Submitted:
11 April 2024
Posted:
12 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
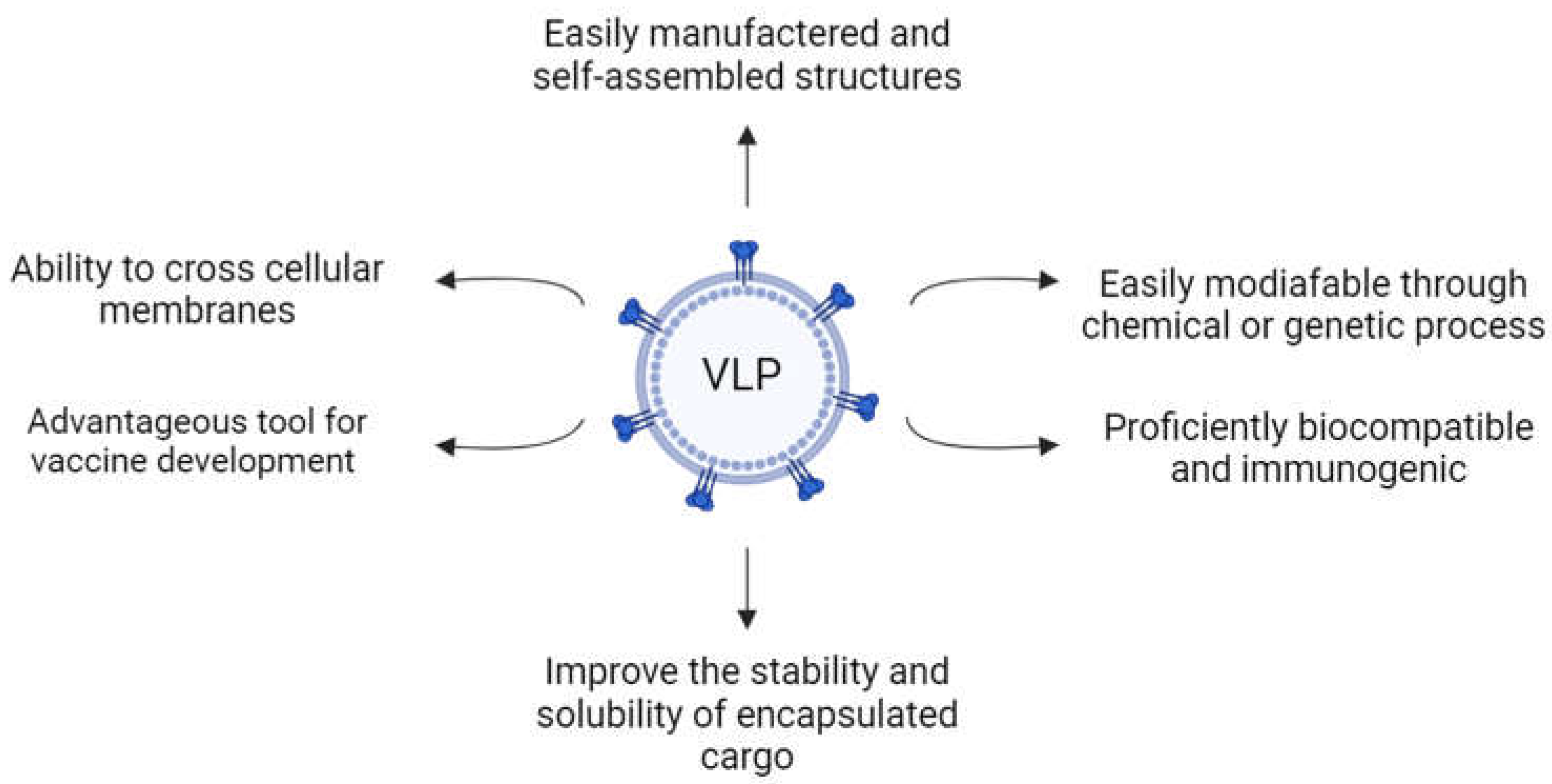
1.1. Virus-like Particles
1.2. VLP Structure
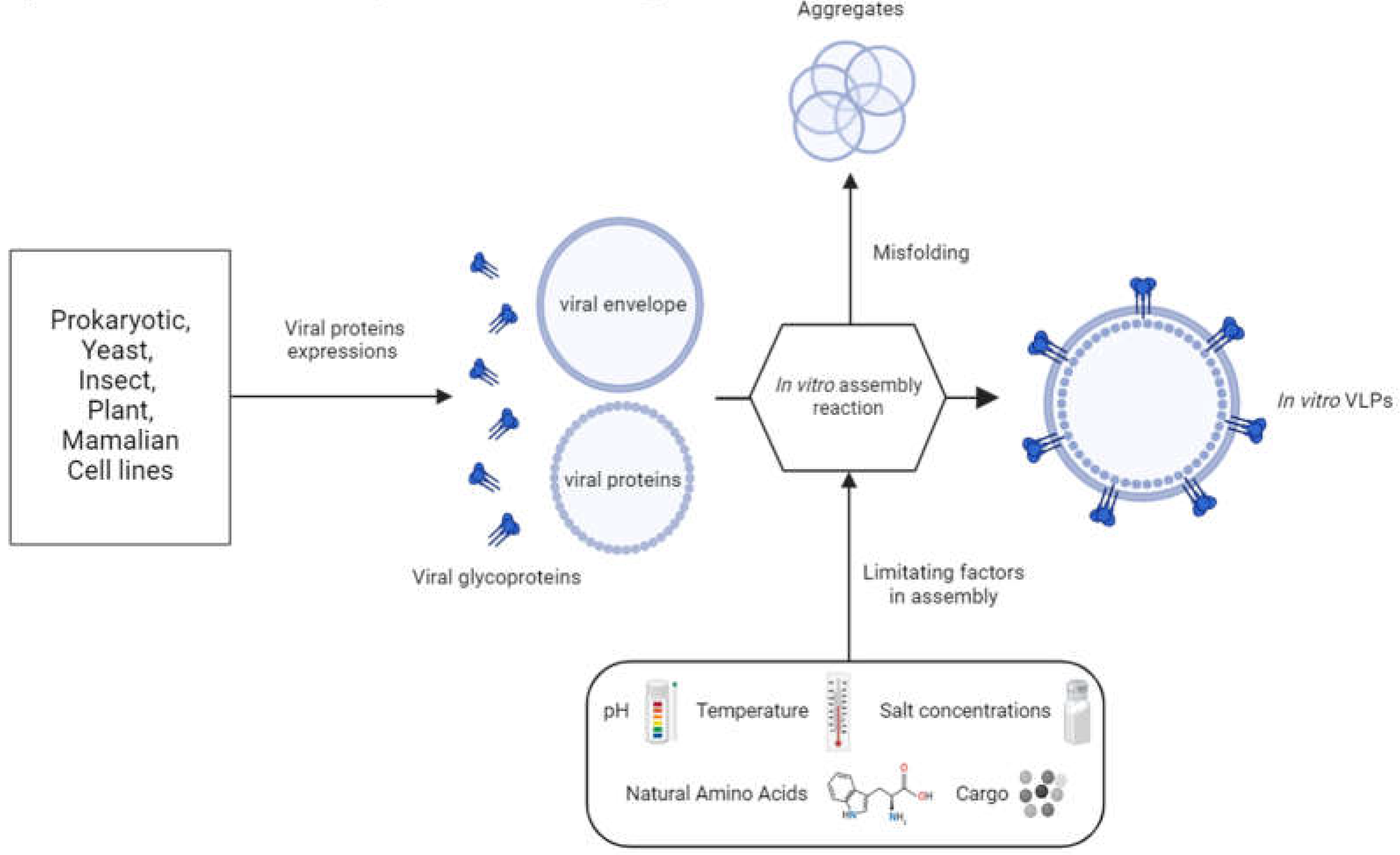
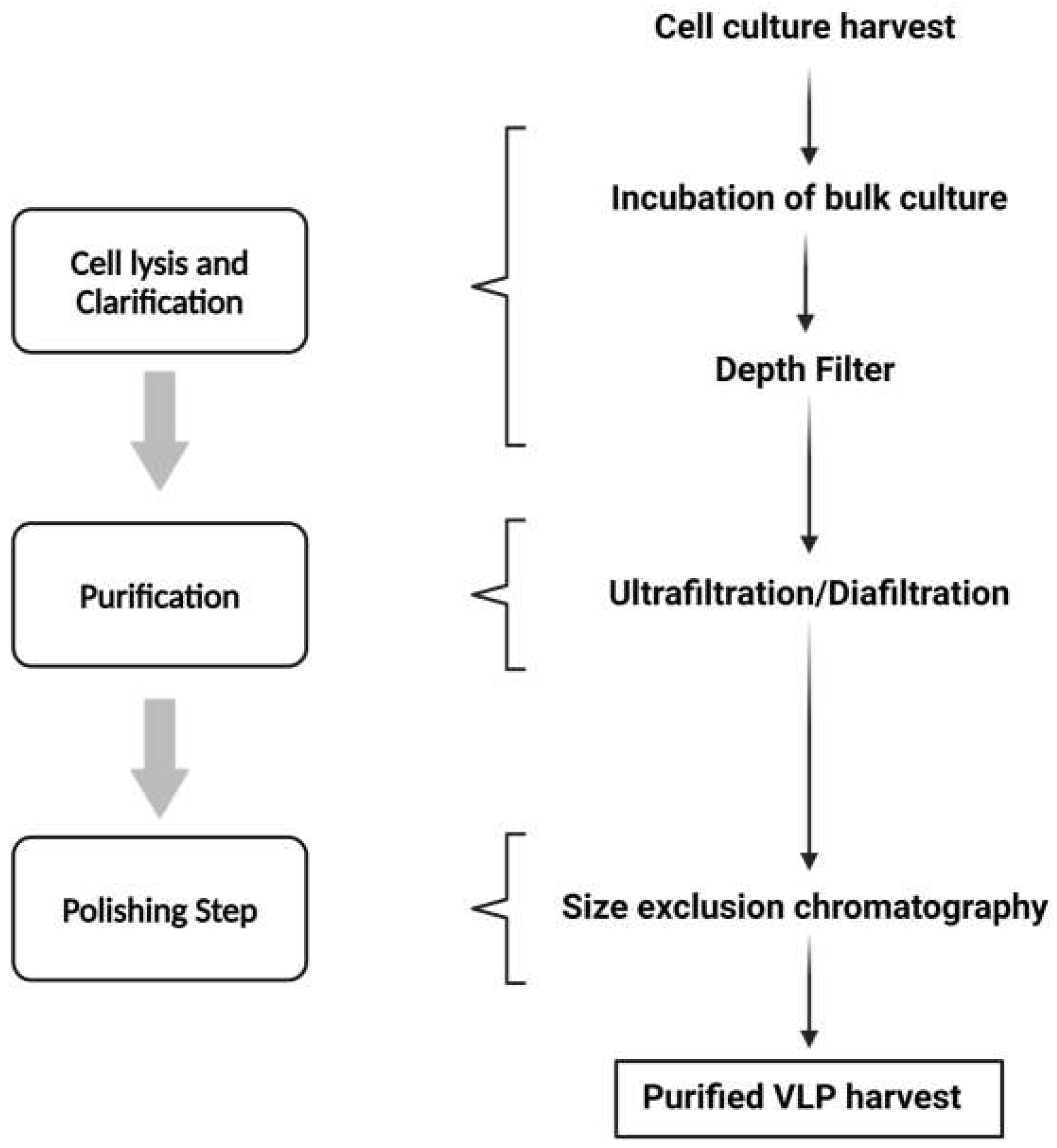
1.3. VLP Downstream Processing
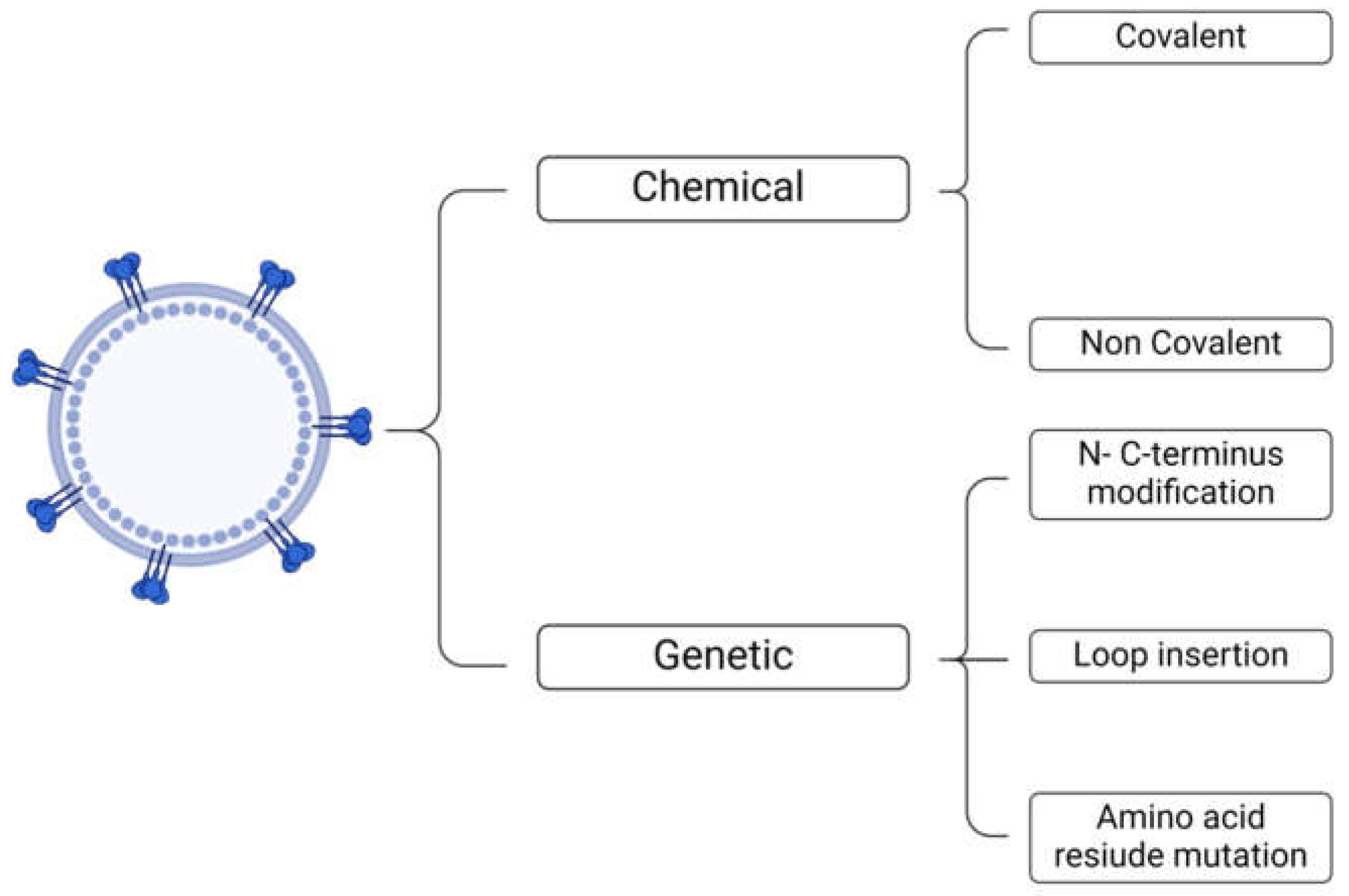
2. Types of Surface Functionalization
3. Biomedical Applications of Functionalized VLPs
3.1. Drug Delivery
3.2. Vaccines
3.3. Imaging
3.4. Biosensing
4. Challenges of VLP-Based Approaches
4.1. Selection of VLP Type and Expression Platform
4.2. VLP Preparation
4.3. Functionalization
4.4. Immunogenicity
5. Emerging Approaches and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater Res 2019, 23, 20. [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N Engl J Med 2010, 363, 2434-2443. [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem Rev 2016, 116, 2826-2885. [CrossRef]
- Chaudhary, P.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Chen, W.-J.; Chen, S. Nanoparticle-Mediated Bioremediation as a Powerful Weapon in the Removal of Environmental Pollutants. Journal of Environmental Chemical Engineering 2023, 109591.
