1. Introduction
In eukaryotes, mitochondria are intracellular organelles that produce energy, maintain calcium and iron homeostasis, and initiate apoptosis [
1]. Recent evidence suggests that mitochondria should no longer be viewed as a static compartment within the cytoplasm but as a mobile plastic entity that regulates signal transmission pathways and supports cell physiological changes [
1]. During this process, mitochondria undergo impressive morphological fission/fusion events [
1]. In addition to their intracellular dynamic nature, the idea that mitochondria could travel outside of cell borders is emerging in light of recent evidence demonstrating intercellular mitochondrial transfer [
2]. This may resemble mitochondria’s bacterial ancestral origins, which colonized eukaryotic cells from the outside. Through this transcellular transfer of mitochondria, it has been postulated that the transferred mitochondria may be incorporated into the endogenous network of the recipient cells, resulting in changes to the bioenergetic profile and other functional properties of the receiving cells. Furthermore, mitochondrial transfer between cells may also involve the horizontal transfer of mitochondrial genes, which has significant implications for mitochondrial dysfunction and physiopathology. The functional mitochondrial transfer was demonstrated for the first time in human stem cells that rescue mitochondrial respiration after mitochondrial depletion [
3]. However, little is known about the nature of mitochondrial release and the metabolic functions of their intercellular transfer in disease. CEMI have been shown to play a role in pathophysiology and regulate inflammation in various disease conditions, although their demonstrated effects are context-dependent. For example, when several cells, including platelets and neural cells, are stimulated by LPS or other toxic proteins, free and encapsulated mitochondria are released, acting as pro-inflammatory signals, thereby driving further inflammation [
4,
5]. Conversely, other studies have reported that the transfer of mitochondria from healthy, unstimulated cells may have anti-inflammatory effects on the recipient cells or repair damaged cells and facilitate recovery following stroke and ischemic injury [
6,
7,
8,
9,
10]. Additionally, the extracellular release of mitochondria has been linked to the maintenance of homeostasis through mitochondrial quality control. Normally, dysfunctional mitochondria are broken down intracellularly by lysosomes through degradation pathways. However, when lysosomal function is compromised, an increased secretion of dysfunctional mitochondria occurs extracellularly. The secreted mitochondria are attacked and captured by circulating macrophages without the activation of inflammatory pathways, ensuring homeostasis is maintained [
11].
Cardiometabolic disease (CMD) describes a continuum of cardio-structural and metabolic diseases characterized by insulin resistance, metabolic syndrome, pre-diabetes, and ultimately cardiovascular disease (CVD) and type 2 diabetes (T2DM) [
12,
13]. CVD is inextricably linked to mitochondrial dysfunction, which contributes to calcium dysregulation, oxidative stress, proteotoxicity, and cardiomyocyte death [
14,
15,
16]. CMD affects a multitude of tissues, including but not limited to the myocardium, vascular endothelium, liver, kidney, adipose, and pancreas, causing metabolic dysregulation, a key component of which is mitochondrial dysfunction [
15,
17]. Those tissues can release and/or receive CEMI under various physiological or pathological conditions [
18]. For example, it has been demonstrated that exogenously derived mitochondria injected or perfused into ischemic hearts are rapidly internalized by the cardiac cells and transported to endosomes and lysosomes. Exogenous mitochondria can then 1) escape from these compartments and fuse with endogenous mitochondrial networks, 2) be hydrolyzed and destroyed, or 3) affect cardiomyocyte metabolism and oxidative homeostasis [
19,
20]. Moreover, fc-mtDNA is released under several pathologic conditions, and its levels in circulation have been associated with T2DM, inflammation, and CVD [
21,
22]. Although extracellular mitochondria have been detected in circulation under disease conditions, it is still unclear whether these result from functional intercellular transfer, cellular waste, or byproducts of cell death. For example, extracellular Cell-free mitochondrial DNA (cf-mtDNA) is released from cells under stress and its levels have been correlated with several cardiometabolic conditions such as hypertension [
23], diabetes [
24], coronary artery disease [
21], heart failure [
25] and myocardial inflammation [
26]. Nonetheless, CEMI have shown potential not only for prognostic and diagnostic purposes, but also for therapeutic applications in CMD [
27,
28]. Circulating mitochondria may confer cellular protection and restore bioenergetics after ischemic or metabolic cellular stress. For example, transplantation of autologous skeletal muscle-derived functional mitochondria to ischemic myocardium led to cardioprotection and a significant reduction in the size of the infarction after four weeks of recovery in animal models [
29]. Aside from direct mitochondrial transplantation, MSCs have also been shown to be capable of rescuing ischemia-exposed cardiomyoblasts from cell death through mitochondrial donation in cocultures [
30]. However, questions remain regarding the diagnostic, prognostic, or therapeutic potential role of CEMI in cardiometabolic disease. Here, we summarize their different conformations and functions under physiological and pathological cardiometabolic conditions. We also outline potential diagnostic and prognostic utility and provide perspectives on how extracellular mitochondria can be employed for targeted therapy of CMD.
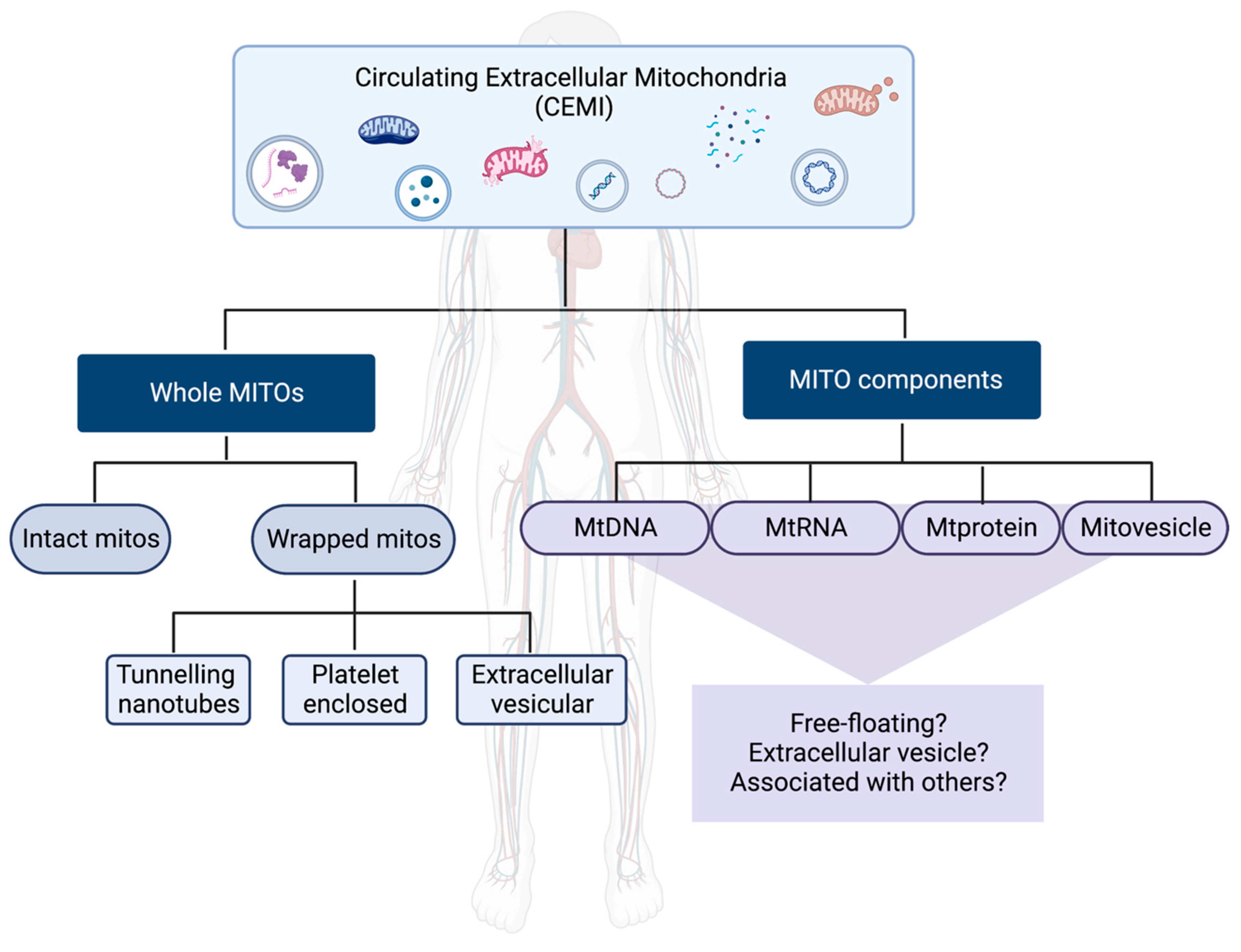
2. Different forms of CEMI
Mitochondria are essential for all living cells except mature RBCs and, more recently, have been attributed to play important functions as an intercellular signaling entity. Given the plethora of CEMI, it is necessary to classify their forms in the circulating milieu and the mode of secretion in order to understand their context and role in diseases. In this section, we attempt to categorize the forms of CEMI, keeping in mind both the possible function and the evolving fields with respect to biomarkers such as extracellular vesicles and RNA biomarkers. On the basis of their composition, CEMI are classified into two forms – whole mitos and mito (mitochondrial) components.
2.1. Whole Mitos
Whole mitochondria or whole mitos includes different sub-categories of forms of CEMI, which have the whole mitochondria in circulation and are involved in intercellular communication to be taken up by recipient cells. Interestingly, this category is currently most applied specifically in the case of the therapeutic use of mitochondria. This form is further classified as (1) Circulating intact mitos and (2) Wrapped mitos on the basis of how they are transferred between cells.
