Submitted:
14 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Standards and the Tested Product
2.2. Birds and Diets
2.3. Hen Performance Assessment and Egg Collection
2.4. Measurements of Egg Quality Parameters
2.5. Chemical Analyses
2.5.1. Vitamins A and E Determinations
2.5.2. Phenolic Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Productive Performance of Laying Hens
3.2. Egg Internal Quality
3.3. Egg External Quality
3.4. Bioactive Compounds Measurements
3.4.1. Vitamins A and E Determinations
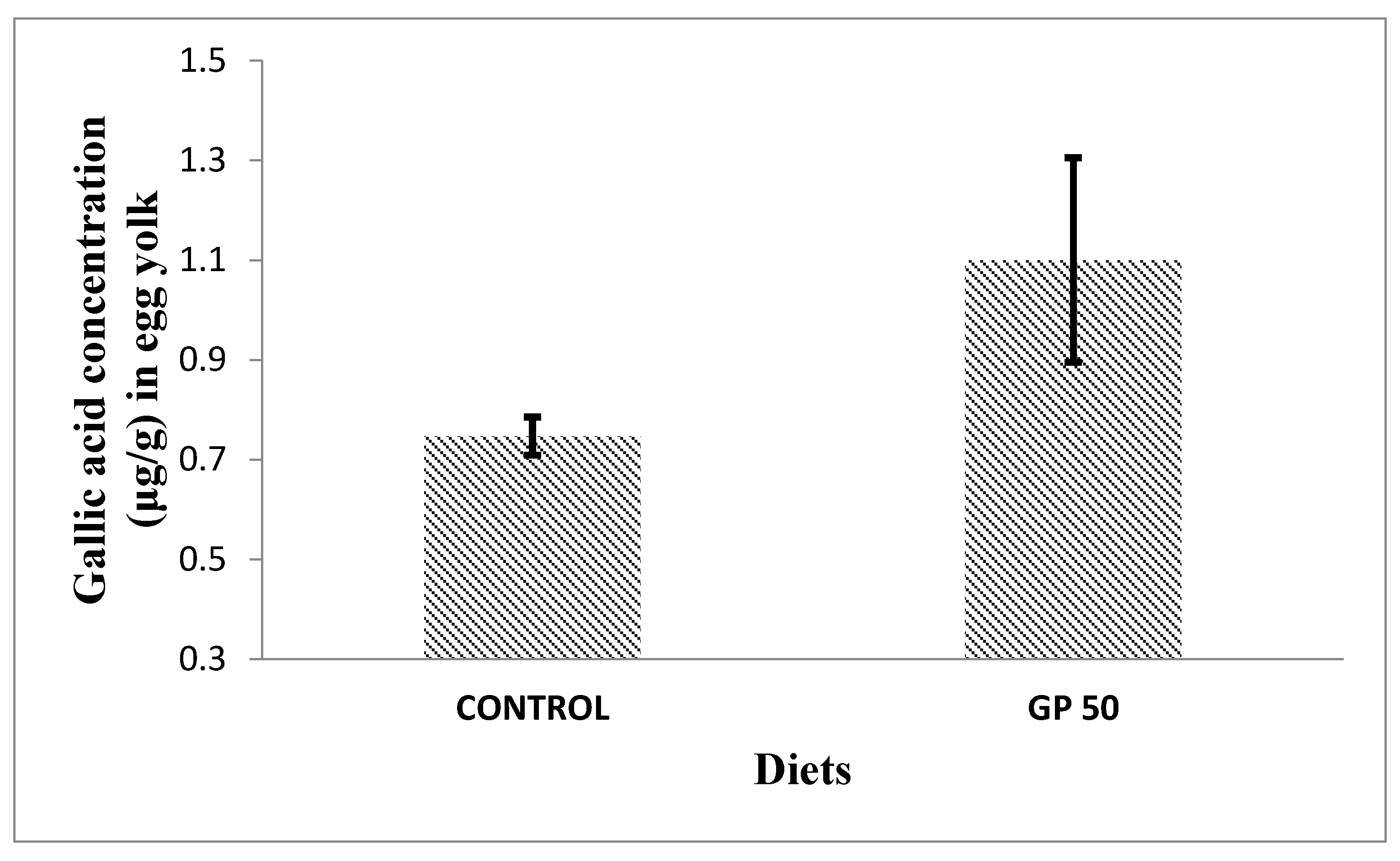
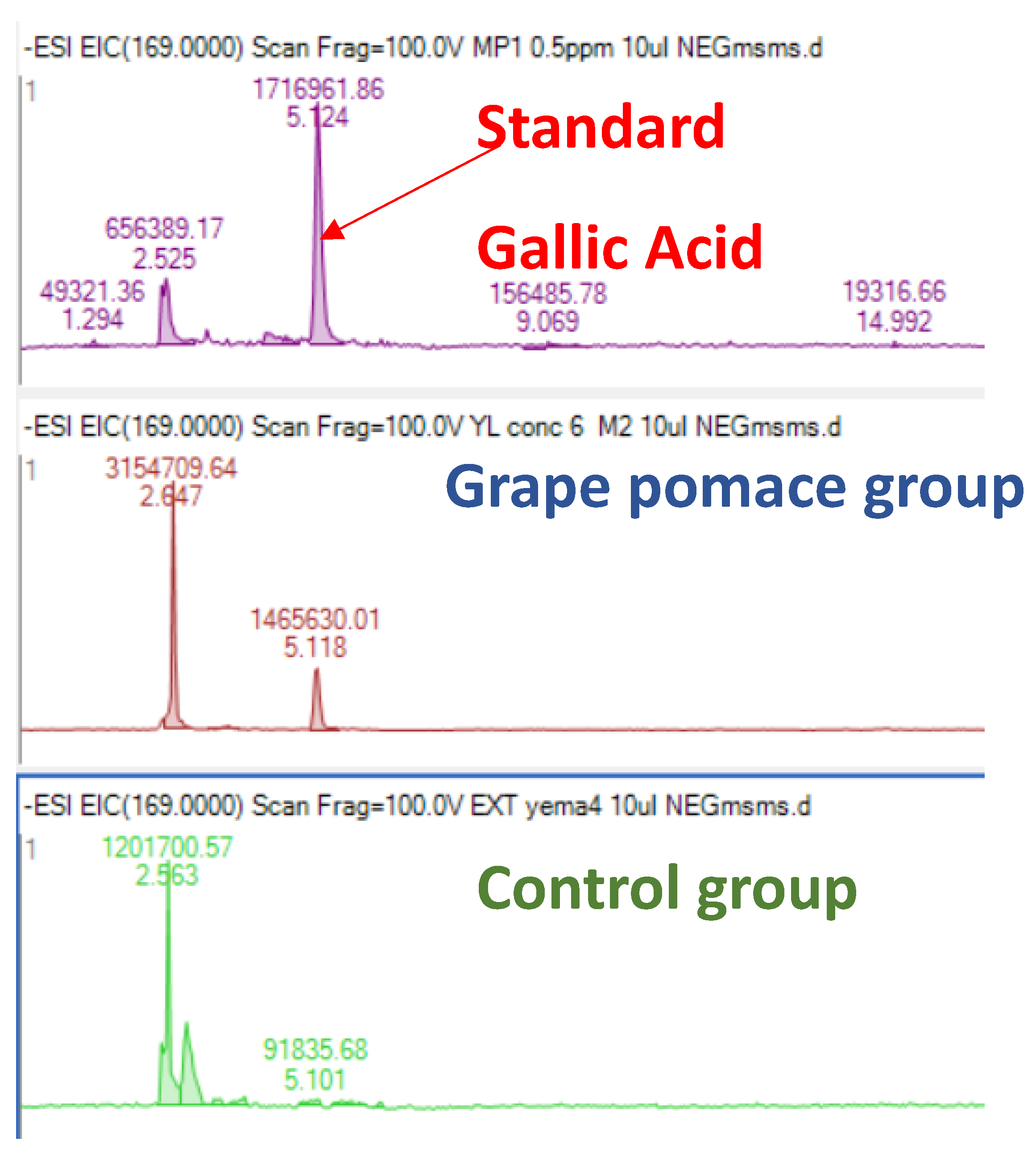
3.4.2. Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dao, H.T.; Sharma, N.K.; Swick, R.A.; Moss, A.F. Feeding recycled food waste improved feed efficiency in laying hens from 24 to 43 weeks of age. Sci. Rep. 2023, 13, 8261–8273. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Saura-Calixto, F.; Arija, I. Effect of grape seed extract on growth performance, protein and polyphenol digestibilities and antioxidant activity in chickens. Span. J. Agric. Res. 2010, 8, 326–333. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Arija, I.; Romero, C.; Álvarez, I.; Rey, A.; Brenes, A. Addition of exogenous enzymes to diets containing grape pomace: Effects on intestinal utilization of catechins and antioxidant status of chickens. Food Res. Int. 2017, 96, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Nardoia, M.; Arija, I.; Viveros, A.; Rey, A.I.; Prodanov, M.; Chamorro, S. Feeding broiler chickens with grape seed and skin meals to enhance α- and γ-tocopherol content and meat oxidative stability. Antioxid. 2021, 10, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Nardoia, M.; Brenes, A.; Arija, I.; Viveros, A.; Chamorro, S. Combining grape byproducts to maximise biological activity of polyphenols in chickens. Animals 2021, 11, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Romero, C.; Brenes, A.; Sánchez-Patán, F.; Bartolomé, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef]
