Submitted:
22 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Plant Material
- DHL-190849: Hull-less, two-rowed, normal endosperm, and yellow grain double-haploid advanced breeding line.
- Rajapani®: Hull-less, six-rowed, normal endosperm, and blue grain registered Spanish variety.
- DHL-151340: Hull-less, two-rowed, normal endosperm, and purple grain double-haploid advanced breeding line.
- DHL-191250: Hull-less, two-rowed, normal endosperm, and black grain double-haploid advanced breeding line.
2.3. Phenolic Compounds and Anthocyanin analysis by Ultra-Performance Liquid Chromatography (UPLC-MS/MS)
2.4. In-Vitro Antioxidant Capacity
2.5. Statistical Analysis
3. Results and Discussion
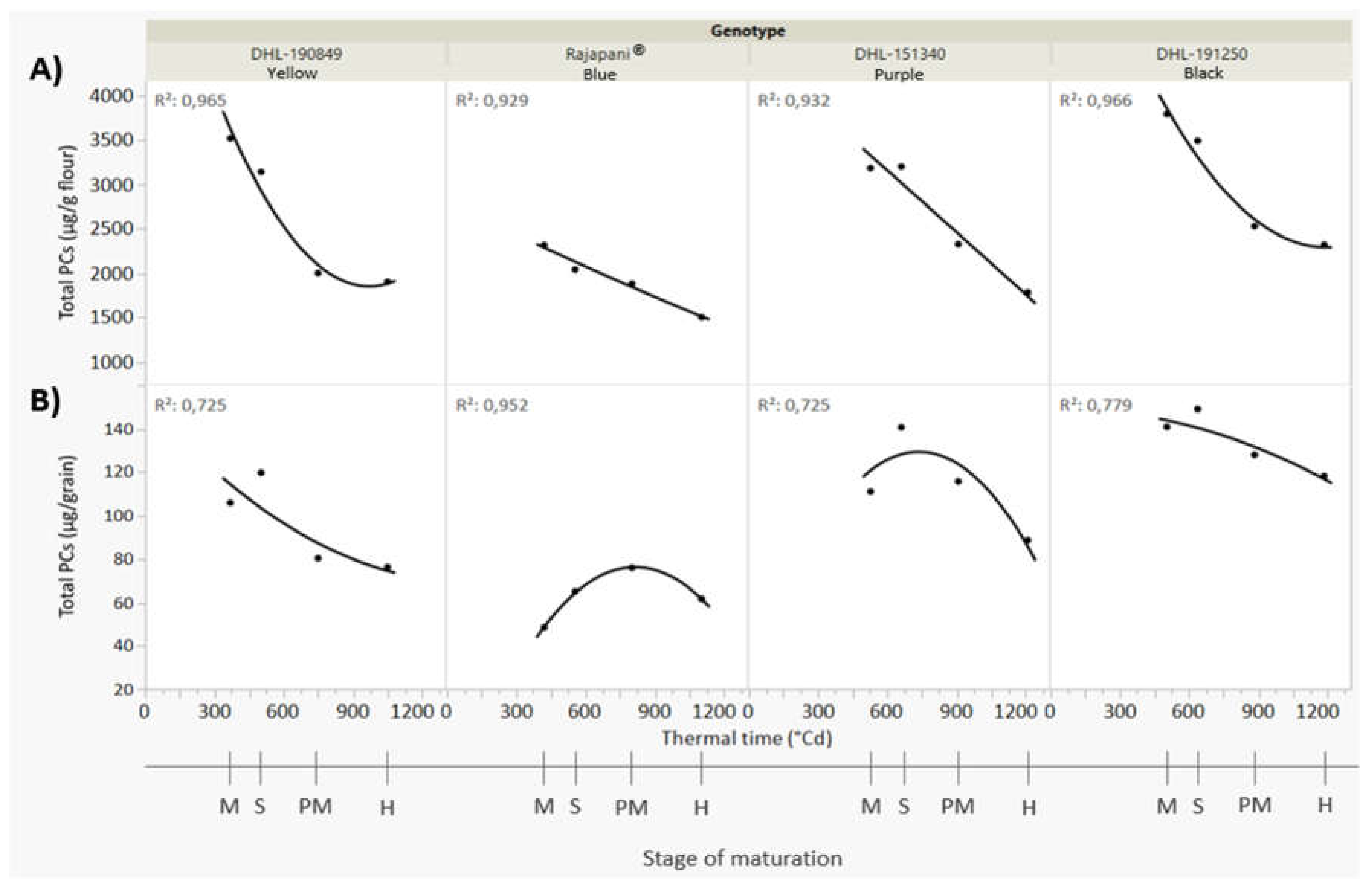
3.1. Deposition Pattern of Total Phenolic Compounds
3.2. Dynamics of Specific Phenolic Compounds across Grain Development Stages
