Submitted:
20 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Reagents and Apparatus
2.2. Synthesis of L-Lys-Boc2
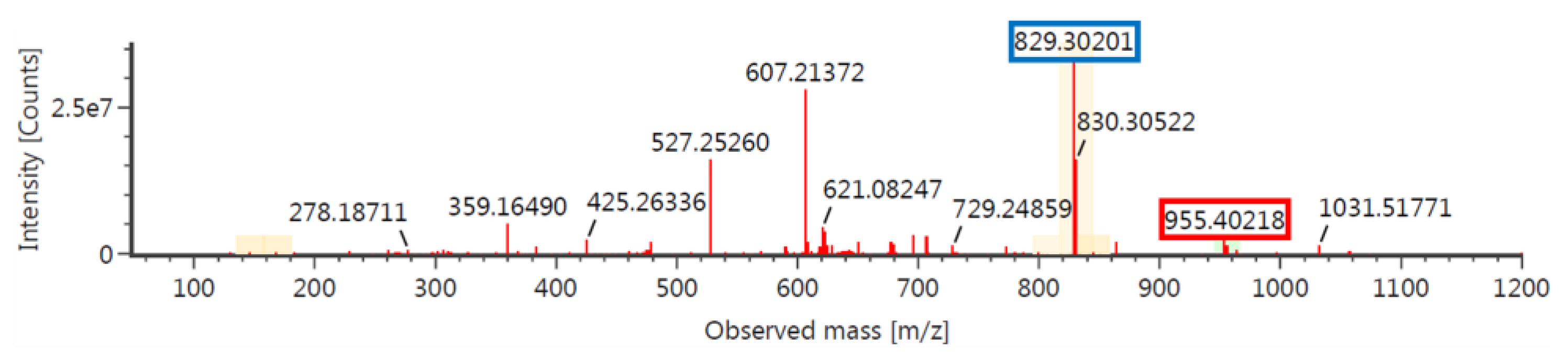
2.3. Synthesis of (L, R)-1
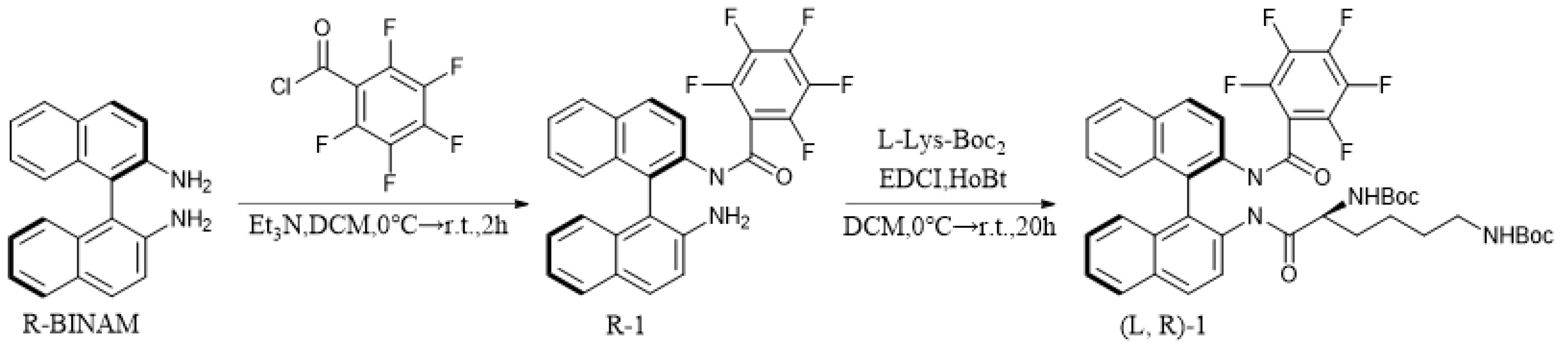
2.4. Preparation of Samples for Fluorescence Analysis
3. Result and Discussion
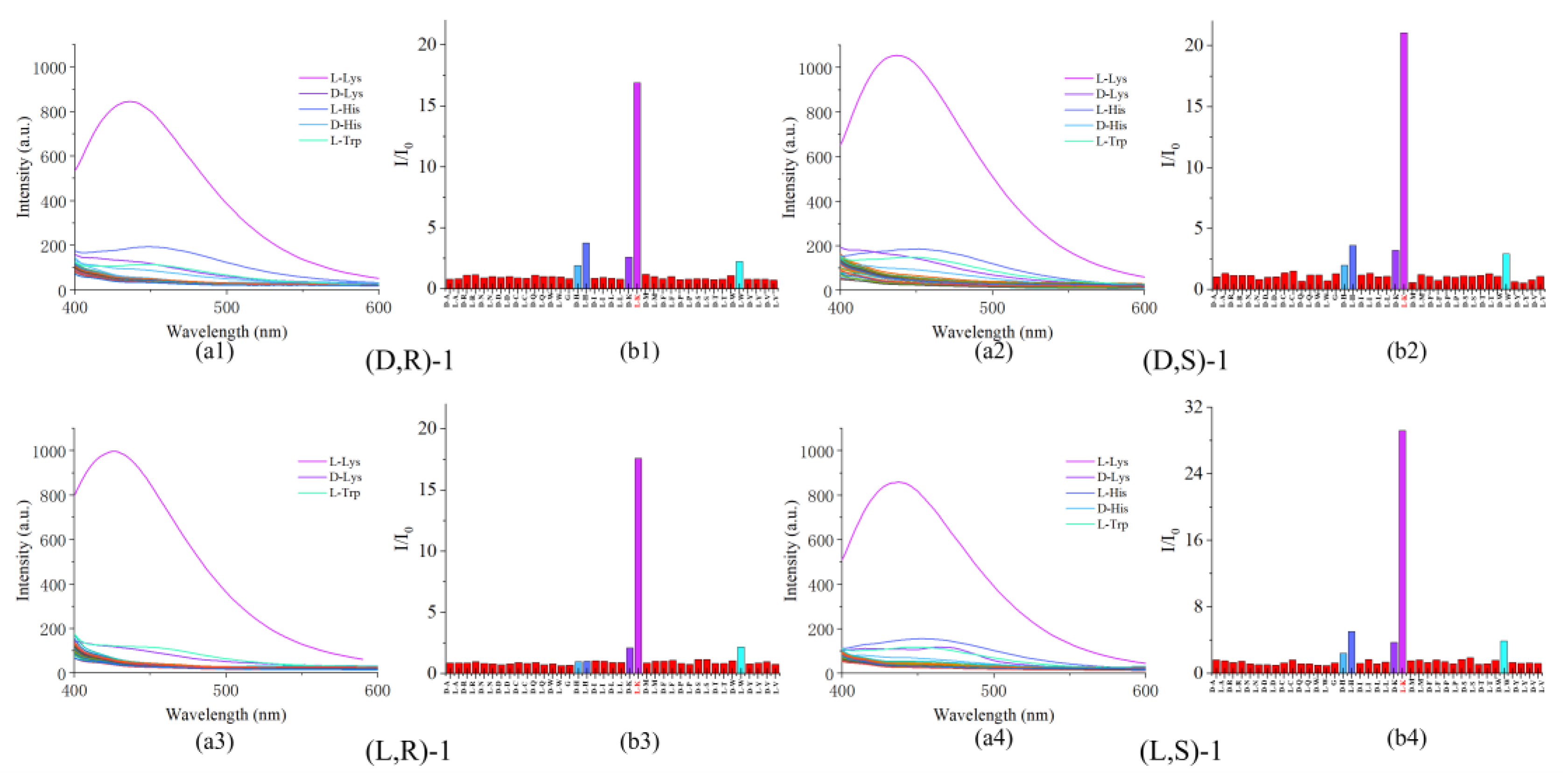
3.1. Chiral Recognition of D/L-Lysine
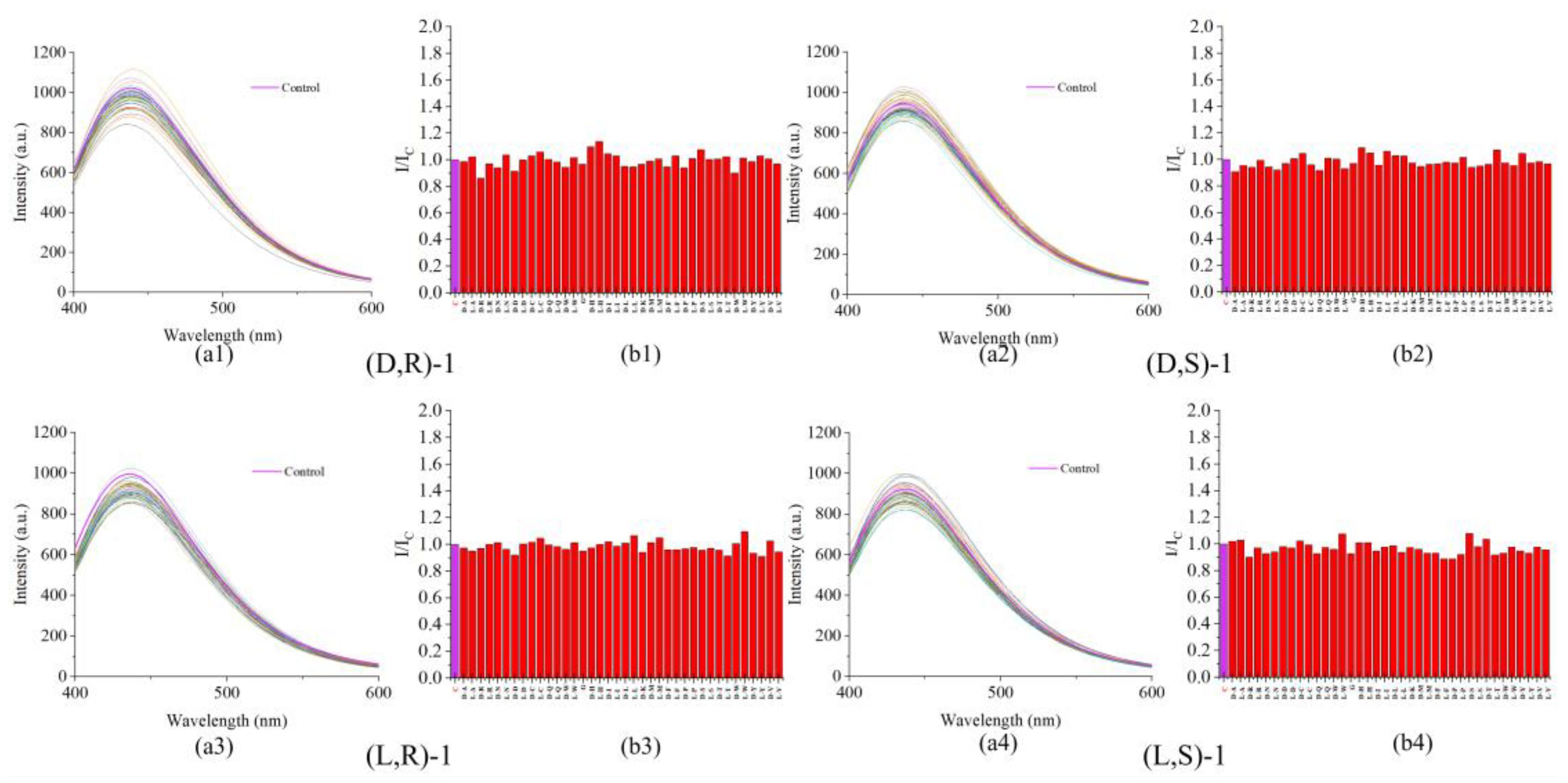
3.2. Interference by other Amino Acids in Probe Recognition
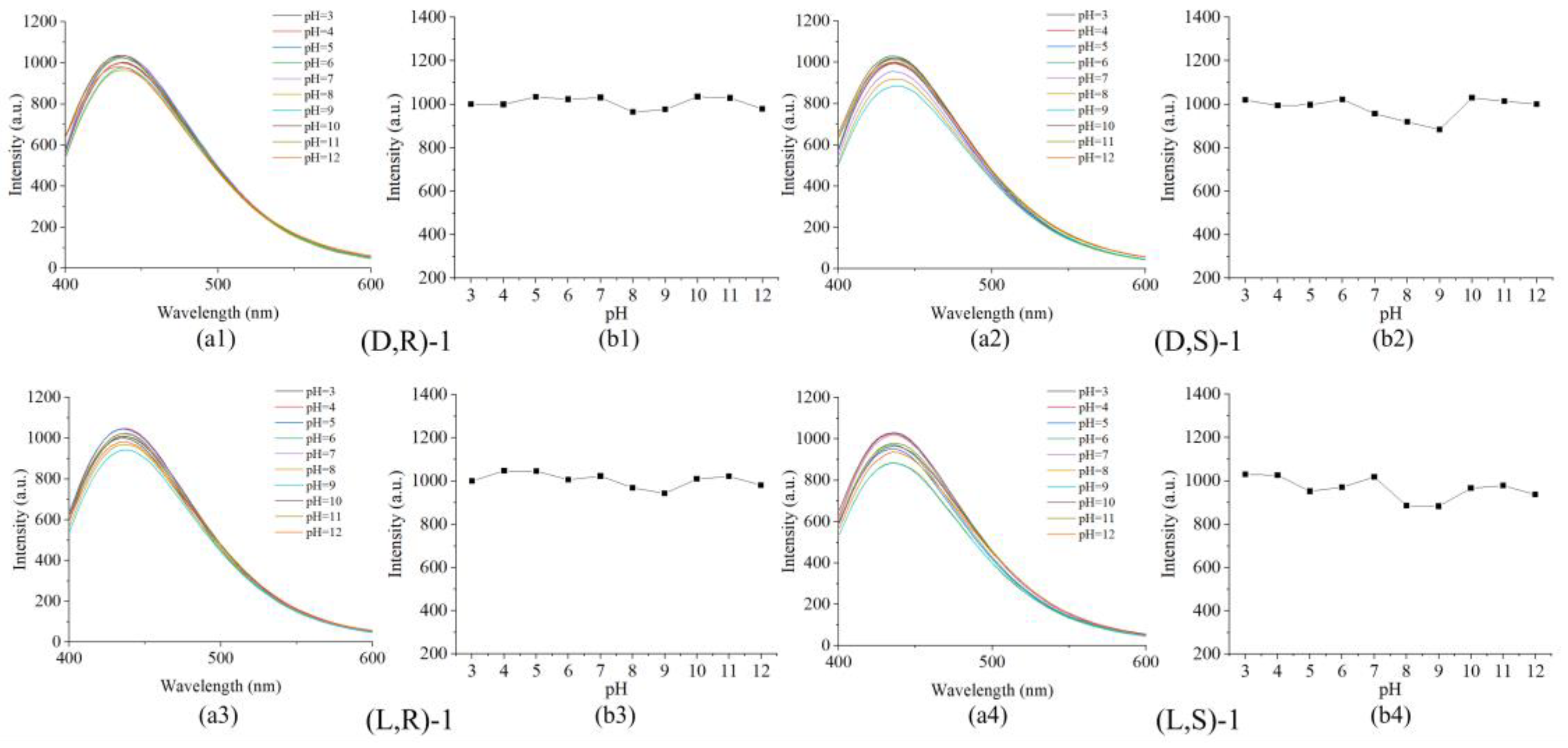
3.3. Recognition in different pH
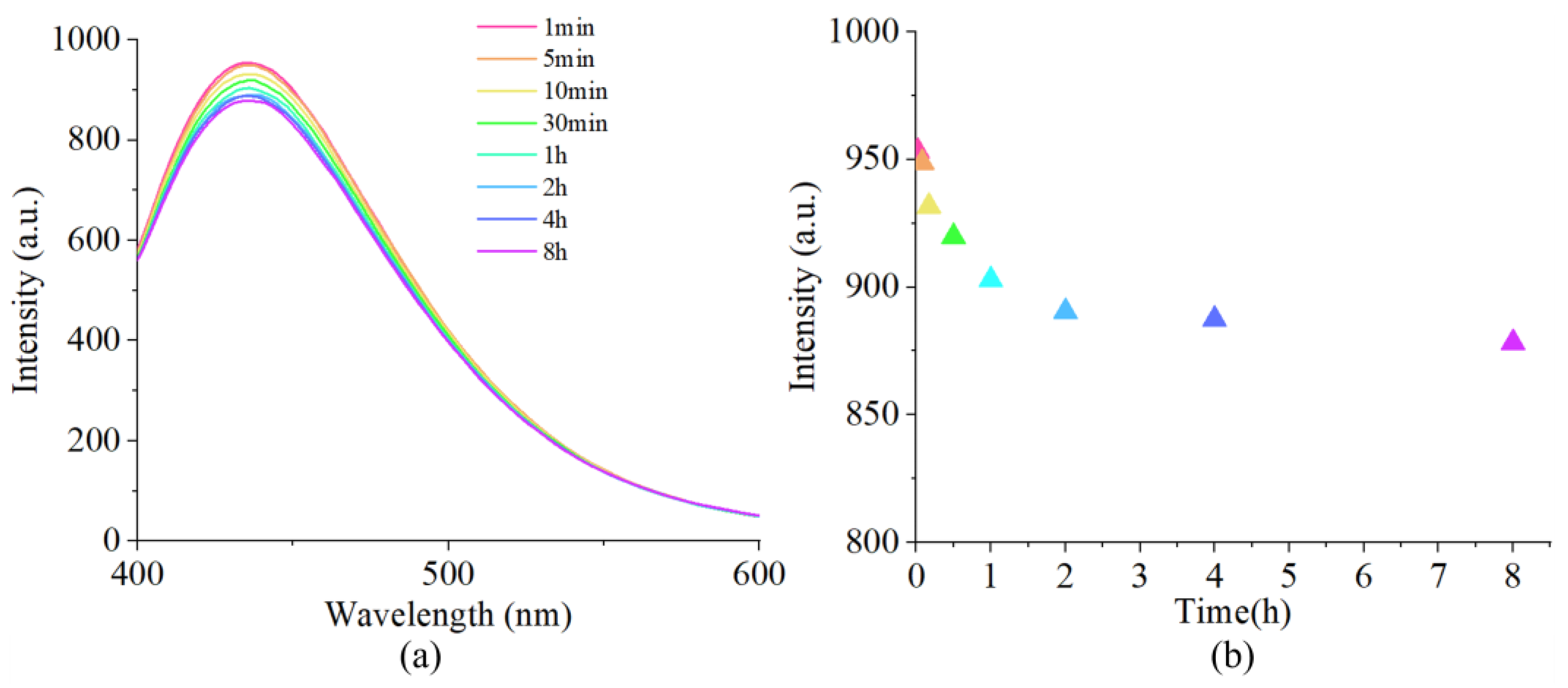
3.4. The reaction Time of the Probes
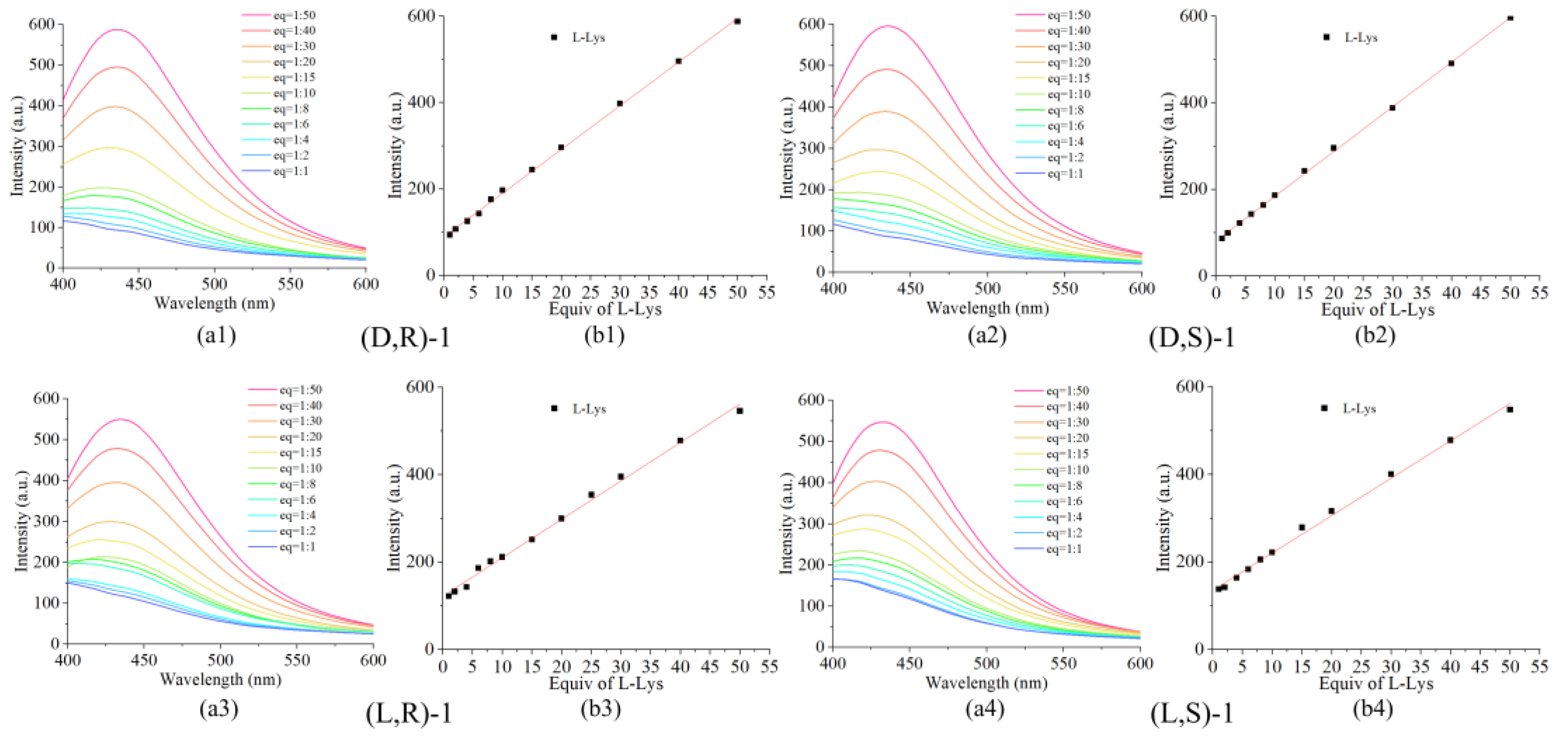
3.5. Limit of Detection
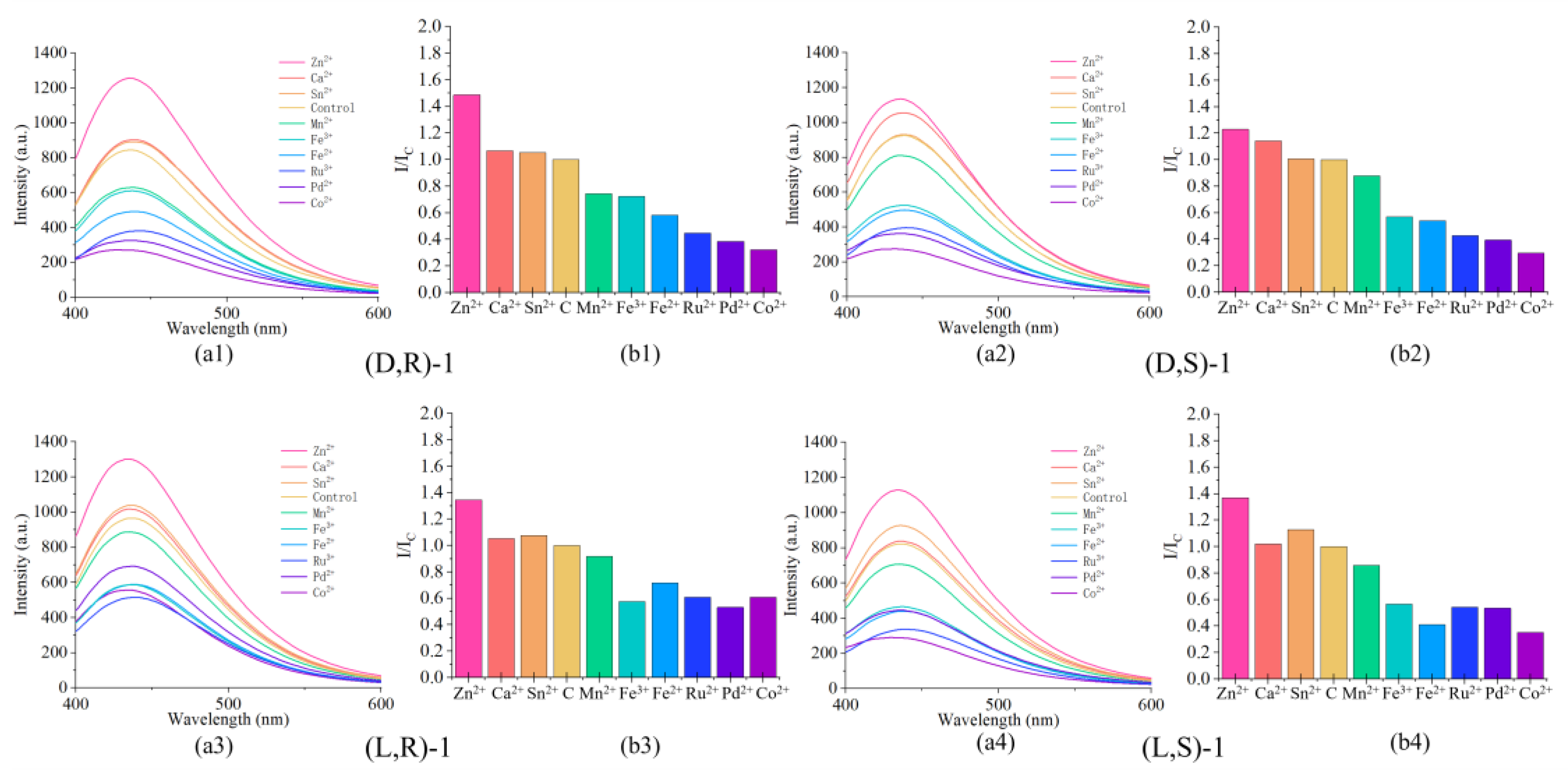
3.6. The Effect of Metal Ions
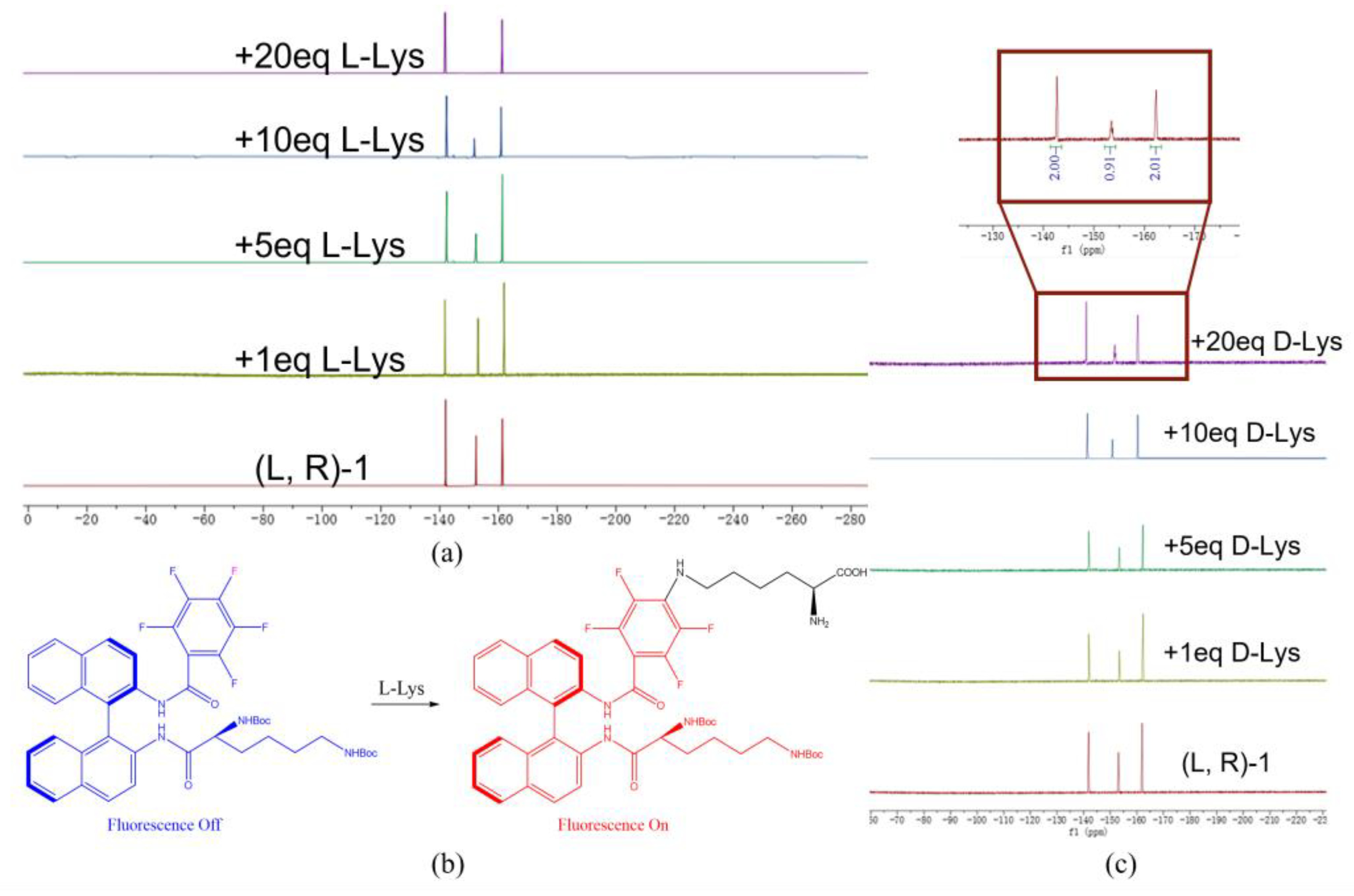
3.7. Confirm the Reaction Site by 19F NMR
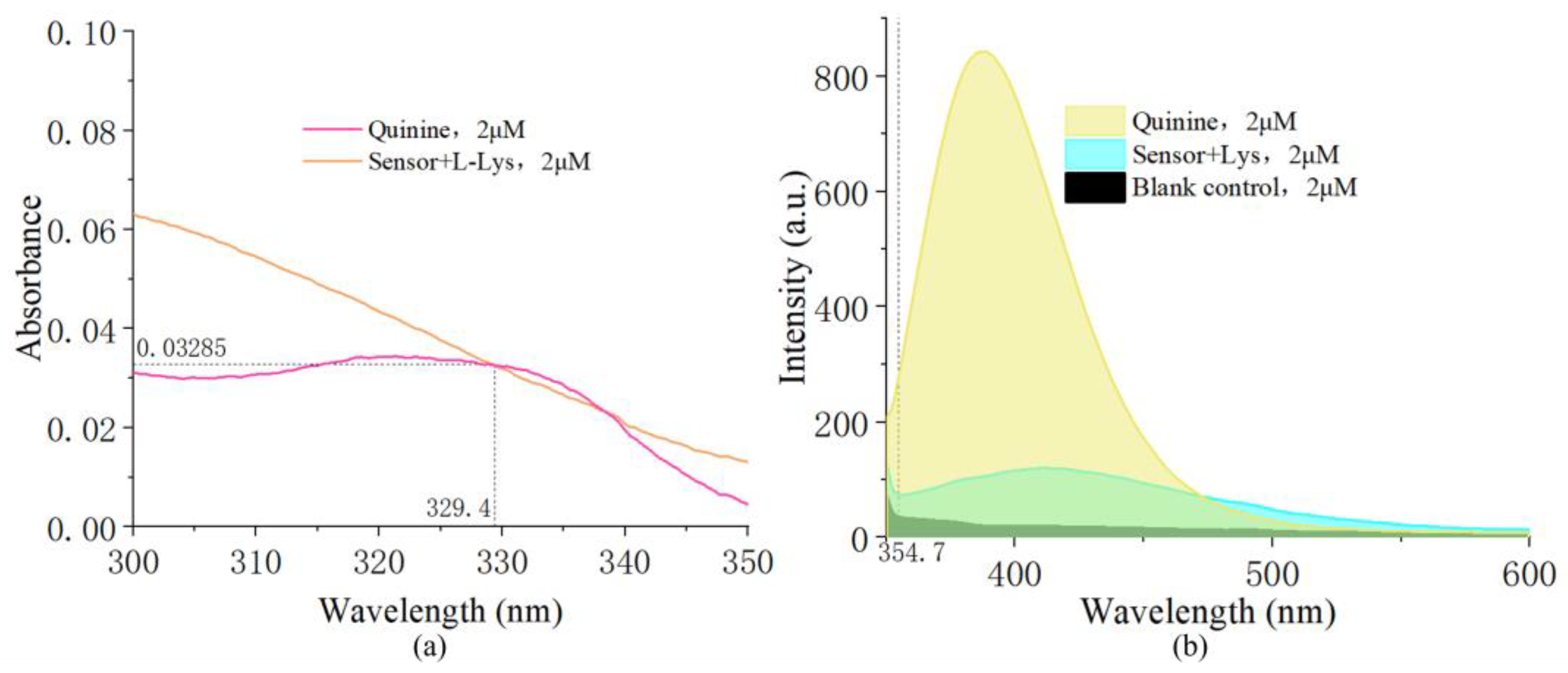
3.8. Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ozturk, S.F. and D.D. Sasselov. On the origins of life’s homochirality: Inducing enantiomeric excess with spin-polarized electrons. Proceedings of the National Academy of Sciences 2022, 119. [Google Scholar]