- Muñoz-Gonzalez, I.; Chamorro, S.; Pérez-Jiménez, J.; López-Andrés, P.; Álvarez. Acero, I.; Herrero, A.M.; Nardoia, M.; Brenes, A.; Viveros, A.; Arija, I.; Rey, A.; Ruiz-Capillas, C. Phenolic metabolites in plasma and thigh meat of chickens supplemented with grape byproducts. J. Agric. Food Chem. 2019, 67, 4463–4471. [Google Scholar] [CrossRef]
- Romero, C.; Arija, I.; Viveros, A.; Chamorro, S. Productive performance, egg quality and yolk lipid oxidation in laying hens fed diets including grape pomace or grape extract. Animals 2022, 12, 1076–1091. [Google Scholar] [CrossRef]
- Kara, K.; Kocaoğlu Güçlü, B.; Baytok, E.; Şentürk, M. Effects of grape pomace supplementation to laying hen diet on performance, egg quality, egg lipid peroxidation and some biochemical parameters. J. Appl. Anim. Res. 2016, 44, 303–310. [Google Scholar] [CrossRef]
- Barbe, A.; Mellouk, N.; Ramé, C.; Grandhaye, J.; Staub, C.; Venturi, E.; Cirot, M.; Petit, A.; Anger, K.; Chahnamian, M.; Ganier, P.; Callut, O.; Cailleau-Audouin, E.; Metayer-Coustard, S.; Riva, A.; Froment, P.; Dupont, J. A grape seed extract maternal dietary supplementation in reproductive hens reduces oxidative stress associated to modulation of plasma and tissue adipokines expression and improves viability of offsprings. Plos One 2020, 15, e0231131–e0231154. [Google Scholar] [CrossRef] [PubMed]
- Grandhaye, J.; Douard, V.; Rodriguez-Mateos, A.; Xu, Y.; Cheok, A.; Riva, A.; Guabiraba, R.; Zemb, O.; Philippe, C.; Monnoye, M.; Staub, C.; Venturi, E.; Barbe, A.; Ramé, C.; Dupont, J.; Froment, P. Microbiota changes due to grape seed extract diet improved intestinal homeostasis and decreased fatness in parental broiler hens. Microorganisms 2020, 8, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, Y.; Kreuzer, M.; Messikommer, R.E. Late laying hens deposit dietary antioxidants preferentially in the egg and not in the body. J. Appl. Poult. Res. 2014, 23, 647–660. [Google Scholar] [CrossRef]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Esteve, R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility and susceptibility to meat lipid oxidation in chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Galobart, J.; Barroeta, A.C.; Cortinas, L.; Baucells, M.D.; Codony, R. Accumulation of alpha-tocopherol in eggs enriched with omega3 and omega6 polyunsaturated fatty acids. Poult. Sci. 2002, 81, 1873–1876. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Vít, T.; Skřivanová, E. Hempseed increases gamma-tocopherol in egg yolks and the breaking strength of tibias in laying hens. Plos One 2019, 14, e0217509–e0217517. [Google Scholar] [CrossRef] [PubMed]
- Guilland, J.C. Les interactions entre les vitamines A, D, E et K : synergie et/ou compétition. OCL 2011, 18, 59–67. [Google Scholar] [CrossRef]
- Eisen, E.J.; Bohren, B.B.; Mckean, H.E. The Haugh unit as a measure of egg albumen quality. Poult. Sci. 1962, 41, 1461–1468. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 1995. [Google Scholar]
- Claeys, E.; Vossen, E.; de Smet, S. Determination of α-tocopherol by reversed-phase HPLC in feed and animal-derived foods without saponification. J. Sci. Food Agr. 2015, 96, 522–529. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, H.K. Effects of energy and protein levels on laying performance, egg quality, blood parameters, blood biochemistry, and apparent total tract digestibility on laying hens in an aviary system. Animals 2022, 12, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Calik, J.; Obrzut, J. Influence of genotype on productivity and egg quality of three hen strains included in a biodiversity program. Animals 2023, 13, 1848–1861. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Qiu, N.; Ma, M.H.; Jin, Y.G.; Yang, H.; Geng, F.; Sun, S.H. Estimation of egg freshness using S-ovalbumin as an indicator. Poult. Sci. 2012, 91, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Wang, J.P.; Ding, X.M.; Bai, S.P.; Qi, S.R.N.; Zeng, Q.F.; Xuan, Y.; Zhang, K.Y. Effect of different tea polyphenol products on egg production performance, egg quality and antioxidative status of laying hens. Anim. Feed Sci. Tech. 2020, 267, 114544–114551. [Google Scholar] [CrossRef]
- Ortiz, D.; Lawson, T.; Jarrett, R.; Ring, A.; Scoles, K.L.; Hoverman, L.; Rocheford, E.; Karcher, D.M.; Rocheford, T. The impact of orange corn in laying hen diets on yolk pigmentation and xanthophyll carotenoid concentrations on a percent inclusion rate basis. J. Appl. Poult. Res. 2022, 31, 100218–100225. [Google Scholar] [CrossRef]