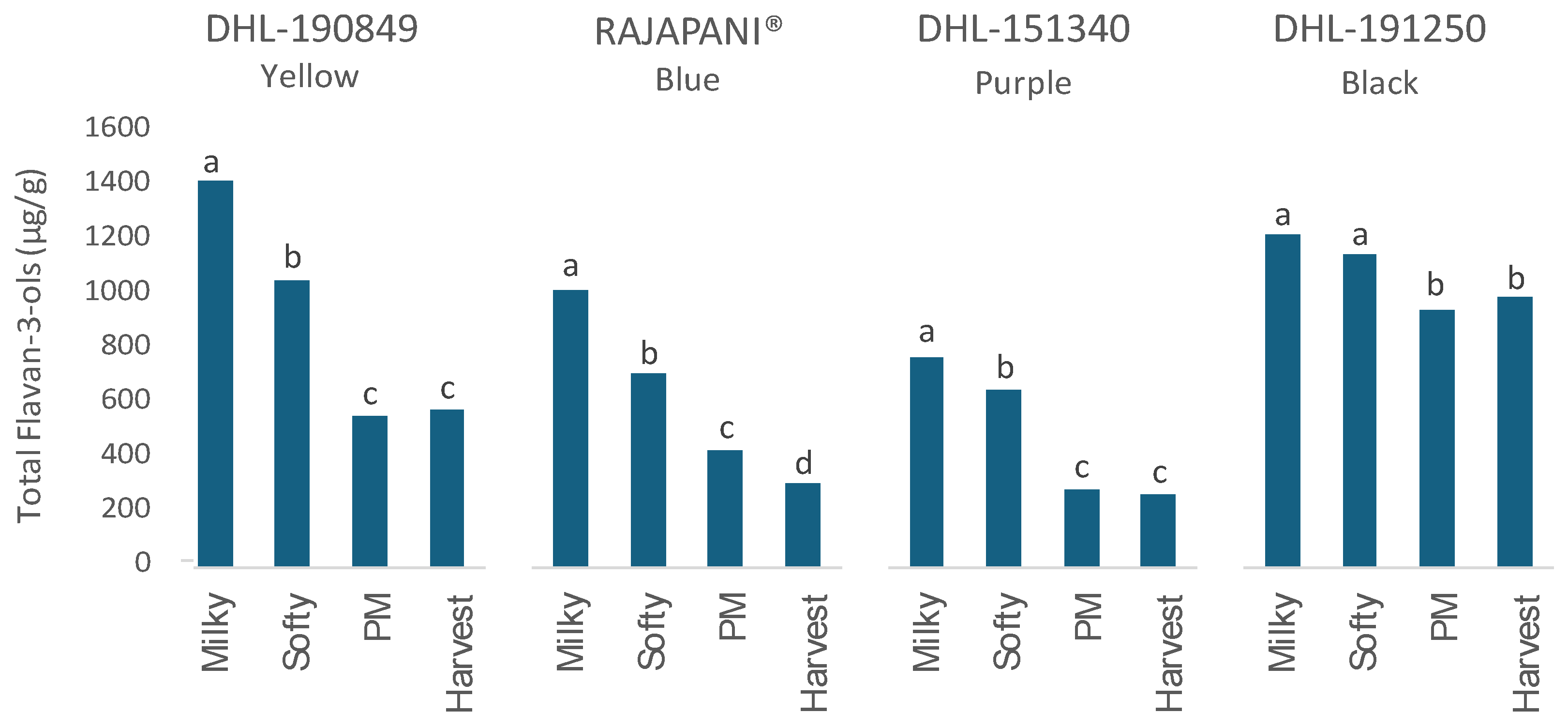
3.2.1. Flavan-3-ols
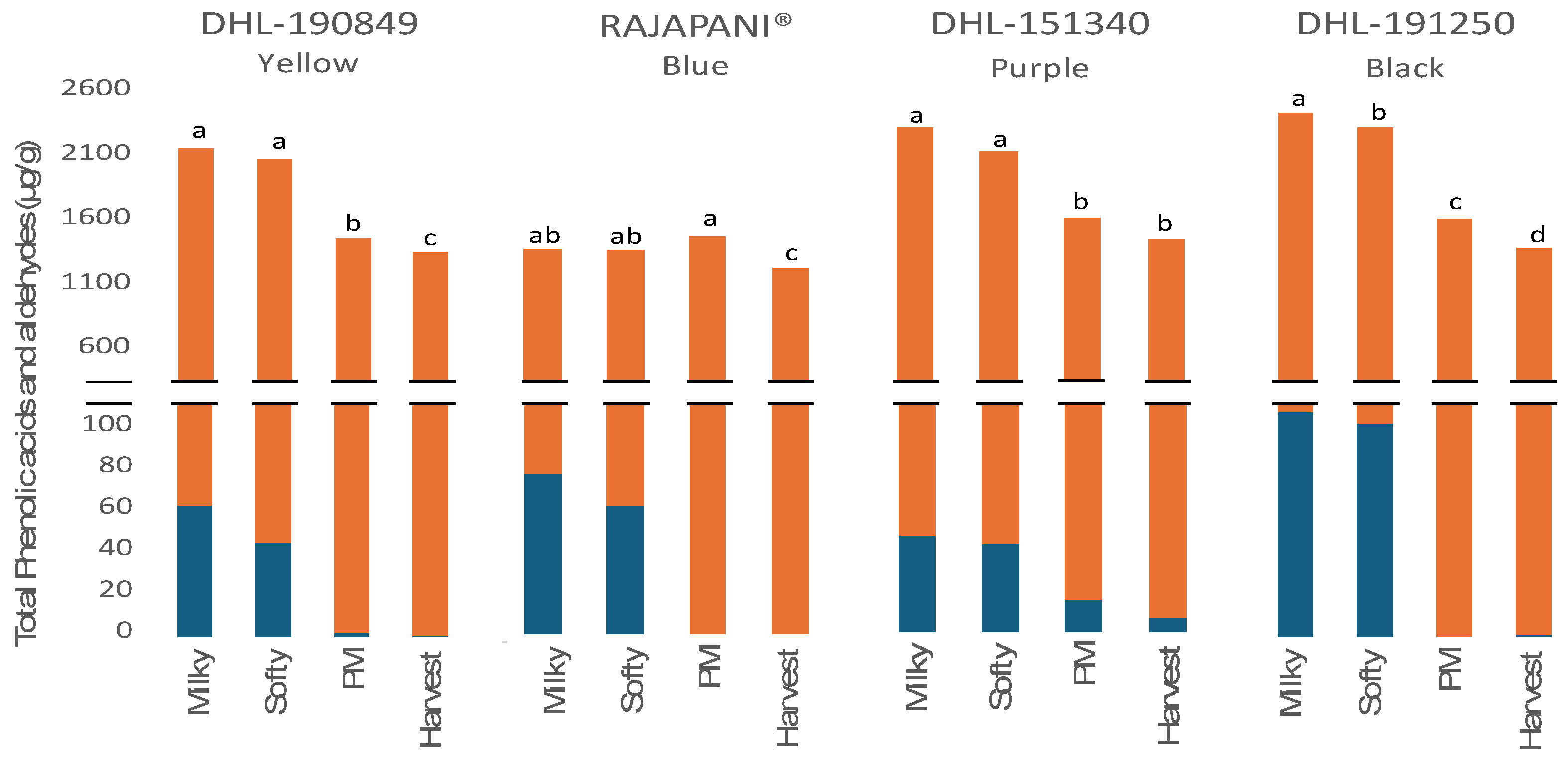
3.2.2. Phenolic Acids and Aldehydes
3.2.3. Flavone Glycosides
3.2.4. Anthocyanins
3.3. Dynamics of Antioxidant Capacity during Barley Grain Development
3.4. Correlations between Phenolic Compound Families and Antioxidant Capacity across Grain Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baik, B.-K.; Ullrich, S.E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008, 48, 233–242. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. Food labeling: Health claims; soluble fiber from certain foods and risk of coronary heart disease. Interim final rule. Fed Regist. 2008, 73, 9938–9947. [Google Scholar]
- EFSA. Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 EFSA [Internet]. 2011 [cited 2024 February 20]. Available from: https://www.efsa.europa.eu/en/efsajournal/pub/2207.
