Submitted:
29 May 2024
Posted:
30 May 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
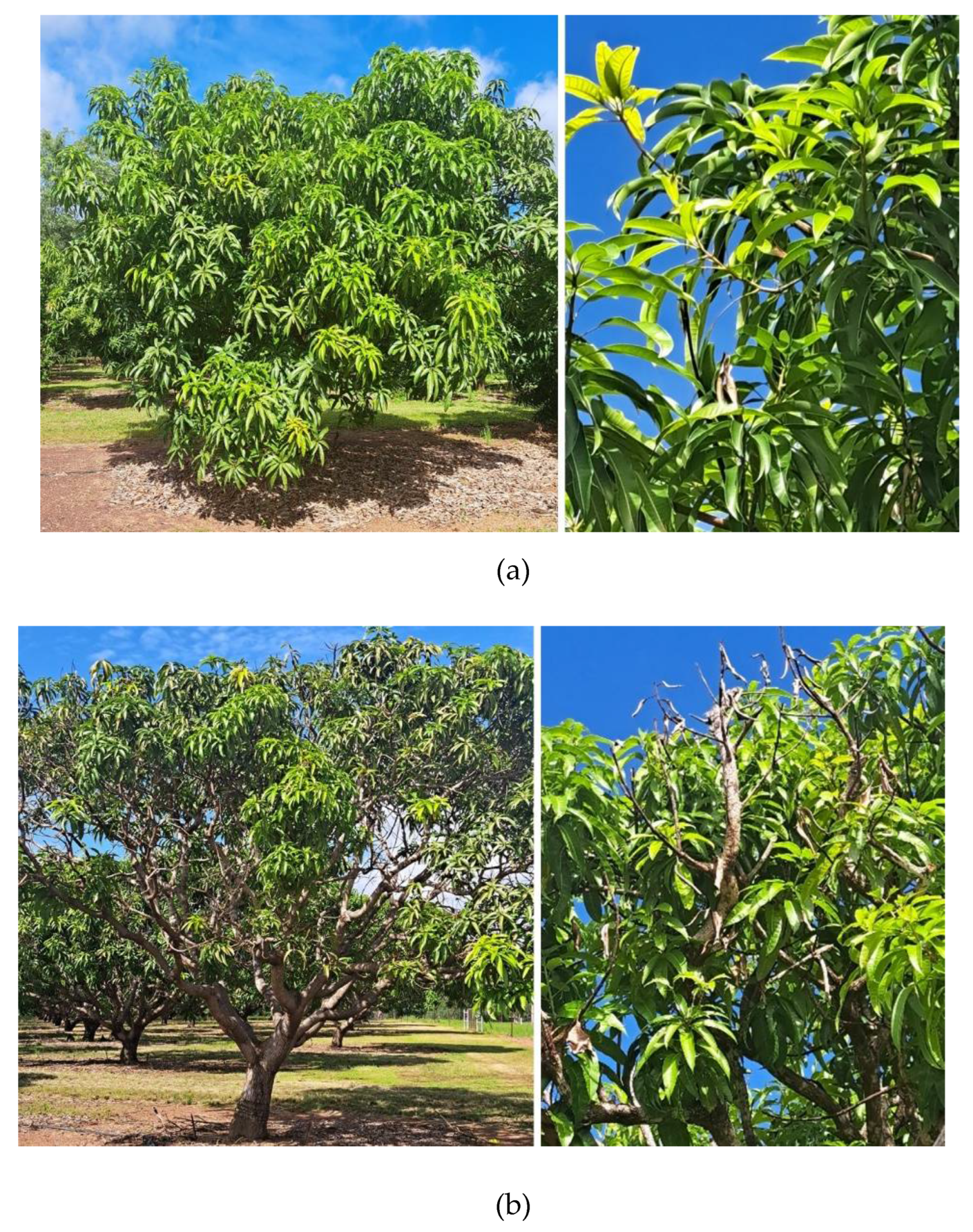
2.1. Study Site
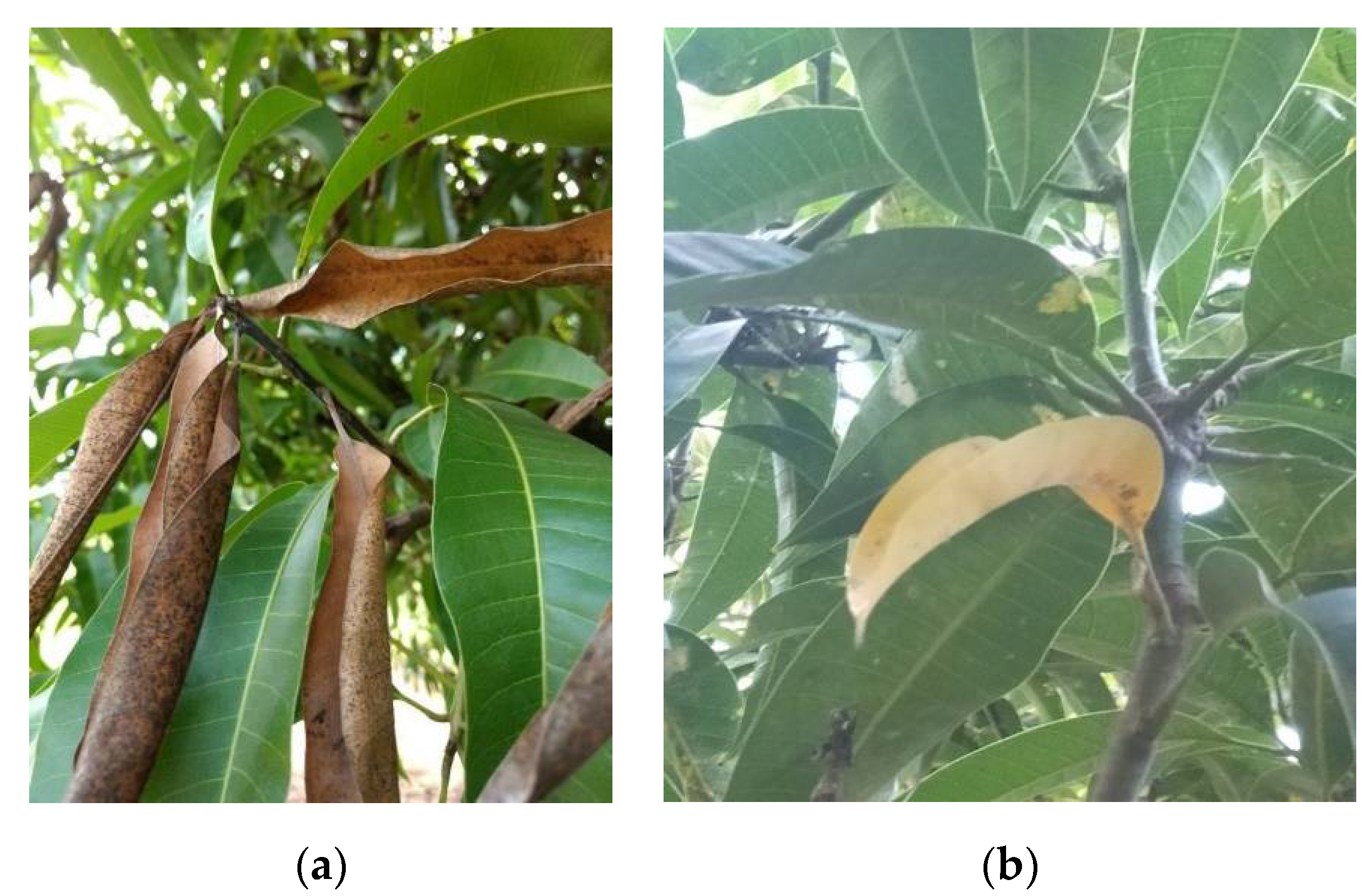
2.2. Twig-Tip Dieback Evaluation
2.3. Sample Collection
2.4. Sample Processing and Analysis
2.5. Sufficient Levels of Nutrient Status of the Leaves
2.6. Deviation from the Optimum Percentage (DOP) Index
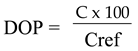
2.7. Nutrient Resorption Efficiency
2.8. Statistical Analysis
3. Results
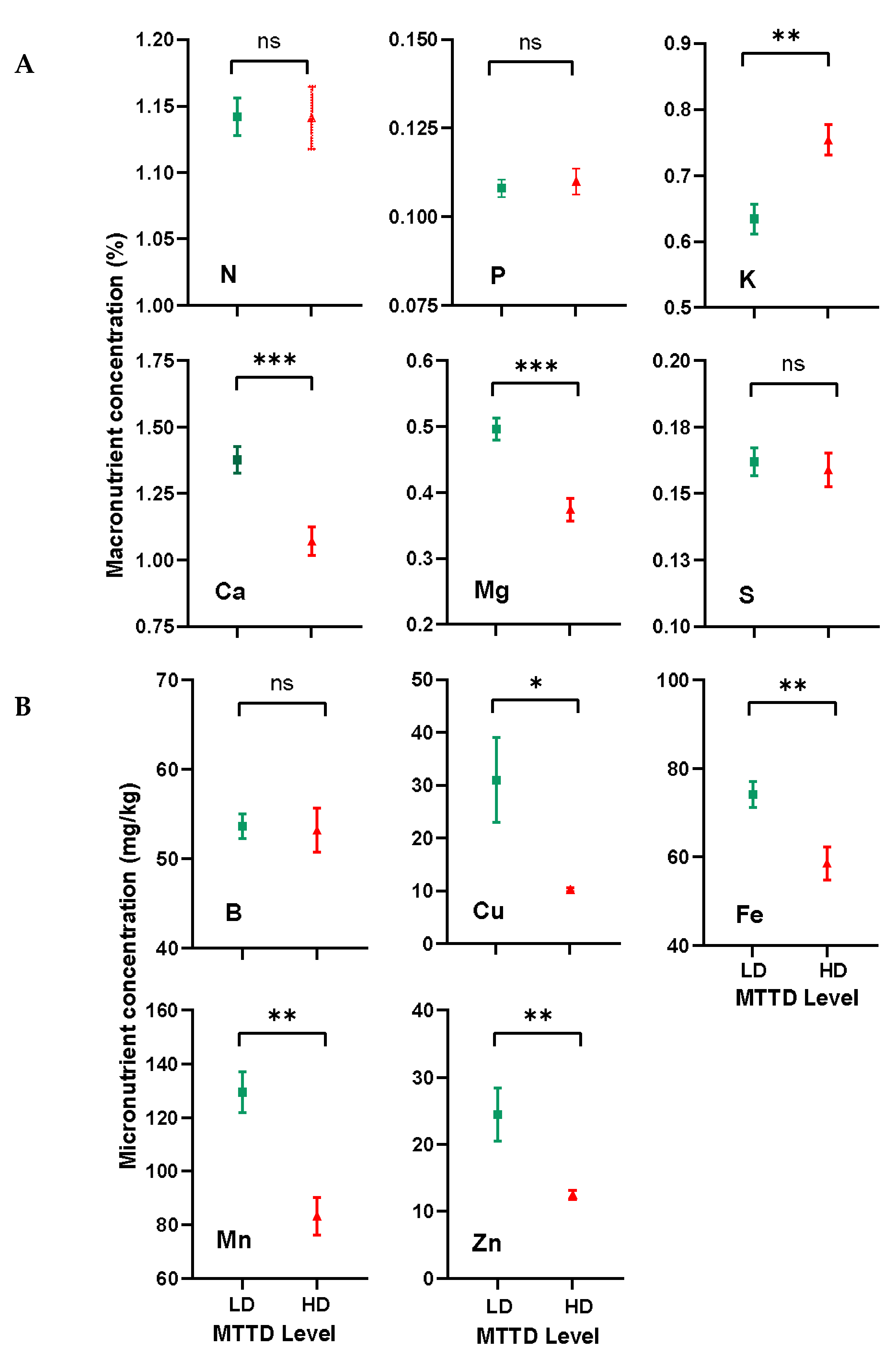
3.1. Soils and Lef Nutrient Analysis
3.2. DOP Index
3.3. Nutrient Resorption Efficiency
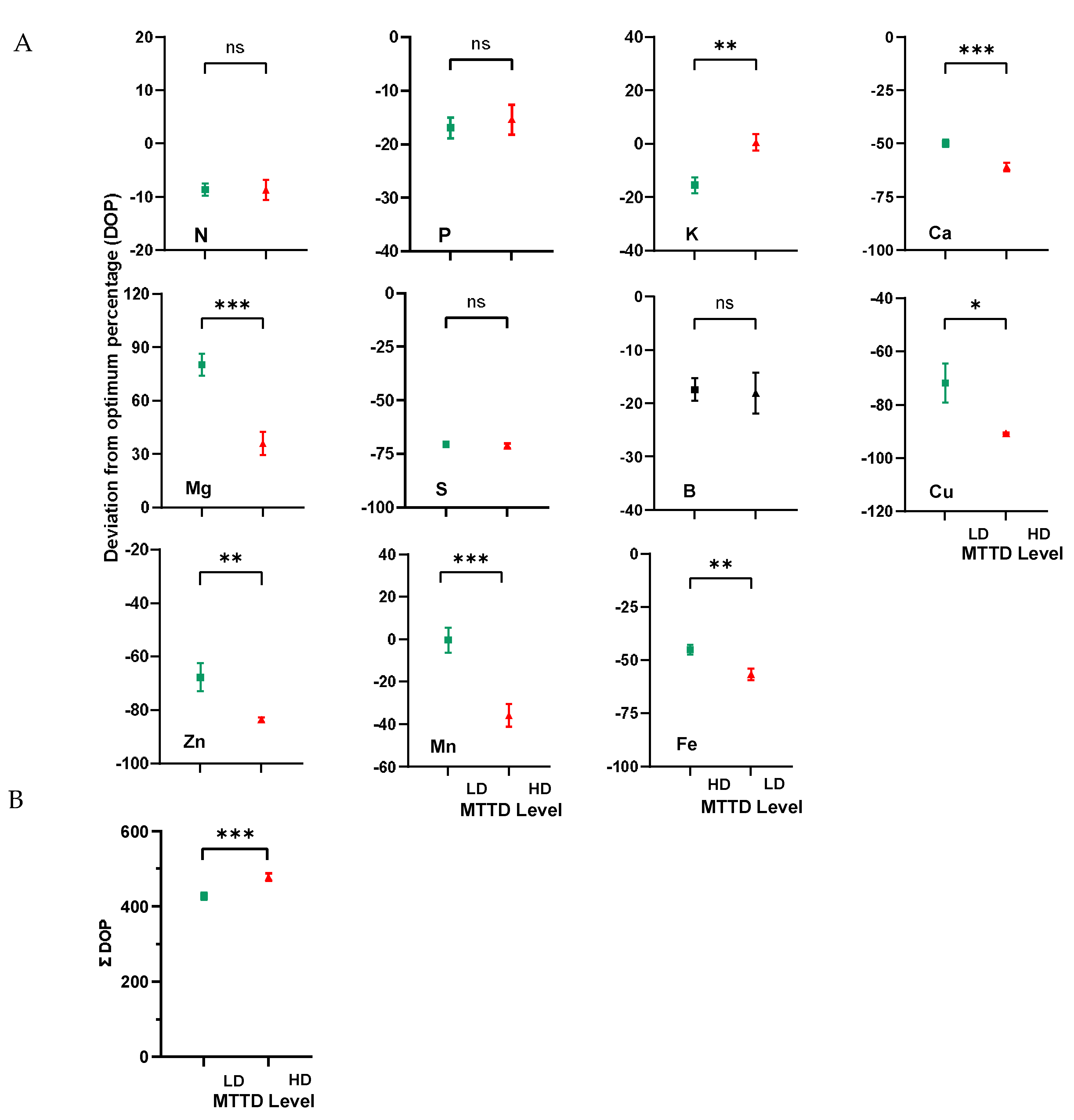
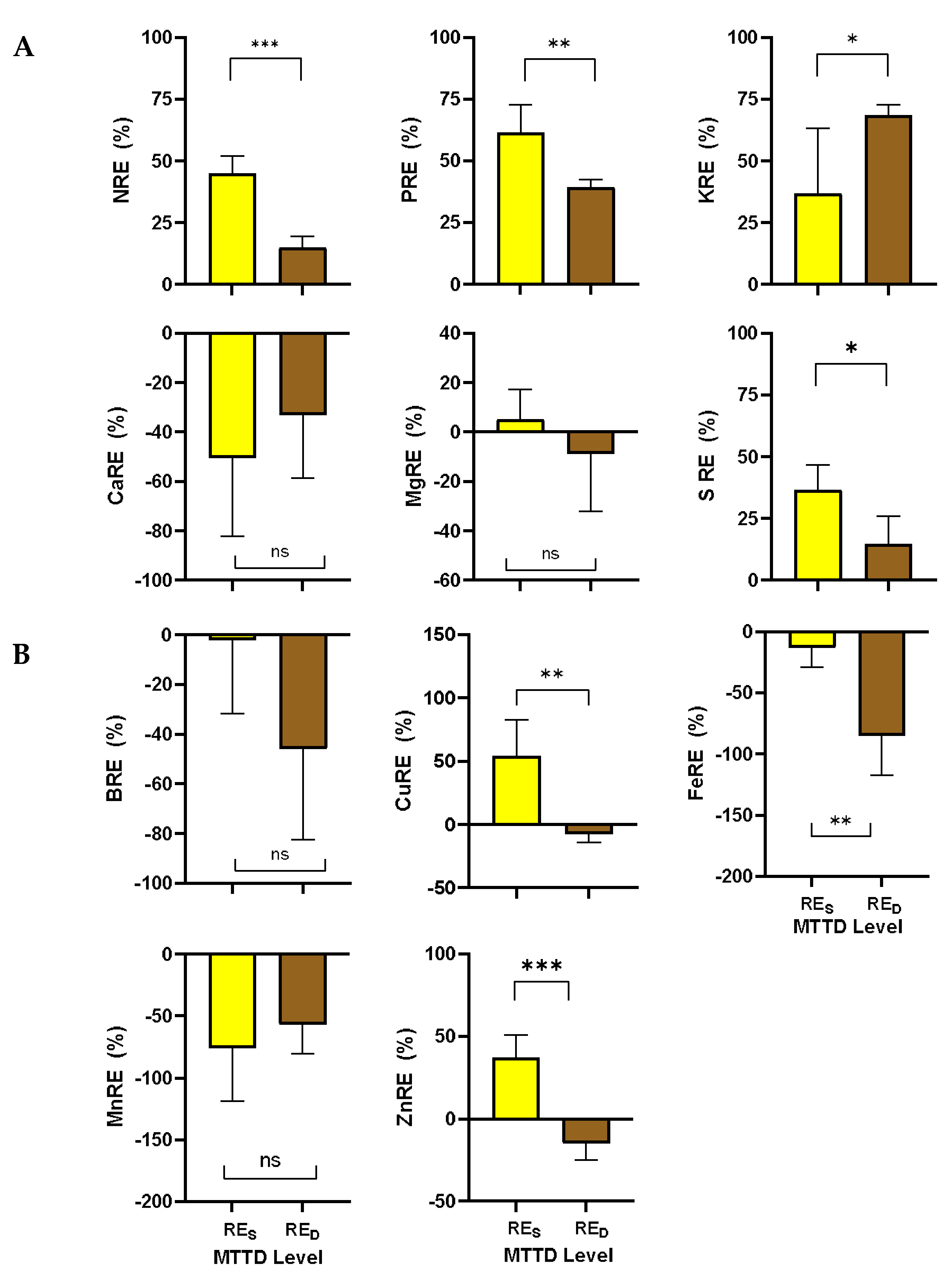
