Submitted:
22 June 2024
Posted:
24 June 2024
Read the latest preprint version here
Abstract
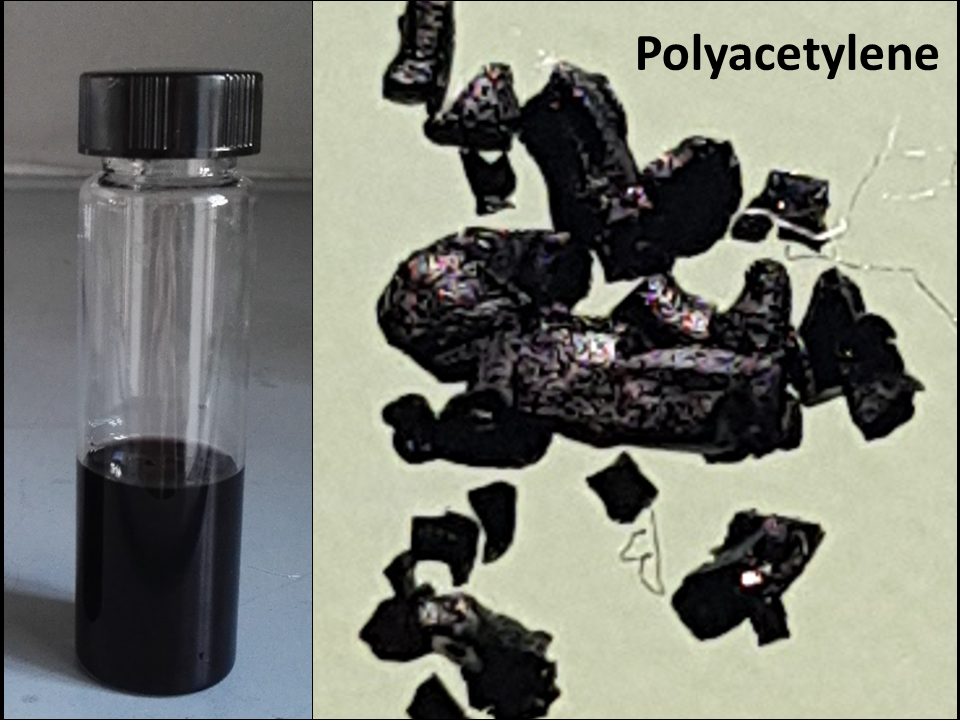