2.1.1. Intact mitos
This category is mainly composed of whole intact mitochondria that are free-floating in circulation, previously known as FreeMitos. One of the first studies to propose the intercellular transfer of mitochondria1, primarily mediated by secreted mitochondria and its active uptake in recipient cells, was assessed by checking the mtDNA and the mitochondrial function in the recipient cells. Since then, many studies have not only explored if intact whole mitochondria are secreted but, more importantly, examined the physiological mechanism of how the intercellular transfer of this form of CEMI occurs. Plasma-derived, whole, intact mitochondria were undeniably demonstrated using electron microscopy by Pollara et al. Further, Dache et al., for the first time, showed that human plasma contains respiration-competent intact functional mitochondria, seen using electron microscopy and electron flow analysis by seahorse. The presence of whole intact mitos was corroborated by many subsequent studies, although whether these mitos are respiration-competent has contrasting reports. However, the function of these intact mitos appears to be context-dependent. For instance, plasma-derived intact mitos observed by Pollara et al. from donors with brain death or cardiac death were inflammation-inducing and correlated with early allograft dysfunction in patients receiving liver transplants. A similar immune-activating phenotype of this form was observed in other studies as well, albeit using other mechanisms, with the ability of these intact mitos to enter cells. Contrastingly, other studies suggest that respiration-competent intact mitos could have protective roles in the recipient cells. Interestingly, there are numerous studies using these intact mitos as a mode of therapy for various diseases ranging from cardiovascular diseases to neurodegenerative diseases and several mitochondrial disorders, even though studies claiming the transfer of intact mitochondria are comparatively fewer.
2.1.2. Wrapped Mitos
Whole mitochondria or whole mitos includes different sub-categories of forms of CEMI, which have the whole mitochondria in circulation and are involved in intercellular communication to be taken up by recipient cells. Interestingly, this category is currently most applied specifically in the case of the therapeutic use of mitochondria. This form is further classified as (1) Circulating intact mitos and (2) Wrapped mitos on the basis of how they are transferred between cells. When whole mitochondria are encapsulated by a membrane so as to be transported from a donor cell to a recipient cell, they are termed wrapped mitos. These are further subcategorized based on the nature of the membrane enclosing the mitos into the following types:
Tunneling mitos are those intact mitochondria that are transferred by tunneling nanotubes between a donor and a recipient cell. Interestingly, mitochondrial transfer due to tunneling is probably one of the first discovered modes of intercellular mitochondrial transfer and is evolutionarily conserved across different kingdoms ranging from bacteria to plant and animal cells. Tunneling nanotubes are nanosized (40-200nm in width and >1um length) tubular protrusions of the cell membrane that encompass F-actin filaments connecting two cells (usually a donor and a recipient cell) which exchange mitochondria and other cargo in a unidirectional or a bidirectional manner. This phenomenon has been observed in almost all types of cells – normal cells and tumor cells (both in vitro and in vivo) primarily to transport mitochondria. With respect to function, tunneling mitos could be transferring functional mitochondria from donor to recipient cells. In several cancers, these tunneling mitos bestow chemoresistance and invasiveness to the recipient cells.
-
b.
Platelet mitos
Platelets are one of the largest extracellular mitochondria carriers. They are 2-4 mm in size and are anucleate cells involved in processes including coagulation, wound healing, thrombosis, and hemostasis. Each platelet has around 4-6 mitochondria which regulate platelet activation. Interestingly, mtDNA is the only genetic material present in these cells. While there has been evidence showing the importance of mitochondria in platelet function and how this correlates with the pathogenesis of several diseases, it is only through the study of Boudreau et al., that platelets have been viewed as a carrier for mitochondria. In fact, this study shows how platelets use mitochondria as mediators of inflammation by either directly releasing mitochondria or via microparticles upon activation and actively taken up by neutrophils.
-
c.
EV mitos
Extracellular vesicle (EV) mitos are whole mitochondria that are packaged into EVs, 30 nm- 10 um-sized bilipid membraneous structures that carry a variety of cargo, including nucleic acids, proteins, lipids, and in this case, even whole organelles such as mitochondria. There are several studies substantiated by electron microscopic evidence that have reported the presence of whole intact mitochondria in EVs. In fact, smaller EVs with a size range of 0.1-1um have been termed as microparticles (MP) when blebbing from apoptotic bodies, and many studies have reported intact mitos being housed in them. Interestingly, one of the first studies demonstrating the platelet mitos also showed that the EVs secreted by platelets carry intact mitochondria 44. Subsequently, several studies emerged since then showing that EVs are an important carrier of whole mitos. However, whether these whole mitochondria are pro-inflammatory or beneficial is inconclusive and depends upon the context of their identification. EV mitos are being increasingly identified to be an important form of CEMI and merit further investigation.
2.2. MITO Components
Apart from whole mitos, mitochondrial components are found in circulation, either by themselves or often associated with specific carriers such as EVs. The presence as well as the levels of these components in circulation could be indicative of the physiological condition of the cell and has been observed to be altered upon being stressed. As the name suggests, the primary circulating MITO components are mtDNA, mtRNA, and mtproteins. We also include mitovesicles, as a separate category, which originate from mitochondria that go into circulation and have been implicated in several pathologies ranging from chronic diseases, including cancers, systemic metabolic diseases, and neurodegenerative diseases, to acute diseases, such as infections and sepsis. Here, we discuss the above-mentioned four types of MITO components.
2.2.1. Circulating mtDNA
MtDNA is the most studied form of CEMI, often considered to represent the complete mitochondria by itself. Interestingly, the evolutionarily conserved bacterial genomic origin of mitochondria is recapitulated in the ability of mtDNA to trigger inflammatory pathways through mitochondria-derived damage-associated molecular patterns (mtDAMPs). MtDNA can exist in its naked form or as encapsulated by EVs in circulation. Its ability to trigger inflammation when in circulation makes it an important biomarker in several diseases. In fact, circulating mtDNA was first one of the first forms of CEMI to be considered as a biomarker in various pathologies, from cancer to cardiovascular disease, from allergy to autoimmune diseases such as systemic lupus erythematosus and even infections including COVID-19 as well as trauma. Recent research on circulating mtDNA suggest an increasing association with aging and mortality.
Moreover, EV mtDNA levels have been associated with race and ancestry, with one study reporting higher concentration levels of mtDNA in African American participants with African ancestry haplogroup compared with White participants and European ancestry haplogroup. However, mtDNA haplogroups reflect genetic ancestry whereas race is a social construct. mtDNA haplogroups are maternally inherited and influenced by population migration, resulting in a haplogroup of one ethnicity becoming dispersed among another ethnic group that can yield in concordance between ancestry and race [
31]. This sheds light on the importance of considering ancestry and race in biomarker concentrations and further studies.
2.2.2. Circulating mtRNA
It is important to mention that most studies primarily view the transfer of mitochondria through either the mtDNA or mt protein. However, the extracellular mtRNA content has been identified to behave as another source of DAMPs in inflammation. Another repertoire of mtRNA composed of non-coding RNAs, including lncRNAs, circRNAs, and miRNAs derived or associated with mitochondria, are only being recently investigated for their role in pathogenesis. While the mitochondrial genome is composed primarily of the protein-coding machinery and genes (mRNAs, rRNAs and tRNAs), nearly 15% is made up of non-coding RNAs and could have important roles as biomarkers. For instance, LIPCAR, a circulating lncRNA originating from mitochondria, was found to be a biomarker in heart failure51. However, the literature on circulating miRNAs, circular RNAs, and other non-coding RNAs are limited and warrant further investigation.
2.2.3. Circulating mtproteins
The presence of mtproteins, representative of the different mitochondrial compartments, has been recorded in the extracellular milieu, many times indicative of different pathologies. However, from recent studies, we observe that while there is evidence of selective packaging of mtprotein in EVs, there are also mtprotein expressing vesicles (called “mitovesicles”) that carry other components of the mitochondria in circulation. Interestingly, ATP synthase, a mitochondrial enzyme, has been observed to be functional in circulating EVs of melanoma patients. In another study, the different polymorphic variant of mtproteins and even mtDNA have been associated with ageing-associated diseases, and could emerge as prognostic/diagnostic biomarker for metabolic states. Certainly, detailed exploration of these aspects should be critically performed to provide newer insights into this emerging field.
2.2.4. Circulating Mitovesicles
Circulating mitovesicles, an emerging newer class of CEMI, are extracellular vesicles that are secreted by the mitochondria consisting of components of mitochondria. These mitovesicles are crucial, especially in pathologies, given that they provide a snapshot of mitochondria during the development of the disease. There has been considerable literature regarding the generation of mitochondrial-derived vesicles that transport mitochondrial cargo between different cellular organelles. One of the primary causes for the generation of these vesicles is thought to be due to mitophagy and is, therefore, a reflection of mitochondrial health. In fact, these intracellular vesicles have been considered a measure of the quality control system as observed in cardiac cells and show an increase under oxidative stress. However, the role of these vesicles in the extracellular milieu is only being recently explored. One of the first reports regarding mitovesicles was in the context of neurodegenerative disorders such as Alzheimer’s disease and Down’s syndrome wherein the brain -derived cargo were altered in the disease state [
32]. A noteworthy mention is the functional effect of mitochondrial components in EVs that are transferred between cells. For instance, mitochondrial cargo in EVs from adipocytes to cardiac tissues can confer a protective effect on the cardiomyocytes. However, the transfer of mitochondrial components isn’t always to our benefit. Recent evidence suggests that several viruses target mitochondria to evade the immune response by hitching a ride in mitovesicles. More specifically, viruses associated with myocarditis have been shown to replicate and spread through mitovesicles, which can activate an autoimmune response [
31]. Whether these are mitovesicles or just mitochondrial components packaged in multivesicular bodies (MVBs) that form an integral part of the EV packaging remains to be seen. Nevertheless, the functional and biomarker role of these mitovesicles needs to be thoroughly investigated to glean information on the ability to track different pathologies.
Interestingly, there have been reports of the extrusion of fragmented mitochondria that activate inflammatory pathways through cytoplasmic vacuoles in plasma membranes by cells undergoing atypical tumor necrosis factor α-induced caspase-dependent apoptosis. Fragmentation of mitochondria occurs as a response to cellular stress, and this case could be a cellular mechanism of coping during the stress condition. In this case, multiple mitochondrial components are found in the extracellular space and are taken up by neighboring cells to activate inflammation pathways. Furthermore, other evidence suggests that mechanically induced cellular stress activates inflammatory reactions. Mechanical overload applied to tendon cells results in disruption of the mitochondrial network, triggering the release of the deformed mitochondria outside of the cell, called extracellular mitochondrial (ExtraMito) particles. Once released, these ExtraMito particles then activate macrophage chemotaxis and increase the production of proinflammatory cytokines that contribute to the inflammatory response [
33]. However, this is a nascent field, and certainly, techniques like electron microscopy and confocal microscopy add tremendous clarity to both the generation process and the nature of CEMI.