- Neelam, *!!! REPLACE !!!*; Khatkar, A. ; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2019, 60, 2655–2675. [Google Scholar] [CrossRef]
- Raj, R.; Shams, R.; Pandey, V.K.; Dash, K.K.; Singh, P.; Bashir, O. Barley phytochemicals and health promoting benefits: A comprehensive review. J. Agric. Food Res. 2023, 14. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Labudda, M.; Muszynska, E.; Gietler, M.; Rozanska, E.; Rybarczyk-Plonska, A.; Fidler, J.; Prabucka, B.; Dababat, A.A. Efficient antioxidant defence systems of spring barley in response to stress induced jointly by the cyst nematode parasitism and cadmium exposure. Plant Soil 2020, 456, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; Learmonth, A.; Hassan, A.S.; Lahnstein, J.; Looseley, M.; Little, A.; Waugh, R.; Burton, R.A.; Halpin, C. Natural variation in HvAT10 underlies grain cell wall-esterified phenolic acid content in cultivated barley. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xiang, Z.; Lin, C.; Zhu, Y.; Yang, K.; Liu, T.; Xia, C.; Chen, J.; Zhang, W.; Zhang, Y.; et al. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT 2021, 151, 112001. [Google Scholar] [CrossRef]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchioni, N.; Marciello, U.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in colored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef]
- Nowak, R.; Szczepanek, M.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Dziedziński, M.; Błaszczyk, K. Profile of phenolic compounds and antioxidant activity of organically and conventionally grown black-grain barley genotypes treated with biostimulant. PLoS ONE 2023, 18, e0288428. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Mursalimov, S.R.; Gracheva, N.V.; Glagoleva, A.Y.; Börner, A.; Khlestkina, E.K. Melanin formation in barley grain occurs within plastids of pericarp and husk cells. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Martínez-Subirà, M.; Romero, M.P.; Puig, E.; Macià, A.; Romagosa, I.; Moralejo, M. Purple, high β-glucan, hulless barley as valuable ingredient for functional food. LWT 2020, 131, 109582. [Google Scholar] [CrossRef]
- Celestina, C.; Hunt, J.; Brown, H.; Huth, N.; Andreucci, M.; Hochman, Z.; et al. Scales of development for wheat and barley specific to either single culms or a population of culms. European Journal of Agronomy , 2023, 147, 126824. [Google Scholar] [CrossRef]
- Ranathunga, R.A.A.; Suwannaporn, P. Young cereal grains as a new source of healthy and hypoallergenic foods: A review. J. Food Sci. Technol. 2021, 59, 3336–3348. [Google Scholar] [CrossRef]
- Paradiso, A.; Cecchini, C.; De Gara, L.; D'Egidio, M.G. Functional, antioxidant and rheological properties of meal from immature durum wheat. J. Cereal Sci. 2006, 43, 216–222. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Lima, L.R.d.S.; Nascimento, F.R.; Nascimento, T.P.D.; Cameron, L.C.; Ferreira, M.S.L. Metabolomic approach for characterization of phenolic compounds in different wheat genotypes during grain development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.-M.; Shin, J.-A.; Cho, J.-W.; Lee, K.-T. Nutritional evaluation of immature grains in two Korean rice cultivars during maturation. Food Sci. Biotechnol. 2013, 22, 903–908. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q.; Wang, X.; Luo, J.; Liu, Y.; Tian, C. Changes in the Main Nutrients, Phytochemicals, and Antioxidant Activity in Yellow Corn Grain during Maturation. J Agric Food Chem. 2010, 58, 5751–5756. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Subirà, M.; Moralejo, M.; Puig, E.; Romero, M.-P.; Savin, R.; Romagosa, I. Impact of Rising Temperature in the Deposition Patterns of Bioactive Compounds in Field Grown Food Barley Grains. Plants 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Samson, M.-F.; Bonicel, J.; Morel, M.-H. Relationship between endosperm cells redox homeostasis and glutenin polymers assembly in developing durum wheat grain. Plant Physiol. Biochem. 2012, 61, 36–45. [Google Scholar] [CrossRef]
- Martínez, M.; Motilva, M.J.; López de las Hazas, M.C.; Romero, M.P.; Vaculova, K.; Ludwig, I.A. Phytochemical composition and β-glucan content of barley genotypes from two different geographic origins for human health food production. Food Chemistry 2018, 245, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2013, 143, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, J.; Zhang, S.; Xiao, W.; Guan, C.; Wang, G.; Wang, Y. Changes in the phenolic compound content and antioxidant activity in developmental maize kernels and expression profiles of phenolic biosynthesis-related genes. J. Cereal Sci. 2020, 96, 103113. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hyun, J.N.; Kim, J.A.; Park, J.C.; Kim, M.Y.; Kim, J.G.; et al. Relationship between Phenolic Compounds, Anthocyanins Content and Antioxidant Activity in Colored Barley Germplasm. J Agric Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of Phenolic Compounds and Expression Profiles of Phenolic Acid Biosynthesis-Related Genes in Developing Grains of White, Purple, and Red Wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Baumgartner, B.; Özkeser, I.; Köksel, H. Changes in the functional constituents and phytic acid contents of firiks produced from wheats at different maturation stages. Food Chem. 2018, 246, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiol. 2005, 140, 637–646. [Google Scholar] [CrossRef]
- Schulman, A.H.; Runeberg-Roos, P.; Jääskeläinen, M. Grain filling and starch synthesis in barley. In Developments in Crop Science; Gupta, A.K., Kaur, N., Eds.; Elsevier; 2000; pp. 147–67. (Carbohydrate Reserves in Plants; vol. 26).