- Willner, M.R.; Vikesland, P.J. Nanomaterial Enabled Sensors for Environmental Contaminants. J Nanobiotechnology 2018, 16, 95. [CrossRef]
- Gómez-López, P.; Puente-Santiago, A.; Castro-Beltrán, A.; do Nascimento, L.A.S.; Balu, A.M.; Luque, R.; Alvarado-Beltrán, C.G. Nanomaterials and Catalysis for Green Chemistry. Current Opinion in Green and Sustainable Chemistry 2020, 24, 48-55.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J Nanobiotechnology 2018, 16, 71. [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of Nanoparticles in Biomedical Imaging. Nanoscale 2019, 11, 799-819. [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9. [CrossRef]
- Mejias, J.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Frontiers in Environmental Science 2021, 9, 52.
- Ghosh, T.; Raj, G.B.; Dash, K.K. A Comprehensive Review on Nanotechnology Based Sensors for Monitoring Quality and Shelf Life of Food Products. Measurement: Food 2022, 100049.
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat Nanotechnol 2022, 16, 45-66. [CrossRef]
- McNamara, K.; Tofail, S.A. Nanoparticles in Biomedical Applications. Advances in Physics: X 2017, 2, 54-88.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat Rev Drug Discov 2021, 20, 101-124. [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current Approaches of Nanomedicines in the Market and Various Stage of Clinical Translation. Acta Pharm Sin B 2022, 12, 3028-3048. [CrossRef]
- Namiot, E.D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schioth, H.B. Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int J Mol Sci 2023, 24. [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-Like Particles: Next-Generation Nanoparticles for Targeted Therapeutic Delivery. Bioeng Transl Med 2017, 2, 43-57. [CrossRef]
- Benjamin, C.; Brohlin, O.; Shahrivarkevishahi, A.; Gassensmith, J.J. Virus Like Particles: Fundamental Concepts, Biological Interactions, and Clinical Applications. Nanoparticles for Biomedical Applications 2020, 153-174.
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-Like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J Nanobiotechnology 2021, 19, 59. [CrossRef]
- Zeltins, A. Construction and Characterization of Virus-Like Particles: A Review. Mol Biotechnol 2013, 53, 92-107. [CrossRef]
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles Associated with Australia Antigen in the Sera of Patients with Leukaemia, Down's Syndrome and Hepatitis. Nature 1968, 218, 1057-1059. [CrossRef]
- Lee, L.A.; Niu, Z.; Wang, Q. Viruses and Virus-Like Protein Assemblies—Chemically Programmable Nanoscale Building Blocks. Nano Research 2009, 2, 349-364.
- Mejia-Mendez, J.L.; Vazquez-Duhalt, R.; Hernandez, L.R.; Sanchez-Arreola, E.; Bach, H. Virus-Like Particles: Fundamentals and Biomedical Applications. Int J Mol Sci 2022, 23. [CrossRef]
- Wang, J.W.; Roden, R.B. Virus-Like Particles for the Prevention of Human Papillomavirus-Associated Malignancies. Expert Rev Vaccines 2013, 12, 129-141. [CrossRef]
- Martins, S.A.; Santos, J.; Silva, R.D.; Rosa, C.; Cabo Verde, S.; Galamba Correia, J.D.; Melo, R. How Promising Are HIV-1-Based Virus-Like Particles for Medical Applications? Frontiers in Cellular and Infection Microbiology 2022, 1526. [CrossRef]
- Herbst-Kralovetz, M.; Mason, H.S.; Chen, Q. Norwalk Virus-Like Particles as Vaccines. Expert Rev Vaccines 2010, 9, 299-307. [CrossRef]
- Pushko, P.; Tretyakova, I. Influenza Virus Like Particles (VLPs): Opportunities for H7N9 Vaccine Development. Viruses 2020, 12. [CrossRef]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-Derived Virus-Like Particles in Vaccine Development. NPJ Vaccines 2017, 2, 3. [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular Pharming - VLPs Made in Plants. Curr Opin Biotechnol 2016, 37, 201-206. [CrossRef]
- Gopal, R.; Schneemann, A. Production and Application of Insect Virus-Based VLPs. Methods Mol Biol 2018, 1776, 125-141. [CrossRef]
- Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery. Vaccines (Basel) 2023, 11. [CrossRef]
- Pulix, M.; Lukashchuk, V.; Smith, D.C.; Dickson, A.J. Molecular Characterization of HEK293 Cells as Emerging Versatile Cell Factories. Curr Opin Biotechnol 2021, 71, 18-24. [CrossRef]
- Rodriguez-Limas, W.A.; Sekar, K.; Tyo, K.E. Virus-Like Particles: The Future of Microbial Factories and Cell-Free Systems as Platforms for Vaccine Development. Curr Opin Biotechnol 2013, 24, 1089-1093. [CrossRef]
- Wang, Y.; Douglas, T. Bioinspired Approaches to Self-Assembly of Virus-like Particles: From Molecules to Materials. Acc Chem Res 2022, 55, 1349-1359. [CrossRef]
- Ikwuagwu, B.; Hartman, E.; Mills, C.E.; Tullman-Ercek, D. Systematic Engineering of Virus-Like Particles to Identify Self-Assembly Rules for Shifting Particle Size. Virology 2023, 579, 137-147. [CrossRef]
- Le, D.T.; Muller, K.M. In Vitro Assembly of Virus-Like Particles and Their Applications. Life (Basel) 2021, 11. [CrossRef]
- Derdak, S.V.; Kueng, H.J.; Leb, V.M.; Neunkirchner, A.; Schmetterer, K.G.; Bielek, E.; Majdic, O.; Knapp, W.; Seed, B.; Pickl, W.F. Direct Stimulation of T Lymphocytes by Immunosomes: Virus-Like Particles Decorated with T Cell Receptor/CD3 Ligands Plus Costimulatory Molecules. Proc Natl Acad Sci U S A 2006, 103, 13144-13149. [CrossRef]
- Patel, K.G.; Swartz, J.R. Surface Functionalization of Virus-Like Particles by Direct Conjugation Using Azide-Alkyne Click Chemistry. Bioconjug Chem 2011, 22, 376-387. [CrossRef]
- Li, X.; Pan, C.; Sun, P.; Peng, Z.; Feng, E.; Wu, J.; Wang, H.; Zhu, L. Orthogonal Modular Biosynthesis of Nanoscale Conjugate Vaccines for Vaccination Against Infection. Nano Res 2022, 15, 1645-1653. [CrossRef]
- Kraj, P.; Selivanovitch, E.; Lee, B.; Douglas, T. Polymer Coatings on Virus-like Particle Nanoreactors at Low Ionic Strength-Charge Reversal and Substrate Access. Biomacromolecules 2021, 22, 2107-2118. [CrossRef]
- Cayetano-Cruz, M.; Coffeen, C.F.; Valadez-Garcia, J.; Montiel, C.; Bustos-Jaimes, I. Decoration of Virus-Like Particles with an Enzymatic Activity of Biomedical Interest. Virus Res 2018, 255, 1-9. [CrossRef]
- Carrico, Z.M.; Farkas, M.E.; Zhou, Y.; Hsiao, S.C.; Marks, J.D.; Chokhawala, H.; Clark, D.S.; Francis, M.B. N-Terminal Labeling of Filamentous Phage to Create Cancer Marker Imaging Agents. ACS Nano 2012, 6, 6675-6680. [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major Findings and Recent Advances in Virus-Like Particle (VLP)-Based Vaccines. Semin Immunol 2017, 34, 123-132. [CrossRef]
- Naskalska, A.; Pyrc, K. Virus Like Particles as Immunogens and Universal Nanocarriers. Pol J Microbiol 2015, 64, 3-13.