- Grashorn, M.; Juergens, J.; Bessei, W. Effects of storage conditions on egg quality. Lohmann Information 2016, 50, 22–27. [Google Scholar]
- de Araújo Soares, R.; Vilela Borges, S.; Vilela Dias, M.; Hilsdorf Piccoli, R.; Fassani, E.J.; Cunha da Silva, E.M. Impact of whey protein isolate/sodium montmorillonite/sodium metabisulfite coating on the shelf life of fresh eggs during storage. LWT 2021, 139, 110611. [Google Scholar] [CrossRef]
- Shan, Y.; Tang, D.; Wang, R.; Tu, A.; Yi, Y.; Wang, X.; Liu, B.; Zhou, Y.; Huang, Q.; Lü, X. Rheological and structural properties of ovomucin from chicken eggs with different interior quality. Food Hydrocolloid. 2020, 100, 105393. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Huang, Q.; Cheng, L.; Gan, R.; Liu, L.; Wu, D.; Li, H.; Peng, L.; Geng, F. Underlying mechanism for the differences in heat-induced gel properties between thick egg whites and thin egg whites: Gel properties, structure and quantitative proteome analysis. Food Hydrocolloid. 2020, 106, 105873. [Google Scholar] [CrossRef]
- Nelson, T.S.; Baptist, J.N. Feed pigments: 2. The influence of feeding single and combined sources of red and yellow pigments on egg yolk color. Poult. Sci. 1968, 47, 924–931. [Google Scholar] [CrossRef]
- Wen, C.; Xu, X.; Zhou, D.; Yu, Q.; Wang, T.; Zhou, Y. The effects of canthaxanthin microencapsulation on yolk color and canthaxanthin deposition in egg yolk of laying hens. Poult. Sci. 2022, 101, 101889–101893. [Google Scholar] [CrossRef] [PubMed]
- Grashorn, M. Feed additives for influencing chicken meat and egg yolk color. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R., Eds.; Publisher: Woodhead Publishing, Cambridge, UK, 2016; pp. 283–302. [Google Scholar]