Keywords:
1. Introduction
2. Experimental Part
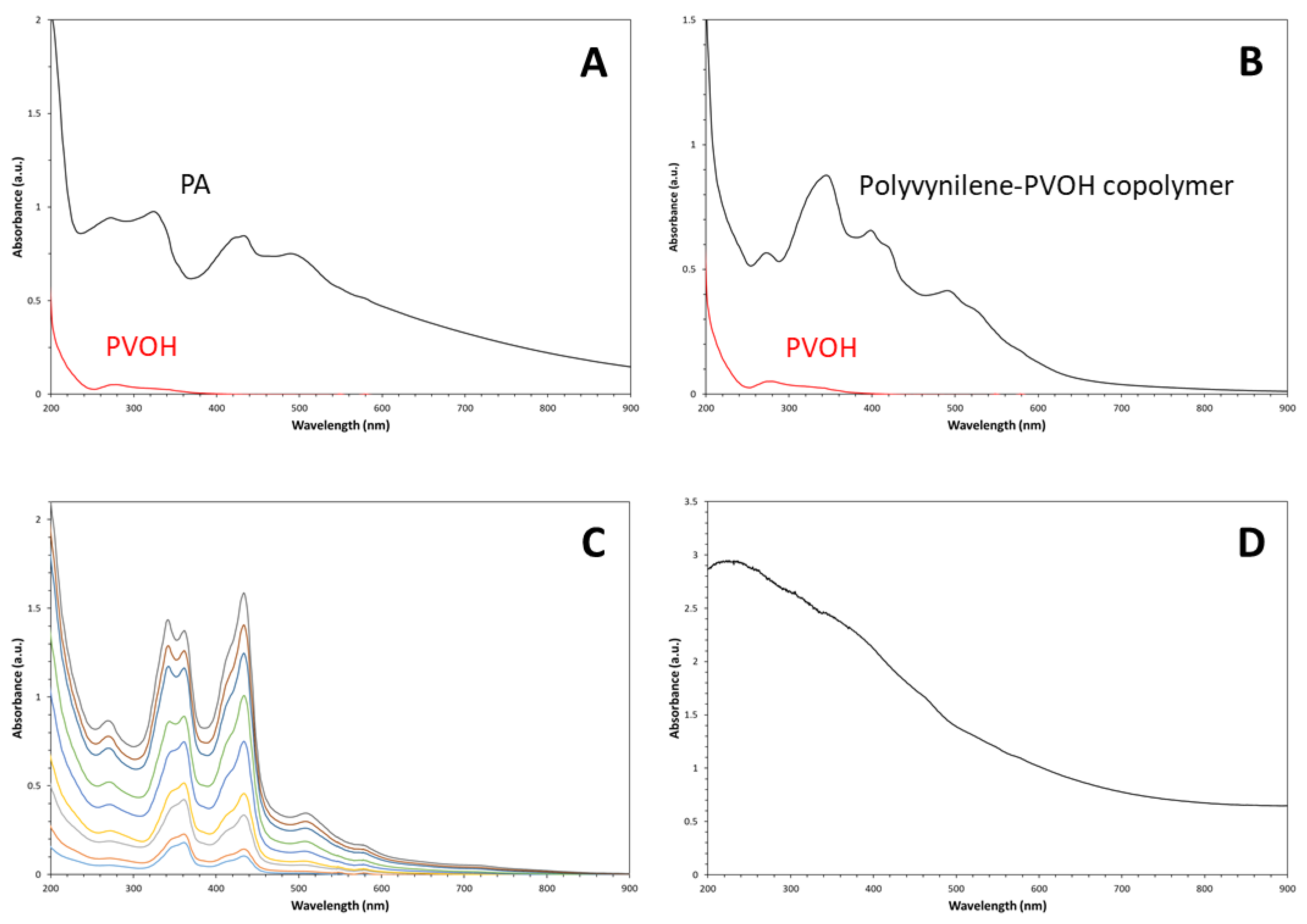
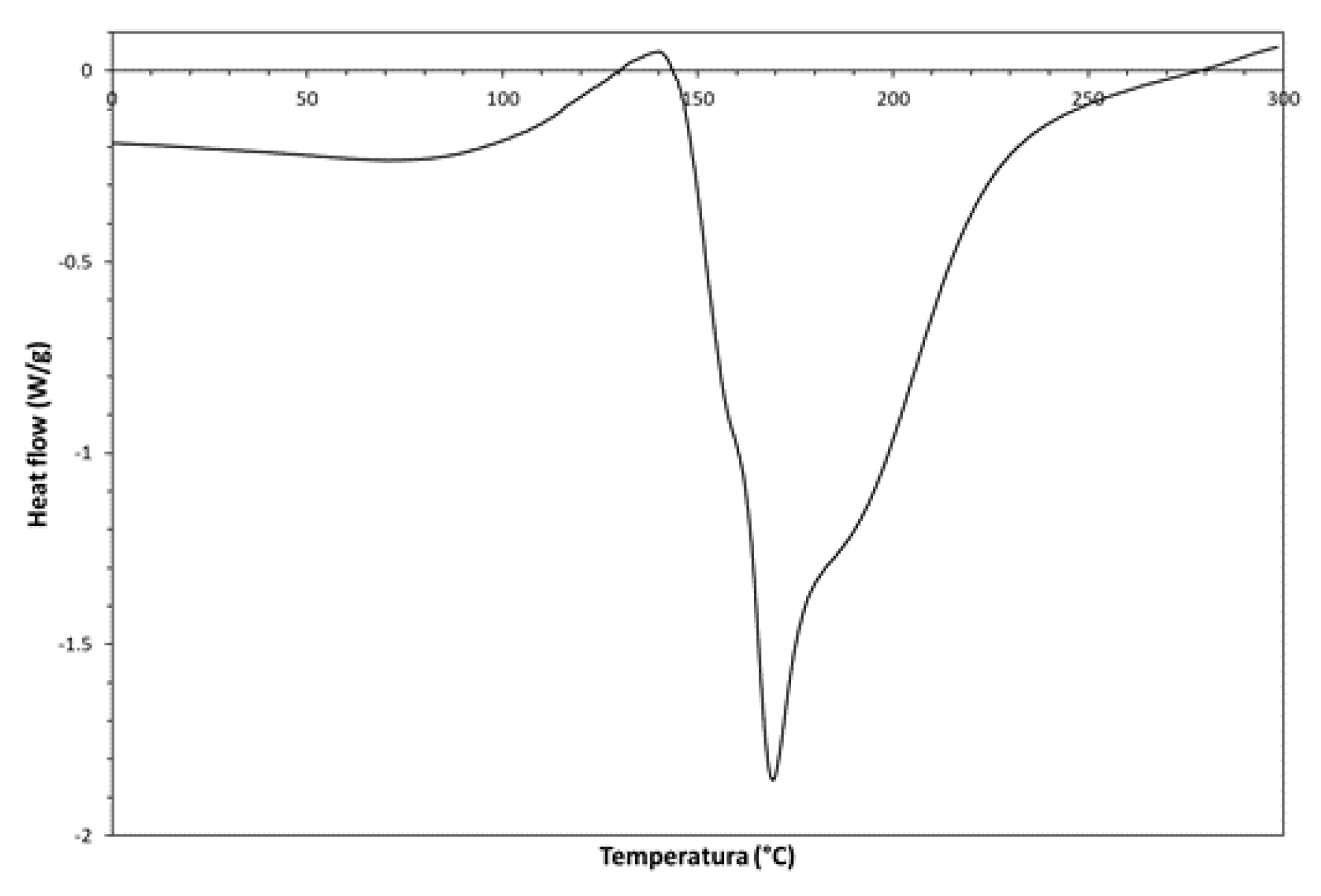