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2016, 25, 148–161. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; et al. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. Journal of Cereal Science 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Šimić, G.; Horvat, D.; Lalić, A.; Komlenić, D.K.; Abičić, I.; Zdunić, Z. Distribution of β-Glucan, Phenolic Acids, and Proteins as Functional Phytonutrients of Hull-Less Barley Grain. Foods 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.; Olsen, H.; Færgestad, E.; Uhlen, A.; Knutsen, S. Variations in water absorption capacity and baking performance of barley varieties with different polysaccharide content and composition. LWT 2008, 41, 2085–2091. [Google Scholar] [CrossRef]
- Gamel, T.; Abdel-Aal, E.S.M. Phenolic acids and antioxidant properties of barley wholegrain and pearling fractions. Agricultural and Food Science 2012, 21, 118–131. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. Journal of the Science of Food and Agriculture 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Pan, Z.-F.; Li, Q.; Deng, G.-B.; Long, H.; Tashi, N.; Zhao, Y.; Yu, M.-Q. Nutritional components, in vitro digestibility, and textural properties of cookies made from whole hull-less barley. Cereal Chem. 2020, 97, 39–52. [Google Scholar] [CrossRef]
- Butsat, S.; Weerapreeyakul, N.; Siriamornpun, S. Changes in Phenolic Acids and Antioxidant Activity in Thai Rice Husk at Five Growth Stages during Grain Development. J. Agric. Food Chem. 2009, 57, 4566–4571. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chemistry. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Knievel, D.; Abdel-Aal, E.-S.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Kohyama, N.; Ono, H.; Yanagisawa, T. Changes in Anthocyanins in the Grains of Purple Waxy Hull-less Barley during Seed Maturation and after Harvest. J. Agric. Food Chem. 2008, 56, 5770–5774. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.V.; Riegel, R.; Calderini, D.F. Anthocyanin content of grains in purple wheat is affected by grain position, assimilate availability and agronomic management. J. Cereal Sci. 2012, 55, 257–264. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Lǚ, X.; Wang, X.; Ji, N.; Zhang, M.; Ren, M. Upregulated structural and regulatory genes involved in anthocyanin biosynthesis for coloration of purple grains during the middle and late grain-filling stages. Plant Physiol. Biochem. 2018, 130, 235–247. [Google Scholar] [CrossRef]
- Wang, F.; Ji, G.; Xu, Z.; Feng, B.; Zhou, Q.; Fan, X.; Wang, T. Metabolomics and Transcriptomics Provide Insights into Anthocyanin Biosynthesis in the Developing Grains of Purple Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2021, 69, 11171–11184. [Google Scholar] [CrossRef]
- Bellido, G.G.; Beta, T. Anthocyanin Composition and Oxygen Radical Scavenging Capacity (ORAC) of Milled and Pearled Purple, Black, and Common Barley. J. Agric. Food Chem. 2009, 57, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, W.; Wen, T.; Li, T.; Guo, X.B.; et al. Anthocyanin accumulation, biosynthesis and antioxidant capacity of black sweet corn (Zea mays L.) during kernel development over two growing seasons. Journal of Cereal Science 2020, 95, 103065. [Google Scholar] [CrossRef]
