1. Introduction
Piroplasmoses are obligately transmissible diseases of animals caused by non-pigmented endoglobular blood parasites. These diseases affect numerous species of domestic and wild animals, with cattle and small ruminants being particularly susceptible. The disease is characterized by fever, anemia, jaundice of the mucous membranes, dysfunction of the cardiovascular and digestive systems, hemoglobinuria, and a sharp decline in productivity.
Additionally, piroplasmoses occur in seasonal outbreaks, which are associated with the life cycle of specific tick vectors. In cattle, the causative agents include
Piroplasma bigeminum (piroplasmosis), Francaiella colchica (francaiellosis), and
Babesia bovis (babesiosis) [
1].
Kazakhstan is experiencing an unfavorable epizootic situation regarding piroplasmoses in cattle, which cause significant damage to the region's livestock industry. Four species of piroplasmoses pathogens have been identified in livestock farms: Babesia bigemina, Babesia bovis, Theileria annulata, and Anaplasma marginale. The dynamics of disease manifestations caused by these pathogens depend on the geographical location of farms within the country's natural and agricultural zones [2, 3].
This problem is particularly acute in the southern regions of Kazakhstan, where favorable climatic conditions promote the development of parasites and their vectors - ixodid ticks. During the summer-autumn period, diseases collectively referred to as piroplasmoses pose a serious threat to livestock farming, requiring constant attention from farmers and veterinarians. Piroplasmoses in cattle represent a significant challenge to the development of the livestock sector. In recent years, there has been an increase in the number of affected animals, the emergence of new infection hotspots, and mass cattle mortality, leading to a decline in both milk and meat production [4, 5].
Piroplasmoses in animals are also widespread in West Kazakhstan, where environmental conditions favor the development of both the parasites themselves and their arthropod vectors. During the spring-summer-autumn period, these diseases pose a serious threat to livestock farmers, causing significant economic losses. These losses manifest as high mortality rates in the absence of timely treatment, prolonged recovery of vital functions, reduced productivity, the birth of weakened offspring, deteriorating reproductive performance, and substantial expenses for treatment and disease prevention.
Mixed forms of piroplasmoses, which involve various combinations of piroplasmosis, francaiellosis, and theileriosis, are particularly dangerous. Mortality rates for these mixed infections can reach 40-50%, even with timely treatment.
Ixodid ticks, the vectors of piroplasmoses pathogens, pose a significant threat to livestock. They reduce productivity, cause stress, dermatitis, and skin lesions, and create a constant risk of infectious disease outbreaks. Effective control requires regular epizootological monitoring, including the identification of dominant tick species (Boophilus, Rhipicephalus, Haemaphysalis, Dermacentor, Hyalomma) and the registration of primary infection vectors.
West Kazakhstan region is epizootically unfavorable for both ixodid ticks and piroplasmoses due to favorable conditions for their development, a high abundance of hosts, and the high density of livestock on pastures. Therefore, comprehensive prevention and control measures are necessary to reduce disease incidence and economic losses in the livestock industry [6, 7].
The most reliable methods for diagnosing piroplasmoses infestation in animals and ticks are polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA). PCR offers high sensitivity and specificity, allowing for the detection of pathogen DNA even at low concentrations in blood or tissue samples. This method is particularly effective for diagnosing chronic infections, where parasite levels may be minimal. ELISA is used to detect specific antibodies against piroplasmoses, aiding in determining the stage of infection and the presence of an immune response in the animal. Both methods are widely employed in veterinary practice for the timely detection and control of piroplasmoses [8, 9].
Research purpose. The research purpose is a comprehensive investigation of the epizootic situation regarding piroplasmidosis and ixodidosis in cattle in western Kazakhstan, including an analysis of disease incidence dynamics, factors of spread, and pathogen identification using modern diagnostic methods.
2. Materials and Methods
The Research plan includes field and laboratory research.
1. Field Research:
1.1. Field visits to farms in West Kazakhstan region located in different natural and climatic zones (steppe, semi-desert, and desert) during various seasons (winter, spring, summer, autumn).
1.2. Collection of Ixodid ticks, vectors of piroplasmosis, from different body parts of cattle across various age groups (up to 1 year, 1-3 years, 4-5 years, 6 years and older).
1.3. Determination of the occurrence index (prevalence) of Ixodid ticks in different natural zones of West Kazakhstan region across different seasons (winter, spring, summer, autumn).
1.4. Assessment of the abundance index and total stock of Ixodid ticks in various natural zones of West Kazakhstan region across different seasons (winter, spring, summer, autumn).
1.5. Evaluation of the seasonal dynamics of cattle infestation by Ixodid ticks across different seasons (winter, spring, summer, autumn).1.6. Analysis of the age-related dynamics of cattle infestation by Ixodid ticks.
1.7. Collection of Ixodid ticks from vegetation in pastures of the studied farms during different seasons (spring, summer, autumn).
1.8 Blood collection from cattle across different age groups (up to 1 year, 1-3 years, 4-5 years, 6 years and older).
2. Laboratory Research:
2.1. Identification of Ixodid ticks based on morphological characteristics at the genus and species levels.
2.2. Determination of Ixodid tick infestation with piroplasmoses using the PCR method.
2.3. Examination of blood samples from cattle of different age groups (up to 1 year, 1-3 years, 4-5 years, 6 years and older) for piroplasmoses infestation using the PCR method.
3. Analysis of research results and preparation of a manuscript for publication.
According to the physical-geographical zoning and natural zonation, the territory of West Kazakhstan region is divided into three natural zones: steppe, semi-desert, and desert. Considering the natural and climatic zonation, farms located in different natural zones of West Kazakhstan region were selected for the study.
The seasonal dynamics of cattle infestation with ticks were determined through quarterly examinations of animals in winter, spring, summer, and autumn. Age-related infestation dynamics were assessed by examining different age groups: up to 1 year, 1-3 years, 4-5 years, and 6 years and older.