- Durous, L.; Rosa-Calatrava, M.; Petiot, E. Advances in Influenza Virus-Like Particles Bioprocesses. Expert Rev Vaccines 2019, 18, 1285-1300. [CrossRef]
- Marzi, A.; Feldmann, H. Ebola Virus Vaccines: An Overview of Current Approaches. Expert Rev Vaccines 2014, 13, 521-531. [CrossRef]
- Shang, W.; Liu, J.; Yang, J.; Hu, Z.; Rao, X. Dengue Virus-Like Particles: Construction and Application. Appl Microbiol Biotechnol 2012, 94, 39-46. [CrossRef]
- Pumpens, P.; Grens, E. The True Story and Advantages of the Famous Hepatitis B Virus Core Particles: Outlook 2016. Mol Biol (Mosk) 2016, 50, 558-576. [CrossRef]
- Fu, Y.; Li, J. A Novel Delivery Platform Based on Bacteriophage MS2 Virus-Like Particles. Virus Res 2016, 211, 9-16. [CrossRef]
- Essus, V.A.; Souza Junior, G.S.E.; Nunes, G.H.P.; Oliveira, J.D.S.; de Faria, B.M.; Romao, L.F.; Cortines, J.R. Bacteriophage P22 Capsid as a Pluripotent Nanotechnology Tool. Viruses 2023, 15. [CrossRef]
- Schwarz, B.; Douglas, T. Development of Virus-Like Particles for Diagnostic and Prophylactic Biomedical Applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2015, 7, 722-735. [CrossRef]
- Palucha, A.; Loniewska, A.; Satheshkumar, S.; Boguszewska-Chachulska, A.M.; Umashankar, M.; Milner, M.; Haenni, A.L.; Savithri, H.S. Virus-Like Particles: Models for Assembly Studies and Foreign Epitope Carriers. Prog Nucleic Acid Res Mol Biol 2005, 80, 135-168. [CrossRef]
- Sanchez-Rodriguez, S.P.; Munch-Anguiano, L.; Echeverria, O.; Vazquez-Nin, G.; Mora-Pale, M.; Dordick, J.S.; Bustos-Jaimes, I. Human Parvovirus B19 Virus-Like Particles: In Vitro Assembly and Stability. Biochimie 2012, 94, 870-878. [CrossRef]
- Jaballah, S.A.; Bailey, G.D.; Desfosses, A.; Hyun, J.; Mitra, A.K.; Kingston, R.L. In Vitro Assembly of the Rous Sarcoma Virus Capsid Protein Into Hexamer Tubes at Physiological Temperature. Sci Rep 2017, 7, 2913. [CrossRef]
- Xu, J.; Guo, H.C.; Wei, Y.Q.; Dong, H.; Han, S.C.; Ao, D.; Sun, D.H.; Wang, H.M.; Cao, S.Z.; Sun, S.Q. Self-Assembly of Virus-Like Particles of Canine Parvovirus Capsid Protein Expressed from Escherichia coli and Application as Virus-Like Particle Vaccine. Appl Microbiol Biotechnol 2014, 98, 3529-3538. [CrossRef]
- Barklis, E.; Alfadhli, A.; McQuaw, C.; Yalamuri, S.; Still, A.; Barklis, R.L.; Kukull, B.; Lopez, C.S. Characterization of the In Vitro HIV-1 Capsid Assembly Pathway. J Mol Biol 2009, 387, 376-389. [CrossRef]
- Zhao, Q.; Chen, W.; Chen, Y.; Zhang, L.; Zhang, J.; Zhang, Z. Self-Assembled Virus-Like Particles From Rotavirus Structural Protein VP6 for Targeted Drug Delivery. Bioconjug Chem 2011, 22, 346-352. [CrossRef]
- Le, D.T.; Radukic, M.T.; Muller, K.M. Adeno-Associated Virus Capsid Protein Expression in Escherichia coli and Chemically Defined Capsid Assembly. Sci Rep 2019, 9, 18631. [CrossRef]
- Vicente, T.; Roldao, A.; Peixoto, C.; Carrondo, M.J.; Alves, P.M. Large-Scale Production and Purification of VLP-Based Vaccines. J Invertebr Pathol 2011, 107 Suppl, S42-48. [CrossRef]
- Lai, C.C.; Cheng, Y.C.; Chen, P.W.; Lin, T.H.; Tzeng, T.T.; Lu, C.C.; Lee, M.S.; Hu, A.Y. Process Development for Pandemic Influenza VLP Vaccine Production Using a Baculovirus Expression System. J Biol Eng 2019, 13, 78. [CrossRef]
- Hillebrandt, N.; Vormittag, P.; Bluthardt, N.; Dietrich, A.; Hubbuch, J. Integrated Process for Capture and Purification of Virus-Like Particles: Enhancing Process Performance by Cross-Flow Filtration. Front Bioeng Biotechnol 2020, 8, 489. [CrossRef]
- Pniewski, T.; Kapusta, J.; Bociag, P.; Wojciechowicz, J.; Kostrzak, A.; Gdula, M.; Fedorowicz-Stronska, O.; Wojcik, P.; Otta, H.; Samardakiewicz, S.; et al. Low-Dose Oral Immunization with Lyophilized Tissue of Herbicide-Resistant Lettuce Expressing Hepatitis B Surface Antigen for Prototype Plant-Derived Vaccine Tablet Formulation. J Appl Genet 2011, 52, 125-136. [CrossRef]
- Hutchins, B.; Sajjadi, N.; Seaver, S.; Shepherd, A.; Bauer, S.R.; Simek, S.; Carson, K.; Aguilar-Cordova, E. Working Toward an Adenoviral Vector Testing Standard. Mol Ther 2000, 2, 532-534. [CrossRef]
- Peixoto, C.; Sousa, M.F.; Silva, A.C.; Carrondo, M.J.; Alves, P.M. Downstream Processing of Triple Layered Rotavirus Like Particles. J Biotechnol 2007, 127, 452-461. [CrossRef]
- Kaczmarczyk, S.J.; Sitaraman, K.; Young, H.A.; Hughes, S.H.; Chatterjee, D.K. Protein Delivery Using Engineered Virus-Like Particles. Proc Natl Acad Sci U S A 2011, 108, 16998-17003. [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-Specific Delivery of Diverse Cargos by Bacteriophage MS2 Virus-Like Particles. ACS Nano 2011, 5, 5729-5745. [CrossRef]
- Li, F.; Zhang, Z.P.; Peng, J.; Cui, Z.Q.; Pang, D.W.; Li, K.; Wei, H.P.; Zhou, Y.F.; Wen, J.K.; Zhang, X.E. Imaging Viral Behavior in Mammalian Cells with Self-Assembled Capsid-Quantum-Dot Hybrid Particles. Small 2009, 5, 718-726. [CrossRef]
- Pokorski, J.K.; Hovlid, M.L.; Finn, M.G. Cell Targeting with Hybrid Qβ Virus-Like Particles Displaying Epidermal Growth Factor. Chembiochem 2011, 12, 2441-2447. [CrossRef]
- Bustos-Jaimes, I.; Soto-Roman, R.A.; Gutierrez-Landa, I.A.; Valadez-Garcia, J.; Segovia-Trinidad, C.L. Construction of Protein-Functionalized Virus-Like Particles of Parvovirus B19. J Biotechnol 2017, 263, 55-63. [CrossRef]
- Zackova Suchanova, J.; Neburkova, J.; Spanielova, H.; Forstova, J.; Cigler, P. Retargeting Polyomavirus-Like Particles to Cancer Cells by Chemical Modification of Capsid Surface. Bioconjug Chem 2017, 28, 307-313. [CrossRef]
- Smith, M.T.; Hawes, A.K.; Bundy, B.C. Reengineering Viruses and Virus-Like Particles Through Chemical Functionalization Strategies. Curr Opin Biotechnol 2013, 24, 620-626. [CrossRef]
- Miller, R.A.; Presley, A.D.; Francis, M.B. Self-Assembling Light-Harvesting Systems from Synthetically Modified Tobacco Mosaic Virus Coat Proteins. J Am Chem Soc 2007, 129, 3104-3109. [CrossRef]
- Destito, G.; Yeh, R.; Rae, C.S.; Finn, M.G.; Manchester, M. Folic Acid-Mediated Targeting of Cowpea Mosaic Virus Particles to Tumor Cells. Chem Biol 2007, 14, 1152-1162. [CrossRef]
- Tong, G.J.; Hsiao, S.C.; Carrico, Z.M.; Francis, M.B. Viral Capsid DNA Aptamer Conjugates as Multivalent Cell-Targeting Vehicles. J Am Chem Soc 2009, 131, 11174-11178. [CrossRef]
- Windram, O.P.; Weber, B.; Jaffer, M.A.; Rybicki, E.P.; Shepherd, D.N.; Varsani, A. An Investigation Into the Use of Human Papillomavirus Type 16 Virus-Like Particles as a Delivery Vector System for Foreign Proteins: N- and C-Terminal Fusion of GFP to the L1 and L2 Capsid Proteins. Arch Virol 2008, 153, 585-589. [CrossRef]
- Guo, Y.; Guo, R.; Ma, Y.; Chang, W.; Ming, S.; Yang, G.; Guo, Y. Chimeric Virus-Like Particles of Universal Antigen Epitopes of Coronavirus and Phage Qβ Coat Protein Trigger the Production of Neutralizing Antibodies. Curr Top Med Chem 2021, 21, 1235-1250. [CrossRef]
- Kostiainen, M.A.; Hiekkataipale, P.; Jose, Á.; Nolte, R.J.; Cornelissen, J.J. Electrostatic Self-Assembly of Virus–Polymer Complexes. Journal of materials chemistry 2011, 21, 2112-2117.