- Dang, B.; Zhang, W.-G.; Zhang, J.; Yang, X.-J.; Xu, H.-D. Evaluation of Nutritional Components, Phenolic Composition, and Antioxidant Capacity of Highland Barley with Different Grain Colors on the Qinghai Tibet Plateau. Foods 2022, 11, 2025. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as Antioxidants. J Nat Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Mekinić, I.G. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a Grapevine R2R3-MYB Transcription Factor That Regulates the Phenylpropanoid Pathway. Plant Physiol. 2005, 140, 499–511. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.J.; Qu, D.; Zhu, Z.Z.; Yang, Y.Z.; Zhao, Z.Y. The MdBBX22–miR858–MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel. Plant Biotechnology Journal. 2022, 20, 1683–1700. [Google Scholar] [CrossRef]
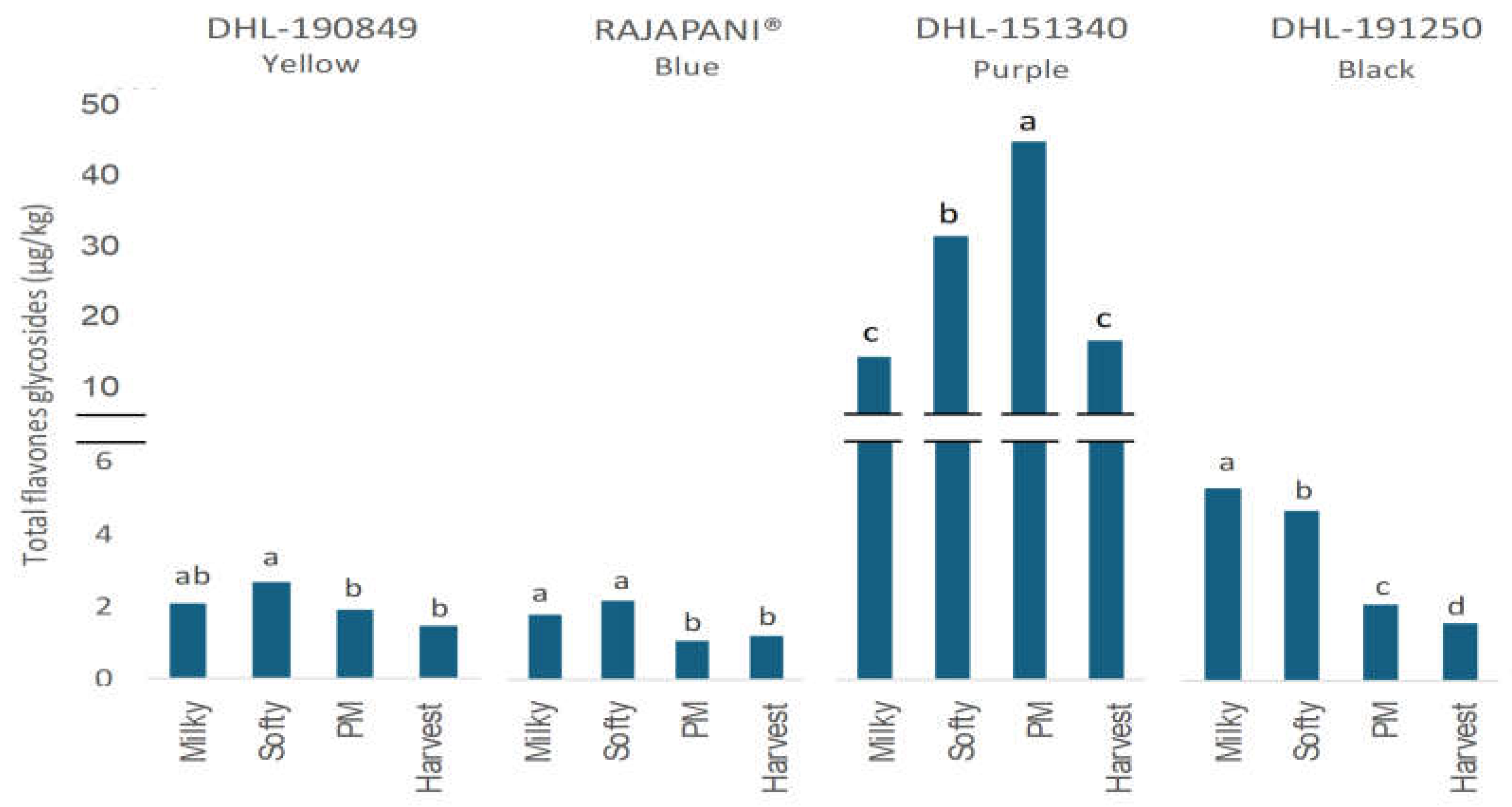
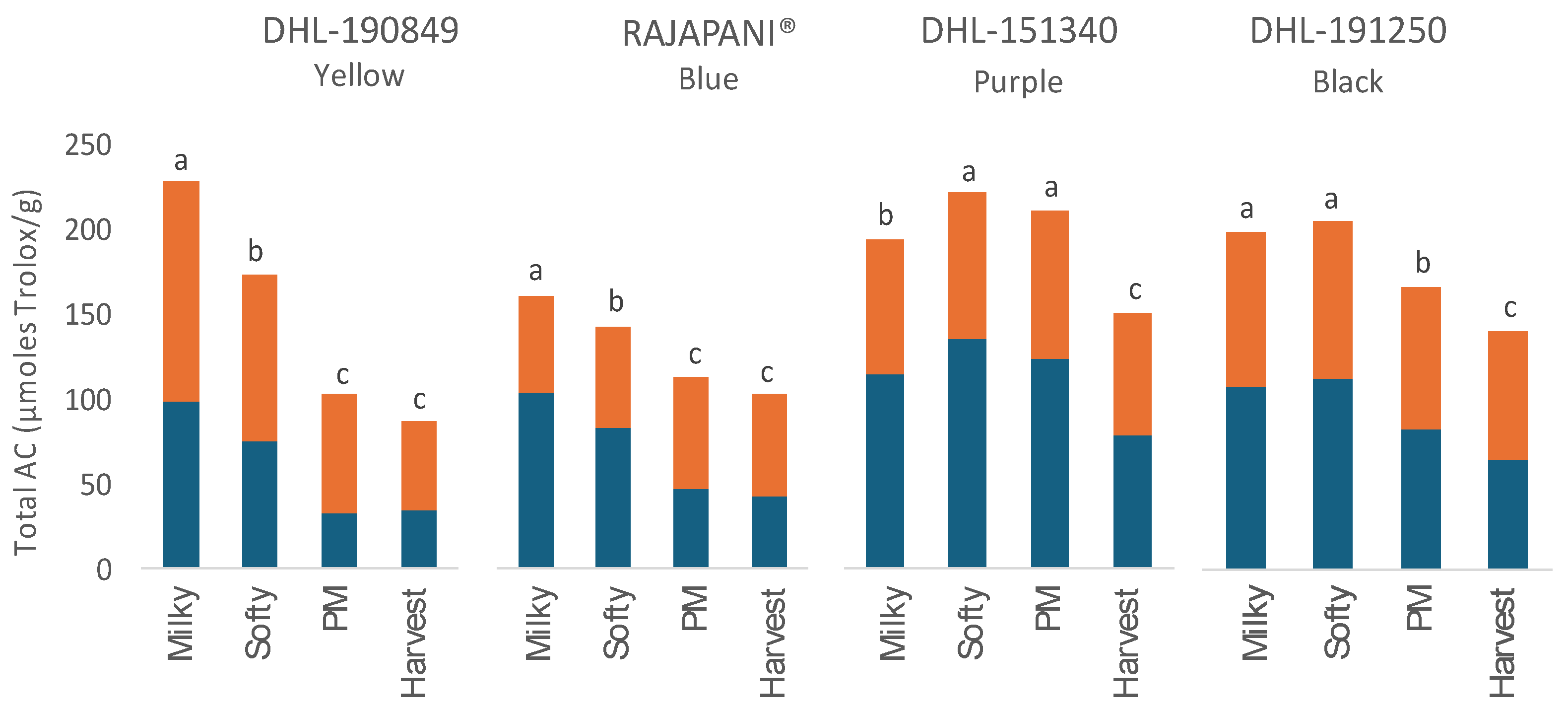
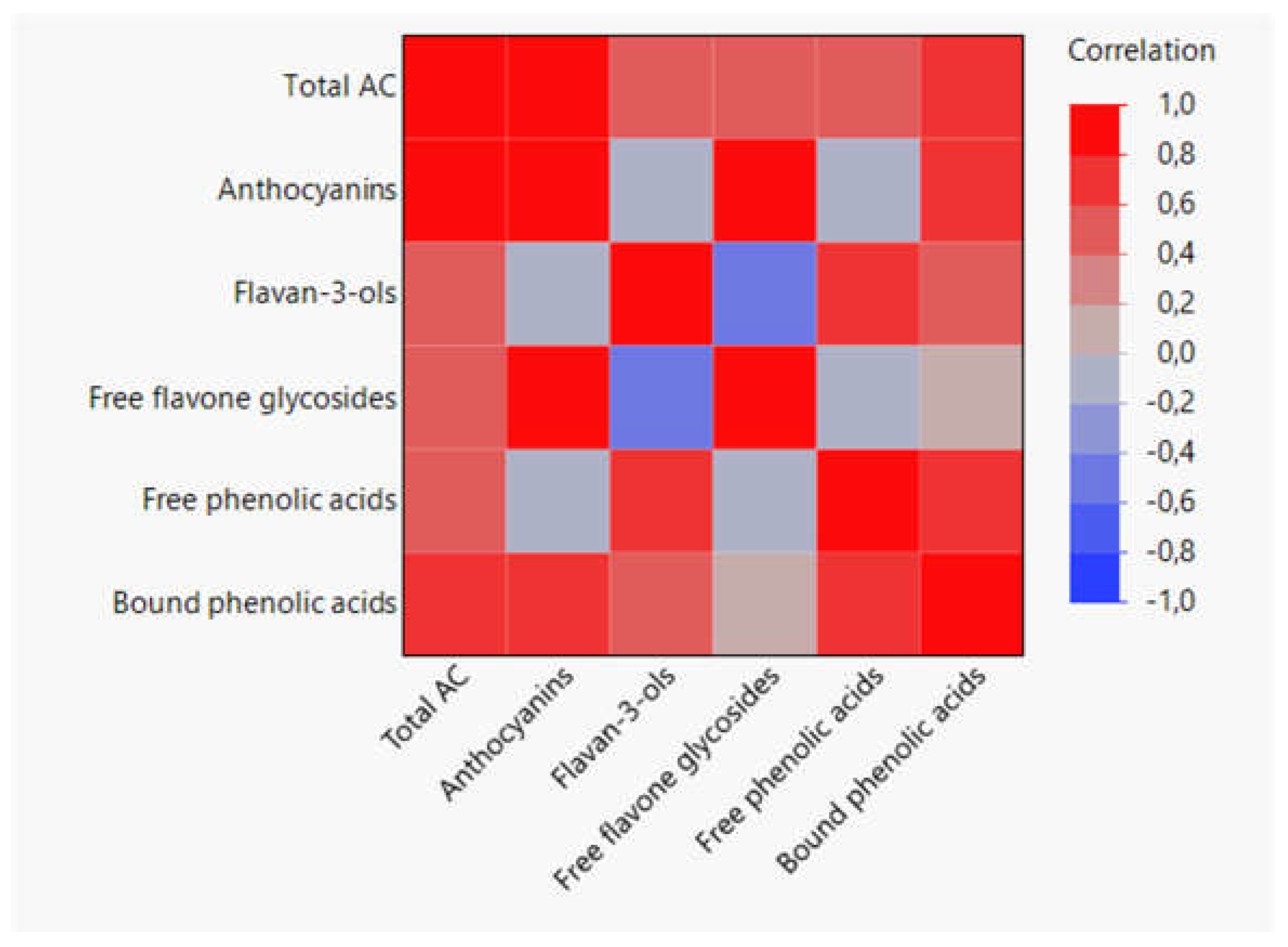
| DHL-190849 Yellow |
Rajapani® Blue |
DHL-151340 Purple |
DHL-191250 Black |
|
|---|---|---|---|---|
| Free phenolic compounds | ||||
| Milky | 1431 A a | 1039 A c | 922 B c | 1284 A b |
| Softy | 1071 B b | 757 B c | 1036 A b | 1223 A a |
| PM | 549 C c | 430 C d | 689 C b | 927 B a |
| Harvest | 568 C b | 308 D c | 342 D c | 972 B a |
| Bound phenolic compounds | ||||
| Milky | 2106 A b | 1282 AB c | 2371 A ab | 2509 A a |
| Softy | 2079 A b | 1289 AB c | 2168 A ab | 2287 B a |
| PM | 1457 B b | 1456 A b | 1643 B a | 1606 C ab |
| Harvest | 1341 C b | 1201 B c | 1462 B a | 1362 D ab |
| Total phenolic compounds | ||||
| Milky | 3537 A ab | 2321 A c | 3193 A b | 3793 A a |
| Softy | 3150 B b | 2047 B c | 3204 A b | 3493 B a |
| PM | 2006 C b | 1886 B b | 2332 B a | 2534 C a |
| Harvest | 1909 C b | 1509 C d | 1788 C c | 2325 C a |
| Mean values followed by different letters are significantly different according to Tukey–Kramer`s HSD for α = 0.05. Uppercase letters show the results of tests for differences among stages of maturation within each genotype. Lowercase letters compared mean phenolic compound content across genotypes for individual stages of maturation. PM: Physiological Maturity. | ||||
| Flavan-3-ols | Phenolic acids | Flavone glycosides | Total free phenols* | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Cat-g | Pc B3 | Pc B2 | Pd B4 | Pd B3 | Pc-d | Pc C2 | C-C-GCs | Ferulic | isoF | TriF | p-Cm | Caff | Sinap-hexo | p-OHB | Vanill | Syring | Lut-g | Isosc-g | ||
| DHL-190849 (Yellow) | |||||||||||||||||||||
| Milky | 469.6 a | 87.0 a | 346.3 a | 14.2 a | 476.2 a | 21.3 a | nd | 10.0 a | nq | 15.7 b | 7.6 a | 1.4 a | 1.2 b | nd | 31.5 a | 1.3 a | 3.1 a | nd | nq | 2.1 ab | 1430.8 a |
| Softy | 216.6 b | 75.0 b | 303.9 b | 6.3 b | 403.8 b | 20.6 a | nd | 7.8 a | nq | 19.2 a | 6.1 b | 1.7 a | 1.6 a | nd | 13.3 b | 1.2 a | 3.0 a | nd | nq | 2.7 a | 1070.5 b |
| PM | 81.8 c | 41.0 c | 181.2 c | 4.0 c | 252.1 c | 10.6 b | 4.7b | 4.2 b | nd | 2.4 c | nd | nd | nd | nd | nd | nd | 1.7 b | nd | nq | 1.9 b | 549.3 c |