- Kljak, K.; Carović-Stanko, K.; Kos, I.; Janječić, Z.; Kiš, G.; Duvnjak, M.; Safner, T.; Bedeković, D. Plant carotenoids as pigment sources in laying hen diets: Effect on yolk color, carotenoid content, oxidative stability and sensory properties of eggs. Foods 2021, 10, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Baghban-Kanani, P.; Azimi-Youvalari, S.; Hosseintabar-Ghasemabad, B.; Slozhenkina, M.; Gorlov, I.; Viktoronova, F.M.; Seidavi, A.; Laudadio, V. Effect of dietary flaxseed meal supplemented with dried tomato and grape pomace on performance traits and antioxidant status of laying hens. Anim. Biotechnol. 2022, 33, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Grčević, M.; Kralik, Z.; Kralik, G.; Galović, D.; Radišić, Z.; Hanžek, D. Quality and oxidative stability of eggs laid by hens fed marigold extract supplemented diet. Poult. Sci. 2019, 98, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- O'Connell, J.E.; Fox, P.F. Effect of extracts of oak (Quercus petraea) bark, oak leaves, aloe vera (Curacao aloe), coconut shell and wine on the colloidal stability of milk and concentrated milk. Food Chem. 1999, 66, 93–96. [Google Scholar] [CrossRef]
- Squires, M.W.; Naber, E.C. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: vitamin A study. Poult. Sci. 1993, 72, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Cherian, G.; Wolfe, F.W.; Sim, J.S. Dietary oils with added tocopherols: effects on egg or tissue tocopherols, fatty acids, and oxidative stability. Poult. Sci. 1996, 75, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Naber, E.C. Modifying vitamin composition of eggs: a review. J. Appl. Poult. Res. 1993, 2, 385–393. [Google Scholar] [CrossRef]
- Rey, A.I.; de-Cara, A.; Rebolé, A.; Arija, I. Short-term spirulina (Spirulina platensis) supplementation and laying hen strain effects on eggs' lipid profile and stability. Animals 2021, 11, 1944–1959. [Google Scholar] [CrossRef]
- Guedes de Pinho, P.; Silva Ferreira, A.C.; Mendes Pinto, M.; Benitez, J.G.; Hogg, T.A. Determination of carotenoid profiles in grapes, musts, and fortified wines from Douro varieties of Vitis vinifera. J. Agr. Food Chem. 2001, 49, 5484–5488. [Google Scholar] [CrossRef]
- Sharma, M.K.; McDaniel, C.D.; Kiess, A.S.; Loar, R.E.; Adhikari, P. Effect of housing environment and hen strain on egg production and egg quality as well as cloacal end eggshell microbiology in laying hens. Poult. Sci. 2022, 101, 101595–101612. [Google Scholar] [CrossRef] [PubMed]