- Ng, B.C.; Chan, S.T.; Lin, J.; Tolbert, S.H. Using Polymer Conformation to Control Architecture in Semiconducting Polymer/Viral Capsid Assemblies. ACS Nano 2011, 5, 7730-7738. [CrossRef]
- Parent, K.N.; Deedas, C.T.; Egelman, E.H.; Casjens, S.R.; Baker, T.S.; Teschke, C.M. Stepwise Molecular Display Utilizing Icosahedral and Helical Complexes of Phage Coat and Decoration Proteins in the Development of Robust Nanoscale Display Vehicles. Biomaterials 2012, 33, 5628-5637. [CrossRef]
- Patterson, D.P.; Schwarz, B.; El-Boubbou, K.; van der Oost, J.; Prevelige, P.E.; Douglas, T. Virus-Like Particle Nanoreactors: Programmed Encapsulation of the Thermostable CelB Glycosidase Inside the P22 Capsid. Soft Matter 2012, 8, 10158-10166.
- Galaway, F.A.; Stockley, P.G. MS2 Virus-Like Particles: A Robust, Semisynthetic Targeted Drug Delivery Platform. Mol Pharm 2013, 10, 59-68. [CrossRef]
- Musick, M.A.; McConnell, K.I.; Lue, J.K.; Wei, F.; Chen, C.; Suh, J. Reprogramming Virus Nanoparticles to Bind Metal Ions Upon Activation with Heat. Biomacromolecules 2011, 12, 2153-2158. [CrossRef]
- Minten, I.J.; Wilke, K.D.; Hendriks, L.J.; van Hest, J.C.; Nolte, R.J.; Cornelissen, J.J. Metal-Ion-Induced Formation and Stabilization of Protein Cages Based on the Cowpea Chlorotic Mottle Virus. Small 2011, 7, 911-919. [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-Like Particles: Passport to Immune Recognition. Methods 2006, 40, 60-65. [CrossRef]
- Carvalho, S.B.; Freire, J.M.; Moleirinho, M.G.; Monteiro, F.; Gaspar, D.; Castanho, M.; Carrondo, M.J.T.; Alves, P.M.; Bernardes, G.J.L.; Peixoto, C. Bioorthogonal Strategy for Bioprocessing of Specific-Site-Functionalized Enveloped Influenza-Virus-Like Particles. Bioconjug Chem 2016, 27, 2386-2399. [CrossRef]
- Martins, S.A.; Santos, J.; Cabo Verde, S.; Correia, J.D.G.; Melo, R. Construction of HER2-Specific HIV-1-Based VLPs. Bioengineering (Basel) 2022, 9. [CrossRef]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, Immunogenicity, and Efficacy of a Recombinantly Produced Plasmodium falciparum circumsporozoite Protein-Hepatitis B Surface Antigen Subunit Vaccine. J Infect Dis 1995, 171, 1576-1585. [CrossRef]
- Boxus, M.; Fochesato, M.; Miseur, A.; Mertens, E.; Dendouga, N.; Brendle, S.; Balogh, K.K.; Christensen, N.D.; Giannini, S.L. Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles. J Virol 2016, 90, 6314-6325. [CrossRef]
- Laurens, M.B. RTS,S/AS01 Vaccine (MosquirixTM): An Overview. Hum Vaccin Immunother 2020, 16, 480-489. [CrossRef]
- Ramazi, S.; Zahiri, J. Posttranslational Modifications in Proteins: Resources, Tools and Prediction Methods. Database (Oxford) 2021, 2021. [CrossRef]
- Farley, A.R.; Link, A.J. Identification and Quantification of Protein Posttranslational Modifications. Methods Enzymol 2009, 463, 725-763. [CrossRef]
- Muller, M.M. Post-Translational Modifications of Protein Backbones: Unique Functions, Mechanisms, and Challenges. Biochemistry 2018, 57, 177-185. [CrossRef]
- Ojha, R.; Prajapati, V.K. Cognizance of Posttranslational Modifications in Vaccines: A Way to Enhanced Immunogenicity. J Cell Physiol 2021, 236, 8020-8034. [CrossRef]
- Vellosillo, P.; Minguez, P. A Global Map of Associations Between Types of Protein Posttranslational Modifications and Human Genetic Diseases. iScience 2021, 24, 102917. [CrossRef]
- Liu, Y.P.; Zhang, T.N.; Wen, R.; Liu, C.F.; Yang, N. Role of Posttranslational Modifications of Proteins in Cardiovascular Disease. Oxid Med Cell Longev 2022, 2022, 3137329. [CrossRef]
- Vanuopadath, M.; Nair, D.; Gopalakrishnan Nair, B.; Sadasivan Nair, S. Post-Translational Modifications of Proteins: Biomarkers and Therapeutic Targets for Diabetes Related Complications. Current Proteomics 2016, 13, 251-270.