| Harvest | 94.7 c | 46.2 c | 149.4 d | 5.7 bc | 234.3 c | 11.4 b | 7.3 a | 3.9 b | nd | 1.5 c | nd | nd | nd | nd | nd | nd | 1.3 b | nd | nq | 1.5 b | 567.8 c |
| SED | 11.89 | 2.80 | 9.28 | 0.42 | 12.90 | 0.96 | 0.29 | 0.65 | 0.35 | 0.43 | 0.21 | 0.05 | 2.09 | 0.18 | 0.30 | 0.23 | 28.06 | ||||
| Rajapani® (Blue) | |||||||||||||||||||||
| Milky | 441.5 a | 43.3 ab | 330.8 a | 13.0 a | 138.8 a | 6.4 ab | nd | 9.0 ab | nd | 36.4 a | 14.7 a | 11.6 a | 2.1 a | nd | nd | 3.5 a | 7.3 a | 1.2 a | nd | 1.7 a | 1038.9 a |
| Softy | 308.8 b | 32.9 c | 211.0 b | 8.9 b | 112.7 b | 7.9 a | nd | 7.5 b | nd | 30.4 b | 16.6 a | 7.3 b | 2.5 a | nd | nd | 2.2 b | 4.7 b | 1.0 a | nd | 2.1 a | 754.7 b |
| PM | 41.7 c | 45.3 a | 199.6 b | 7.0 bc | 105.9 b | 4.3 b | 8.3 a | 9.8 a | nd | 3.2 c | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.0 b | 423.3 c |
| Harvest | 33.3 c | 42.0 b | 138.0 c | 5.7 c | 71.4 c | 1.5 b | 7.2 a | 7.5 b | nd | 0.9 c | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.2 b | 306.9 d |
| SED | 12.92 | 0.99 | 10.12 | 0.73 | 5.72 | 0.54 | 0.86 | 0.55 | 0.79 | 1.32 | 0.76 | 0.30 | 0.31 | 0.51 | 0.12 | 0.13 | 29.34 | ||||
| DHL-151340 (Purple) | |||||||||||||||||||||
| Milky | 195.0 a | 56.4 a | 233.8 a | 11.0 a | 242.5 a | 1.9 a | nd | 9.7 a | nd | 31.8 a | 4.9 a | 2.2 a | 3.5 a | 0.7 b | nd | 2.0 b | 4.7 ab | nd | 10.5 c | 2.5 a | 815.0 a |
| Softy | 108.5 b | 58.5 a | 258.9 a | 8.7 b | 188.7 b | 9.7 b | 3.9 a | 7.4 b | nd | 25.9 b | 3.8 b | 1.2 b | 2.7 a | 2.1 a | nd | 3.1 a | 6.7 a | nd | 26.7 b | 3.1 a | 711.6 b |
| PM | 38.4 c | 35.8 b | 134.2 b | 4.1 c | 55. 8 d | 5.5 c | 3.3 a | 6.0 bc | nd | 10.6 c | 1.4 c | nd | 1.5 b | 0.6 b | nd | 1.8 b | 4.2 b | nd | 42.3 a | 2.4 ab | 344.2 c |
| Harvest | 24.8 c | 43.7 b | 129.9 b | 4.6 c | 81.1 c | 1.9 d | 6.2 a | 4.7 c | nd | 6.3 d | nd | nd | nd | nd | nd | 0.9 c | 3.2 b | nd | 13.5 c | 1.9 b | 311.0 d |
| SED | 4.87 | 3.00 | 7.53 | 0.47 | 3.04 | 0.65 | 0.73 | 0.49 | 0.99 | 0.24 | 0.08 | 0.31 | 0.17 | 0.14 | 0.55 | 1.44 | 0.18 | 7.98 | |||
| DHL-191250 (Black) | |||||||||||||||||||||
| Milky | 170.5 a | 115.6 a | 434.3 a | 13.7 ab | 414.9 a | 24.3 a | nd | 13.6 b | 9.7 a | 28.0 a | 14.7 a | 3.4 a | 2.9 a | nd | 47.9 a | 1.9 a | 4.9 a | nd | nd | 5.6 a | 1283.9 a |
| Softy | 117.5 b | 114.2 a | 420.4 a | 15.4 a | 395.0 ab | 23.4 ab | nd | 16.3 a | 7.5 b | 30.6 a | 15.5 a | 1.7 b | 2.7 a | nd | 41.6 b | 0.9 b | 5.4 a | nd | nd | 4.9 a | 1222.6 a |
| PM | 82.2 c | 97.8 a | 311.2 b | 12.0 ab | 396.0 ab | 19.7 c | 17.8 a | 12.0 b | 10.3 a | 1.0 b | 1.9 b | nd | nd | nd | nd | nd | nd | nd | nd | 2.2 b | 927.3 b |
| Harvest | 89.8 c | 114.6 a | 324.2 b | 11.1 b | 375.3 b | 20.7 bc | 19.0 a | 13.8 ab | 9.1 ab | 1.5 b | 1.3 b | nd | nd | nd | nd | nd | nd | nd | nd | 1.7 b | 972.0 b |
| SED | 4.87 | 5.10 | 13.19 | 1.03 | 6.99 | 0.74 | 1.15 | 0.72 | 0.50 | 1.07 | 0.80 | 0.34 | 0.46 | 1.22 | 0.15 | 0.41 | 0.23 | 31.32 | |||