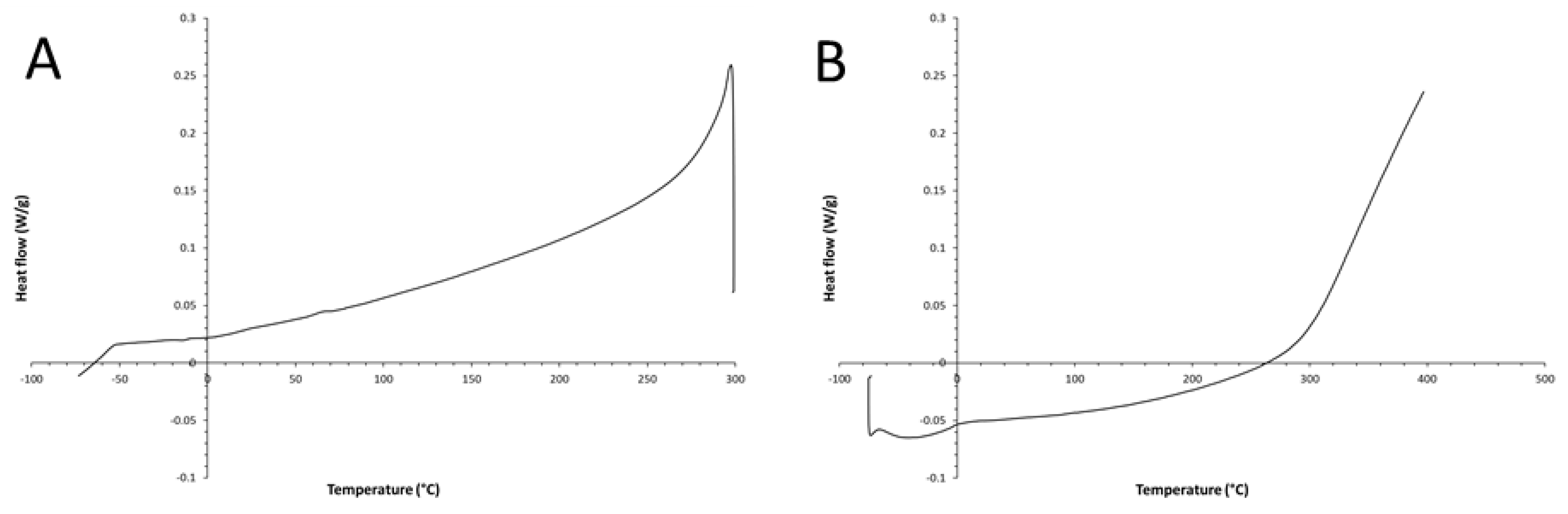
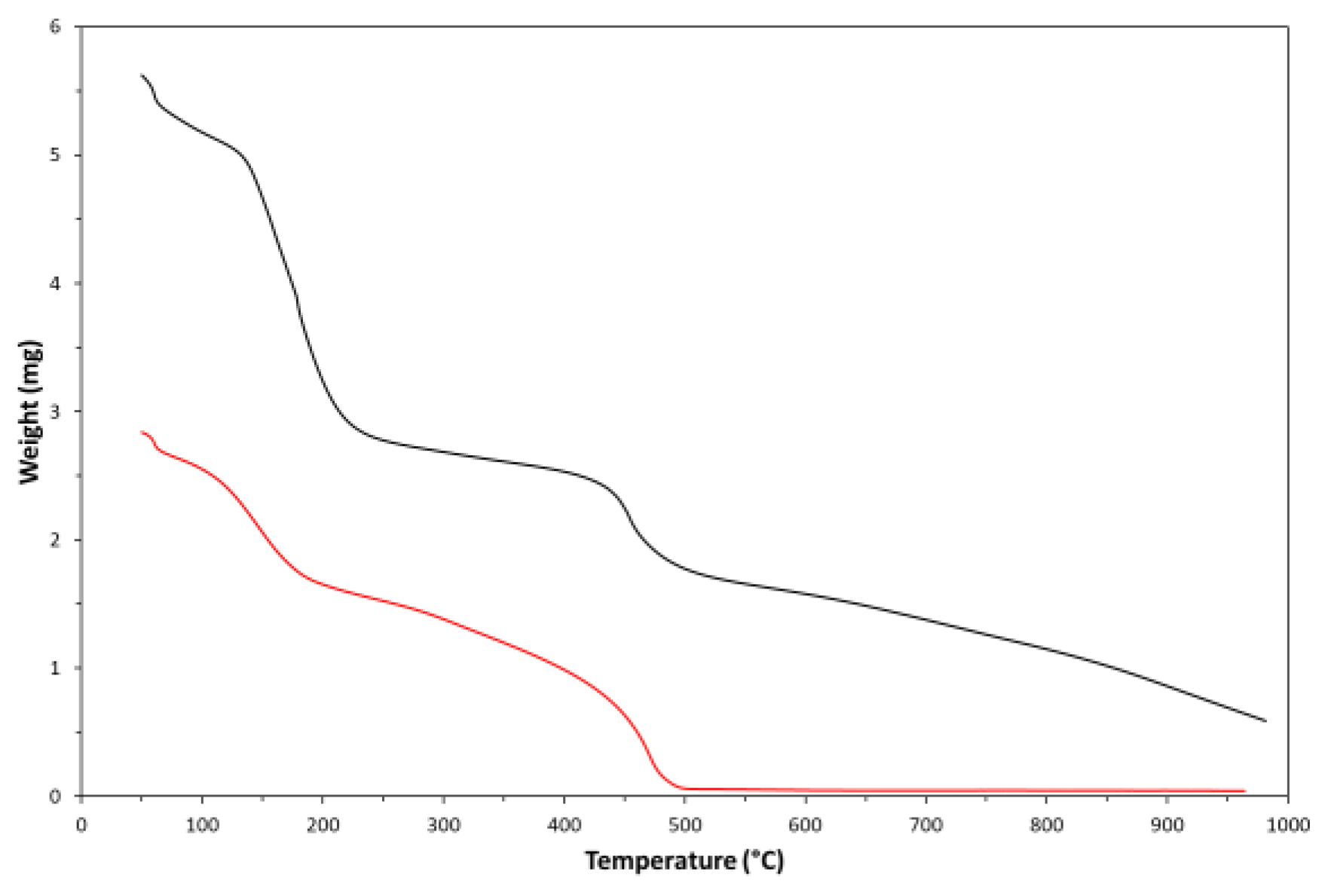
3. Results and Discussion
4. Conclusion
Acknowledgments
References
- Olabisi, Olagoke, and Kolapo, Adewale, eds. Handbook of thermoplastics. Vol. 41. CRC press, 2016. Cap. 34.