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- NT (Northern Territory) Farmers. NT Mangoes. Available online: https://ntfarmers.org.au/commodities/mangoes/ (accessed on 2 November 2023).
- Jabeen, A. 2016. Field and Postharvest Biology of Dendritic Spot and Stem End Rot of Mango. PhD Thesis, School of Agriculture and Food Sciences, The University of Queensland, Gatton, Queensland, Australia, 2016. [CrossRef]
- Umar, M.; McConchie, C.; Tran-Nguyen, L.; Asis, C.A.; Eyles, A.; Stanley, R; Gracie, A. Perception of Australian consumers on resin canal discouloration (RCD) in mango. Acta Hortic 2020, 1299, 13-18. [CrossRef]
- Grice, K.R.E.; Bally, I.S.E.; Wright, C.L.; Maddox, C.; Ali, A.; Dillon, N.L. Mango germplasm screening for the identification of sources of tolerance to anthracnose. Australasian Plant Pathol 2023 52, 27-41. [CrossRef]
- Giblin, F.R.; Tan, Y.P.; Mitchell, R.; Coates, L.M.; Irwin, J.A.G.; Shivas, R.G. Colletotrichum species associated with pre-and post-harvest diseases of avocado and mango in eastern Australia. Australasian Plant Pathol 2018, 47, 269–276. [CrossRef]
- Johnson, G.I.; Cooke, A.W.; Mead, A.J.; Wells, I.A. Stem end rot of mango in Australia: Causes and control. Acta Hortic 1991, 291, 288-295. [CrossRef]
- Asis, C.A.; Meschiari, L.; McConchie, C. Ionome balance analysis of mango fruit from orchard with and without resin canal discolouration. Acta Hortic 2019, 1244, 221-228. [CrossRef]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E. StJ.; Burges, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur J Plant Pathol 2011, 130, 379–391. [CrossRef]