Species identification was performed using the Atlas of Ixodid Ticks by Ganiyev and Aliverdiyev. The stereoscopic microscope “Micromed MS-1 var. 1C LED” was used to determine the species of adult ticks.
Study site
Field research was conducted in 2024 on farms in West Kazakhstan region, located in different natural and climatic zones (steppe, semi-desert, and desert).
In the steppe zone, the study was carried out in Taskala District of West Kazakhstan region at Oyan farm, located at 51°06'28.5" N latitude and 50°17'31.2" E longitude.
In the semi-desert zone, research was conducted in Akzhaik District of West Kazakhstan region at Sazhida farm, located at 50°11′15″ N latitude and 51°10′02″ E longitude.
In the desert zone, the study was performed in Zhangaly District of West Kazakhstan region at Nur farm, located at 49°12′54″ N latitude and 50°18′10″ E longitude.
Laboratory research was conducted at the Testing Center Laboratory of Zhangir Khan West Kazakhstan Agrarian-Technical University, 090009, West Kazakhstan Region, Uralsk, Zhangir Khan Street 51, Republic of Kazakhstan.
1. Obtaining blood samples
Blood samples were collected from cattle via the coccygeal vein. The animals were restrained in a cattle chute to ensure safety and minimize stress. The tail was held at the middle third and slowly lifted upward to provide optimal access to the vein. The puncture site, located in the region of the 2nd-5th caudal vertebrae, was disinfected using a 70% alcohol solution. Blood was collected in sterile plastic vacuum tubes (vacutainers) containing the anticoagulant ethylenediaminetetraacetic acid (EDTA) K₂ with a volume of 5 mL and a purple cap. After collection, the sample was gently mixed by inverting the tube 5-8 times to prevent coagulation. Blood samples were transported to the laboratory in a TM-9 thermal container within 6 hours, strictly maintaining the required temperature range (+2 to +8°C) and storage conditions to prevent hemolysis and alterations in blood cell composition.
2. Collection of ixodid ticks from cattle.
The collection of Ixodid ticks from cattle was conducted once per quarter for each farm, corresponding to the seasons of the year. A thorough examination of the entire body of each animal was performed systematically. The imaginal stages of ticks were most frequently found on the skin in the posterior body regions, including the inguinal area, udder (in cows), and scrotum (in bulls). Ticks were grasped as close to the skin as possible using forceps and carefully removed by rotating counterclockwise without abrupt movements to prevent proboscis rupture. Collected ticks were placed in 100 mm³ containers, with the bottom lined with a layer of sterile cotton wool moistened with distilled water, covered by two circles of filter paper on the day of collection. Each container was labeled with the date and time of collection, animal identification number, and body site from which the ticks were removed. The ticks were stored at a temperature of +4 to +8°C in a TM-9 thermal container until further examination. They were transported to the laboratory within 24 hours after collection, where they were sorted and counted for further analysis.
3. Collection of ticks from vegetation
Ticks with a pasture-type parasitism were collected using the flagging method, in which a flag was dragged through different vegetation layers. The method relies on the mechanical stimulation of ticks that remain on vegetation in a passive waiting posture. The flag was made of light-colored, plush cotton fabric. The front edge of the flag was sewn to form a pocket, into which a lightweight pole was inserted. The pole length was selected according to the height of the researcher. The flag was dragged parallel to the researcher’s movement, and inspections were performed every 10-20 meters. In the field, collected ticks were placed in 100 mm³ containers, with the bottom lined with a layer of sterile cotton wool moistened with distilled water, covered by two circles of filter paper on the day of collection. At the end of each route, a label was written, specifying the biotope, sample number, date, and time of the survey route. Ticks were stored at a temperature of +4 to +8°C in a TM-9 thermal container until further examination. They were transported to the laboratory within 24 hours after collection, where they were sorted and counted.
The relative abundance of ticks was calculated per one flag unit per distance traveled (1 flag-kilometer) using the following formula (1):
(1) X0 = Ʃ di xi,
where Х0 – weighted average tick abundance
di– the proportion of the area occupied by the i-habitat from the entire area under study, xi is the number of ticks collected in the same habitat.
4. Morphological characteristics of ticks.
The body of a tick consists of the idiosoma (main body) and the gnathosoma (mouthparts or capitulum). The idiosoma bears the walking appendages, with three pairs of legs in larvae and four pairs in nymphs and adults. The dorsal surface of the idiosoma is covered by a scutum (dorsal shield), which completely covers the male's body but only partially covers the anterior region in females.
The cuticle of the idiosoma (excluding the scutum) contains epicuticular microfolds, which unfold as the tick engorges with blood. Sexual dimorphism is pronounced in adult ticks: males are smaller than females, their idiosoma is more sclerotized, and they differ in the structure of the gnathosoma and genital openings.
The nymph and larva are immature stages. The nymph resembles the female morphologically but is smaller in size. The larva is distinguished by its smaller size and the absence of one pair of legs.
The gnathosoma is located at the anterior end of the idiosoma and contains a piercing-sucking mouthpart. The ventral portion of the gnathosoma consists of the hypostome, which is armed with rows of recurved denticles. The dorsal portion forms cheliceral sheaths, within which mobile chelicerae are housed. The cheliceral shafts move in an anteroposterior direction, while their digits perform cutting movements, allowing the tick to penetrate the skin of vertebrate hosts.
Morphological characteristics of the genus Ixodes
1. Absence of eyes
2. Anteriorly positioned anus (analogous anus) – the anal opening is located closer to the anterior margin of the anal region.
