics of animal populations, studies on sex ratio are vital for understanding the biology of populations and the biology of pathogens. Accordingly, arthropod vectors (e.g., ticks) sex ratio could play different roles in pathogen transmission [
4,
5].
Although ticks have been known since time immemorial, their importance in causing livestock troubles initiated in the mid-19th century; due to the world population increase and the nutritional needs, the number of livestock through the industry has increased rapidly. At the same time, concerns and issues related to ticks emerged [
6]. In 1814, piroplasmosis was diagnosed in cattle in the United States, and in 1821 it was discovered that the disease was transmitted to cattle by the ticks' bite called
Boophilus annulatus [
7]. In 1971, Mazloum in Iran conducted studies on the geographical distribution, seasonal activity, preferred ticks hosts, and diseases transmitted to livestock and humans [
8]. Pourmand et al. have also conducted a study to determine the frequency and species diversity of hard ticks and their sex ratio in equids in Sardasht suburb, West Azerbaijan Province, Iran. Their results indicate the presence of 85.48% male and 14.51% female ticks with the highest frequency of
Hyalomma anatolicum (67.74%),
H. marginatum (8.01%),
Rhipicephalus bursa (21.94%), and
Dermacentor marginatus (2.29%), respectively [
9]. Therefore, considering that tick bites are a manner of transmitting the disease to livestock and poultry, it seems that identifying the dominant ticks by host and sex ratio of ticks can be a practical way to oppose ticks and prevent transmitted diseases by them stop economic failures due to livestock losses. This study aimed to determine the sex and identify ticks in different hosts, including camels, sheep, cattle, dogs, chickens, and pigeons in Tehran province during 2019.
Materials and Methods
Geographical Area
This study was performed in 20 selected villages of Tehran province with an area of about 185.956 square kilometers, located between 34 to 5.36 degrees north latitude and 50 to 53 degrees east longitude.
Sampling Size and Method
The sample size was determined using the [
10] procedure, in which (d=0/045, p=0 /3, (1-p) = 0/7). Accordingly, 685 hard ticks and 121 soft ticks were collected from 1623 livestock and poultry.
Sample Collection and Identification
The feeding ticks on the animal body were separated from different parts such as earlobes, groin, tail base, and abdomen and transferred to particular cans using a pence. Then the characteristics of the animal (age, sex, livestock owner, and livestock code), village name, and date of tick collection, collector name, and the number of caught ticks were recorded on the can and transferred to a unique flask to maintain the humidity and temperature required by the ticks (
Figure 1). The ticks were then transferred to the laboratory for diagnosis and placed under a loop (stereomicroscope) to identify the sex and species using the valid diagnostic keys of the world and the region [
11].
Results
Identification and Distribution of Livestock Ticks by Sex
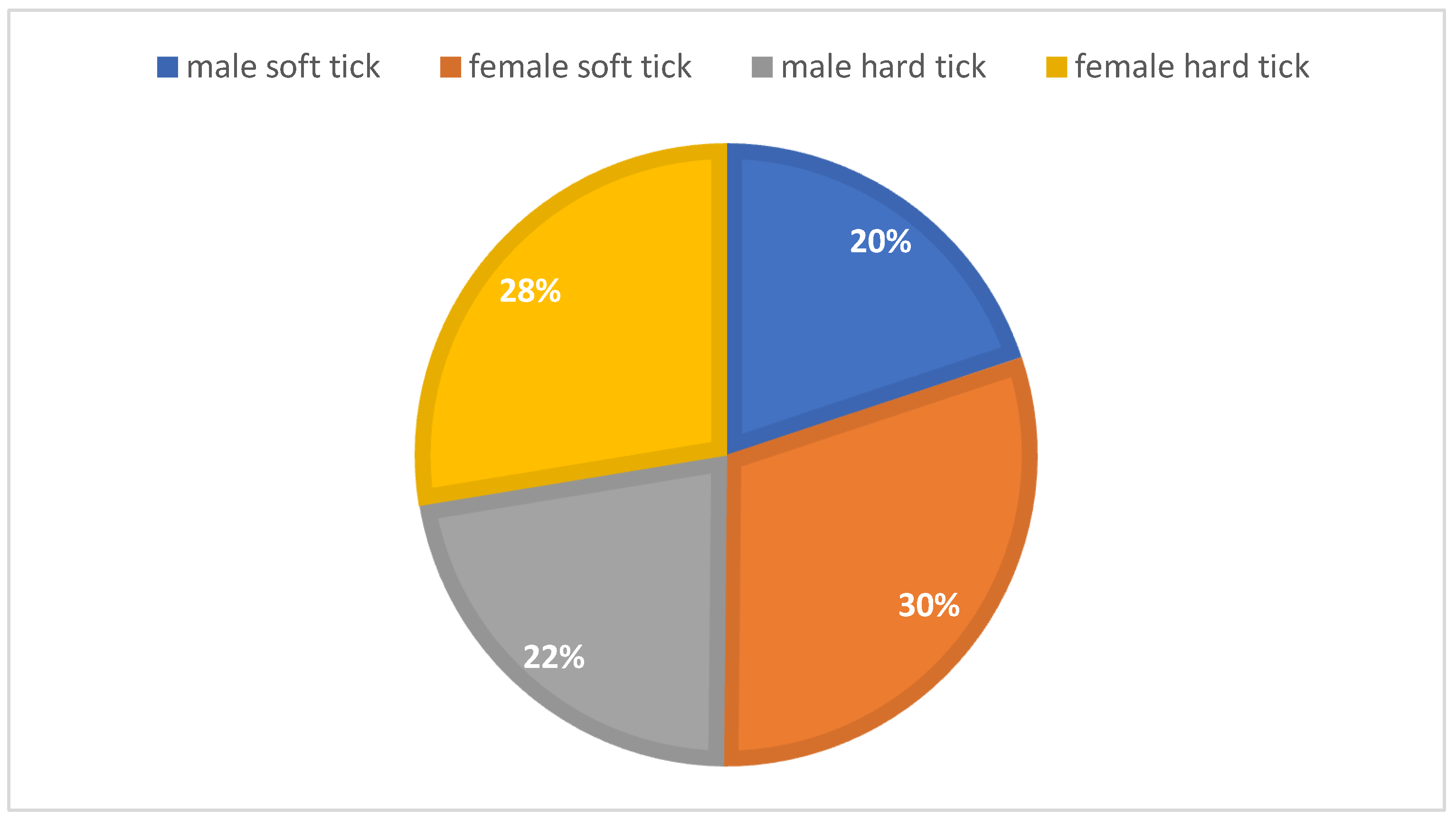
This study gathered 685 hard ticks and 121 soft ticks from a total of 1623 domestic animals, including camels, sheep, cows, dogs, chickens, and pigeons infected with ticks. Regarding sex segregation among all caught ticks, the results showed that 42.01% of ticks were male, 57.99% female, and 15.01% soft ticks (