There are a few considerations that are pertinent to the nature of CEMI that should be mentioned. The field of CEMI is evolving, and the methodology dictates the type of form identified. Given that mitochondria are inseparable from the cellular process of apoptosis, there is no definite way to rule out if some types of CEMI originate from an apoptotic cell or intact cells. Certainly,
in vitro experiments could shed light on the mechanism of how CEMI are released – however, one needs to be cautious in the type of media used as recent reports suggest serum (FBS, HBS) contains freemitos. Evidence has even suggested that mitochondria themselves play an important role in the development and release of EVs, which may house CEMI [
34]. Nevertheless, for each of the forms, it would be necessary to experimentally validate using multiple methods to eliminate any other misleading representation.
Now that we have delineated the different forms of CEMI, we now describe the specific CEMI and their role specifically in CMDs classified based on different aberrant metabolic pathways in the following sections.
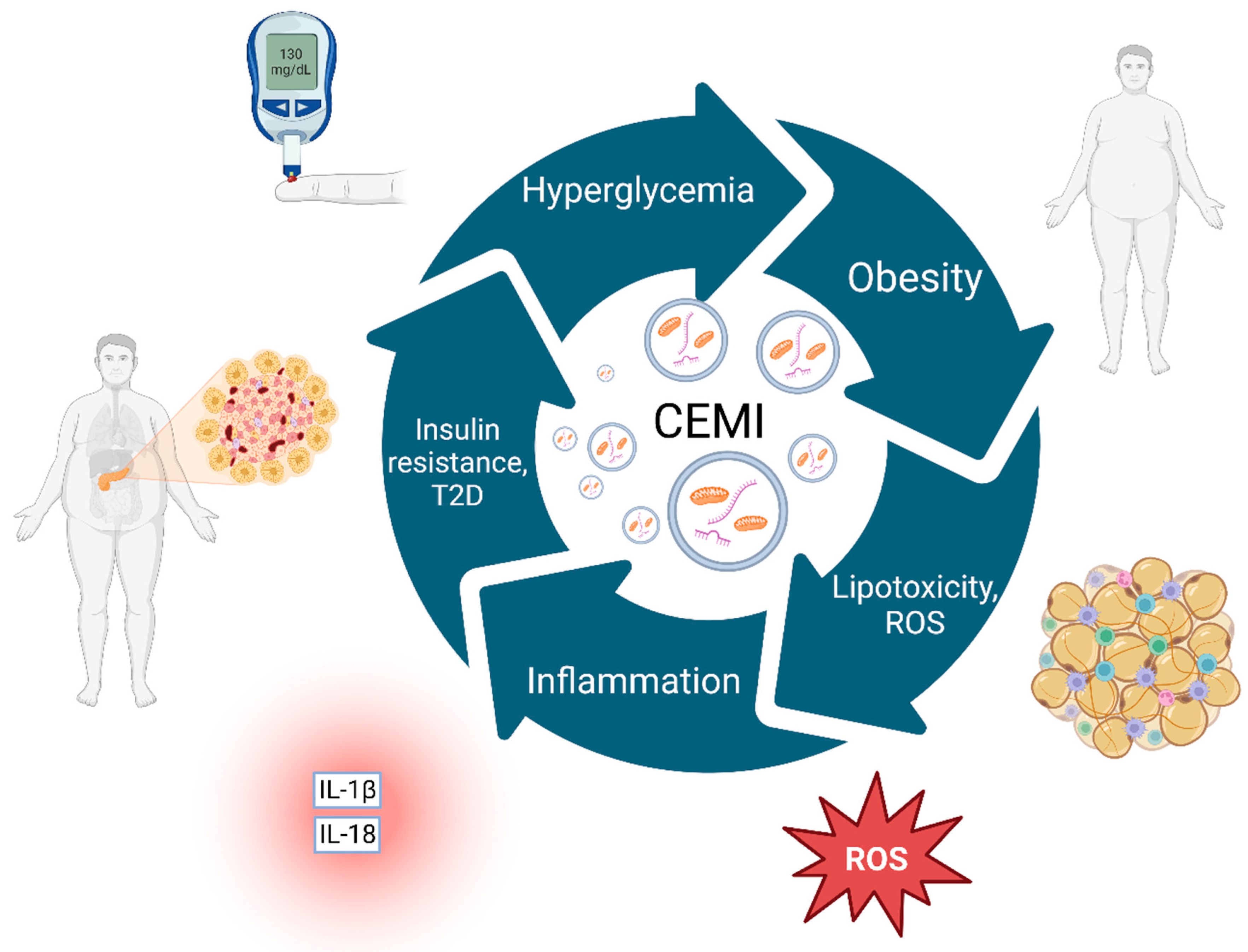
3. T2D, Obesity, and Insulin Resistance
T2D, Obesity, and insulin resistance are intertwined, forming a vicious cycle at the basis of CMD pathophysiology (
Figure 2). Obesity often leads to fat cells becoming filled with lipids, leaking more fatty acids, and becoming metabolically rigid [
35]. This stress on the endoplasmic reticulum contributes to insulin resistance, inflammation, and fibrosis, causing systemic lipotoxicity and promoting comorbid conditions like CVD [
36,
37]. These are accompanied by inflammation, oxidative stress, mitochondrial and adipose tissue dysfunction. In obesity, adipocytes release CEMI in the form of small EVs containing damaged but respiration-capable mitochondria into the bloodstream [
20]. While some cells, like cardiomyocytes, might handle this without significant damage [
20], other tissues were observed to be negatively affected. For example, mitochondrial dysfunction induced by oxidative stress contributes to obesity-related insulin resistance [
38].
Obesity leads to lipotoxicity and ROS accumulation, resulting in heightened inflammation levels as evidenced by inflammatory markers such as IL-1β and IL-18. Lipotoxicity, inflammation, and other comorbid factors subsequently foster insulin resistance, paving the way for the development of T2D. T2D exacerbates the condition by further increasing blood glucose levels, a condition termed "hyperglycemia," which feeds back into the cycle, enhancing obesity. At the center of the cycle, CEMI represent key elements that, in various forms, interact with all stages of this pathophysiological process. They can originate from several sources and play a vital role in driving and maintaining this vicious cycle through their involvement in inflammation, oxidative stress, and other metabolic dysfunctions. CEMI; Circulating extracellular mitochondria, ROS; Reactive Oxygen Species, T2D; Type 2 Diabetes. Created with BioRender.com
T2D is characterized by hyperglycemia, which triggers endoplasmic reticulum stress and mitochondrial dysfunction, generating reactive oxygen species (ROS). This, in turn, contributes to increased systemic inflammation [
39]. Hyperglycemia and obesity can also impair autophagy, a crucial mechanism for mtDNA clearance [
40,
41]. Impaired autophagy and reduction in disulfide-bond A oxidoreductase-like protein (DsbA-L), an antioxidant enzyme that preserves the mitochondrial-associated ER membrane, could lead to the release of mtDNA and activate inflammatory pathways such as the cGAS-cGAMP-STING pathway [
42,
43]. It’s worth noting that conditions such as hyperglycemia, high-fat diet, obesity, and increased ROS levels can reduce DsbA-L levels [
42,
43,
44]. Several studies have also shown that circulating levels of mtDNA are increased in the plasma of patients with T2D and correlate with inflammation (as evidenced through increased CRP and IL-1β levels) and oxidative stress [
45,
46]. These elevated mtDNA levels are also found in prediabetic patients and are linked with early endothelial cell dysfunction and insulin resistance [
24]. mtDNA has also been linked with diabetic complications like diabetic retinopathy, making it a potential prognostic biomarker for T2D [
47,
48]. The harmful impact of mtDNA in T2D may stem from intracellular mitochondrial dysfunction and the activation of Pattern recognition receptor (PRR) inflammatory pathways. PRRs recognize molecules released by injured or transformed cells known as damage-associated molecular patterns (DAMPs) [
49]. Certain PRRs are triggered by mtDNA from T2D patients, lead to the assembly of the inflammasome, an immune complex that induces the secretion of cytokines IL-1β and IL-18 [
24,
45,
50]. Circulating mtDNA from T2D patients activates caspase-1 via the AIM2 inflammasome in macrophages and stimulates endothelial TLR9, which impairs the endothelium [
45].
Obesity has a well-established association with T2D acting as a risk factor and a comorbidity [
51]. Levels of mtDNA were observed to be elevated both in serum and in the urine of obese patients compared to non-obese [
52]. Interestingly, obese T2D patients have significantly higher urinary mtDNA levels compared to obese patients without T2D, potentially indicating intercellular mitochondrial kinetics of the kidneys are affected by T2D in obesity [
52]. Moreover, patients who undergo treatment for obesity and T2D, including bariatric surgery or medication like empagliflozin, show a significant reduction in urinary mtDNA levels [
52,
53]. This is a noteable demonstration of the prognostic potential and/or response-to-treatment biomarker of urinary mtDNA and warrants further investigation in larger patient cohorts. In conclusion, extracellular mtDNA levels may reflect cellular mitochondrial homeostasis [
46], are associated with disease, and return to baseline after treatment, and therefore, may hold significant non-invasive biomarker potential.
4. Atherosclerosis, Cardiac inflammation, and Endothelial Dysfunction
Dysfunction and paradoxical activation of the vascular endothelium, a symptom of chronic and acute inflammation in atherosclerosis, underlies all coronary syndromes – another important branch of CMD [
54]. Different forms of CEMI may contribute to this pathological continuum of atherosclerosis, endothelial dysfunction and eventually myocardial infarction (
Figure 3). Platelets, central to the harmful cycle driving atherosclerosis, endothelial dysfunction, and cardiometabolic disease, can cause hypercoagulability, aberrant thrombosis, and inflammation. These conditions, in turn, can contribute to their activation [
55,
56]. Platelet mitochondria, numbering five to eight per platelet, are vital to platelet metabolism, activation, and apoptosis. Their dysfunction can trigger platelet activation or apoptosis, negatively impacting the endothelium as observed in CVD, diabetes, and sepsis [
57,
58]. Additionally, activated platelets can release CEMI within microparticles, as free organelles, or as circulating mtDNA [
59]. The mitochondrial membrane can be hydrolyzed by secreted phospholipase A2 IIA (sPLA2-IIA), producing inflammatory mediators and triggering leukocyte activation and inflammatory responses [
4]. Regardless of the originating cell, rising mtDNA levels are associated with age and pro-inflammatory cytokines like IL-6, TNF-α, and IL-1 receptor antagonists [
60].