- Skřivan, M.; Englmaierová, M. The deposition of carotenoids and α-tocopherol in hen eggs produced under a combination of sequential feeding and grazing. Anim. Feed Sci. Tech. 2014, 190, 79–86. [Google Scholar] [CrossRef]
- Bennett, D.C.; Cheng, K.M. Selenium enrichment of table eggs. Poult. Sci. 2010, 89, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Sato, T.; Harada, H.; Takita, T. Transfer of soy isoflavone into the egg yolk of chickens. Biosci. Biotechnol. Biochem. 2001, 65, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Saitoh, S.; Sato, T.; Harada, H.; Matsuda, T. Biotransformation of soy isoflavone-glycosides in laying hens: intestinal absorption and preferential accumulation into egg yolk of equol, a more estrogenic metabolite of daidzein. Biochim. Biophys. Acta 2004, 1674, 122–130. [Google Scholar] [CrossRef]
- Medina, M.; Iuga, C.; Alvarez-Idaboy, J.R. Antioxidant activity of propyl gallate in aqueous and lipid media: a theoretical study. Phys. Chem. Chem. Phys. 2013, 31, 13137–13146. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kanellos, P.T.; Kalogeropoulos, N. Gallic acid bioavailability in humans. In Handbook on Gallic Acid; Thompson, M.A., Collins, P.B., Eds.; Publisher: Nova Science Publishers, New York, USA, 2013; pp. 301–312. [Google Scholar]
- Obianwuna, U.E.; Oleforuh-Okoleh, V.U.; Wang, J.; Zhang, H.J.; Qi, G.H.; Qiu, K.; Wu, S.G. Potential implications of natural antioxidants of plant origin on oxidative stability of chicken albumen during storage: A review, Antioxid. , 2022, 11, 630–649. [Google Scholar] [CrossRef]
| Nutrients | Grape pomace composition |
|---|---|
| Ether extract | 7.5 |
| Crude protein | 10.4 |
| Crude fibre | 25.3 |
| Ashes | 5.1 |
| Total extractable polyphenols (g gallic acid equivalents/100 g DM) |
2.10 |
| Compound | Molecular formula | Mass | mg/g |
|---|---|---|---|
| Gallic acid | C7H6O5 | (-)169,0149 | 0.12 |
| Delphinidin-3-glucoside | C21H21O12 | (+)465,1005 | 0.09 |
| Cyanidin-3-glucoside | C21H21O11 | (+)449,1099 | 0.13 |
| Procyanidin B2 | C30H26O12 | (-)577,1367 | 0.80 |