- Farrington, G.C.; Huq, R. Polyacetylene electrodes for non-aqueous lithium batteries. J. Power Sources 1985, 14, 3–9. [CrossRef]
- T. Nagatomo, C. Ichikawa, O. Omoto, “All-plastic batteries with polyacetylene electrodes”, J. Electrochem. Soc. 134(2)(1987)305-308. [CrossRef]
- S.E. Eusyukov, Yu P. Kudryavtsev, Yu V. Korshak, “Chemical dehydroalogenation of halogen-containing polymers”, Russian Chemical Reviews 60(4)(1991)373-390. [CrossRef]
- Prosanov, I.Y.; Uvarov, N.F. Electrical properties of dehydrated polyvinyl alcohol. Phys. Solid State 2012, 54, 421–424. [CrossRef]
- Prosanov, I.Y.; Matvienko, A.A. Study of PVA thermal destruction by means of IR and Raman spectroscopy. Phys. Solid State 2010, 52, 2203–2206. [CrossRef]
- Prosanov, I.Y.; Matvienko, A.A.; Bokhonov, B.B. Influence of urea on polyvinyl alcohol molecular superstructure formation. Phys. Solid State 2011, 53, 1302–1306. [CrossRef]
- Mainthia, S.B.; Kronick, P.L.; Labes, M.M. Electrical Measurements on Polyvinylene and Polyphenylene. J. Chem. Phys. 1962, 37, 2509–2510. [CrossRef]
- Ward, D.J.; Saccomando, D.J.; Walker, G.; Mansell, S.M. Sustainable routes to alkenes: applications of homogeneous catalysis to the dehydration of alcohols to alkenes. Catal. Sci. Technol. 2023, 13, 2638–2647. [CrossRef]