Another form of CEMI, circulating mtDNA, can act as a proinflammatory signal, stimulate cytokine production in monocytes and potentially contribute to age-related cardiovascular diseases such as atherosclerosis and ischemic heart disease [
60]. Increased mtDNA levels are linked to higher mortality in various pathological contexts. In patients with chronic illnesses or acute coronary syndromes, mtDNA has been identified as a predictor of fatal outcomes [
61,
62]. Notably, coronary artery disease (CAD) patients exhibit a two-fold increase in circulating mtDNA, while CAD patients with T2D show a five-fold increase [
63]. MtDNA levels have been found to change dynamically in response to treatment in acute coronary syndromes, returning to normal levels within two days post-percutaneous coronary intervention (PCI) [
64].
Besides mtDNA, other types of CEMI, such as mitochondrial long noncoding RNAs (lncRNAs), have also been implicated in CVD. These RNAs, composed of more than 200 nucleotides, exist in various cytoplasmic organelles, including mitochondria. Mitochondrial lncRNAs can be released extracellularly, but the molecular mechanisms underlying the extracellular trafficking of mitochondria-encoded lncRNAs in humans are poorly understood. For instance, the mitochondrial lncRNA LIPCAR has been found to be elevated in the plasma of patients with HF, and its levels are independently associated with an increased risk of left ventricular remodeling, cardiovascular death, coronary artery disease, and increased HF severity after AMI [
65,
66,
67]. Given LIPCAR’s widespread expression across tissues, its presence in plasma samples could reflect the release of mitochondrial components from a range of cells. This release of extracellular mitochondrial lncRNA might reflect pathological conditions in mitochondrial metabolism and structure.
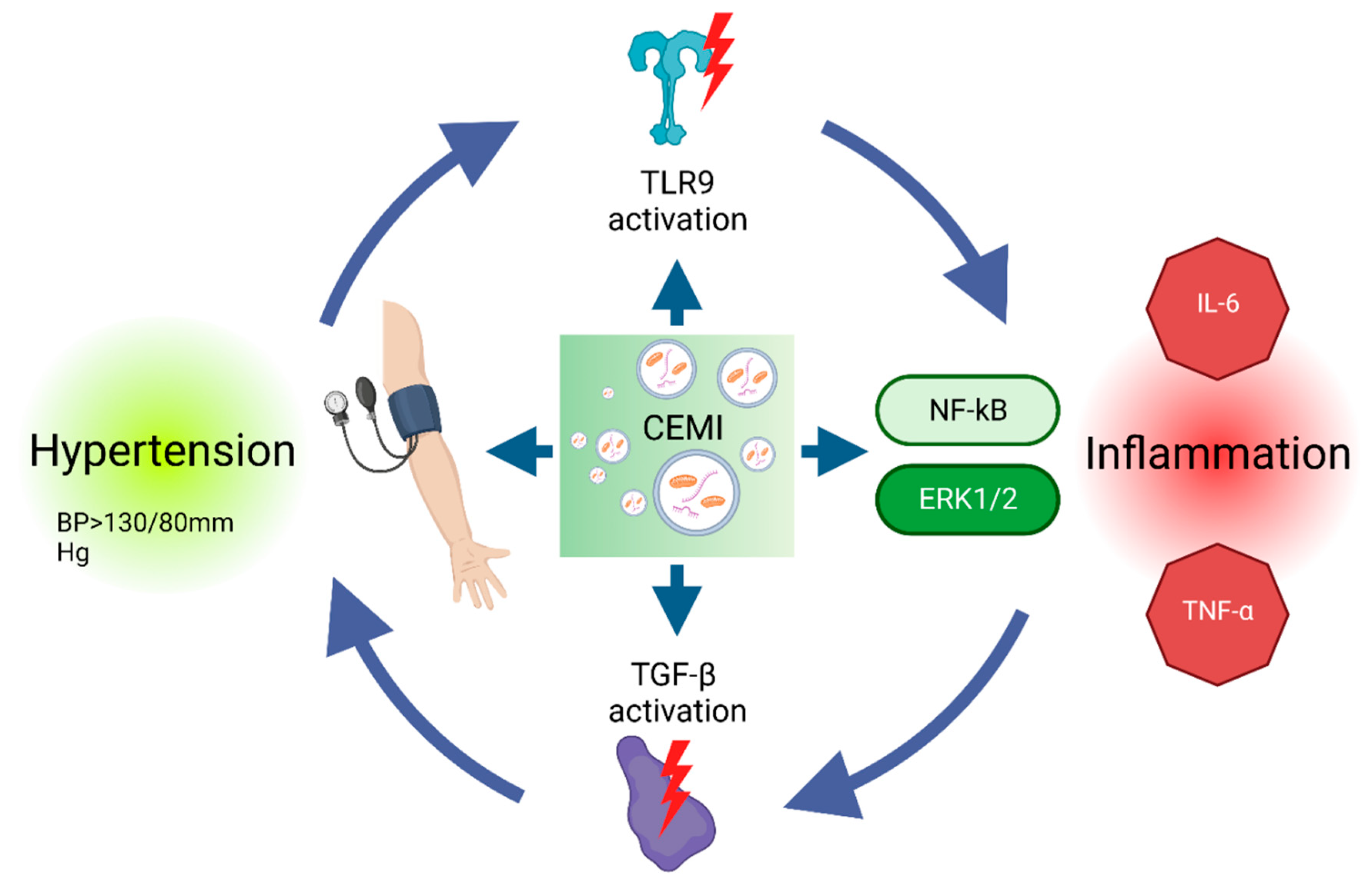
5. Hypertension and Inflammation
According to the 2017 ACC/AHA guidelines, hypertension is identified when blood pressure persistently surpasses 130/80 mm Hg, with a prevalence of 30% in the United States [
68]. Hypertension elevates the risk of CMD and may be influenced by inflammation [
69]. Numerous cytokines and complement-related proteins, including highly sensitive C-reactive protein (CRP), are elevated in individuals with hypertension [
70].
CEMI, such as mitochondrial DNA (mtDNA), have been implicated in the pathogenesis of hypertension by inducing inflammation in essential organs, highlighting the significant role of CEMI contributing to hypertension pathophysiology [
71] (
Figure 4). Transforming growth factor-beta (TGF-β) has been identified as a vital mediator of circulating mtDNA-induced inflammation in obesity-associated hypertension [
23]. Excessive central TGF-β can emulate mtDNA effects, causing hypertension, while TGF-β receptor antagonists can counteract this process. Nuclear factor kappa B (NF-κB) activation, regulated by the central nervous system, may also play a role. MtDNA can activate NF-κB via the Toll-like receptor 9 (TLR9) pathway, and TGF-β can further enhance NF-κB activation by destabilizing the inhibitor of nuclear factor kappa B (IκBα) mRNA [
23]. Since similar observations have been made regarding mtDNA in the circulation of hypertensive animal models and hypertensive patients, there is potential for mtDNA utilization in hypertension prognosis and diagnosis. Furthermore, MtDNA has shown a positive correlation with increased arterial blood pressure in spontaneously hypertensive rat (SHR) models [
72]. In vitro studies have shown that mtDNA augments pro-inflammatory molecules, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) and amplifies the contractile response to phenylephrine in male SHR aortas, but not in females. This effect is associated with heightened phosphorylation of ERK1/2 and can be attenuated by inhibiting TLR9 [
72]. Further research has revealed that increased mtDNA levels in plasma provoked inflammation by activating TLR9 in another SHR model. According to this study, a TLR9-specific CpG oligonucleotide (ODN2395) induced endothelial dysfunction in normotensive rats and led to hypertension [
71].
Renal medullary hypoxia and increased oxidative stress have been identified in hypertensive African American patients [
73]. As mentioned before, urinary mtDNA level exhibs diagnostic and prognostic utility as a non-invasive method for CMD assessment. Urinary mtDNA levels, released at renal mitochondrial injury sites, are significantly elevated in those patients, however it should be underlined that in this study hypertensive patients were also obese [
73]. Nonetheless, in par with those findings, a subsequent study found urinary mtDNA levels to be elevated in patients with renovascular hypertension, highlighting the direct relationship between CEMI and elevated blood pressure [
74].
However, notwithstanding the emerging evidence linking CEMI to hypertension, several knowledge gaps need to be addressed to fully elucidate their role in the diagnosis, prognosis, and management of this disease. For example, the mechanisms underlying the release of CEMI in hypertensive conditions, their interactions with the vasculature, and the role of inflammation, possibly as a catalyst, remain to be clarified. Additionally, it is essential to determine the specificity of CEMI as biomarkers for hypertension, considering the potential confounding effects of other factors such as age, sex, and other comorbidities on their circulating levels. For instance, research has demonstrated that serum mtDNA levels are approximately four times higher in male patients with AH compared to females [
72]. Moreover, most studies mentioned included patients with other comorbid conditions that may confound the effect of elevated blood pressure. Overall, by deepening our understanding of the role of CEMI and their components in hypertension and CMD, we may be able to improve the diagnosis, prognosis, and treatment of this prevalent condition and its associated complications.
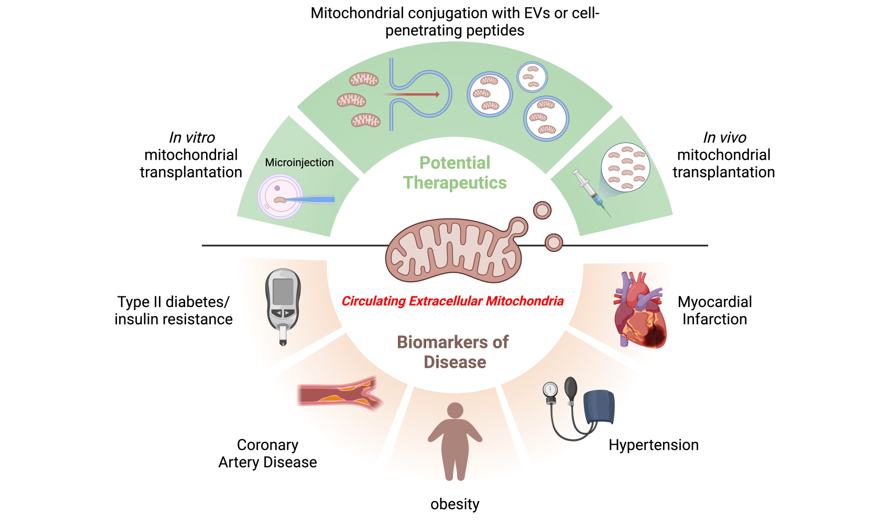
6. Mitochondrial Transplantation for Cardiovascular and Cardiometabolic Diseases: Strategies and Approaches
Mitochondrial dysfunction is a common pathogenesis in various cardiovascular and cardiometabolic diseases. More recently, researchers have explored the possibility of healthy tissue mitochondria transplantation or CEMI delivery into cells to restore their function. This intervention aims to restore cellular bioenergetics, reduce oxidative stress, and promote cellular repair and regeneration. Various strategies and approaches have been explored to optimize the efficiency and applicability of CEMI treatment (
Table 1). Based on the mode of CEMI delivery, two main strategies have been employed: Direct Mitochondrial Transplantation (DMT) and indirect Mitochondrial Delivery (IMD). Based on the source of mitochondria, CEMI transplantation can be further subcategorized as autologous, non-autologous, or interspecies (
Table 1).