| Procyanidin B3 | C30H26O12 | (-)577,1367 | 0.21 |
| Catechin | C15H14O6 | (-)289,0733 | 0.50 |
| Peonidin-3-glucoside | C22H23O11 | (+)463,1217 | 0.90 |
| Epicatechin | C15H14O6 | (-)289,0731 | 0.52 |
| Vitisin A | C26H25O14 | (+)561,1217 | 0.04 |
| Myricetin-3-glucoside | C21H20O13 | (-)479,0848 | 0.06 |
| Malvidin-3-acetylglucoside | C25H27O13 | (+)535,1433 | 0.04 |
| Delphinidin 3-(6-coumaroylglucoside) | C30H27O14+ | (+)611,1399 | 0.03 |
| Malvidin 3-caffeoylglucoside | C32H31O15+ | (+)655,1655 | 0.05 |
| Quercetin-3-glucoside | C21H20O12 | (-)463,0892 | 0.11 |
| Cyanidin 3-(6-coumaroylglucoside) | C30H27O13 | (+)595,1440 | 0.04 |
| Quercetin-3-glucuronide | C21H18O13 | (-)477,0684 | 0.14 |
| Malvidin 3-(6-coumaroylglucoside) | C32H31O14 | (+)639,1705 | 0.08 |
| Kaempherol-3-glucoside | C21H20O11 | (-)447,0942 | 0.06 |
| Quercetin | C15H10O7 | (-)301,0340 | 0.07 |
| Kaempherol | C15H10O6 | (-)285,0442 | 0.04 |
| Ingredients | Experimental diets | |
|---|---|---|
| Control | GP 1 50 | |
| Corn | 500.8 | 468.6 |
| Soybean | 295.0 | 293.2 |
| Sunflower oil | 54.0 | 63.1 |
| Grape pomace | - | 50.0 |
| Straw | 25.0 | - |
| Salt | 3.0 | 3.0 |
| Monocalcium phosphate | 12.5 | 12.5 |
| Calcium carbonate | 92.5 | 92.5 |
| Vitamin-mineral premix 2 | 5.0 | 5.0 |
| DL-Methionine | 2.2 | 2.1 |
| Celite 3 | 10.0 | 10.0 |
| Analysed composition | ||
| Crude protein | 166 | 169 |
| Ether extract | 76.0 | 86.0 |
| Crude fibre | 35.0 | 35.0 |
| Total extractable polyphenols (g gallic acid equivalents/kg) |
0.644 | 0.728 |
| Calculated composition | ||
| Grape extractable polyphenols (g gallic acid equivalents/kg) 4 |
- | 1.05 |
| AME 5 (MJ/kg) | 11.4 | 11.4 |
| Calcium | 38.6 | 38.9 |
| Available P | 3.70 | 3.70 |
| Lysine | 8.85 | 8.85 |
| Meth+Cys | 7.86 | 7.74 |
| Genetics | ISA White | ISA Brown | SEM 1 | P | ||||
|---|---|---|---|---|---|---|---|---|
| Diet | Control | GP 50 | Control | GP 50 | Genetics | Diet | Interaction | |
| Daily egg production (%) | 97.2 | 95.7 | 97.6 | 98.1 | 0.839 | 0.10 | 0.50 | 0.20 |
| Average egg weight (g) | 57.0 | 57.3 | 59.4 | 59.1 | 0.691 | 0.002 | 0.95 | 0.65 |