- McTaggart, A.R.; Grice, K.R.; Shivas, R.G. First report of Vialaea minutella in Australia, its association with mango branch dieback and systematic placement of Vialaea in the Xylariales. Australasian Plant Dis Notes 2013, 8, 63–66. [CrossRef]
- Umar, U.D.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients foliar and drench application mitigate mango sudden decline disorder and impact fruit yield. Agron 2022, 12, 2449. [CrossRef]
- Saeed, A.; Shad, M.A.; Nawaz, H.; Shafqat, M.N.; Muneer, Z.; Shaheen, A.; Shah, S.T.A. Quick decline disease disturbs the levels of important phytochemicals and minerals in the stem bark of mango (Mangifera indica). Int J Agron 2016, 8219356. [CrossRef]
- Saeed, E.E.; Sham, A.; AbuZarqa, A.; Al Shurafa, K.A.; Al Naqbi, T.S.; Iratni, R.; El-Tarabily, K.; AbuQamar, S.F. Detection and management of mango dieback disease in the United Arab Emirates. Int J Mol Sci 2017, 18, 2086. PMID: 29053600; PMCID: PMC5666768. [CrossRef]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; Abu Qamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using stretomycete and non-streptomycete Actinobacteria in the United Arab Emirates. Front Microb 2018, 9, 829. [CrossRef]
- NT Rural Review. 2021. Mango common dieback. Available online https://industry.nt.gov.au/publications/primary-industry-publications/newsletters/regional-newsletters/rural-review/nt-rural-review-november-2021/mango-common-dieback (accessed on 5 November 2023).