3. Long proboscis – the gnathosoma is elongated,
4. Hypostome with numerous denticles
5. Lack of ornamentation on the scutum – the dorsal scutum is smooth, without patterns.
Morphological characteristics of the genus Haemaphysalis
1. Short and broad proboscis – the gnathosoma is shorter than in Ixodes,
2. Hypostome with small denticles – the denticles are less pronounced than in Ixodes,
3. Lateral projections at the base of the gnathosoma – characteristic lateral expansions distinguishing Haemaphysalis from other genera of Ixodid ticks.
4. Unornamented dorsal scutum – smooth, without patterns,
5. Presence of eyes – simple eyes are present.
6. Well-defined anal groove – located posterior to the anal opening (postanal anus).
7. Distinctive characteristics of the palps – the second segment of the palps is noticeably wider than the first, serving as an important diagnostic feature.
Morphological characteristics of the genus Dermacentor
1. Large body size – Dermacentor ticks are larger than most other Ixodid ticks.
2. Distinct scutum ornamentation – in adult stages, the scutum is adorned with light-colored spots, creating a marbled pattern.
3. Short and robust proboscis – the gnathosoma is broader and shorter,
4. Hypostome with large denticles – denticles are prominent and arranged in multiple rows
5. Well-developed eyes – located on the lateral sides of the scutum.
6. Postanal anal groove – positioned posterior to the anal opening.
Morphological characteristics of the genus Hyalomma
1. Large body size – Hyalomma ticks are significantly larger than many other Ixodid ticks.
2. Long and slender legs – enable high mobility and rapid movement across the host’s body.
3. Long proboscis – the gnathosoma is elongated,
4. Hypostome with large denticles
5. Well-developed eyes – located on the lateral sides of the scutum,
6. Lack of prominent ornamentation on the scutum – the scutum is dark and uniform in color, sometimes with a faint pattern.
7. Postanal anal groove – positioned posterior to the anal opening,
Morphological characteristics of the genus Rhipicephalus
1. Medium or small body size
2. Short and broad proboscis
3. Hypostome with small denticles – arranged in multiple rows,
4. Postanal anal groove – located posterior to the anal opening.
5. Well-developed eyes – positioned on the lateral sides of the scutum.
6. Lack of prominent ornamentation on the scutum
7. Short and robust legs
5. PCR protocols for testing cattle blood and Ixodes ticks
1.1. Consumables
- PCR kit ScreenMix (Evrogen);
- Nuclease-free water
- Ethidium bromide (EtBr)
- 1× Tris-acetate-EDTA (TAE) buffer
- Agarose
1.2. Biological Samples
- Cattle blood – 507 samples
- Ixodes ticks – 581 samples
1.3. DNA Extraction
DNA extraction of parasitic infection pathogens was performed using the QIAamp DNA Mini Kit (Qiagen).
1.4. PCR amplification of the 18S rRNA gene of parasitic infections
Oligonucleotide primers
Forward and reverse primer sequences:
Direct primer BaF-5ʹ AAT ACC CAA TCC TGA CAC AGGG 3ʹ
Reverse primer BaR- 5ʹ TTA AAT ACG AAT GCC CCC AAC 3ʹ,
The PCR product size was 400 bp for the 18S rRNA fragments.
The PCR reaction was performed using the ScreenMix kit (Evrogen). Preparation of the PCR mixture:
5µ. L of 5X ScreenMix, 1 µL of forward primer (10 mM), 5 µL (concentration from 5 ng/µL to 50 ng/µL) of genomic DNA and nuclease-free water to a final volume of 25 µL.
The reaction mixture was loaded into a thermal cycler (Applied Biosystems, USA), optimized for amplification of the target DNA sequence.
The cycling protocol was programmed as follows:
initial denaturation at 95 ºC for 3 min, then 35 cycles:
denaturation (96 ºC for 15 sec),
annealing (60 ºC for 20 sec)
elongation (72 ºC for 25 sec).
synthesis 72 ºC for 1 min
1.5. PCR Amplification for Theileria annulata
Specific primers for Theileria annulata detection:
Direct primer TanF 3 AAGTTTCTACTGTCCCGTT
Reverse primer TanR3 GATGACTTGCGCATACTAGG
The size of the PCR product of Theileria annulata is 250 bp.
The ScreenMix kit (Eurogen) was used for PCR. The PCR mixture consists of:
5 µl 5X ScreenMix, 1 µl each of forward and reverse primers (10 mM), 5 µl (from 5 ng/µl to 50 ng/µl) genomic DNA and up to 25 µl of nuclease-free water. The reaction mixture was loaded into a thermal cycler (Applied Bio Systems, USA)
Amplification protocol:
initial denaturation at 95 ºC for 3 min, then 35 cycles of:
denaturation (96 ºC for 15 s),
annealing (55 ºC for 20 s)
elongation (72 ºC for 25 s).
synthesis 72 ºC for 1 min
1.6. Setting up PCR for detection of Theileria sergenti
Theileria sergenti-specific primers:
Direct primer Tser-U CACGCTATGTTGTCCAAGAG
Reverse primer Tser-R TGTGAGACTCAATGCGCCTA
The ScreenMix kit (Eurogen) was used for PCR. The PCR mixture consists of:
5 µl 5X ScreenMix, 1 µl each of forward and reverse primers (10 mM), 5 µl (from 5 ng/µl to 50 ng/µl) genomic DNA and up to 25 µl of nuclease-free water. The reaction mixture was loaded into a thermal cycler (Applied Bio Systems, USA)
Amplification protocol:
initial denaturation at 94 ºC for 3 min, then 30 cycles of:
denaturation (94 ºC for 1 min),
annealing (57 ºC for 1 min)
elongation (72 ºC for 1 min).
synthesis 72 ºC for 7 min
1.7. Setting up PCR for detection of Theileria orientalis
Theileria orientalis-specific primers:
Direct primer MPSP-F 5’-CTTTGCCTAGGATACTTCCT-3’
Reverse primer MPSP-R 5’-ACGGCAAGTGGTGAGAACT-3’
The size of the PCR product of Theileria orientalis is 776 bp.