- BEATTY-DESANA, J.W., HOGGARD, M. J., & COOLEDGE, J. W. Letters to nature. Nature 2001, 255, 242–243. [Google Scholar]
- Copur, F., et al. Nanopaper-based photoluminescent enantioselective sensing of L-Lysine by L-Cysteine modified carbon quantum dots. Sensors and Actuators B: Chemical 2019, 279, 305–312. [Google Scholar] [CrossRef]
- Lu, W., et al. Carbon nano-dots as a fluorescent and colorimetric dual-readout probe for the detection of arginine and Cu2+and its logic gate operation. Nanoscale 2017, 9, 11545–11552. [Google Scholar] [CrossRef]
- Bonner, W.A. Parity violation and the evolution of biomolecular homochirality. Chirality 2000, 12, 114–126. [Google Scholar] [CrossRef]
- Gao, P., Z. Xie, and M. Zheng. Chiral carbon dots-based nanosensors for Sn(II) detection and lysine enantiomers recognition. Sensors and Actuators B: Chemical 2020, 319. [Google Scholar]
- Du, S., M. Wey, and D.W. Armstrong. d-Amino acids in biological systems. Chirality 2023, 35, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Matthews, D.E. Review of Lysine Metabolism with a Focus on Humans. The Journal of Nutrition 2020, 150, 2548S–2555S. [Google Scholar] [CrossRef] [PubMed]
- Chang, D. Ratiometric fluorescent carbon dots for enantioselective sensing of L-lysine and pH discrimination in vivo and in vitro. Sensors and Actuators B: Chemical 2022, 362. [Google Scholar] [CrossRef]
- Song, L., et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE 2011, 6. [Google Scholar]
- Payne, A., et al. Lysine mediation of neuroendocrine food regulation in guinea fowl. Poultry Science 2016, 95, 276–286. [Google Scholar] [CrossRef]
- Estaras, M., et al. The lysine derivative aminoadipic acid, a biomarker of protein oxidation and diabetes-risk, induces production of reactive oxygen species and impairs trypsin secretion in mouse pancreatic acinar cells. Food and Chemical Toxicology 2020, 145. [Google Scholar]