- NT Rural Review. 2021. Mango twig tip dieback. Available online https://industry.nt.gov.au/publications/primary-industry-publications/newsletters/regional-newsletters/rural-review/nt-rural-review-november-2021/mango-twig-tip-dieback (accessed on 5 November 2023).
- Ram, R.A.; Rahim, M.A.; Alam, M.S. Diagnosis and management of nutrient constraints in mango. In Fruit Crops: Diagnosis and Management of Nutrient Constraints, Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 629–650. [CrossRef]
- Tripathi, R.; Terwai, R.; Sing, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front Plant Sci 2022, 13, 883970. [CrossRef]
- Kumar, R.; Kumar, V. Physiological disorders in perennial woody tropical and subtropical fruit crops: A review. Indian J Agric Sci 2016, 86, 703–17.
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron Sustain Dev 2008, 28, 33–46. [CrossRef]
- Gupta, N.; Debnath, S.; Sharma, S.; Sharma, P.; Purohit, J. Role of nutrients in controlling plant diseases in sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture, Meena, V.S., Mishra, P., Bisht, J., Pattanayak, A. Eds., Springer, Singapore. 2017; Volume 2, pp. 217-2162. [CrossRef]
- Gonzalez de Andrés, E.; Gazol, A.; Querejeta, J.I.; Igual, J.M.; Colangelo, M.; Sánchez-Salguero, R.; Linares, J.C.; Camarero, J.J. The role of nutritional impairment in carbon-water balance of silver fir drought-induced dieback. Glob Chang Biol 2022, 28, 4439-4458. [CrossRef]
- Bal, T.L.; Storer, A.J.; Jurgensen, M.F.; Doskey, P.V.; Amacher, M.C. Nutrient stress predisposes and contributes to sugar maple dieback across its northern ranges: A review. Forestry 2015, 18, 64-83. [CrossRef]
- Akillioglu, A. Micronutrient levels of cherry orchards in the Aegean region of Turkey. Acta Hortic 1997, 448, 199-202. [CrossRef]
- Nageli, P.; Grant, J.; Nichols, J.D.; Sheil, D.; Horton, B. Bell miner associated dieback: Nutrient cycling and herbivore crown damage in Eucalytus propinqua. Austral For 2016, 79, 74-82. https://10.1080/00049158.2015.1123565.
- Gerrish, G.; Dombois, D.M.; Bridges, K.W. Nutrient limitation and Metrosideros forest dieback in Hawai’i. Ecol 1988, 69, 723-727. [CrossRef]
- Cao, J.; Cheng, C.; Yang, J.; Wang Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Sci Rep 2015, 5, 14675. [CrossRef]
- Huang, C.; Xu, C.; Ma, Y.; Song, T.; Xu, Z.; Li, S.; Liang, J.; Zhang, L. Nutritional diagnosis of the mineral elements in Tainong mango leaves during flowering in karst areas. Land 2022, 11, 1311. [CrossRef]
- Sarkhosh, A.; Shahkoomahally, S.; Asis, C.; McConchie, C. Influence of rootstocks on scion leaf mineral content in mango tree (Mangifera indica L.). Hortic Environ Biotechnol 2021, 62, 725–735. [CrossRef]
- Ganeshamurthy, A.N.; Reddy, Y.T.N. Fitness of mango for colonization in low fertility soils and dry lands: Examination of leaf life-span, leaf nutrient resorption, and nutrient use efficiency in elite mango varieties. Agric Res 2015, 4, 254–260. [CrossRef]
- Reuter, D.J.; Robinson, J.B. Plant Analysis, an Interpretation Manual, 2nd ed.; CSIRO Publishing, Collingwood, Australia, 1997; pp. 366-367.