Figure 2). In addition, among 685 hard-caught ticks (44.37%), 304 were male, and (55.62%) 381 were female. In both mountain and plain climates,
R. sanguineus has the highest sex ratio of hard ticks (
Table 1).
Identify and Determine the Distribution of Ticks by Host Type
Concerning tick-infested hosts, most ticks were collected from sheep with 60.04% and the lowest from cattle with 0.62%. Among the species of caught ticks, in the family of hard ticks, the genus
Boophilus was collected only from the cattle, the genus
Haemaphysalis from the sheep and goats, and the genera of
Ripisfalus and
Hyalomma were collected from all hosts (except pigeons, chickens, and corral wall). In the soft tick's family, the genus
Ornithodoros was collected only from the cage wall, and the genus
Argas was collected from both pigeon and chicken. Unlike soft ticks, no soft ticks were caught from cattle, sheep, goats, camels, and dogs. (
Table 2). The frequency of caught tick species by host type is such that in cattle, two species of
Hy. Marginatum and
B. annulatus were found in small quantities. In sheep,
R. sanguineus with 242 numerals had the highest, and
R. bursa with 5 numerals had the lowest amount.
R. sanguineus is found with the highest accumulation on the body of goats. In camels, the species
Hy. Marginatum had the highest, and
R. sanguineus had the lowest frequency. In dogs, only
R. sanguineus with 19 numbers was found. In pigeons, only
A. reflexus of the genus
Argas has been collected.
A. persicus was collected in significant abundance from the chicken body, and
O. lahorensis was found only from the corral wall (
Table 3).
Discussion
The current study results show that sex ratios observed in ticks differ among genera and even among host populations. Life-history aspects probably play an essential role, but survival analyses under natural conditions are lacking in practically all tick genera, which are crucial to elucidate general patterns. Prospective molecular methods will provide new routes to determine the vast spectrum of possible performers that affects sex ratios. Previous studies demonstrated that sex ratios could depend on the season and area of collection. Also, in arthropods (e.g., ticks), sex ratios could be skewed towards females by reproductive parasites that appertain to this gender for their transmission (transovarial) [
12,
13]. Our results showed that the highest rate of infected livestock with ticks related to sheep with 60.04%, and the lowest rate related to cattle with 0.62% because most studied livestock was sheep. The study in Ghaemshahr city demonstrated that the highest rate of infected livestock with ticks was observed in sheep with 28.3% and the lowest with 20% in cattle, which is compatible with our results [
14]. In addition, the dominant fauna species was
Rhipicephalus Sanguineus, which was consistent with the results of many previous researchers [
15,
16]. Therefore, in conclusion, the genus and species of dominant ticks in each region are diverse, and the geographical zone and climatic conditions of the area regulate the species and even the sex of active ticks in that province. Therefore, due to the high contamination of sheep, it is necessary that the authorities veterinary personnel and ranchers in the control and control programs against external livestock parasites (mites). At least twice a year (with a maximum interval of 30 days), in addition to corral pesticide spraying, bathing the animals in the anti-tick bath.