The ScreenMix kit (Eurogen) was used for PCR. The PCR mixture consists of:
5 μl 5X ScreenMix, 1 μl each of forward and reverse primers (10 mM), 5 μl (from 5 ng/μl to 50 ng/μl) of genomic DNA and up to 25 μl of nuclease-free water. The reaction mixture was loaded into a thermal cycler (Applied Bio Systems, USA).
Amplification protocol:
initial denaturation at 94 ºC for 10 min, then 35 cycles of:
denaturation (94 ºC for 1 min),
annealing (58 ºC for 1 min)
elongation (72 ºC for 1 min).
synthesis 72 ºC for 4 min
1.8. Setting up PCR for detection of Babesia bovis
Babesia bovis-specific primers:
Direct primer 5’- TGAA-CAAAGCAGGTATCATAGG-3’
Reverse primer 5’- CCAAGGAGATTGTGATAATTCA-3’
The ScreenMix kit (Eurogen) was used for PCR. The PCR mixture consisted of:
5 μl 5X ScreenMix, 1 μl each of forward and reverse primers (10 mM), 5 μl (5 ng/μl to 50 ng/μl) genomic DNA and up to 25 μl nuclease-free water. The reaction mixture was loaded into a thermal cycler (Applied Bio Systems, USA).
Amplification protocol:
Initial denaturation at 95 ºC for 5 min,
then 35 cycles of:
denaturation (95 ºC for 1 min),
annealing (60 ºC for 1 min)
elongation (70 ºC for 1 min).
synthesis 72 ºC for 10 min
1.9. Electrophoretic analysis of PCR products
5 μl of the PCR product together with the DNA marker were subjected to electrophoresis in 1.5% agarose gel containing 0.5 μg/ml ethidium bromide (EtBr) in 1×Tris-acetate-EDTA (TAE) buffer at 120 V for 20 min. The amplicons were then visualized and documented in a gel documentation system.
3. Results
Species composition of ixodid ticks. In West Kazakhstan region, across various natural zones, including steppes, semi-deserts, and deserts, 11 species of ixodid ticks belonging to five genera have been identified: Ixodes, Haemophysalis, Dermacentor, Hyalomma, Rhipicephalus.
The genus Ixodes is represented by a single species, Ixodes ricinus, which lives in the steppe zone.
The genus Haemophysalis includes one species, Haemophysalis erinacei, which is also found in the steppe zone.
The genus Dermacentor comprises three species:
Dermacentor marginatus - a three-host tick, widespread throughout the region;
Dermacentor reticulatus - a three-host species living in the steppe zone;
Dermacentor niveus - a rare three-host tick found in desert areas.
The genus
Hyalomma (
Figure 1) is represented by four species:
Hyalomma marginatum - a three-host tick, common in all natural zones of the region;
Hyalomma scupense - a single-host species, widespread in steppes and semi-deserts;
Hyalomma detritum - a rare species found in desert areas;
Hyalomma asiaticum - a three-host rare species found in deserts.
The genus Rhipicephalus includes two species:
Rhipicephalus rossicus - a small representative of the steppe regions;
Rhipicephalus pumilio - a numerous three-host tick that lives in the desert zones of the region (
Table 1).
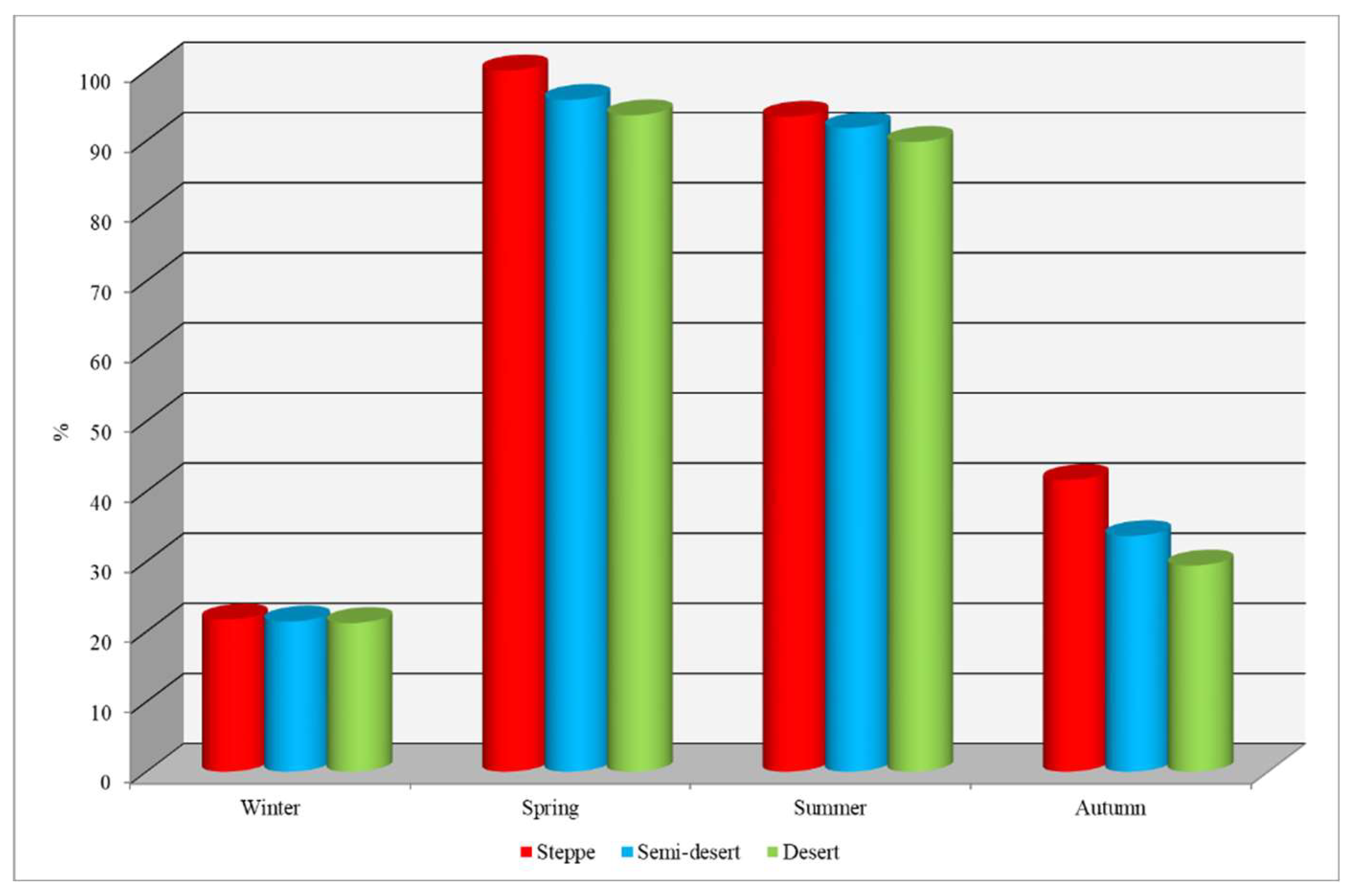
Seasonal dynamics. Analysis of the seasonal dynamics of cattle infestation with ixodid ticks revealed that the prevalence index (PI) of these parasites varied depending on the time of year.