DMT have been performed both
in vitro and
in vivo. The
in vitro transplantation of mitochondria has been conducted through two main methods: microinjection of mitochondria into cells and co-incubation of mitochondria with recipient cells [
18]. King and Attardi (1988) performed an early study where human mitochondria were microinjected into human cells. Several mtDNA and nuclear DNA polymorphisms showed that exogenous mtDNA rapidly replaced the resident mtDNA. This replacement was completed six to ten weeks after the microinjection [
75]. In a more recent study by Ali Pour et al., isolated mitochondria from different sources were co-incubated with healthy rat cardiomyocyte H9c2 cells. The co-incubation strategy is based on the endosymbiotic theory of mitochondrial origin. Enhanced bioenergetics in recipient cardiomyocytes were observed 2 days following co-incubation, as assessed by significant increases in cells’ basal respiration and ATP production. Based on a 28-hour time-lapse study, mitochondrial internalization was observed at various time points starting from the 5-hour timepoint. However, it was hard to prove that the beneficial effects could be solely attributed to the exogenous mitochondria or were due to secondary effects of the transplantation. Moreover, the effects were transient and diminished over time to return to baseline control 28 days post-treatment [
76]. In vivo mitochondrial transplantation has been explored through various methods. Shi et al. (2017) studied the in vivo delivery approach in experimental Parkinson’s disease (PD) model mice by injecting mitochondria isolated from human hepatoma cells intravenously. Mitochondrial replacement improved electron transport chain activity, decreased reactive oxygen species levels, and prevented cell apoptosis and necrosis, preventing PD progression. Interestingly, the same group demonstrated the distribution of intravenously injected mitochondria in various organs, including the brain, liver, kidneys, muscle, and heart [
77]. An alternative strategy involves the intraoperative injection of viable CEMI directly into the ischemia-affected tissue. Using this technique, which is the first of its kind to be used on a pediatric population, autologous mitochondria were injected intraoperatively into the myocardium of patients who required ECMO support as a result of ischemia-reperfusion injury (IRI). All patients demonstrated improvement in ventricular function within several days following mitochondrial injection without any adverse short-term complications [
78]. Intraoperative injection of autologous CEMI directly into the myocardium has also been studied on IRI porcine and rabbit models [
29,
79]. In response to CEMI treatment, oxygen consumption, synthesis of high-energy phosphates, and modulation of cytokine mediators and proteomic pathways were increased, all of which contribute to preserving the myocardium’s function, retaining its viability, and improving cardiac function following ischemic injury [
29,
79,
80]. Using coronary arteries as conduits for mitochondrial perfusion is a less invasive alternative to direct myocardial muscle tissue injection [
81]. Intracoronary perfusion of autologous mitochondria decreased infarct size and significantly enhanced post-ischemic myocardial function. This allows mitochondria to be distributed rapidly throughout the heart, providing immediate cardioprotection [
81].
IMD exploits the natural homing and integrative capabilities of delivery systems such as nanoparticles (EVs), cells, and peptides to achieve efficient mitochondrial delivery to tissues. For instance, CEMI encapsulated in EVs has shown potential for effective and rapid delivery to injured myocardial tissue. EVs derived from mesenchymal stem cells (MSCs) were studied for their therapeutic effects in patients with anthracycline-induced cardiomyopathy (AIC). It was found that large EVs enriched in mitochondrial components improved cardiomyocyte viability and inhibited apoptosis. Furthermore, EV treatment improved contractility, reactive oxygen species production, ATP production, and mitochondrial biogenesis in injured cardiomyocytes [
82]. Similarly, another study examined the feasibility of using mitochondria-rich EVs derived from human induced pluripotent stem cell-derived cardiomyocytes (iCMs) to enhance cardiac function and restore myocardial bioenergetics. Large EVs contained healthy mitochondria and were found to be capable of transferring them into recipient iCMs. This study demonstrated increased intracellular ATP production and improved contractile profiles of hypoxia-injured iCMs when treated with these EVs [
83]. Cell-penetrating peptide conjugation is another approach that has been investigated for its therapeutic role [
84]. Chang et al. conjugated mitochondria with the Pep-1 peptide (Pep-1-mito), which facilitated mitochondria internalization. As part of this study, the researchers used Pep-1-mito to treat a genetic mitochondrial disease cell model (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes, or MELAS disease). Pep-1-mito treatment led to mitochondrial function restoration after four days, as well as increases in mitochondrial mass, mitochondrial biogenesis, and the ratio of mitochondrial fusion to fission [
84].
Depending on the source of mitochondria, mitochondrial transplantation may be classified as autologous, non-autologous (allogeneic), or interspecies. Using autologous transplantation, mitochondria are isolated from a patient’s own tissues [
78]. Non-autologous transplantation, on the other hand, involves isolating mitochondria from a donor’s tissues [
76]. Finally, interspecies transplantation involves isolating mitochondria from different species [
76]. All these above-mentioned mitochondrial transfer methods have successfully showcased mitochondria’s therapeutic potential in the context of CMDs. However, this is a preliminary approach that requires an in-depth exploration to be successfully incorporated as a treatment option.
7. Integration of CEMI in Everyday Clinical Practice: Challenges and Limitations
The incorporation of CEMI into everyday clinical practice can revolutionize the management of cardiovascular diseases. However, several aspects need to be considered before fully integrating CEMI into routine clinical settings. To ensure the accuracy and reliability of CEMI as diagnostic and prognostic biomarkers, it is crucial to establish standardized protocols for isolating and quantifying CEMI from blood samples. These standardized procedures will enable the comparison of results across different studies and facilitate the development of CEMI-based diagnostic tests and prognostic tools. Furthermore, clinical interpretation of CEMI levels requires the establishment of reference values for healthy individuals and different disease states. Age, sex, and ethnicity-based reference ranges should be defined to account for potential variations in CEMI levels among diverse populations. Understanding the kinetics and dynamics of CEMI release, uptake, and clearance in healthy as well as diseased states is essential for the successful integration of CEMI into clinical practice. This knowledge will help determine the optimal time points for CEMI assessment and their relevance as diagnostic and prognostic biomarkers of CMDs. Finally, the diagnostic, prognostic, and therapeutic potential of CEMI should be validated in large-scale clinical studies, including randomized controlled trials. These studies will help assess the efficacy and safety of CEMI-based interventions and provide a solid foundation for their integration into everyday clinical practice.
Regarding production, carrier, and delivery, one of the major limitations is the lack of standardized protocols for CEMI isolation and quantification. Currently, there is no consensus on the best method for isolating and quantifying CEMI, which may result in variability and inconsistency in the results obtained by various research groups. This highlights the need for standardization and validation of methods for isolation and quantification to ensure reliable and reproducible results. Furthermore, the precise roles of extracellular mitochondria in intercellular communication and disease pathogenesis are not yet fully understood. Indeed, further research is needed to elucidate the mechanisms underlying CEMI-mediated effects in cardiovascular diseases and other pathological conditions. Regarding CEMI carriers and forms, EVs containing mitochondria are a heterogeneous population, which may influence their diagnostic and therapeutic potential. The development of techniques to selectively isolate and characterize subpopulations of mitochondria-rich EVs is necessary to optimize their clinical applications. Another major limitation is the CEMI scalability and cost-effectiveness as the large-scale production of certain classes of CEMI, eg. mitochondria-rich EVs for therapeutic purposes, poses a significant challenge. Advances in bioprocessing technologies and the development of cost-effective methods for isolation and purification are required to facilitate the widespread adoption of CEMI-based therapies. Finally, the use of CEMI in clinical practice must adhere to strict regulatory and ethical guidelines, particularly when utilizing stem cell-derived products and considering the immunogenic nature of several of these CEMI. Ensuring the safety, quality, and efficacy of CEMI-based interventions is paramount for their successful integration into everyday clinical practice.
8. Future Perspectives
As our knowledge of the biological functions and mechanisms of CEMI expands, researchers will be better equipped to harness their diagnostic, prognostic, and therapeutic potential. A deeper understanding of CEMI biology will also enable the development of targeted interventions that modulate their release, uptake, and clearance to optimize clinical outcomes. Future developments in isolation, characterization, and quantification techniques for CEMI and other extracellular vesicles will improve their clinical utility. The advent of high-throughput, cost-effective, and reliable methodologies will facilitate the integration of CEMI-based biomarkers and therapeutics into routine clinical practice. The integration of CEMI into clinical practice has the potential to contribute significantly to personalized medicine. By assessing individual patients’ CEMI profiles, healthcare providers may be better able to tailor diagnostic and therapeutic strategies to optimize patient outcomes. This personalized approach may result in more effective treatments, reduced adverse effects, and improved overall patient care. Moreover, future research may explore the potential of combining CEMI-based therapies with existing treatments for cardiovascular diseases to maximize their efficacy. Such combination therapies may help overcome limitations associated with current treatment options and improve patient outcomes. In fact, as our understanding of CEMI grows, their potential application may extend beyond cardiovascular and cardiometabolic diseases to other pathological conditions, such as neurodegenerative disorders, metabolic diseases, and cancer. These new applications may provide novel diagnostic and therapeutic opportunities to improve patient care across a wide range of clinical domains. Surely enough, future developments in regulatory and ethical frameworks will be crucial to ensure the safe and effective integration of CEMI-based interventions into clinical practice. As new therapeutic modalities and diagnostic tools emerge, these frameworks will need to evolve to accommodate advances in CEMI research and maintain the highest standards of patient safety and care.