- Jin, C.-l., et al. mTORC1-Mediated Satellite Cell Differentiation Is Required for Lysine-Induced Skeletal Muscle Growth. Journal of Agricultural and Food Chemistry 2020, 68, 4884–4892. [Google Scholar] [CrossRef]
- Tang, S., et al. Enantioselective Recognition of L-Lysine by ICT Effect with a Novel Binaphthyl-Based Complex. Micromachines 2023, 14. [Google Scholar] [CrossRef]
- Loaëc, G., et al. Impact of Variety and Agronomic Factors on Crude Protein and Total Lysine in Chicory; Nε-Carboxymethyl-lysine-Forming Potential during Drying and Roasting. Journal of Agricultural and Food Chemistry 2015, 63, 10295–10302. [Google Scholar] [CrossRef] [PubMed]
- Jin, C. and J. Bao. Lysine Production by Dry Biorefining of Wheat Straw and Cofermentation of Corynebacterium glutamicum. Journal of Agricultural and Food Chemistry 2021, 69, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. and C.E. Levin. Nutritional and medicinal aspects of d-amino acids. Amino Acids 2011, 42, 1553–1582. [Google Scholar]
- Abbasov, M.E., et al. A proteome-wide atlas of lysine-reactive chemistry. Nature Chemistry 2021, 13, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, A., R. Fukada, and D. Funamoto. A luminol chemiluminescence method for sensing histidine and lysine using enzyme reactions. Analytical Biochemistry 2013, 443, 22–26. [Google Scholar] [CrossRef]
- Namera, A., Yashiki, M., Nishida, M., & Kojima, T. Direct extract derivatization for determination of amino acids in human urine by gas chromatography and mass spectrometry. Journal of Chromatography B 2002, 776, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, T., et al. Differential Adsorption of l- and d-Lysine on Achiral MFI Zeolites as Determined by Synchrotron X-Ray Powder Diffraction and Thermogravimetric Analysis. Angewandte Chemie International Edition 2019, 59, 1093–1097. [Google Scholar]
- Du, G. and L. Pu. Micelle-Encapsulated Fluorescent Probe: Chemoselective and Enantioselective Recognition of Lysine in Aqueous Solution. Organic Letters 2019, 21, 4777–4781. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.J., et al. Highly Enantioselective Kinetic Resolution of Axially Chiral BINAM Derivatives Catalyzed by a Brønsted Acid. Angewandte Chemie International Edition 2014, 53, 3684–3687. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. Fluorescence of Organic Molecules in Chiral Recognition. Chemical Reviews 2004, 104, 1687–1716. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. Enantioselective Fluorescent Sensors: A Tale of BINOL. Accounts of Chemical Research 2012, 45, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y., et al. Free Amino Acid Recognition: A Bisbinaphthyl-Based Fluorescent Probe with High Enantioselectivity. Journal of the American Chemical Society 2018, 141, 175–181. [Google Scholar]
- Averin, A.D., et al. Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors. Pure and Applied Chemistry 2020, 92, 1367–1386. [Google Scholar] [CrossRef]
- Zhao, H., et al. Spectroscopic studies of a BINAM-based sensor: Highly selective fluorescent recognition of lysine in water solution through a nucleophilic substitution reaction. Tetrahedron Letters 2019, 60, 1238–1242. [Google Scholar] [CrossRef]
- Jiao, S.-Y., et al. Making pyrophosphate visible: the first precipitable and real-time fluorescent sensor for pyrophosphate in aqueous solution. The Analyst 2015, 140, 174–181. [Google Scholar] [CrossRef]
- Kondo, S.-i., Y. Nakadai, and M. Unno. Recognition of dicarboxylates in aqueous acetonitrile by a dinuclear zinc(II) complex of 2,2’-binaphthalene-based receptor. Supramolecular Chemistry 2018, 31, 9–18. [Google Scholar]
- Sasaki, Y., et al. Accurate chiral pattern recognition for amines from just a single chemosensor. Chemical Science 2020, 11, 3790–3796. [Google Scholar] [CrossRef]
- Huang, Z., et al. Zn(ii) promoted dramatic enhancement in the enantioselective fluorescent recognition of functional chiral amines by a chiral aldehyde. Chem. Sci. 2014, 5, 3457–3462. [Google Scholar] [CrossRef]
- Wang, S., et al. Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sensors and Actuators B: Chemical 2010, 145, 826–831. [Google Scholar] [CrossRef]
- Song, T., et al. Fluorescent Recognition of Zn2+ by Two Diastereomeric Salicylaldimines: Dramatically Different Responses and Spectroscopic Investigation. Inorganic Chemistry, 2017, 56, 4395–4399. [Google Scholar] [CrossRef]