| Daily egg mass (g/d) | 55.5 | 54.9 | 58.0 | 58.0 | 0.975 | 0.004 | 0.71 | 0.73 |
| Feed intake (g/d) | 111 | 111 | 124 | 123 | 1.41 | < 0.001 | 0.81 | 0.82 |
| Feed conversion ratio (g feed/ g egg mass) |
2.01 | 2.03 | 2.14 | 2.13 | 0.047 | 0.012 | 0.90 | 0.75 |
| Genetics | ISA White | ISA Brown | SEM 1 | P | ||||
|---|---|---|---|---|---|---|---|---|
| Diet | Control | GP 50 | Control | GP 50 | Genetics | Diet | Interaction | |
| Shell (%) | 10.6 | 10.4 | 10.1 | 10.2 | 0.137 | 0.017 | 0.97 | 0.24 |
| Shell thickness (μm) | 368 | 373 | 380 | 392 | 6.0 | 0.038 | 0.21 | 0.56 |
| Haugh units | 93.8 | 96.3 | 98.6 | 99.0 | 1.30 | 0.003 | 0.23 | 0.38 |
| Egg yolk colour | ||||||||
| Lightness, L* | 43.5 | 45.6 | 43.5 | 44.5 | 1.06 | 0.61 | 0.15 | 0.58 |
| Redness, a* | 3.35 | 2.51 | 3.72 | 2.95 | 0.14 | 0.008 | < 0.001 | 0.82 |
| Yellowness, b* | 27.2 | 25.1 | 27.8 | 26.7 | 0.89 | 0.23 | 0.07 | 0.59 |
| Storage time | 0 days | 15 days | 21 days | 31 days | SEM 1 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Control | GP 50 | Control | GP 50 | Control | GP 50 | Control | GP 50 | Storage time | Diet | Interaction | |
| Haugh units | 95.1 | 98.4 | 93.3 | 93.6 | 85.1 | 89.8 | 69.9 | 69.3 | 0.70 | < 0.001 | 0.06 | 0.22 |
| Yolk colour score | 9.62 | 10.4 | 9.56 | 10.5 | 9.29 | 10.7 | 9.59 | 10.2 | 0.217 | 0.41 | < 0.001 | 0.27 |
| Diet | Control | GP 50 | SEM 1 | P |
|---|---|---|---|---|
| Average egg weight (g) | 63.5 | 63.2 | 0.29 | 0.68 |
| Shell thickness (μm) | 424 | 361 | 8.6 | < 0.001 |
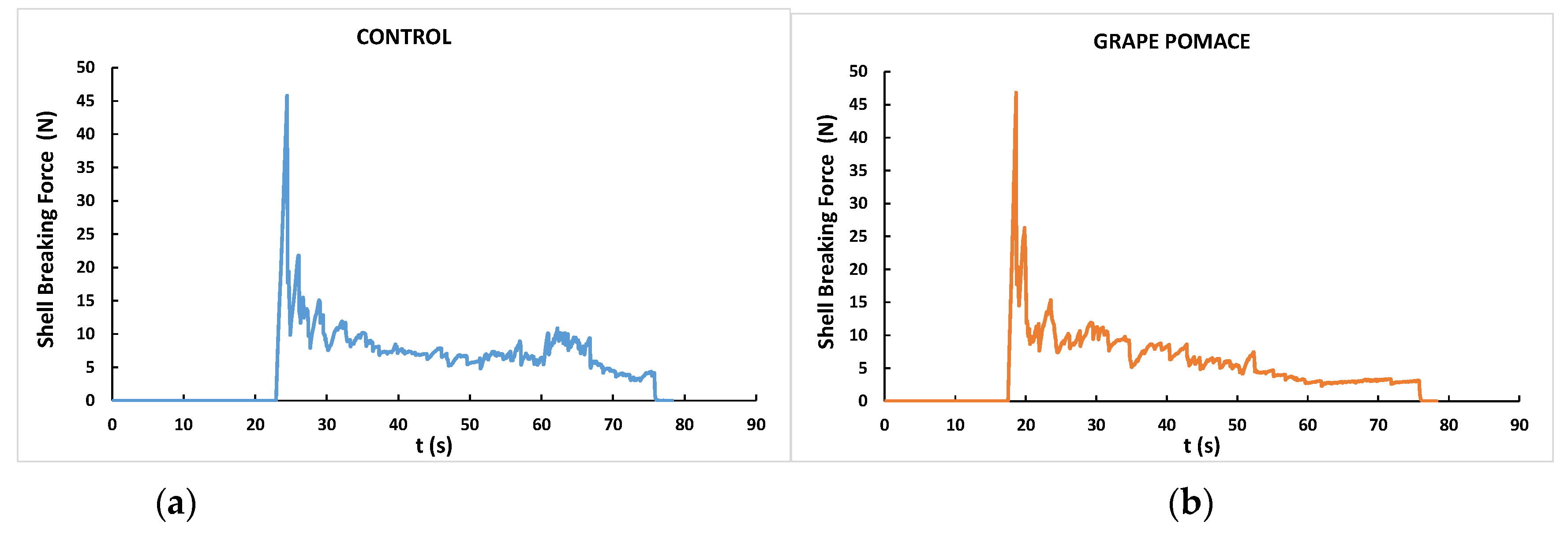
| Shell breaking strength (N) | 46.4 | 45.6 | 1.50 | 0.55 |
| Total rupture area (N.s) | 441 | 413 | 15.5 | 0.21 |
| Shell rupture force peaks | 92.6 | 82.6 | 3.84 | 0.06 |
| Genetics | ISA White | ISA Brown | SEM 1 | P | ||||
|---|---|---|---|---|---|---|---|---|
| Diet | Control | GP 50 | Control | GP 50 | Genetics | Diet | Interaction | |
| α-tocopherol | 87.7 | 104 | 139 | 142 | 14.8 | 0.005 | 0.55 | 0.65 |
| γ-tocopherol | 35.3 | 41.0 | 22.1 | 22.5 | 5.03 | 0.004 | 0.56 | 0.60 |
| retinol | 42.1 | 42.0 | 57.2 | 47.5 | 6.64 | 0.14 | 0.44 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