During winter, infestation rates were minimal across all natural zones, with a prevalence of 21.4%.
In spring, the highest level of infestation was observed: in the steppe zone, all examined animals were infected (100%); in the semi-desert zone, the infestation rate was 95.8%; and in the desert zone, 93.6% of animals were affected (Figure. 2).
In summer, the infection intensity decreased: in the steppe zone, 93.4% of animals were affected; in the semi-desert zone, 91.8% were infected; and in the desert zone, the infection rate was 89.8%.
In autumn, the infestation level was lower than in the spring-summer period: in the steppe zone, 41.6% of animals were infected with ticks; in the semi-desert zone, infestation was recorded in 33.6% of cattle; and in the desert zone, 29.4% of animals were affected.
Thus, the highest infestation rate of cattle with ixodid ticks occurs during the spring-summer period, while the lowest is observed in the autumn-winter season.
The abundance index (AI) of ixodid ticks (number of specimens per head) in cattle across different natural zones of West Kazakhstan region depends on the tick species.
The highest abundance was observed for Hyalomma scupense (49.8 specimens per head on average across the region), Hyalomma asiaticum (47.5 specimens per head), and Hyalomma detritum (45.0 specimens per head), with their maximum values recorded in the desert zone.
Among Dermacentor species, D. marginatus was the most prevalent (24.9 specimens per head), while D. niveus and D. reticulatus had similar values, ranging from 8.6 to 8.9 specimens per head.
Within the Rhipicephalus genus, R. pumilio was more common (36.2 specimens per head) compared to R. rossicus (9.4 specimens per head).
Overall, the tick abundance index increased from the desert zone (24.7 specimens per head) to the steppe zone (29.6 specimens per head), indicating more favorable conditions for ticks in arid landscapes.
Age dynamics. The study of the age-related dynamics of cattle infestation with ixodid ticks revealed that parasites were detected in all age groups, with an average prevalence index (PI) of 61.0%. However, the number of ticks per animal (abundance index, AI) varied depending on age and natural zone.
In the steppe zone, the highest tick abundance was recorded in young animals under one year old (18.3 specimens per head) and in cattle aged 1-3 years (49.8 specimens per head).
A similar trend was observed in the semi-desert zone, with the highest infestation rates in young animals under one year old (11.7 specimens per head) and in those aged 1-3 years (21.4 specimens per head).
In the desert zone, the highest tick infestation was found in animals aged 1-3 years (17.8 specimens per head) and in the 4-5-year age group (13.5 specimens per head).
On average, across West Kazakhstan region, the highest abundance index was observed in cattle aged 1-3 years (44.1 specimens per head), while the lowest was recorded in animals aged six years and older (13.5 specimens per head) (
Table 2).
Results of laboratory tests of ixodid ticks and blood of cattle. The detection of hemoparasitic infections in cattle and ixodid ticks was conducted using PCR. In the first stage of the study, 507 blood samples from cattle were analyzed by PCR using specific primers targeting 18S rRNA to detect hemoparasite DNA. As a result of amplification, PCR products of 400 bp were obtained in 162 samples (32%), indicating the presence of hemoparasite DNA in these samples.
To identify hemoparasitic infections in vectors, 581 ixodid ticks were examined, grouped into 29 pools. PCR analysis using specific 18S rRNA primers revealed that two tick pools tested positive for the presence of hemoparasite DNA.
For the final identification of pathogens, 162 positive cattle blood samples and two positive tick pools were further analyzed by PCR using species-specific primers for Theileria annulata, Theileria sergenti, Theileria orientalis, and Babesia bovis. The results showed that 159 cattle blood samples and both tick pools contained 250 bp PCR products characteristic of Theileria annulata. No DNA of Theileria sergenti, Theileria orientalis, or Babesia bovis was detected in the analyzed samples.
Thus, out of the 507 examined cattle blood samples and 581 ixodid ticks, Theileria annulata was identified in 159 blood samples (31.4%) and in 2 tick pools (6.9%). These findings confirm the significant prevalence of Theileria annulata, the causative agent of theileriosis, among the cattle population and its natural vectors in the studied area.
4. Discussion
The conducted study provided valuable data on the prevalence of Ixodid ticks and piroplasmoses pathogens among cattle in West Kazakhstan region. Based on the obtained results, several important conclusions can be drawn, and measures can be proposed to mitigate the epizootic threat.