- Balieu, J.; Jung, J.W.; Chan, P.; Lomonossoff, G.P.; Lerouge, P.; Bardor, M. Investigation of the N-Glycosylation of the SARS-CoV-2 S Protein Contained in VLPs Produced in Nicotiana benthamiana. Molecules 2022, 27. [CrossRef]
- Lee, C.D.; Yan, Y.P.; Liang, S.M.; Wang, T.F. Production of FMDV Virus-Like pPrticles by a SUMO Fusion Protein Approach in Escherichia coli. J Biomed Sci 2009, 16, 69. [CrossRef]
- Ikwuagwu, B.; Tullman-Ercek, D. Virus-Like Particles for Drug Delivery: A Review of Methods and Applications. Curr Opin Biotechnol 2022, 78, 102785. [CrossRef]
- Yuan, B.; Liu, Y.; Lv, M.; Sui, Y.; Hou, S.; Yang, T.; Belhadj, Z.; Zhou, Y.; Chang, N.; Ren, Y.; et al. Virus-Like Particle-Based Nanocarriers as an Emerging Platform for Drug Delivery. J Drug Target 2023, 31, 433-455. [CrossRef]
- Pan, Y.; Zhang, Y.; Jia, T.; Zhang, K.; Li, J.; Wang, L. Development of a MicroRNA Delivery System Based on Bacteriophage MS2 Virus-Like Particles. FEBS J 2012, 279, 1198-1208. [CrossRef]
- Yao, Y.; Jia, T.; Pan, Y.; Gou, H.; Li, Y.; Sun, Y.; Zhang, R.; Zhang, K.; Lin, G.; Xie, J.; et al. Using a Novel MicroRNA Delivery System to Inhibit Osteoclastogenesis. Int J Mol Sci 2015, 16, 8337-8350. [CrossRef]
- Pan, Y.; Jia, T.; Zhang, Y.; Zhang, K.; Zhang, R.; Li, J.; Wang, L. MS2 VLP-Based Delivery of MicroRNA-146a Inhibits Autoantibody Production in Lupus-Prone Mice. Int J Nanomedicine 2012, 7, 5957-5967. [CrossRef]
- Chang, L.; Wang, G.; Jia, T.; Zhang, L.; Li, Y.; Han, Y.; Zhang, K.; Lin, G.; Zhang, R.; Li, J.; et al. Armored Long Non-Coding RNA MEG3 Targeting EGFR Based on Recombinant MS2 Bacteriophage Virus-Like Particles Against Hepatocellular Carcinoma. Oncotarget 2016, 7, 23988-24004. [CrossRef]
- Wang, G.; Jia, T.; Xu, X.; Chang, L.; Zhang, R.; Fu, Y.; Li, Y.; Yang, X.; Zhang, K.; Lin, G.; et al. Novel miR-122 Delivery System Based on MS2 Virus Like Particle Surface Displaying Cell-Penetrating Peptide TAT for Hepatocellular Carcinoma. Oncotarget 2016, 7, 59402-59416. [CrossRef]
- Kato, T.; Yui, M.; Deo, V.K.; Park, E.Y. Development of Rous sarcoma Virus-Like Particles Displaying hCC49 scFv for Specific Targeted Drug Delivery to Human Colon Carcinoma Cells. Pharm Res 2015, 32, 3699-3707. [CrossRef]
- Hartzell, E.J.; Lieser, R.M.; Sullivan, M.O.; Chen, W. Modular Hepatitis B Virus-like Particle Platform for Biosensing and Drug Delivery. ACS Nano 2020, 14, 12642-12651. [CrossRef]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and Characterization of a Novel Peptide Ligand of Epidermal Growth Factor Receptor for Targeted Delivery of Therapeutics. FASEB J 2005, 19, 1978-1985. [CrossRef]
- Berasain, C.; Avila, M.A. The EGFR Signalling System in the Liver: From Hepatoprotection to Hepatocarcinogenesis. J Gastroenterol 2014, 49, 9-23. [CrossRef]
- Braconi, C.; Kogure, T.; Valeri, N.; Huang, N.; Nuovo, G.; Costinean, S.; Negrini, M.; Miotto, E.; Croce, C.M.; Patel, T. MicroRNA-29 Can Regulate Expression of the Long Non-Coding RNA Gene MEG3 in Hepatocellular Cancer. Oncogene 2011, 30, 4750-4756. [CrossRef]
- Nakao, K.; Miyaaki, H.; Ichikawa, T. Antitumor Function of microRNA-122 Against Hepatocellular Carcinoma. J Gastroenterol 2014, 49, 589-593. [CrossRef]
- D'Souza, A.A.; Devarajan, P.V. Asialoglycoprotein Receptor Mediated Hepatocyte Targeting - Strategies and Applications. J Control Release 2015, 203, 126-139. [CrossRef]
- Brune, K.D.; Leneghan, D.B.; Brian, I.J.; Ishizuka, A.S.; Bachmann, M.F.; Draper, S.J.; Biswas, S.; Howarth, M. Plug-and-Display: Decoration of Virus-Like Particles Via Isopeptide Bonds for Modular Immunization. Sci Rep 2016, 6, 19234. [CrossRef]
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-Like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15. [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-Like Particles as a Highly Efficient Vaccine Platform: Diversity of Targets and Production Systems and Advances in Clinical Development. Vaccine 2012, 31, 58-83. [CrossRef]
- Keating, G.M.; Noble, S. Recombinant Hepatitis B Vaccine (Engerix-B®): A Review of Its Immunogenicity and Protective Efficacy Against Hepatitis B. Drugs 2003, 63, 1021-1051. [CrossRef]
- Hussain, Z.; Ali, S.S.; Husain, S.A.; Raish, M.; Sharma, D.R.; Kar, P. Evaluation of Immunogenicity and Reactogenicity of Recombinant DNA Hepatitis B Vaccine Produced in India. World J Gastroenterol 2005, 11, 7165-7168. [CrossRef]
- Tele, S.A.; Martins, R.M.; Lopes, C.L.; dos Santos Carneiro, M.A.; Souza, K.P.; Yoshida, C.F. Immunogenicity of a Recombinant Hepatitis B Vaccine (Euvax-B) in Haemodialysis Patients and Staff. Eur J Epidemiol 2001, 17, 145-149. [CrossRef]
- Shivananda; Somani, V.; Srikanth, B.S.; Mohan, M.; Kulkarni, P.S. Comparison of Two Hepatitis B Vaccines (GeneVac-B and Engerix-B) in Healthy Infants in India. Clin Vaccine Immunol 2006, 13, 661-664. [CrossRef]
- Batdelger, D.; Dandii, D.; Dahgwahdorj, Y.; Erdenetsogt, E.; Oyunbileg, J.; Tsend, N.; Bayarmagnai, B.; Jirathitikal, V.; Bourinbaiar, A.S. Clinical Experience with Therapeutic Vaccines Designed for Patients with Hepatitis. Curr Pharm Des 2009, 15, 1159-1171. [CrossRef]
- Hieu, N.T.; Kim, K.H.; Janowicz, Z.; Timmermans, I. Comparative Efficacy, Safety and Immunogenicity of Hepavax-Gene and Engerix-B, Recombinant Hepatitis B Vaccines, in Infants Born to HBsAg and HBeAg Positive Mothers in Vietnam: An Assessment at 2 Years. Vaccine 2002, 20, 1803-1808. [CrossRef]
- Venters, C.; Graham, W.; Cassidy, W. Recombivax-HB: Perspectives Past, Present and Future. Expert Rev Vaccines 2004, 3, 119-129. [CrossRef]
- Lakshmi, G.; Reddy, R.P.; Kumar, K.K.; Bhavani, N.V.; Dayanand, M. Study of the Safety, Immunogenicity and Seroconversion of a Hepatitis-B Vaccine in Malnourished Children of India. Vaccine 2000, 18, 2009-2014. [CrossRef]
- Abraham, P.; Mistry, F.P.; Bapat, M.R.; Sharma, G.; Reddy, G.R.; Prasad, K.S.; Ramanna, V. Evaluation of a New Recombinant DNA Hepatitis B Vaccine (Shanvac-B). Vaccine 1999, 17, 1125-1129. [CrossRef]
- Shi, L.; Sings, H.L.; Bryan, J.T.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.W.; Sitrin, R.; Barr, E. GARDASIL®: Prophylactic Human Papillomavirus Vaccine Development—From Bench Top to Bed-Side. Clin Pharmacol Ther 2007, 81, 259-264. [CrossRef]
- Monie, A.; Hung, C.F.; Roden, R.; Wu, T.C. Cervarix™: A Vaccine for the Prevention of HPV 16, 18-Associated Cervical Cancer. Biologics 2008, 2, 97-105.
- Herzog, C.; Hartmann, K.; Kunzi, V.; Kursteiner, O.; Mischler, R.; Lazar, H.; Gluck, R. Eleven Years of Inflexal V® - A Virosomal Adjuvanted Influenza Vaccine. Vaccine 2009, 27, 4381-4387. [CrossRef]
- Palladini, A.; Thrane, S.; Janitzek, C.M.; Pihl, J.; Clemmensen, S.B.; de Jongh, W.A.; Clausen, T.M.; Nicoletti, G.; Landuzzi, L.; Penichet, M.L.; et al. Virus-Like Particle Display of HER2 Induces Potent Anti-Cancer Responses. Oncoimmunology 2018, 7, e1408749. [CrossRef]
- Salazar-Gonzalez, J.A.; Ruiz-Cruz, A.A.; Bustos-Jaimes, I.; Moreno-Fierros, L. Expression of Breast Cancer-Related Epitopes Targeting the IGF-1 Receptor in Chimeric Human Parvovirus B19 Virus-Like Particles. Mol Biotechnol 2019, 61, 742-753. [CrossRef]
- Zhang, P.; Falcone, S.; Tsybovsky, Y.; Singh, M.; Gopan, V.; Miao, H.; Seo, Y.; Rogers, D.; Renzi, I.; Lai, Y.T.; et al. Increased Neutralization Potency and Breadth Elicited by a SARS-CoV-2 mRNA Vaccine Forming Virus-Like Particles. Proc Natl Acad Sci U S A 2023, 120, e2305896120. [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv Drug Deliv Rev 2020, 156, 214-235. [CrossRef]
- Sun, X.; Cui, Z. Virus-Like Particles as Theranostic Platforms. Advanced Therapeutics 2020, 3, 1900194.