| Results are presented as the mean. Means within a column followed by different letters indicate significant differences according to Tukey–Kramer`s HSD for α = 0.05. SED: standard error of the difference between means. Flavan-3-ols: (Cat: Catechin, Cat-g: Catechin-glucoside, Pc B3: Procyanidin, Pc B2: Procyanidin B2, Pd B4: Prodelphinidin B4, Pd B3: Prodelphinidin B3, Pc-d: Procyanidin-diglucoside, Pc C2: Procyanidin C2, C-C-GCs: sum of C-C-GC/GC-C-C and C-GC-C); Phenolic acids (Ferulic: Ferulic acid, isoF: Iso-Ferulic acid, TriF: Tri-Ferulic acid, p-Cm: p-Coumaric acid, Caff: Caffeic acid, Sinap-hexo: Sinapoyl-hexose, p-OHB: p-hydroxybenzoic acid, Vanill: Vanillic acid, Syring: Syringic acid); Flavone glycosides: (Lut-g: Luteolin-O-glucoside, Isosc-g: Isoscoparin-7-glucoside). nd: not detected. nq: not quantified. PM: Physiological Maturity. *Anthocyanins not included. | |||||||||||||||||||||
| Phenolic acids | Flavone glycosides | Total bound phenlols | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferulic | isoF | DiF | TriF | DC DiF | p-Cm | m-Cm | Sinapic | Caff | Cinna | Ferul-pen | p-OHB | Vanillic | Syring | Syngaral | Isosc-g | |||||
| DHL-190849 (Yellow) | ||||||||||||||||||||
| Milky | 1364.8 a | 377.9 a | 249.5 ab | 21.4 c | 28.6 b | 9.9 b | 0.8 b | nd | 0.3 a | nd | nd | 1.5 b | 1.6 d | 0.5 c | 0.6 c | nd | 2106.3 a | |||
| Softy | 1418.2 a | 423.6 a | 255.1 a | 37.0 b | 36.2 a | 9.4 b | 0.9 b | nd | 0.1 a | nd | 0.3 ab | 1.6 b | 3.0 c | 0.7 bc | 1.1 b | 0.2 a | 2078.6 a | |||
| PM | 866.6 b | 258.6 b | 211.8 bc | 51.8 a | 36.5 a | 16.5 a | 3.8 a | nd | 0.2 a | 0.4 a | 0.4 a | 2.5 a | 7.0 a | 0.9 ab | 1.3 a | 0.3 a | 1457.1 b | |||
| Harvest | 833.3 b | 243.0 b | 180.3 c | 33.2 b | 33.1 ab | 7.2 c | 0.8 b | nd | 0.2 a | 0.3 a | 0.2 b | 1.8 b | 5.3 b | 1.1 a | 1.2 a | 0.2 a | 1341.1 c | |||
| SED | 70.59 | 24.57 | 11.16 | 1.75 | 1.94 | 0.64 | 0.06 | 0.04 | 0.04 | 0.04 | 0.10 | 0.31 | 0.10 | 0.05 | 0.03 | 28.01 | ||||
| Rajapani® (Blue) | ||||||||||||||||||||
| Milky | 884.8 ab | 229.3 a | 122.0 b | 10.4 c | 21.0 b | 12.7 b | 1.5 a | nd | 0.2 a | nd | nd | 1.4 c | 1.5 b | 0.6 b | 1.3 b | 0.1 a | 1282.4 ab | |||
| Softy | 823.5 ab | 228.3 a | 133.7 b | 14.5 bc | 23.2 b | 12.9 b | 1.9 a | nd | 0.2 a | nd | nd | 1.8 b | 2.4 b | 0.8 ab | 0.5 c | 0.1 a | 1289.3 ab | |||
| PM | 944.4 a | 227.0 a | 188.3 a | 49.2 a | 24.6 b | 15.4 a | 1.5 a | nd | 0.2 a | nd | 0.2 a | 2.2 a | 5.2 a | 1.1 a | 2.4 a | 0.2 a | 1456.1 a | |||
| Harvest | 799.2 b | 192.2 a | 133.5 b | 25.0 b | 35.2 a | 13.7 ab | 1.5 a | nd | 0.2 a | nd | 0.2 a | 2.1 ab | 4.9 a | 1.2 a | 2.3 a | 0.1 a | 1200.6 b | |||
| SED | 38.87 | 13.34 | 10.23 | 2.63 | 2.66 | 0.66 | 0.15 | 0.02 | 0.03 | 0.10 | 0.28 | 0.14 | 0.12 | 0.03 | 62.58 | |||||
| DHL-151340 (Purple) | ||||||||||||||||||||
| Milky | 1461.6 a | 414.1 a | 345.8 b | 56.8 b | 50.0 b | 27.0 bc | 2.0 c | 3.8 a | 0.2 a | 3.5 a | nd | 1.3 d | 2.7 d | 0.8 a | 1.3 b | 0.04 b | 2371.1 a | |||
| Softy | 1240.0 b | 328.1 b | 398 .9 a | 82.3 a | 63.1 a | 32.3 a | 3.1 b | 4.1 a | 0.2 a | 3.3 a | 1.6 a | 2.8 c | 5.2 c | 0.9 a | 1.8 ab | 0.07 ab | 2167.8 a | |||
| PM | 1006.0 c | 165.4 c | 299.4 c | 62.0 b | 51.6 b | 31.7 ab | 4.0 a | 3.6 a | 0.1 a | 2.2 b | 0.7 b | 5.9 b | 8.4 b | 1.0 a | 2.1 a | 0.08 a | 1642.8 b | |||