- Dolson, D.A.; Battino, R.; Letcher, T.M.; Pegel, K.H.; Revaprasadu, N. Carbohydrate Dehydration Demonstrations. J. Chem. Educ. 1995, 72. [CrossRef]
- B.S. Minhas, D.G. Peiffer, J.L. Soto, D.L. Stern, “Process for the recovery of sulfuric acid using polymeric membranes, PTC patent, WO 2004/074811 A2 (2 September 2004).
- K. Iwahana, P. Knoll, H. Kuzmany, M. Riegler, B. Hubmann, “Luminescence from trans-polyacetylene degraded by laser irradiation”. In: Kuzmany; H., Mehring, M.; Roth, S. (eds.) Electronic Properties of Polymers and Related Compounds. Springer Series in Solid-State Sciences, Vol. 63, Springer, Berlin, Heidelberg (1985). [CrossRef]
- Hill, C.; Altgen, M.; Penttilӓ, P.; Rautkari, L. Review: interaction of water vapour with wood and other hygro-responsive materials. J. Mater. Sci. 2024, 59, 7595–7635. [CrossRef]
- Shirakawa, H.; Ikeda, S. Infrared Spectra of Poly(acetylene). Polym. J. 1971, 2, 231–244. [CrossRef]
- Cataldo, F. A spectroscopic study of polyacetylene prepared by using Rh(I) catalysts. Polymer 1994, 35, 5235–5240. [CrossRef]
- Kim, J.-Y.; Kim, J.-T.; Kwon, M.-H.; Han, D.-K.; Kwon, S.-J. ATR-Infrared spectroscopic study of n-doped polyacetylene films. Macromol. Res. 2007, 15, 5–9. [CrossRef]
- Gibson, H.W.; Kaplan, S.; Mosher, R.A.; Prest, W.M.; Weagley, R.J. Isomerization of polyacetylene films of the Shirakawa type - spectroscopy and kinetics. J. Am. Chem. Soc. 1986, 108, 6843–6851. [CrossRef]