Another important aspect is also our growing understanding of mitochondrial biology through the lens of quantum biology, wherein mitochondrial bioenergetics has been investigated for incorporating quantum phenomena such as quantum tunneling [
85,
86]. While this field is nascent currently, scientific advancements in this direction could not only accelerate the understanding of normal physiology but also how complex diseases such as CMDs, cancers, and other aging-associated degenerative diseases are driven by the physiological disturbances in this energy flux. This will also impact our approach to therapy and possibly have a meaningful impact on such complicated disorders. This adds an important layer in the field of the practical application of CEMI both in the realm of biomarker and a therapeutic agent. Therefore, while the journey of mitochondrial research is certainly an exciting one, its future also seems to be heading in a deeply impactful direction.
9. Conclusions
The core role of mitochondria in various diseases has long been recognized, as they play a crucial part in cellular bioenergetics, metabolism, and regulation of cell death. Mitochondrial dysfunction has been implicated in the pathogenesis of numerous diseases, including cardiovascular, neurodegenerative, and metabolic disorders. The recent expansion of our understanding to include the extracellular realm of mitochondria has opened new avenues for research and clinical applications. CEMI and their associated forms, such as mitochondria-rich exosomes, have emerged as important mediators of intercellular communication, with significant implications in diagnosis, prognosis, and treatment of various diseases. By transferring functional mitochondria and other bioactive molecules between cells, CEMI can modulate cellular function and response to injury, providing novel therapeutic opportunities for a wide range of clinical conditions. As glimpsed in a few studies, the integration of CEMI into clinical practice has the potential to revolutionize patient care, paving the way for personalized medicine and targeted interventions based on individual patients’ CEMI profiles. However, this potential can only be realized through continued research to deepen our understanding of CEMI biology, refine isolation and characterization techniques, and develop effective therapies that harness the full potential of extracellular mitochondria. In the future, CEMI-based diagnostic, prognostic, and therapeutic strategies are poised to play an increasingly significant role in the management of various diseases. As our understanding of the underlying mechanisms involving newly developing fields and potential applications of CEMI continues to evolve, it is crucial to conduct rigorous clinical trials that will establish the safety and efficacy of these novel approaches - all of which will facilitate the development of more refined and targeted interventions. This will enable clinicians to maximize the utilization of extracellular mitochondria for diagnosis, prognosis, and treatment in a way that complements existing treatment modalities, ultimately improving patient outcomes.
Funding
Saumya Das is funded by grants from NHLBI (R35HL150807, 1RO1HL150401).
Conflicts of Interest
Saumya Das is a co-founder for Switch Therapeutics and Thryv Therapeutics and received research funding from Bristol Myers Squibb and Abbott Laboratories, none of which were relevant for this manuscript.
References
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular Communication: Diverse Structures for Exchange of Genetic Information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial Transfer between Cells Can Rescue Aerobic Respiration. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Duchez, A.-C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T.; et al. Platelets Release Mitochondria Serving as Substrate for Bactericidal Group IIA-Secreted Phospholipase A2 to Promote Inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W.; Mochly-Rosen, D. Fragmented Mitochondria Released from Microglia Trigger A1 Astrocytic Response and Propagate Inflammatory Neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal Stem Cells Use Extracellular Vesicles to Outsource Mitophagy and Shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural Stem Cells Traffic Functional Mitochondria via Extracellular Vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of Mitochondria from Astrocytes to Neurons after Stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.A.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Khutornenko, A.A.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells. Stem Cells Transl. Med. 2015, 4, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Mol. Basel Switz. 2018, 23, 687. [Google Scholar] [CrossRef]
- Liang, W.; Sagar, S.; Ravindran, R.; Najor, R.H.; Quiles, J.M.; Chi, L.; Diao, R.Y.; Woodall, B.P.; Leon, L.J.; Zumaya, E.; et al. Mitochondria Are Secreted in Extracellular Vesicles When Lysosomal Function Is Impaired. Nat. Commun. 2023, 14, 5031. [Google Scholar] [CrossRef]
- Guo, F.; Moellering, D.R.; Garvey, W.T. The Progression of Cardiometabolic Disease: Validation of a New Cardiometabolic Disease Staging System Applicable to Obesity. Obes. Silver Spring Md 2014, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- De Waard, A.-K.M.; Hollander, M.; Korevaar, J.C.; Nielen, M.M.J.; Carlsson, A.C.; Lionis, C.; Seifert, B.; Thilsing, T.; De Wit, N.J.; Schellevis, F.G.; et al. Selective Prevention of Cardiometabolic Diseases: Activities and Attitudes of General Practitioners across Europe. Eur. J. Public Health 2019, 29, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial Dysfunction in Pathophysiology of Heart Failure. J. Clin. Invest. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Wieckowski, M.R.; Sinclair, D.A.; Kroemer, G.; Pinton, P.; Galluzzi, L. Targeting Mitochondria for Cardiovascular Disorders: Therapeutic Potential and Obstacles. Nat. Rev. Cardiol. 2019, 16, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.-F.; Orekhov, A.N. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: An Organ-Based Analysis. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Lee, Y.-S. Mitochondria as Secretory Organelles and Therapeutic Cargos. Exp. Mol. Med. 2024, 56, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D. Transit and Integration of Extracellular Mitochondria in Human Heart Cells. Sci. Rep. 2017, 7, 17450. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Funcke, J.-B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, X.; Xie, L.; Tang, Y.; Cheng, J.; Wang, J.; Wang, L.; Gong, J. Circulating Cell Free Mitochondrial DNA Is a Biomarker in the Development of Coronary Heart Disease in the Patients with Type 2 Diabetes. Clin. Lab. 2015, 61, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Lu, J.; Wang, L.; Gao, M. Pro-inflammatory Role of cell-free Mitochondrial DNA in Cardiovascular Diseases. IUBMB Life 2020, 72, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Alé, A.; Zhang, Y.; Han, C.; Cai, D. Obesity-Associated Extracellular mtDNA Activates Central TGFβ Pathway to Cause Blood Pressure Increase. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E161–E174. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vásquez, N. Circulating Cell-Free Mitochondrial DNA as the Probable Inducer of Early Endothelial Dysfunction in the Prediabetic Patient. Exp. Gerontol. 2015, 69, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA That Escapes from Autophagy Causes Inflammation and Heart Failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.-Y.; Zhang, H.-W.; Gu, J.; Xu, F.; Liang, H.-M.; Fan, K.-J.; Shen, J.-Y.; Xiao, Z.-H.; Zhang, E.-Y.; Hu, J. Mitochondrial DNA-induced Inflammatory Damage Contributes to Myocardial Ischemia Reperfusion Injury in Rats: Cardioprotective Role of Epigallocatechin. Mol. Med. Rep. 2017, 16, 7569–7576. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, M.; van Marion, D.M.S.; Bouman, E.J.; Li, J.; Zhang, D.; Ramos, K.S.; Lanters, E.A.H.; de Groot, N.M.S.; Brundel, B.J.J.M. Cell-Free Circulating Mitochondrial DNA: A Potential Blood-Based Marker for Atrial Fibrillation. Cells 2020, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Kumari, R.; Kamthan, A.; Tiwari, R.; Srivastava, R.K.; Van Der Westhuizen, F.H.; Mishra, P.K. Cell-Free Circulating Mitochondrial DNA: An Emerging Biomarker for Airborne Particulate Matter Associated with Cardiovascular Diseases. Free Radic. Biol. Med. 2023, 195, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of Autologously Derived Mitochondria Protects the Heart from Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef] [PubMed]
- Cselenyák, A.; Pankotai, E.; Horváth, E.M.; Kiss, L.; Lacza, Z. Mesenchymal Stem Cells Rescue Cardiomyoblasts from Cell Death in an in Vitro Ischemia Model via Direct Cell-to-Cell Connections. BMC Cell Biol. 2010, 11, 29. [Google Scholar] [CrossRef]
- Byappanahalli, A.M.; Omoniyi, V.; Noren Hooten, N.; Smith, J.T.; Mode, N.A.; Ezike, N.; Zonderman, A.B.; Evans, M.K. Extracellular Vesicle Mitochondrial DNA Levels Are Associated with Race and Mitochondrial DNA Haplogroup. iScience 2024, 27, 108724. [Google Scholar] [CrossRef] [PubMed]