- Heras, L.; Montañés, L. Desviación del óptimo porcentual (DOP): Nuevo índice para la interpretación del análisis vegetal. An Estac Exp Aula Dei 1991, 20, 93–108.
- Kumar, P.; Sharma, S.K.; Kumar, A. Foliar nutritive fluids affect generative potential of apples: Multilocation DOP indexing and PCA studies under dry temperate agro-climatic conditions of north-west Himalaya. Sci Hortic 2017, 218, 265–274. [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gómez-Aparisi, J.; Betrán, J.; Moreno, M. Influence of almond× peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigour of two peach cultivars. Sci Hortic 2005, 106, 502–514. [CrossRef]
- Zhang, Y., Yang, J., Wei, X., Ni, X., & Wu, F. Monthly dynamical patterns of nitrogen and phosphorus resorption efficiencies and C: N: P stoichiometric ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests 2022, 13, 1458.
- Montañés, L.; Heras, L.; Abadía, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J Plant Nutr 1993, 16, 1289–308. [CrossRef]
- Tadayon, M.S.; Sadeghi, S. The relation between compositional nutrient diagnosis indices and susceptibility of Valencia orange to citrus decline. J Plant Nutr 2023, 46, 261-274. [CrossRef]
- Milošević, T., Moreno, M.Á., Milošević, N., Milinković, M. Regulation of yield, fruit size, and leaf mineral nutrients of the ‘Šumadinka’ sour cherry cultivar with help of rootstocks. J Plant Growth Regul 2023, 42, 5587–5599. [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct Ecol 2003, 17, 10–19. [CrossRef]
- Prieto, I.; Querejeta, J.I. Simulated climate change decreases nutrient resorption from senescing leaves. Glob Change Biol 2020, 26, 1795-1807.
- Zhang, M.; Zhang, L.; Yao, X.; Li, J.; Deng, Q. Co-evaluation of plant leaf nutrient concentrations and resorption in response to fertilization under different nutrient-limited conditions. Diversity 2022, 14, 385. [CrossRef]
- Smith, S.; Hill, J. Supporting sustainable development–risks and impacts of plant industries on soil condition. Tech Bull 2011, 340, 1-27. https://industry.nt.gov.au/__data/assets/pdf_file/0005/233258/tb340.pdf.
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on citrus and huanglongbing. Proc Fla State Hort Soc 2009, 22, 169-171. https://swfrec.ifas.ufl.edu/hlb/database/pdf/00001871.pdf.
- Azim Nejad, Z.; Badehian, Z.; Rezaei Nejad, A.; Bazot, S. Do soil properties and ecophysiological responses of aak (Quercus brantii Lindl.) correlate with the rate of dieback? Trees 2021, 35, 1639–1650. [CrossRef]
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between plant nutrition and symptom expression in mandarin trees infected with the disease huanglongbing. Australas Plant Dis Notes 2008, 3, 112-115. [CrossRef]
- Mathew, J.; Haris, A.A.; Bhat, R.; Kumar, V.K.; Muralidharan, K.; John, K.S.; Surendran, U. A comparative assessment of nutrient partitioning in healthy and root (wilt) disease affected coconut palms grown in an Entisol of humid tropical Kerala. Trees 2021, 35, 621-635. [CrossRef]
- Sousa Filho, H.R.; Jesus, R.M.D.; Bezerra, M.A.; da Silva, V.H.; da Silva Jr, A.L.; Alves, J.P.; Santana, G; Souza Jr, J.O.D. Mineral nutrients and plant-fungal interaction in cocoa trees (Theobroma cacao L.). J Braz Chem Soc 2021, 32, 337-346. [CrossRef]
- Soares, T.P.; Pozza, E.A.; Pozza, A.A.; Mafia, R.G.; Ferreira, M.A. Calcium and potassium imbalance favours leaf blight and defoliation caused by Calonectria pteridis in Eucalyptus plants. Forests 2018, 9, 782. [CrossRef]
- Pervez, H.; Ashraf, M.; Makhdum, M.I.; Mahmood, T. Potassium nutrition of cotton (Gossypium hirsutum L.) in relation to cotton leaf curl virus disease in aridisols. Pak J Bot 2007, 39, 529-539. https://www.pakbs.org/pjbot/PDFs/39(2)/PJB39(2)529.pdf.