- Alvarez, B.; Sarmiento-Santos, A.; Vera-López, E. Acetylene polymerization in plasma of direct current. J. Physics: Conf. Ser. 2019, 1386, 012044. [CrossRef]
- Li, H.; Chen, G.; Duchesne, P.N.; Zhang, P.; Dai, Y.; Yang, H.; Wu, B.; Liu, S.; Xu, C.; Zheng, N. A nanoparticulate polyacetylene-supported Pd(II) catalyst combining the advantages of homogeneous and heterogeneous catalysts. Chin. J. Catal. 2015, 36, 1560–1572. [CrossRef]
- Zhao, W.; Yamamoto, Y.; Seki, S.; Tagawa, S. Formation of Conjugated Double Bonds in Poly(vinyl alcohol) Film under Irradiation with γ-Rays at Elevated Temperature. Chem. Lett. 1997, 26, 183–184. [CrossRef]
- Kleist, F.D.; Byrd, N.R. Preparation and properties of polyacetylene. J. Polym. Sci. Part A-1: Polym. Chem. 1969, 7, 3419–3425. [CrossRef]
- Yen, H.-J.; Shan, C.; Wang, L.; Xu, P.; Zhou, M.; Wang, H.-L. Development of Conjugated Polymers for Memory Device Applications. Polymers 2017, 9, 25. [CrossRef]
- Luo, T.; Xu, X.; Jiang, M.; Lu, Y.-Z.; Meng, H.; Li, C.-X. Polyacetylene carbon materials: facile preparation using AlCl3 catalyst and excellent electrochemical performance for supercapacitors. RSC Adv. 2019, 9, 11986–11995. [CrossRef]