- Li, K.; Chen, Y.; Li, S.; Nguyen, H.G.; Niu, Z.; You, S.; Mello, C.M.; Lu, X.; Wang, Q. Chemical Modification of M13 Bacteriophage and Its Application in Cancer Cell Imaging. Bioconjug Chem 2010, 21, 1369-1377. [CrossRef]
- Sun, X.; Li, W.; Zhang, X.; Qi, M.; Zhang, Z.; Zhang, X.E.; Cui, Z. In Vivo Targeting and Imaging of Atherosclerosis Using Multifunctional Virus-Like Particles of Simian Virus 40. Nano Lett 2016, 16, 6164-6171. [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int J Prev Med 2014, 5, 927-946.
- Mulder, W.J.; Jaffer, F.A.; Fayad, Z.A.; Nahrendorf, M. Imaging and Nanomedicine in Inflammatory Atherosclerosis. Sci Transl Med 2014, 6, 239sr231. [CrossRef]
- Cassette, E.; Helle, M.; Bezdetnaya, L.; Marchal, F.; Dubertret, B.; Pons, T. Design of New Quantum Dot Materials for Deep Tissue Infrared Imaging. Adv Drug Deliv Rev 2013, 65, 719-731. [CrossRef]
- Aanei, I.L.; ElSohly, A.M.; Farkas, M.E.; Netirojjanakul, C.; Regan, M.; Taylor Murphy, S.; O'Neil, J.P.; Seo, Y.; Francis, M.B. Biodistribution of Antibody-MS2 Viral Capsid Conjugates in Breast Cancer Models. Mol Pharm 2016, 13, 3764-3772. [CrossRef]
- ElSohly, A.M.; Netirojjanakul, C.; Aanei, I.L.; Jager, A.; Bendall, S.C.; Farkas, M.E.; Nolan, G.P.; Francis, M.B. Synthetically Modified Viral Capsids as Versatile Carriers for Use in Antibody-Based Cell Targeting. Bioconjug Chem 2015, 26, 1590-1596. [CrossRef]
- Hu, H.; Masarapu, H.; Gu, Y.; Zhang, Y.; Yu, X.; Steinmetz, N.F. Physalis Mottle Virus-like Nanoparticles for Targeted Cancer Imaging. ACS Appl Mater Interfaces 2019, 11, 18213-18223. [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors (Basel) 2021, 21. [CrossRef]
- Mieloch, A.A.; Mleczko, A.M.; Samelak-Czajka, A.; Jackowiak, P.; Rybka, J.D. Biomimetic Virus-Like Particles with Magnetic Core. From Bioactivity to an Immunodiagnostic Tool. Chemical Engineering Journal 2024, 149714. [CrossRef]
- Zhang, Q.; Su, C.; Qiu, J.-G.; Jiang, B.-H.; Zhang, C.-y. Advances in Enzyme-Free Nucleic Acid Amplification-Based Fluorescent Biosensors for Real-Time Imaging of DNA Repair Enzymes in Living Cells. Coordination Chemistry Reviews 2023, 496, 215406.
- Ye, M.; Hu, S.; Zhou, L.; Tang, X.; Zhao, S.; Zhao, J. Fluidic Membrane Accelerating the Kinetics of Photoactivatable Hybridization Chain Reaction for Accurate Imaging of Tumor-Derived Exosomes. Anal Chem 2022, 94, 17645-17652. [CrossRef]
- Dirks, R.M.; Pierce, N.A. Triggered Amplification by Hybridization Chain Reaction. Proceedings of the National Academy of Sciences 2004, 101, 15275-15278. [CrossRef]
- Chen, Y.; Song, Y.; Wang, X.; Tang, H.; Li, C. Genetically Engineered Virus-Like Particle-Armoured and Multibranched DNA Scaffold-Corbelled Ultra-Sensitive Hierarchical Hybridization Chain Reaction for Targeting-Enhanced Imaging in Living Biosystems Under Spatiotemporal Light Powering. Biosens Bioelectron 2024, 247, 115943. [CrossRef]
- Wang, J.; Zhang, L.; Yan, G.; Cheng, L.; Zhang, F.; Wu, J.; Lei, Y.; An, Q.; Qi, H.; Zhang, C.; et al. Modified Exfoliated Graphene Functionalized with Carboxylic Acid-Group and Thionine on a Screen-Printed Carbon Electrode as a Platform for an Electrochemical Enzyme Immunosensor. Mikrochim Acta 2024, 191, 143. [CrossRef]
- Lua, L.H.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P. Bioengineering Virus-Like Particles as Vaccines. Biotechnol Bioeng 2014, 111, 425-440. [CrossRef]
- Hillebrandt, N.; Hubbuch, J. Size-Selective Downstream Processing of Virus Particles and Non-Enveloped Virus-Like Particles. Front Bioeng Biotechnol 2023, 11, 1192050. [CrossRef]
- Roldao, A.; Mellado, M.C.; Lima, J.C.; Carrondo, M.J.; Alves, P.M.; Oliveira, R. On the Effect of Thermodynamic Equilibrium on the Assembly Efficiency of Complex Multi-Layered Virus-Like Particles (VLP): the Case of Rotavirus VLP. PLoS Comput Biol 2012, 8, e1002367. [CrossRef]
- Zhao, Q.; Allen, M.J.; Wang, Y.; Wang, B.; Wang, N.; Shi, L.; Sitrin, R.D. Disassembly and Reassembly Improves Morphology and Thermal Stability of Human Papillomavirus Type 16 Virus-Like Particles. Nanomedicine 2012, 8, 1182-1189. [CrossRef]
- Zhao, Q.; Modis, Y.; High, K.; Towne, V.; Meng, Y.; Wang, Y.; Alexandroff, J.; Brown, M.; Carragher, B.; Potter, C.S.; et al. Disassembly and Reassembly of Human Papillomavirus Virus-Like Particles Produces More Virion-Like Antibody Reactivity. Virol J 2012, 9, 52. [CrossRef]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, Advances, and Technical Challenges in Virus-Like Particles-Based Vaccines. Front Immunol 2023, 14, 1123805. [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front Microbiol 2021, 12, 790121. [CrossRef]
- Fuenmayor, J.; Godia, F.; Cervera, L. Production of Virus-Like Particles for Vaccines. N Biotechnol 2017, 39, 174-180. [CrossRef]
- Fernandes, B.; Correia, R.; Alves, P.M.; Roldao, A. Intensifying Continuous Production of Gag-HA VLPs at High Cell Density Using Stable Insect Cells Adapted to Low Culture Temperature. Front Bioeng Biotechnol 2022, 10, 917746. [CrossRef]
- Fontana, D.; Kratje, R.; Etcheverrigaray, M.; Prieto, C. Immunogenic Virus-Like Particles Continuously Expressed in Mammalian Cells as a Veterinary Rabies Vaccine Candidate. Vaccine 2015, 33, 4238-4246. [CrossRef]
- Comas-Garcia, M.; Kroupa, T.; Datta, S.A.; Harvin, D.P.; Hu, W.S.; Rein, A. Efficient Support of Virus-Like Particle Assembly by the HIV-1 Packaging Signal. Elife 2018, 7. [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines (Basel) 2018, 6. [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Virus-Like Particles as Preventive and Therapeutic Cancer Vaccines. Vaccines (Basel) 2022, 10. [CrossRef]
- He, J.; Yu, L.; Lin, X.; Liu, X.; Zhang, Y.; Yang, F.; Deng, W. Virus-Like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses 2022, 14. [CrossRef]