A review of the literature revealed that Ixodidosis and piroplasmoses infections in cattle have been well studied in the southern regions of Kazakhstan; however, research in this field remains limited in the western part of the country. The lack of data on the distribution, seasonality, and species composition of pathogens in West Kazakhstan complicates the development of effective preventive and control measures for these diseases.
An analysis of the species composition of Ixodid ticks demonstrated a high diversity in the region. The most abundant were species of the genus Hyalomma, particularly H. scupense, H. asiaticum, and H. detritum, confirming their primary role in the transmission of blood parasites. Their high numbers in desert and semi-desert zones may be associated with favorable climatic conditions and the availability of suitable host animals, consistent with studies by Kazakhstani researchers [
2].
The seasonal dynamics of infestation showed a pronounced spring-summer peak in cattle infection with Ixodid ticks. The maximum values of the occurrence index (reaching up to 100% in the steppe zone) and the tick abundance index indicate the necessity of active preventive measures during this period. The decline in infestation rates in autumn and winter confirms the influence of climatic factors on the dynamics of vector populations, which is consistent with the findings of several authors [
7].
The age structure of animal infestation revealed that the highest level of invasion was observed in young cattle aged 1-3 years. This may be due to the lower resistance of their organisms to parasite attacks, as well as the conditions under which they are kept. Therefore, special attention should be given to this age group when implementing preventive and therapeutic measures.
The results of molecular genetic analysis confirmed the significant prevalence of Theileria annulata among cattle, indicating a high risk of theileriosis for livestock farming in the region. The detection of the pathogen's DNA in Ixodid tick samples proves their significant role in the epizootiological cycle of the disease, which correlates with research findings from southern Kazakhstan [
4].
Knowledge of the key measures to combat parasitic diseases in cattle and the ability to apply them in practice would contribute to the development of optimal conditions for the effective combination of therapeutic and preventive interventions aimed at controlling parasite spread in agricultural settings [
14].
The direct causative agent of cattle theileriosis was first identified during blood studies of animals in Africa [
15]. No fundamental differences were found in the course of this disease compared to typical piroplasmosis. Subsequent extensive seasonal experiments conducted on cattle demonstrated that the blood of infected animals contained specific plasma-like inclusions, later known as "plasma globules" or "pomegranate bodies" [
16].
Piroplasmoses pose a significant threat to the livestock industry, primarily because the disease occurs during the spring, summer, and autumn periods. Infected cows experience a decline in milk production, reduced weight gain during fattening, and high mortality rates if veterinary intervention is delayed [
17]. The prevention of theileriosis involves substantial financial costs, which are not always feasible for agricultural enterprises [
18]. This, in turn, explains the occasionally unjustifiably high mortality rate from this disease, which, with timely diagnosis and preventive measures, does not appear as dangerous for livestock.
A recent study [
19] confirmed the relative abundance of the pathogen, partly due to the challenges associated with its classification and its variability, which is influenced by a combination of factors. These factors determine both the nature of the disease progression and the susceptibility of specific groups of animals to infection.
Research conducted in West Kazakhstan has identified the presence of 20 species of Ixodid ticks, 10 of which exhibit relatively high population densities. The most prevalent tick species may play a key role in transmitting the causative agents of piroplasmoses and other cattle diseases, highlighting the need for continued monitoring of their populations and epidemiological significance [
20].
Ixodid ticks found in West Kazakhstan act as both reservoirs and vectors of natural focal infections [
21].
As a result of research conducted by Kazakhstani authors in Kyzylorda Region from 2012 to 2022, three species of Ixodes ticks were identified: I. crenulatus, I. occultus and I. Ricinus, I. Crenulatus. The pasture tick I. ricinus was recorded for the first time in this area. This species is a known vector of numerous dangerous diseases affecting both humans and animals [
22].
In recent years, there has been an observed increase in the prevalence of pasture-type Ixodid ticks (Ixodidae) within the boundaries of large cities, in modern biotopes, and particularly in West Kazakhstan Region. In this area, we recorded two species of Ixodid ticks: Ixodes ricinus (Linnaeus, 1758) and Dermacentor reticulatus (Fabricius, 1794) [
23].
Monitoring the epizootic situation regarding cattle piroplasmosis in West Kazakhstan Region, as well as the seasonal patterns of tick parasitism on animals across different climatic and geographical zones, allows for a more rational approach to treatment and prevention strategies for piroplasmosis. This underscores the need for the development and practical implementation of a comprehensive set of preventive measures aimed at strengthening the immune system of cattle. Protection measures should be applied primarily during the spring, summer, and autumn months to prevent piroplasmosis infections and eliminate conditions that could contribute to the subsequent progression of the disease upon its onset.
As the infection rate of cattle with piroplasmoses decreases, the mortality rate will also decline, which is a crucial prerequisite for maintaining the overall cattle population. In this context, special attention should be given to the timely provision of veterinary care for infected animals. This implies the mandatory and regular implementation of therapeutic and preventive measures on all livestock farms in West Kazakhstan Region without exception. Proper execution of all necessary measures, including monitoring the spread dynamics of piroplasmoses and ensuring timely prevention and treatment, would contribute to preserving the cattle population and significantly reducing mortality from these diseases in the region.
Further studies on ticks in various regions are required to assess the epizootic situation across the country.
Based on the obtained data, the following recommendations can be proposed:
Epizootiological monitoring: regular observation of Ixodid tick populations and analysis of their infection rates with blood parasites will help identify epizootically unfavorable areas in a timely manner.
Application of acaricidal preparations: treating animals with insecticidal agents during the spring and summer periods will help reduce infection levels and prevent large-scale disease outbreaks.
Development of a pasture management strategy: moving livestock to less endemic areas during peak tick activity periods may help reduce animal contact with infection vectors.