- V.M. Kobryanskii, “Nanopolyacetylene: optical properties and practical application”, Proceedings of SPIE, Volume 4277, Integrated Optics Devices V, Symposium on Integrated Optics, 2001, San Jose, CA, United States. [CrossRef]
- Lee, H.; Choi, W.I.; Ihm, J. On hydrogen storage in metal-decorated trans-polyacetylene. J. Alloy. Compd. 2007, 446-447, 373–375. [CrossRef]
- J. Liang, C. Song, J. Deng, “Optically active microspheres constructed by helical substituted polyacetylene and used for absorption of organic compounds in aqueous systems”, ACS Appl. Mater. Interfaces 6(21)(2014)19041-19049. [CrossRef]
- Chen, M.; Hu, G.; Shen, T.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Applications of Polyacetylene Derivatives in Gas and Liquid Separation. Molecules 2023, 28, 2748. [CrossRef]
- Li, H.; Chen, G.; Duchesne, P.N.; Zhang, P.; Dai, Y.; Yang, H.; Wu, B.; Liu, S.; Xu, C.; Zheng, N. A nanoparticulate polyacetylene-supported Pd(II) catalyst combining the advantages of homogeneous and heterogeneous catalysts. Chin. J. Catal. 2015, 36, 1560–1572. [CrossRef]
- Sebastian, A. Raghavan, “Advanced polymer composite with graphene content for EMI shielding”, International Research Journal on Advanced Engineering Hub 2(5)(2024)1334-1340. [CrossRef]
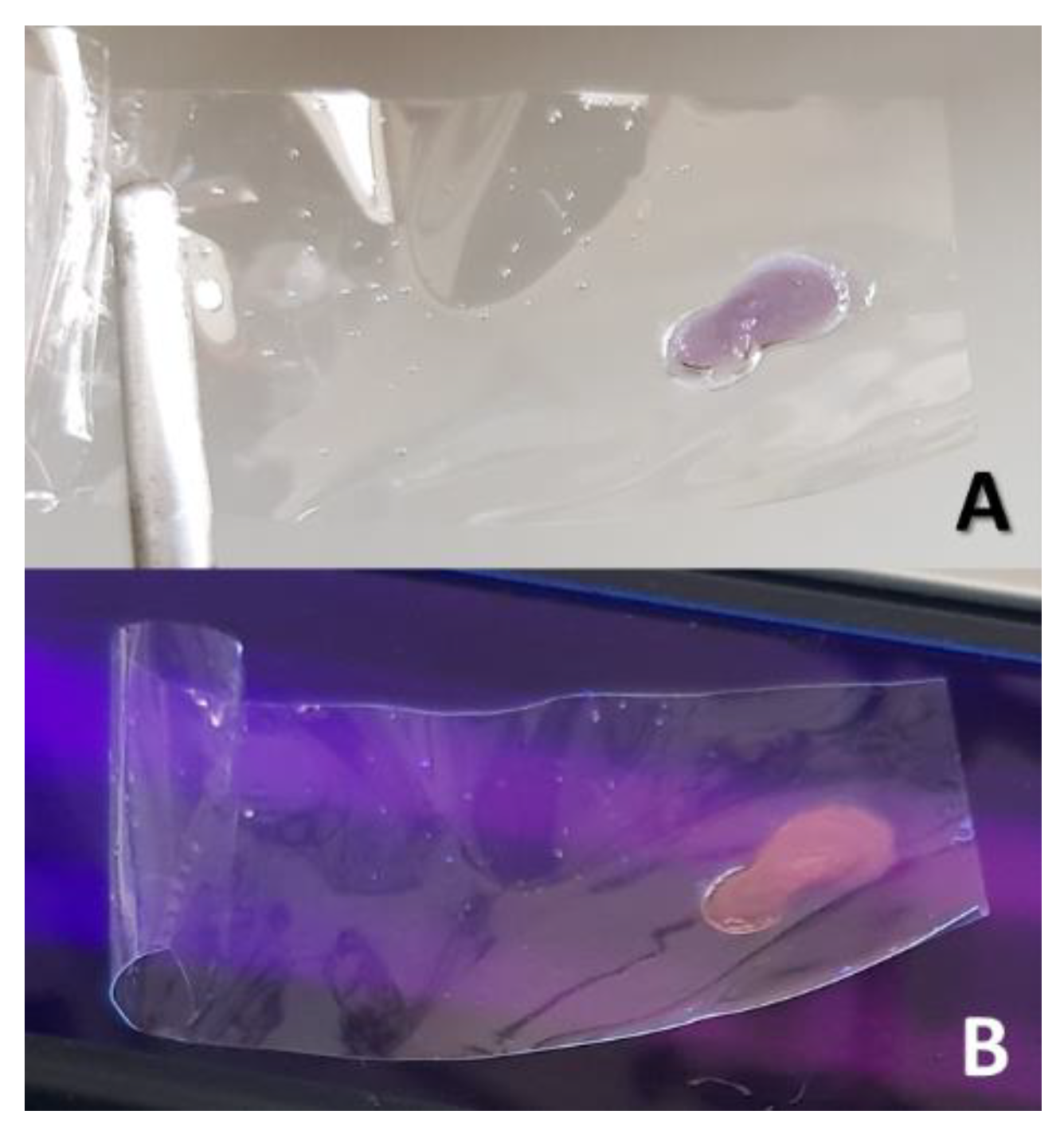
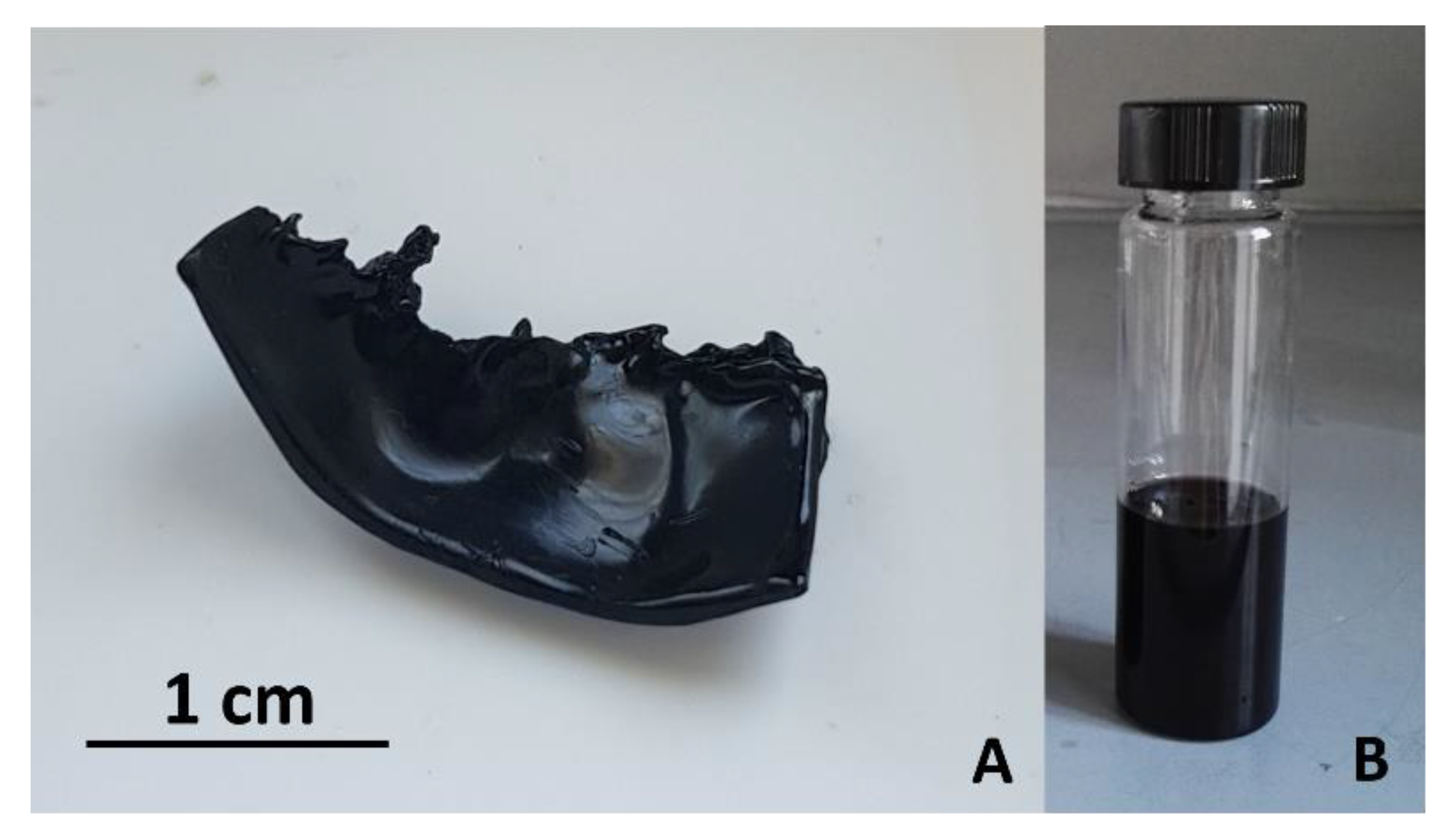
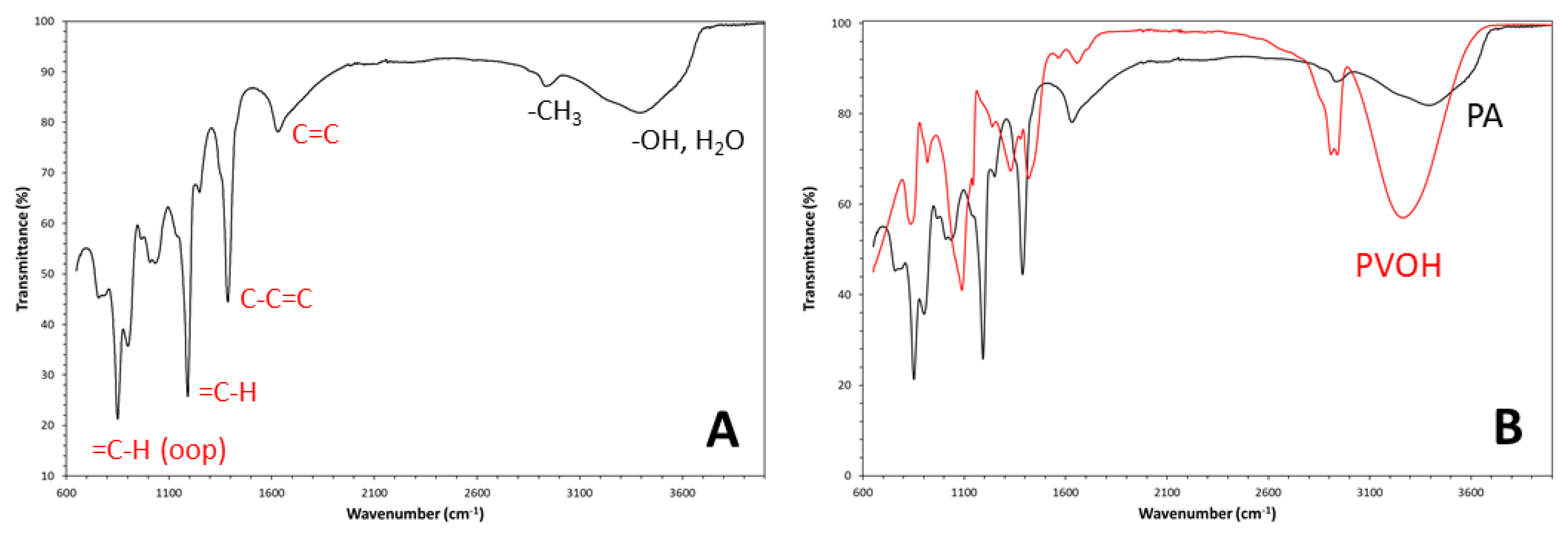
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