- Graça, R.N.; Alfenas, A.C.; Maffia, L. A.; Titon, M.; Alfenas, R.F.; Lau, D.; Rocabado, J.M.A. Factors influencing infection of eucalypts by Cylindrocladium pteridis. Plant Pathol 2009, 58, 971-981. [CrossRef]
- Maity, A.; Sharma, J.; Sarkar, A.; More, A.K.; Pal, R.K. Nutrient imbalance indices are closely related with susceptibility of pomegranate to bacterial blight disease. Sci Hortic 2016, 211, 79-86. [CrossRef]
- Gopi, R.; Madhavi, G.B.; Kapoor, C.; Raj, C.; Singh, S.; Ramprakash, T. Role of mineral nutrients in the management of plant diseases. In Plant Disease Management Strategies, Nehra, S., Trivedi, P.C., Ed., Agrobios Research: Rajasthan, India, 2021; Chapter 4, pp. 87-117.
- Weinmann, M.; Bradáčová, K.; Nikolic, M.. Relationship between mineral nutrition, plant diseases, and pests. In Marschner's Mineral Nutrition of Higher Plants, 4th ed.; Marschner, P., Ed.; Elsevier/Academic Press: Amsterdam, Netherlands, 2023; Chapter 10, pp. 445-476. [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.M.; Yvin ,J.C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci 2015, 6, 317. [CrossRef]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture, 2018, 8, 14. [CrossRef]
- Achat, D.L.; Pousse, N.; Nicolas, M.; Augusto, L. Nutrient remobilization in tree foliage as affected by soil nutrients and leaf life span. Ecol Monogr 2018, 88, 408-428. [CrossRef]
- McIntire, C.D.; Huggett, B.A.; Dunn, E.; Munck, I.A.; Vadeboncoeur, M.A.; Asbjornsen, H. Pathogen-induced defoliation impacts on transpiration, leaf gas exchange, and non-structural carbohydrate allocation in eastern white pine (Pinus strobus). Trees 2021, 35, 357-373. [CrossRef]
- Gortari, F.; Guiamet, J.J.; Cortizo, S.C.; Graciano, C. Poplar leaf rust reduces dry mass accumulation and internal nitrogen recycling more markedly under low soil nitrogen availability, and decreases growth in the following spring, Tree Physiol 2019, 39, 19–30. [CrossRef]
- Boercker, M.A. The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis thaliana. Master's Thesis, University of Tennessee, Knoxville, Tennessee, USA, December 2006. https://trace.tennessee.edu/utk_gradthes/1506.
- Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; Yavaş, İ.; Hussain, S. Plant secondary metabolites and abiotic stress tolerance: Overview and implications. In Plant Abiotic Stress Responses and Tolerance Mechanisms, Hussain, S., Hussain Awan, T., Ahmad Waraich, E., Iqbal Awan, M., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 3, pp. 133-206. [CrossRef]
- McMahon, P. Effect of nutrition and soil function on pathogens of tropical tree crops. In Plant Pathology, Cumagon, C.J.R., Ed., IntechOpen: Rijeka, Croatia, 2012; Chapter 10, pp. 241–272. https://www.intechopen.com/chapters/34847.
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Faisal Hayat, F.; Deng, L.; Narejo, M.; Bozdar, B.; Tu, P. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci Hortic 2024, 323, 112512. [CrossRef]

| Soil Properties | Dieback Level | p value | |
|---|---|---|---|
| LD | HD | ||
| Soil pH (H2O) | 7.36±0.13 | 7.26±0.05 | 0.49 ns |
| Organic C (%) | 1.06±0.11 | 0.93±0.04 | 0.33 ns |
| EC (dS/m) | 0.04±0.008 | 0.03±0.003 | 0.46 ns |
| N (mg/kg) | 1.23±0.36 | 2.42±0.39 | 0.06 ns |
| P (mg/kg) | 10.80±1.93 | 12.6±2.44 | 0.57 ns |
| S (mg/kg) | 4.64±1.71 | 5.22±1.42 | 0.80 ns |
| K (cmol(+)/kg) | 0.16±0.04 | 0.11±0.01 | 0.23 ns |
| Ca (cmol(+)/kg) | 4.01±0.55 | 3.66±0.31 | 0.59 ns |
| Mg (cmol(+)/kg) | 2.70±0.52 | 2.45±0.27 | 0.68 ns |
| B (mg/kg) | 0.22±0.02 | 0.21±0.01 | 0.94 ns |
| Cu (mg/kg) | 2.19±0.20 | 2.44±0.31 | 0.54 ns |
| Fe (mg/kg) | 14.84±0.75 | 16.26±0.74 | 0.21 ns |
| Mn (mg/kg) | 6.74±2.38 | 4.97±0.66 | 0.49 ns |
| Zn (mg/kg) | 8.26±4.37 | 0.93±0.04 | 0.33 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