Thus, the results of this study confirm the significant epidemiological threat posed by piroplasmosis to livestock farming in West Kazakhstan. A comprehensive approach to controlling Ixodid ticks and blood parasitic infections will help reduce economic losses and ensure the health of the cattle population.
5. Conclusions
The conducted studies revealed a high species diversity of ixodid ticks in West Kazakhstan region, where 11 species belonging to five genera (Ixodes, Haemaphysalis, Dermacentor, Hyalomma, Rhipicephalus) were identified. Ticks of the genus Hyalomma demonstrated the highest prevalence, particularly Hyalomma scupense, Hyalomma asiaticum, and Hyalomma detritum, with their highest abundance recorded in the desert zone.
An analysis of the seasonal and age-related dynamics of cattle infestation with ixodid ticks revealed that the peak of infestation occurs during the spring-summer period, with the highest infection rate observed in spring (up to 100% in the steppe zone). The tick abundance index varied depending on environmental conditions and the age of the animals, reaching its highest values in the steppe zone and among young cattle aged 1-3 years.
PCR analysis of blood parasitic infections detected the presence of blood parasite DNA in 32% of the examined cattle blood samples and in 6.9% of tick pools. Molecular genetic analysis confirmed that the primary causative agent of infection was Theileria annulata, which was identified in 159 cattle blood samples (31.4%) and 2 tick pools (6.9%). Theileria sergenti, Theileria orientalis, and Babesia bovis DNA were not detected in the analyzed samples.
The obtained results indicate a significant prevalence of theileriosis among cattle in the region, as well as the role of ixodid ticks in pathogen transmission. The highest infestation rates in cattle during the spring-summer period and the high tick abundance, particularly in arid natural zones, confirm the need for preventive measures aimed at controlling tick populations and reducing the risk of theileriosis spread among livestock.
Author Contributions
Conceptualization, Rashid Karmaliyev, Balaussa Yertleuova; methodology, Asylzhan Myrzakhmet, Farida Nurzhanova; software, Yerbol Sengaliyev, Nurzhan Sariyev; validation, Bekzhassar Sidikhov, Zhangeldi Ussenov; formal analysis Aiman Ichshanova, Bekzhassar Sidikhov, Zhangeldi Ussenov; investigation Rashid Karmaliyev, Bekzhassar Sidikhov; resources Balaussa Yertleuova, Asylzhan Myrzakhmet; data curation Zhangeldi Ussenov, Rashid Karmaliyev; writing—original draft preparation Zhangeldi Ussenov, Rashid Karmaliyev, Bekzhassar Sidikhov; writing—review and editing, Yerbol Sengaliyev, Farida Nurzhanova; visualization Aiman Ichshanova, Asylzhan Myrzakhmet; project administration Bekzhassar Sidikhov; funding acquisition Rashid Karmaliyev, Bekzhassar Sidikhov. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted as part of the Grant Funding Project for 2024-2026 of the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan AP23487588. The project is titled: "Improving the prevention of piroplasmosis of cattle transmitted by ixodid ticks in the steppe, semi-desert and desert zones of West Kazakhstan region"
Institutional Review Board Statement
The animal study protocol was approved by the Local Committee on Biological Ethics of the West Kazakhstan Research Institute for Veterinary Sanitation (a branch of LLP "KazNIVI") on November 17, 2023 (Protocol No. 3). The committee members present at the meeting included Dr. S.G. Kanatbayev (DSc), Senior Researcher Dr. E.K. Tuyashev (PhD, Veterinary Sciences), and Senior Researcher Dr. R.A. Amanzhol (PhD, Veterinary Sciences). The protocol submission for the research project titled " Improving the prevention of piroplasmosis of cattle transmitted by ixodid ticks in the steppe, semi-desert and desert zones of West Kazakhstan region" (Grant Funding Project 2024–2026, Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, AP23487588) was reviewed in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986), as well as Recommendation 2007/526/EC of June 18, 2007, on the accommodation and care of animals used for experimental and other scientific purposes. Additionally, the study complies with the WHO Guidelines for Ethics Committees Reviewing Biomedical Research (Geneva, 2000). Based on expert evaluation, the Committee approved the protocol submission. Reports on study progress must be submitted annually (November, 2022).
Acknowledgments
This study was conducted as part of the Grant Funding Project for 2024-2026 of the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan AP23487588. The project is titled: " Improving the prevention of piroplasmosis of cattle transmitted by ixodid ticks in the steppe, semi-desert and desert zones of West Kazakhstan region."
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PCR |
Polymerase chain reaction |
| DNA |
Deoxyribonucleic acid |
| RNA |
Ribonucleic acid |
| AI |
abundance index |
| PI |
prevalence index |
| ELISA |
enzyme-linked immunosorbent assay |
| EDTA |
ethylenediaminetetraacetic acid |
References
- Blagoveshchensky, D. I. Materials on the fauna of external parasites of animals of Kazalinsky and some other regions of South Kazakhstan / D. I. Blagoveshchensky // Proceedings of KazFAN. Issue. - 1937. - No. 2. – pp. 11-84.
- Kozhabayev, M.K. , Amanzhol R., Tuleukhanov A. Spread of ixodid ticks in the South of Kazakhstan // Theory and practice of combating parasitic diseases 2024 No. 25pp. 242-244.
- Taurbayeva, S. N. , Tokpan S. S., Shevtsov A. B., Lider L. A. Theileriosis of cattle: distribution and diagnostics in the conditions of Kyzylorda region // Bulletin of Science of Seifullin Kazakh Agrotechnical University (interdisciplinary). - 2017. - No. 4 (95). - pp.73-78.
- Taurbayeva, S.N. , Lider L.A. The extent of the spread of theileriosis in cattle in South Kazakhstan // Proceedings of the Republican scientific-theoretical conference "Seifullin readings - 13: preserving traditions, creating the future", dedicated to the 60th anniversary of Seifullin Kazakh Agrotechnical University. - 2017. - V.І, P.2. - pp.241-244.