- D’Acunzo, P.; Argyrousi, E.K.; Ungania, J.M.; Kim, Y.; DeRosa, S.; Pawlik, M.; Goulbourne, C.N.; Arancio, O.; Levy, E. Electrophysiological Effects of Mitochondria-derived Extracellular Vesicles: Implications for Memory Dysfunction in Neurodegenerative Disorders. Alzheimers Dement. 2023, 19, e076549. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Chen, P.; Tai, A.; Li, J.; Bassonga, E.L.; Gao, J.; Liu, D.; Wood, D.; Kennedy, B.F.; et al. Mechanical Overload-Induced Release of Extracellular Mitochondrial Particles from Tendon Cells Leads to Inflammation in Tendinopathy. Exp. Mol. Med. 2024, 56, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Morganti, C.; Van Gastel, N.; Ito, K.; Calura, E.; Zanolla, I.; Ferroni, L.; Zhang, Y.; Jung, Y.; Sales, G.; et al. A Mitochondrial NADPH-Cholesterol Axis Regulates Extracellular Vesicle Biogenesis to Support Hematopoietic Stem Cell Fate. Cell Stem Cell 2024, 31, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The Ominous Triad of Adipose Tissue Dysfunction: Inflammation, Fibrosis, and Impaired Angiogenesis. J. Clin. Invest. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Laddu, D.; Arena, R.; Ortega, F.B.; Alpert, M.A.; Kushner, R.F. Healthy Weight and Obesity Prevention. J. Am. Coll. Cardiol. 2018, 72, 1506–1531. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The Role of Adipose Tissue in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zheng, N.; Ding, X. Mitochondrial Abnormalities: A Hub in Metabolic Syndrome-Related Cardiac Dysfunction Caused by Oxidative Stress. Heart Fail. Rev. 2022, 27, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-Induced Oxidative Stress and Its Role in Diabetes Mellitus Related Cardiovascular Diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Rockhold, J.D.; Conway, R. Selective Autophagy in Hyperglycemia-Induced Microvascular and Macrovascular Diseases. Cells 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting Autophagy in Obesity: From Pathophysiology to Management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, L.; Gao, P.; Zhu, X.; Han, Y.; Chen, X.; Li, L.; Xiao, Y.; Wei, L.; Li, C.; et al. DsbA-L Ameliorates High Glucose Induced Tubular Damage through Maintaining MAM Integrity. EBioMedicine 2019, 43, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cervantes, C.; Liu, J.; He, S.; Zhou, H.; Zhang, B.; Cai, H.; Yin, D.; Hu, D.; Li, Z.; et al. DsbA-L Prevents Obesity-Induced Inflammation and Insulin Resistance by Suppressing the mtDNA Release-Activated cGAS-cGAMP-STING Pathway. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 12196–12201. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, S.; Jiang, N.; Wang, X.; Han, Y.; Zhao, H.; Xiong, X.; Liu, Y.; Zhao, C.; Zhu, X.; et al. DsbA-L Ameliorates Renal Injury Through the AMPK/NLRP3 Inflammasome Signaling Pathway in Diabetic Nephropathy. Front. Physiol. 2021, 12, 659751. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Jo, S.I.; Kim, S.J.; Lee, J.M.; Jeong, J.H.; Kang, J.S.; Cho, N.-J.; Kim, S.S.; Lee, E.Y.; Moon, J.-S. Circulating Cell-Free mtDNA Contributes to AIM2 Inflammasome-Mediated Chronic Inflammation in Patients with Type 2 Diabetes. Cells 2019, 8, 328. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; Pastukh, V.M.; Ruchko, M.V.; Simmons, J.D.; Richards, W.O.; Rachek, L.I. Plasma Mitochondrial DNA Is Elevated in Obese Type 2 Diabetes Mellitus Patients and Correlates Positively with Insulin Resistance. PLoS ONE 2019, 14, e0222278. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Parsade, C.K.; Ajaz, S.; Crosby-Nwaobi, R.; Gnudi, L.; Czajka, A.; Sivaprasad, S. Altered Circulating Mitochondrial DNA and Increased Inflammation in Patients with Diabetic Retinopathy. Diabetes Res. Clin. Pract. 2015, 110, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Silzer, T.; Barber, R.; Sun, J.; Pathak, G.; Johnson, L.; O’Bryant, S.; Phillips, N. Circulating Mitochondrial DNA: New Indices of Type 2 Diabetes-Related Cognitive Impairment in Mexican Americans. PLoS ONE 2019, 14, e0213527. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Martinon, F. The AIM2 Inflammasome: Sensor of Pathogens and Cellular Perturbations. Immunol. Rev. 2018, 281, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies - EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Kim, H.; Noh, H.; Jeon, J.S.; Byun, D.W.; Kim, S.H.; Kim, H.J.; Suh, K.; Park, H.K.; Kwon, S.H. Effect of Bariatric Surgery on Circulating and Urinary Mitochondrial DNA Copy Numbers in Obesity with or without Diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001372. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Park, R.; Byun, D.W.; Kim, H.J.; Suh, K.; Park, H.K.; Kwon, S.H. Empagliflozin Suppresses Urinary Mitochondrial DNA Copy Numbers and Interleukin-1β in Type 2 Diabetes Patients. Sci. Rep. 2022, 12, 19103. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial Dysfunction and Platelet Hyperactivity in Type 2 Diabetes Mellitus: Molecular Insights and Therapeutic Strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.-P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.-L.; Zhang, R.; et al. Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci. Rep. 2016, 6, 36222. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; Cevenini, E.; Nasi, M.; De Biasi, S.; Salvioli, S.; Monti, D.; Benatti, S.; Gibellini, L.; Cotichini, R.; Stazi, M.A.; et al. Circulating Mitochondrial DNA Increases with Age and Is a Familiar Trait: Implications for “Inflamm-Aging”. Eur. J. Immunol. 2014, 44, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.S.; Huh, J.-W.; Schenck, E.J.; Nakahira, K.; Siempos, I.I.; Choi, A.M.K. Circulating Mitochondrial DNA as Predictor of Mortality in Critically Ill Patients. Chest 2019, 156, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Sudakov, N.P.; Apartsin, K.A.; Lepekhova, S.A.; Nikiforov, S.B.; Katyshev, A.I.; Lifshits, G.I.; Vybivantseva, A.V.; Konstantinov, Y.M. The Level of Free Circulating Mitochondrial DNA in Blood as Predictor of Death in Case of Acute Coronary Syndrome. Eur. J. Med. Res. 2017, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, Y.; Tang, Y.; Xi, M.; Xie, L.; Zhang, Q.; Gong, J. Circulating Cell-Free Mitochondrial Deoxyribonucleic Acid Is Increased in Coronary Heart Disease Patients with Diabetes Mellitus. J. Diabetes Investig. 2016, 7, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, L.; Zhang, Q.; Cai, X.; Tang, Y.; Wang, L.; Hang, T.; Liu, J.; Gong, J. Plasma Nuclear and Mitochondrial DNA Levels in Acute Myocardial Infarction Patients. Coron. Artery Dis. 2015, 26, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients with Heart Failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, W.; Long, Q.-Q.; Zhang, J.; Li, Y.-F.; Liu, D.-C.; Yan, J.-J.; Yang, Z.-J.; Wang, L.-S. Increased Plasma Levels of lncRNA H19 and LIPCAR Are Associated with Increased Risk of Coronary Artery Disease in a Chinese Population. Sci. Rep. 2017, 7, 7491. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Wang, M.; Wang, L.; Zhang, W.; Ge, Z.-R. Circulating LIPCAR Is a Potential Biomarker of Heart Failure in Patients Post-Acute Myocardial Infarction. Exp. Biol. Med. Maywood NJ 2021, 246, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and Cardiovascular Risk: General Aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rahimi, K.; Bautista, L.E.; Nazarzadeh, M.; Zargar, M.S.; Shab-Bidar, S. Inflammation Markers and Risk of Developing Hypertension: A Meta-Analysis of Cohort Studies. Heart Br. Card. Soc. 2019, 105, 686–692. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.G.; Wenceslau, C.F.; Goulopoulou, S.; Ogbi, S.; Baban, B.; Sullivan, J.C.; Matsumoto, T.; Webb, R.C. Circulating Mitochondrial DNA and Toll-like Receptor 9 Are Associated with Vascular Dysfunction in Spontaneously Hypertensive Rats. Cardiovasc. Res. 2015, 107, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Echem, C.; da Costa, T.J.; Oliveira, V.; Giglio Colli, L.; Landgraf, M.A.; Rodrigues, S.F.; Franco, M. do C.P.; Landgraf, R.G.; Santos-Eichler, R.A.; Bomfim, G.F.; et al. Mitochondrial DNA: A New Driver for Sex Differences in Spontaneous Hypertension. Pharmacol. Res. 2019, 144, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Saad, A.; Woollard, J.R.; Juncos, L.A.; Calhoun, D.A.; Tang, H.; Lerman, A.; Textor, S.C.; Lerman, L.O. Glomerular Hyperfiltration in Obese African American Hypertensive Patients Is Associated With Elevated Urinary Mitochondrial-DNA Copy Number. Am. J. Hypertens. 2017, 30, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Herrmann, S.M.; Saad, A.; Abumoawad, A.; Tang, H.; Lerman, A.; Textor, S.C.; Lerman, L.O. Urinary Mitochondrial DNA Copy Number Identifies Renal Mitochondrial Injury in Renovascular Hypertensive Patients Undergoing Renal Revascularization: A Pilot Study. Acta Physiol. Oxf. Engl. 2019, 226, e13267. [Google Scholar] [CrossRef] [PubMed]
- King, M.P.; Attardi, G. Injection of Mitochondria into Human Cells Leads to a Rapid Replacement of the Endogenous Mitochondrial DNA. Cell 1988, 52, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Ali Pour, P.; Kenney, M.C.; Kheradvar, A. Bioenergetics Consequences of Mitochondrial Transplantation in Cardiomyocytes. J. Am. Heart Assoc. 2020, 9, e014501. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous Administration of Mitochondria for Treating Experimental Parkinson’s Disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Kaza, A.K.; Wamala, I.; Friehs, I.; Kuebler, J.D.; Rathod, R.H.; Berra, I.; Ericsson, M.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; et al. Myocardial Rescue with Autologous Mitochondrial Transplantation in a Porcine Model of Ischemia/Reperfusion. J. Thorac. Cardiovasc. Surg. 2017, 153, 934–943. [Google Scholar] [CrossRef]
- McCully, J.D.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B. Mitochondrial Transplantation for Therapeutic Use. Clin. Transl. Med. 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016, 11, e0160889. [Google Scholar] [CrossRef]
- O’Brien, C.G.; Ozen, M.O.; Ikeda, G.; Vaskova, E.; Jung, J.H.; Bayardo, N.; Santoso, M.R.; Shi, L.; Wahlquist, C.; Jiang, Z.; et al. Mitochondria-Rich Extracellular Vesicles Rescue Patient-Specific Cardiomyocytes From Doxorubicin Injury: Insights Into the SENECA Trial. JACC CardioOncology 2021, 3, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.-H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell–Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-C.; Hoel, F.; Liu, K.-H.; Wei, Y.-H.; Cheng, F.-C.; Kuo, S.-J.; Tronstad, K.J.; Liu, C.-S. Peptide-Mediated Delivery of Donor Mitochondria Improves Mitochondrial Function and Cell Viability in Human Cybrid Cells with the MELAS A3243G Mutation. Sci. Rep. 2017, 7, 10710. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G. Chronobiology Meets Quantum Biology: A New Paradigm Overlooking the Horizon? Front. Physiol. 2022, 13, 892582. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Sim, W.J.; Namgung, B.; Choi, Y.; Li, B.; Lee, L.P. Quantum Biological Tunnel Junction for Electron Transfer Imaging in Live Cells. Nat. Commun. 2019, 10, 3245. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Schematic representation of different forms of circulating extracellular mitochondrial components (CEMI). Principally CEMI are classified into two broad categories based on the nature of mitochondria. First, being whole mitochondria (Whole MITOs), that are in turn composed of intact mitochondria (intact mitos) or mitochondria wrapped (wrapped mitos) in one the following cellular structures such as tunneling nanotubes, platelets or extracellular vesicles. The second category is composed of mitochondrial components (MITO components) which could either be mitochondrial DNA (mtDNA), mitochondrial RNA (mtRNA), mitochondrial protein (mtprotein) or mitochondria-derived vesicles (mitovesicles) with one or more of these components which could circulate by themselves (free-floating), enclosed in extracellular vesicles or associated with other cellular structures or proteins. Created with BioRender.com.
Figure 1.