Contributions of the Authors
E.A. designed and collected the ticks, and identified tick species, recorded geographic coordinates and area information, wrote the manuscript, and confirmed and sent the articles.
Funding
This study received no grants from commercial, public, or nonprofit entities.
Data Availability Statements
All data obtained from this research are included in the article main text.
Acknowledgments
The authors thank Tehran province Veterinary Office and the Research Vice-chancellor of Shiraz University of Medical Sciences for their cooperation.
Competing Interests
The authors declare that they have no competing interests.
Consent to publication
The author fully consents to the publication of the article. The authors acknowledge the Tehran province Veterinary Office for help and cooperation
References
- Charrier, N.P.; Hermouet, A.; Hervet, C.; Agoulon, A.; Barker, S.C.; Heylen, D.; Toty, C.; McCoy, K.D.; Plantard, O.; Rispe, C. A transcriptome-based phylogenetic study of hard ticks (Ixodidae). Sci. Rep. 2019, 9, 1–13, . [CrossRef]
- Boulanger N, Boyer P, Talagrand-Reboul E, Hansmann Y. Ticks and tick-borne diseases. Medecine et maladies infectieuses. 2019;49(2):87-97.
- Abubakar M, Perera PK, Iqbal A, Manzoor S. Introductory Chapter: Ticks and Tick-Borne Pathogens. Ticks and Tick-Borne Pathogens: IntechOpen; 2018.
- Eberhart-Phillips, L.J.; Küpper, C.; Carmona-Isunza, M.C.; Vincze, O.; Zefania, S.; Cruz-López, M.; Kosztolányi, A.; Miller, T.E.X.; Barta, Z.; Cuthill, I.C.; et al. Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat. Commun. 2018, 9, 1–8, . [CrossRef]
- Van Oosten, A.R.; Duron, O.; Heylen, D.J. Sex ratios of the tick Ixodes arboricola are strongly female-biased, but there are no indications of sex-distorting bacteria. Ticks Tick-borne Dis. 2018, 9, 307–313, . [CrossRef]
- Bennett, R.J.; Baker, K.S. Looking Backward To Move Forward: the Utility of Sequencing Historical Bacterial Genomes. J. Clin. Microbiol. 2019, 57, . [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Eltaysh, R.; Igarashi, I. MMV020275 and MMV020490, promising compounds from malaria box for the treatment of equine piroplasmosis. Ticks Tick-borne Dis. 2022, 13, 101904, . [CrossRef]
- MAZLOUM Z. Differents Ticks occurring in Iran (geographical distribution, seasonal activities, hosts). 1971.
- Pourmand A, Malekifard F, Yakhvhali M. A survey of hard ticks (Acari: Ixodidae) infestation on equids in Sardasht suburb, Iran. Veterinary Researches & Biological Products. 2021;34(2):77-84.
- Hosseini-Vasoukolaei N, Oshaghi MA, Shayan P, Vatandoost H, Babamahmoudi F, Yaghoobi-Ershadi MR, et al. Anaplasma infection in ticks, livestock and human in Ghaemshahr, Mazandaran Province, Iran. Journal of arthropod-borne diseases. 2014;8(2):204.
- Jongejan, F.; Zivkovic, D.; Pegram, R.G.; Tatchell, R.J.; Fison, T.; Latif, A.A.; Paine, G. Ticks (Acari:Ixodidae) of the Blue and White Nile ecosystems in the Sudan with particular reference to theRhipicephalus sanguineus group. Exp. Appl. Acarol. 1987, 3, 331–346, . [CrossRef]
- Daniels, T.J.; Fish, D.; Falco, R.C. Seasonal Activity and Survival of Adult Ixodes dammini (Acari: Ixodidae) in Southern New York State. J. Med Èntomol. 1989, 26, 610–614, . [CrossRef]
- Tomillo, P.S.; Oro, D.; Paladino, F.V.; Piedra, R.; Sieg, A.E.; Spotila, J.R. High beach temperatures increased female-biased primary sex ratios but reduced output of female hatchlings in the leatherback turtle. Biol. Conserv. 2014, 176, 71–79, . [CrossRef]
- Nasibeh, H.V.; Zakkyeh, T.; Hassan, V.; Reza, Y.E.M.; Morteza, H.V.; Ali, O.M. Survey of tick species parasiting domestic ruminants in Ghaemshahr county, Mazandaran province, Iran. Asian Pac. J. Trop. Med. 2010, 3, 804–806, . [CrossRef]
- Nabian S, Rahbari S, Shayan P, Hadadzadeh H. Current status of tick fauna in north of Iran. 2007.
- Sofizadeh, A.; Telmadarraiy, Z.; Rahnama, A.; Gorganli-Davaji, A.; Hosseini-Chegeni, A. Hard Tick Species of Livestock and their Bioecology in Golestan Province, North of Iran. 2014, 8, 108–116.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).