- Sabanshiyev, M. S. Blood-sucking ticks are carriers of piroplasmosis in the South of Kazakhstan. / M. S. Sabanshiyev, M. Zh. Suleimenov, T. T. Suleimenov // Bulletin of the Kyrgyz Research Institute of Livestock, ArstanbekDuisheev Veterinary Medicine and Pastures. – 2007. – No. 1– pp. 328–329.
- Karmaliyev, R.S. , Sidikhov B.M., Usenov Zh.T., Nurzhanova F.Kh., Maikanov N.S., Arisov M.V., Sengaliyev E.M. Ixodids of cattle in West Kazakhstan region, species composition and distribution // "Science and Education" Scientific and practical journal of Zhangir Khan West Kazakhstan Agrarian and Technical University in Uralsk. 2024. V.1. No. 3-1 (76), pp. 41-55.
- Tanitovsky, V. A. Fauna and distribution of ixodid ticks in West Kazakhstan region / Tanitovsky V. A., Maikanov N. S. // Materials of the interregional scientific and practical conference "Epidemiological surveillance of natural focal infections. Ecology of carriers and transmitters. Biosafety", dedicated to the 130th anniversary of the discovery of the plague pathogen and the 110th anniversary of the formation of the Uralsk anti-plague station, Uralsk. – 2024. - pp. 72-81.
- Georgiou, H., V. V. Belimenko, Babesiosis of cattle caused by Babesia bovis // Russian Veterinary Journal. Farmanimals 2015, No. 2, pp. 32-33.
- Lyapunov, A.V. , Khasanatinov M.A., Manzarova N.A., Bolotova N.A., Danchinova G.A. Application of the real-time PCR method for diagnostics of infections transmitted by ixodid ticks // Bulletin of the East Siberian Scientific Center of the Siberian Branch of the Russian Academy of Medical Sciences, 2016, Vol. 1, No. 6 (112), pp. 161-165.
- Beklemishev, V. N. Terms and concepts necessary for quantitative study of populations of ectoparasites and nidicols / V. N. Beklemishev // Russian Ornithological Journal 2009. - Volume 18, Express Issue 509. – pp. 1527-1540.
- Dantas-Torres, F. Efficiency of flagging and dragging for tick collection / F. Dantas-Torres, R. Paolo Lia, G. Capelli, D. Otranto // Exp Appl Acarol, 2013. Vol. 61. pp. 119-127.
- Bespyatova, L.A. , Bugmyrin S.V. Ixodid ticks of Karelia (distribution, ecology, tick-borne infections) Study guide. Petrozavodsk 2012 p. 87.
- Methods of collection, storage and identification of blood-sucking insects and ticks: a tutorial // F. I. Vasilevich, S. Yu. Pigina, A. M. Nikanorova, R. M. Akbayev.M.,2023. — 296 p.
- Kuotsu, N, Kuotsu K, Ozukum S, Ralte L, Jayathangaraj MG, Das G and Vijayashanthi R, 2022. Thelaziasis and theileriosis in a cow – A clinicalreport from Nagaland, India. IndianVeterinaryJournal 99: 45-47.
- Medjkane, S and Weitzman JB, 2020. Intracellular Theileria parasites pindownhostmetabolism. Frontiers in Cell and DevelopmentalBiology 8: Article # 134. [CrossRef]
- Jaja, IF, Mushonga B, Green E and Muchenje V, 2017. Financiallossestimation of bovinefasciolosis in SlaughteredCattle in SouthAfrica. ParasiteEpidemiology and Control 2: 27- 34. [CrossRef]
- Olsen, A, Berg R, Tagel M, Must K, Deksne G, EnemarkHL,Alban L, Johansen MV, Nielsen HV, Sandberg M, LundenA, Stensvold CR, Pires SM and Jokelainen P, 2019.Seroprevalence of toxoplasma gondii in domestic pigs,sheep, cattle, wild boars, and moose in the Nordic-BalticRegion: A systematic review and meta-analysis. ParasiteEpidemiology and Control 5: Article # e00100. [CrossRef]
- Hayati, MA, Hassan SM, Ahmed SK and Salih DA, 2020. Prevalence of ticks (Acari: Ixodidae) and Theileria annulatainfection of cattle in GeziraState, Sudan. ParasiteEpidemiology and Control 10: Article # e00148. [CrossRef]
- Rana, G, 2018. Inhibitionefficiency of a newlyisolatedflavonoidcompound from Vitexnegundo L. leavesagainstcattle-endosymbiontSetariacervi: Phytomedicineforlymphaticfilariasis. ParasiteEpidemiology and Control 3: 88-95. [CrossRef]
- Tanitovsky, V.A. , Maikanov N.S. Fauna of ixodid ticks of West Kazakhstan and features of their distribution across the territory // Bulletin of WKSU, Uralsk. - 2018. - No. 2 (70) – pp. 294-305.
- Maikanov, N.S. Epidemiological significance of ixodid ticks in Kazakhstan // Proceedings of the International Conf. "Fauna of Kazakhstan and adjacent territories". - Almaty, 2012. – pp. 134 -135.).
- Sayakova, Z.Z. , Abdybekova A.M., et al. Ticks of the genus ixodeslatreille, 1795 (ixodidae, ixodinae) in Kyzylorda region // Journal of Biological Research 2024.- No. 1 (4), pp. 15-25.
- Sarsenova, B.B. , Zholdasbaeva, T.K.Znachenie i biojekologicheskoeizucheniepastbishhnyhkleshhej v suhojstepiZapadno-Kazahstanskoj oblasti (Meaning and bioecologicalstudy of pastureticks in thedrysteppe of Zapadno-Kazakhstan region) // PolishJournal of Science. – 2022. – No 50 (50). – S. 9-13. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).