Schematic representation of different forms of circulating extracellular mitochondrial components (CEMI). Principally CEMI are classified into two broad categories based on the nature of mitochondria. First, being whole mitochondria (Whole MITOs), that are in turn composed of intact mitochondria (intact mitos) or mitochondria wrapped (wrapped mitos) in one the following cellular structures such as tunneling nanotubes, platelets or extracellular vesicles. The second category is composed of mitochondrial components (MITO components) which could either be mitochondrial DNA (mtDNA), mitochondrial RNA (mtRNA), mitochondrial protein (mtprotein) or mitochondria-derived vesicles (mitovesicles) with one or more of these components which could circulate by themselves (free-floating), enclosed in extracellular vesicles or associated with other cellular structures or proteins. Created with BioRender.com.
Figure 2.
The complex cyclical interplay of obesity, insulin resistance, and Type 2 Diabetes (T2D), with CEMI at the core.
Figure 2.
The complex cyclical interplay of obesity, insulin resistance, and Type 2 Diabetes (T2D), with CEMI at the core.
Figure 3.
Platelet-derived and other CEMI participating in different pathologies of the CVD spectrum comprised of Atherosclerosis, CAD, Endothelial Dysfunction, and Myocardial Infarction.The figure illustrates the multifaceted involvement of CEMI in various cardiovascular conditions underlying atherosclerosis and ischemic CVD, with emphasis on their potential origins and influences. The diagram displays three forms of CEMI - two with identifiable origins (e.g., platelet-derived EVs carrying mitochondria and platelet-released mitochondrial DNA (mtDNA) and mitochondrial RNAs and long non-coding RNAs (e.g., LIPCAR) with currently unknown cells of origin. These CEMI entities are shown to be associated with atherosclerosis, CAD, endothelial dysfunction, and myocardial infarction via the mediation of inflammatory cytokines and chemokines such as TNFα, IL-6, and RANTES. However, it’s crucial to note that the diagram illustrates a hypothesized association of these CEMI with the disease states, and further research is required to ascertain whether these relationships are causal or merely associative. CEMI; Circulating extracellular mitochondria, EV; extracellular vesicles, CVD; Cardiovascular Disease, CAD; Coronary Artery Disease, LIPCAR; lncRNA uc022bqs.1, TNFα; Tumor Necrosis Factor α, RANTES; regulated on activation, normal T cell expressed and secreted. Created with BioRender.com.
Figure 3.
Platelet-derived and other CEMI participating in different pathologies of the CVD spectrum comprised of Atherosclerosis, CAD, Endothelial Dysfunction, and Myocardial Infarction.The figure illustrates the multifaceted involvement of CEMI in various cardiovascular conditions underlying atherosclerosis and ischemic CVD, with emphasis on their potential origins and influences. The diagram displays three forms of CEMI - two with identifiable origins (e.g., platelet-derived EVs carrying mitochondria and platelet-released mitochondrial DNA (mtDNA) and mitochondrial RNAs and long non-coding RNAs (e.g., LIPCAR) with currently unknown cells of origin. These CEMI entities are shown to be associated with atherosclerosis, CAD, endothelial dysfunction, and myocardial infarction via the mediation of inflammatory cytokines and chemokines such as TNFα, IL-6, and RANTES. However, it’s crucial to note that the diagram illustrates a hypothesized association of these CEMI with the disease states, and further research is required to ascertain whether these relationships are causal or merely associative. CEMI; Circulating extracellular mitochondria, EV; extracellular vesicles, CVD; Cardiovascular Disease, CAD; Coronary Artery Disease, LIPCAR; lncRNA uc022bqs.1, TNFα; Tumor Necrosis Factor α, RANTES; regulated on activation, normal T cell expressed and secreted. Created with BioRender.com.
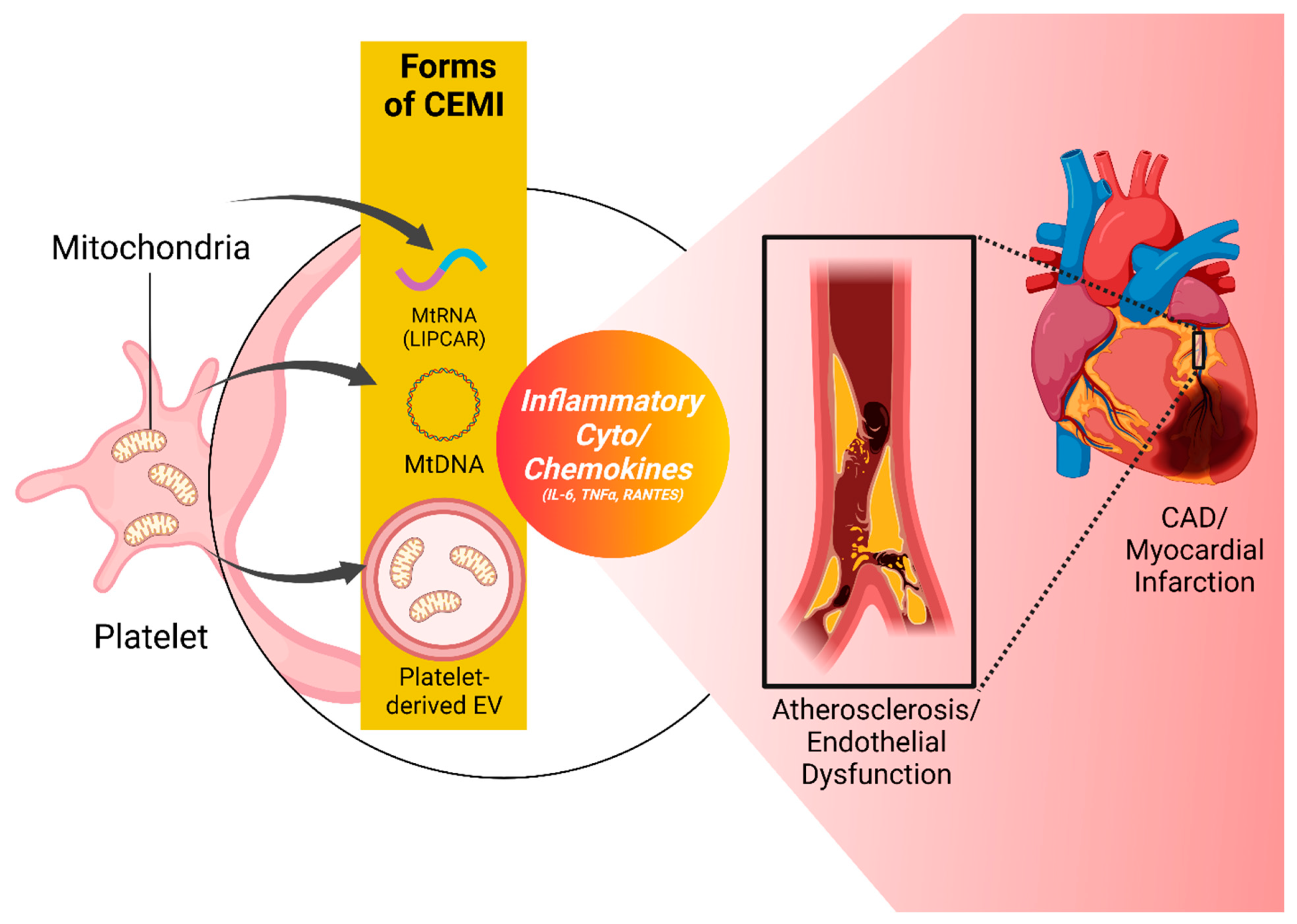
Figure 4.
Interactions of CEMI, Hypertension, and Inflammation This is a representation of the intricate relationships between CEMI, hypertension, and inflammation. It emphasizes the role of CEMI in promoting inflammation and contributing to the pathophysiology of hypertension, as indicated by the circular pathway connecting key mediators including TLR9, TGF-β, two separate pathways that lead to the activation of NF-κB, and ERK1/2. These mediators, activated under hypertensive conditions, can both propagate inflammation and be influenced by it, highlighting a complex interplay. The figure underscores the potential diagnostic and prognostic roles of CEMI in hypertension while emphasizing the need for further research to better understand these interactions. CEMI; Circulating extracellular mitochondria, TLR9; Toll-like Receptor 9, TGF-β; Transforming growth factor β, TNFα; Tumor Necrosis Factor α, IL-6; Interleukin 6. Created with BioRender.com.
Figure 4.
Interactions of CEMI, Hypertension, and Inflammation This is a representation of the intricate relationships between CEMI, hypertension, and inflammation. It emphasizes the role of CEMI in promoting inflammation and contributing to the pathophysiology of hypertension, as indicated by the circular pathway connecting key mediators including TLR9, TGF-β, two separate pathways that lead to the activation of NF-κB, and ERK1/2. These mediators, activated under hypertensive conditions, can both propagate inflammation and be influenced by it, highlighting a complex interplay. The figure underscores the potential diagnostic and prognostic roles of CEMI in hypertension while emphasizing the need for further research to better understand these interactions. CEMI; Circulating extracellular mitochondria, TLR9; Toll-like Receptor 9, TGF-β; Transforming growth factor β, TNFα; Tumor Necrosis Factor α, IL-6; Interleukin 6. Created with BioRender.com.
Table 1.
Categorization of Mitochondrial Transplantation Approaches and Related Studies.
Table 1.
Categorization of Mitochondrial Transplantation Approaches and Related Studies.
| Method |
Mode |
Studies |
|
In vitro mitochondrial transplantation |
Microinjection |
King and Attardi (1988) |
| |
Co-incubation |
Ali Pour et al. (2020) |
|
In vivo mitochondrial transplantation |
Intravenous injection |
Shi et al. (2017) |
| |
Intraoperative injection |
Kaza et al. (2017), Pepe (2017) |
| |
Perfusion via coronary arteries |
Cowan et al. (2016) |
| |
Epicardial injection |
Emani et al. (2017), Emani and McCully (2018) |
| Mitochondrial conjugation with EVs or cell-penetrating peptides |
Mitochondria-enriched extracellular Vesicles |
O’Brien et al. 2021, Ikeda et al. 2021, PMID: 34604804, https://doi.org/10.1016/j.jacc.2020.12.060 |
| |
Pep-1 peptide conjugation |
Chang et al. (2013) |
| Types of mitochondrial transplantation based on the source of mitochondria |
Autologous |
Emani et al. (2017) |
| |
Non-autologous (allogenic) |
Ali et al. 2020 |
| |
Interspecies |
Ali et al. (2020) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).