- Wang, X., et al. Reaction of Zn(II) with a BINOL-amino-acid Schiff base: An unusual off-on-off fluorescence response. Tetrahedron Letters 2018, 59, 2332–2334. [Google Scholar] [CrossRef]
- Zhao, H., et al. Study of the ZnII Complexes of 1,1′-Binaphthyl-Based Schiff Bases: Fluorescent Detection of Thiocyanate. European Journal of Inorganic Chemistry 2018, 2018, 4153–4157. [Google Scholar] [CrossRef]
- Levitus, M. Tutorial: measurement of fluorescence spectra and determination of relative fluorescence quantum yields of transparent samples. Methods and Applications in Fluorescence 2020, 8. [Google Scholar] [CrossRef]
- Melhuish, W.H. QUANTUM EFFICIENCIES OF FLUORESCENCE OF ORGANIC SUBSTANCES: EFFECT OF SOLVENT AND CONCENTRATION OF THE FLUORESCENT SOLUTE1. The Journal of Physical Chemistry 1961, 65, 229–235. [Google Scholar] [CrossRef]
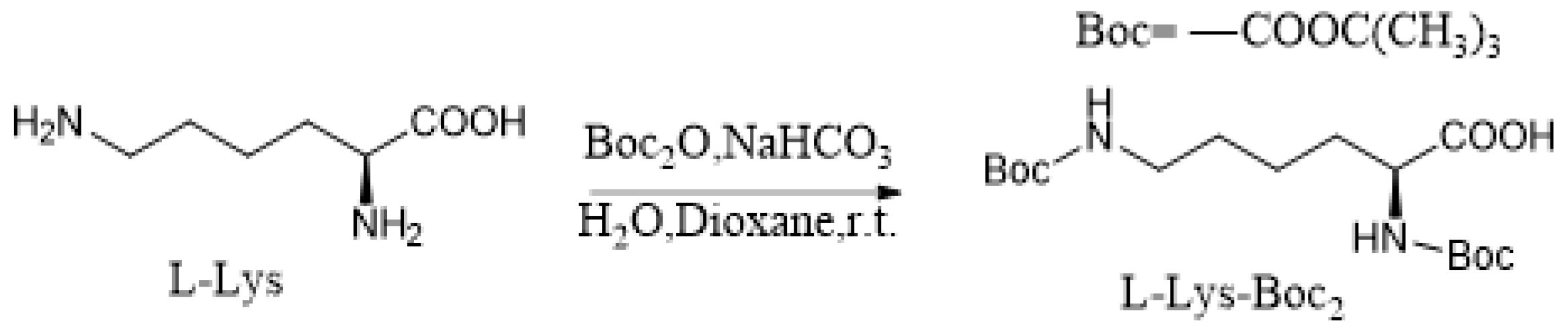
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