- Woolf, T.M. Therapeutic Repair of Mutated Nucleic Acid Sequences. Nat Biotechnol 1998, 16, 341-344. [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-Frequency Off-Target Mutagenesis Induced by CRISPR-Cas Nucleases in Human Cells. Nat Biotechnol 2013, 31, 822-826. [CrossRef]
- Gaj, T.; Sirk, S.J.; Shui, S.L.; Liu, J. Genome-Editing Technologies: Principles and Applications. Cold Spring Harb Perspect Biol 2016, 8. [CrossRef]
- Lindel, F.; Dodt, C.R.; Weidner, N.; Noll, M.; Bergemann, F.; Behrendt, R.; Fischer, S.; Dietrich, J.; Cartellieri, M.; Hamann, M.V.; et al. TraFo-CRISPR: Enhanced Genome Engineering by Transient Foamy Virus Vector-Mediated Delivery of CRISPR/Cas9 Components. Mol Ther Nucleic Acids 2019, 18, 708-726. [CrossRef]
- Lyu, P.; Javidi-Parsijani, P.; Atala, A.; Lu, B. Delivering Cas9/sgRNA Ribonucleoprotein (RNP) by Lentiviral Capsid-Based Bionanoparticles for Efficient ‘Hit-and-Run' Genome Editing. Nucleic Acids Res 2019, 47, e99. [CrossRef]
- Choi, J.G.; Dang, Y.; Abraham, S.; Ma, H.; Zhang, J.; Guo, H.; Cai, Y.; Mikkelsen, J.G.; Wu, H.; Shankar, P.; et al. Lentivirus Pre-Packed with Cas9 Protein for Safer Gene Editing. Gene Ther 2016, 23, 627-633. [CrossRef]
- Knopp, Y.; Geis, F.K.; Heckl, D.; Horn, S.; Neumann, T.; Kuehle, J.; Meyer, J.; Fehse, B.; Baum, C.; Morgan, M.; et al. Transient Retrovirus-Based CRISPR/Cas9 All-in-One Particles for Efficient, Targeted Gene Knockout. Mol Ther Nucleic Acids 2018, 13, 256-274. [CrossRef]
- Cai, Y.; Bak, R.O.; Mikkelsen, J.G. Targeted Genome Editing by Lentiviral Protein Transduction of Zinc-Finger and TAL-Effector Nucleases. Elife 2014, 3, e01911. [CrossRef]
- Habeeb, M.; You, H.W.; Umapathi, M.; Ravikumar, K.K.; Hariyadi; Mishra, S. Strategies of Artificial intelligence Tools in the Domain of Nanomedicine. Journal of Drug Delivery Science and Technology 2024, 91. [CrossRef]
- Li, R.; Wijma, H.J.; Song, L.; Cui, Y.; Otzen, M.; Tian, Y.; Du, J.; Li, T.; Niu, D.; Chen, Y.; et al. Computational Redesign of Enzymes for Regio- and Enantioselective Hydroamination. Nat Chem Biol 2018, 14, 664-670. [CrossRef]
- Cunningham, J.M.; Koytiger, G.; Sorger, P.K.; AlQuraishi, M. Biophysical Prediction of Protein-Peptide Pnteractions and Signaling Networks Using Machine Learning. Nat Methods 2020, 17, 175-183. [CrossRef]
- Madhukar, N.S.; Khade, P.K.; Huang, L.; Gayvert, K.; Galletti, G.; Stogniew, M.; Allen, J.E.; Giannakakou, P.; Elemento, O. A Bayesian Machine Learning Approach for Drug Target Identification Using Diverse Data Types. Nat Commun 2019, 10, 5221. [CrossRef]
- Ianevski, A.; Giri, A.K.; Gautam, P.; Kononov, A.; Potdar, S.; Saarela, J.; Wennerberg, K.; Aittokallio, T. Prediction of Drug Vombination Effects with a Minimal Set of Experiments. Nat Mach Intell 2019, 1, 568-577. [CrossRef]
- Gainza, P.; Sverrisson, F.; Monti, F.; Rodola, E.; Boscaini, D.; Bronstein, M.M.; Correia, B.E. Deciphering Interaction Fingerprints from Protein Molecular Surfaces Using Geometric Deep Learning. Nat Methods 2020, 17, 184-192. [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J Control Release 2015, 200, 138-157. [CrossRef]
- Padma, V.V. An Overview of Targeted Cancer Therapy. Biomedicine (Taipei) 2015, 5, 19. [CrossRef]
- Verhoef, J.J.; Anchordoquy, T.J. Questioning the Use of PEGylation for Drug Delivery. Drug Deliv Transl Res 2013, 3, 499-503. [CrossRef]
- Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Bioinspired Shielding Strategies for Nanoparticle Drug Delivery Applications. Mol Pharm 2018, 15, 2900-2909. [CrossRef]
| Class | VLP | Virus Type | Expression Systems | Applications | References |
|---|---|---|---|---|---|
| Enveloped | HIV | Human virus | Eukaryotic (Mammalian, Yeast, Insect, Plant) | Vaccination and Drug Delivery | [25] |
| Influenza | Human virus | Eukaryotic (Mammalian, Insect, Plant) | Vaccination | [45] | |
| Ebola | Human virus | Eukaryotic (Mammalian, Insect) | Vaccination | [46] | |
| Dengue | Human virus | Eukaryotic (Mammalian, Yeast, Insect, Plant) | Vaccination and Diagnosis | [47] | |
| Non-Enveloped | HPV | Human virus | Prokaryotic and Eukaryotic (Mammalian, Yeast, Insect, Plant) | Vaccination and Drug Delivery | [24] |
| Norwalk | Human virus | Prokaryotic and Eukaryotic (Mammalian, Insect, Plant) | Vaccination | [26] | |
| Hepatitis B (core) | Human virus | Prokaryotic and Eukaryotic (Mammalian, Insect, Plant) | Vaccination, Drug Delivery | [48] | |
| MS2 | Bacteriophage | Prokaryotic and Eukaryotic (Yeast, | Vaccination, Drug Delivery and Imaging | [49] | |
| P22 | Bacteriophage | Prokaryotic | Vaccination, Drug Delivery and Imaging | [50] | |
| Qβ | Bacteriophage | Prokaryotic and Eukaryotic (Yeast) | Vaccination, Drug Delivery and Imaging | [51] |
| Virus | Recombinant Protein | Expression System | VLP Type | Vaccine | Reference |
|---|---|---|---|---|---|
| Hepatitis B virus (HBV) | HBsAg | Eukaryotic Cell (S. cerevisiae) | Non-Enveloped | Engerix-B® | [116] |
| Eukaryotic Cell (P. pastoris) | Non-Enveloped | Enivac HB® | [117] | ||
| Eukaryotic Cell (S. cerevisiae) | Non-Enveloped | Euvax® | [118] | ||
| Eukaryotic Cell (H. polymorpha) | Non-Enveloped | Gene Vac-B® | [119] | ||
| Eukaryotic Cell (P. pastoris) | Non-Enveloped | Heberiovac HB | [120] | ||
| Eukaryotic Cell (H. polymorpha) | Non-Enveloped | Hepavax-Gene® | [121] | ||
| Eukaryotic Cell (S. cerevisiae) | Non-Enveloped | Recombivax HB® | [122] | ||
| Eukaryotic Cell (P. pastoris) | Non- Enveloped | Revac-B® | [123] | ||
| Eukaryotic Cell (P. pastoris) | Non-Enveloped | Shanvac-B® | [124] | ||
| Papillomavirus | HPV6 /11/16/18 L1 | Eukaryotic Cell (S. cerevisiae) | Non-Enveloped | Gardasil® | [125] |
| HPV6 16/18 L1 | Insect Cells | Non-Enveloped | Cervarix® | [126] | |
| Influenza virus A | A (H1N1), A (H3N2), B, HA, NA |
Cell-Free | Enveloped | Inflexal® V | [127] |
| Expression System | Advantages | Disadvantages |
|---|---|---|
| Bacteria |
|
|
| Yeast |
|
|
| Insect cells |
|
|
| Mammalian cells |
|
|
| Plant cells |
|
|
| Cell-free systems |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).