| Harvest | 954.8 c | 106.4 d | 242.3 d | 58.7 b | 48.5 b | 22.5 c | 1.5 c | 4.2 a | 0.2 a | 2.1 b | 0.0 b | 7.6 a | 9.8 a | 1.1 a | 1.9 a | nd | 1461.5 b | |||
| SED | 39.67 | 8.97 | 7.91 | 4.65 | 2.51 | 1.34 | 0.19 | 0.04 | 0.03 | 0.22 | 0.18 | 0.28 | 0.35 | 0.10 | 0.12 | 0.01 | 61.57 | |||
| DHL-191250 (Black) | ||||||||||||||||||||
| Milky | 1526.0 a | 663.6 a | 231.2 b | 21.0 b | 46.3 b | 13.7 b | 2.1 b | nd | 0.2 a | nd | nd | 1.4 a | 2.3 d | 0.9 a | 0.8 c | 0.2 a | 2509.3 a | |||
| Softy | 1320.0 b | 438.4 b | 396.4 a | 36.5 a | 58.4 a | 20.3 a | 3.2 a | 4.1 a | 0.4 a | 0.3 a | 0.6 a | 1.6 a | 3.6 c | 1.0 a | 1.8 b | 0.3 a | 2286.6 b | |||
| PM | 960.7 c | 347.5 c | 220.0 bc | 24.8 b | 36.3 c | 8.5 c | 1.2 c | 3.8 a | 0.2 a | 0.2 b | 0.3 b | 1.4 a | 6.4 a | 1.0 a | 2.5 a | 0.3 a | 1606.3 c | |||
| Harvest | 851.4 d | 247.2 d | 186.2 c | 21.2 b | 31.2 c | 10.5 bc | 1.3 c | 2.7 b | 0.2 a | 0.2 ab | nd | 1.4 a | 5.3 b | 1.0 a | 1.8 b | nd | 1361.6 d | |||
| SED | 28.59 | 17.86 | 10.75 | 2.37 | 2.52 | 0.87 | 0.17 | 0.27 | 0.07 | 0.01 | 0.04 | 0.12 | 0.21 | 0.13 | 0.07 | 0.05 | 49.85 | |||
| Results are presented as the mean. Means within a column followed by different letters indicate significant differences according to Tukey–Kramer`s HSD for α = 0.05. SED: standard error of the difference between means. Phenolic acids: (Ferulic: Ferulic acid, isoF: Iso-Ferulic acid, DiF: Di-Ferulic acid, TriF: Tri-Ferulic acid, DC DiF: Decarboxylated Di-ferulic acid, p-Cm: p-Coumaric acid, m-Cm: m-Coumaric acid, Sinapic: Sinapic acid, Caff: Caffeic acid, Cinna: Cinnamic acid, Ferul-pen: Feruloyl-pentose, p-OHB: p-hydroxybenzoic acid, Vanillic: Vanillic acid, Syring: Syringic acid, Syngaral: Syngaraldehyde); Flavone glycosides: (Isosc-g: Isoscoparin-7-glucoside). nd: not detected. nq: not quantified. PM: Physiological Maturity. | ||||||||||||||||||||
| Anthocyanins | |||||||
|---|---|---|---|---|---|---|---|
| Cyanidins | Pelargonidins | Peonidins | Delphinidins | Petunidins | Malvidins | Total anthocyanins | |
| Rajapani® (Blue) | |||||||
| Milky | 0.29 d | 0.01 d | 0.01 d | 0.06 d | 0.01 c | 0.001 c | 0.37 d |
| Softy | 1.95 b | 0.10 b | 0.09 b | 0.32 b | 0.05 a | 0.004 b | 2.51 b |
| PM | 5.36 a | 0.25 a | 0.13 a | 0.44 a | 0.06 a | 0.010 a | 6.34 a |
| Harvest | 0.85 c | 0.04 c | 0.02 c | 0.23 d | 0.02 b | 0.001 c | 1.16 c |
| SED | 0.054 | 0.002 | 0.030 | 0.005 | 0.001 | 0.000 | 0.054 |
| DHL-151340 (Purple) | |||||||
| Milky | 103.88 c | 1.93 b | 1.19 c | 0.96 c | 0.19 b | 0.09 c | 108.24 c |
| Softy | 301.87 b | 14.57 a | 2.58 b | 3.66 a | 0.62 a | 0.67 a | 323.97 b |
| PM | 323.60 a | 13.73 a | 3.06 a | 3.41 b | 0.58 a | 0.51 b | 344.89 a |
| Harvest | 27.35 d | 1.60 b | 0.94 d | 1.09 c | 0.14 c | 0.10 c | 31.21 d |
| SED | 2.588 | 0.213 | 0.038 | 0.054 | 0.011 | 0.016 | 2.769 |
| Results are presented as the mean. Means within a column followed by different letters indicate significant differences according to Tukey–Kramer`s HSD for α = 0.05. SED: standard error of the difference between means. PM: Physiological Maturity. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





