Submitted:
28 April 2024
Posted:
30 April 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Search Method and Results
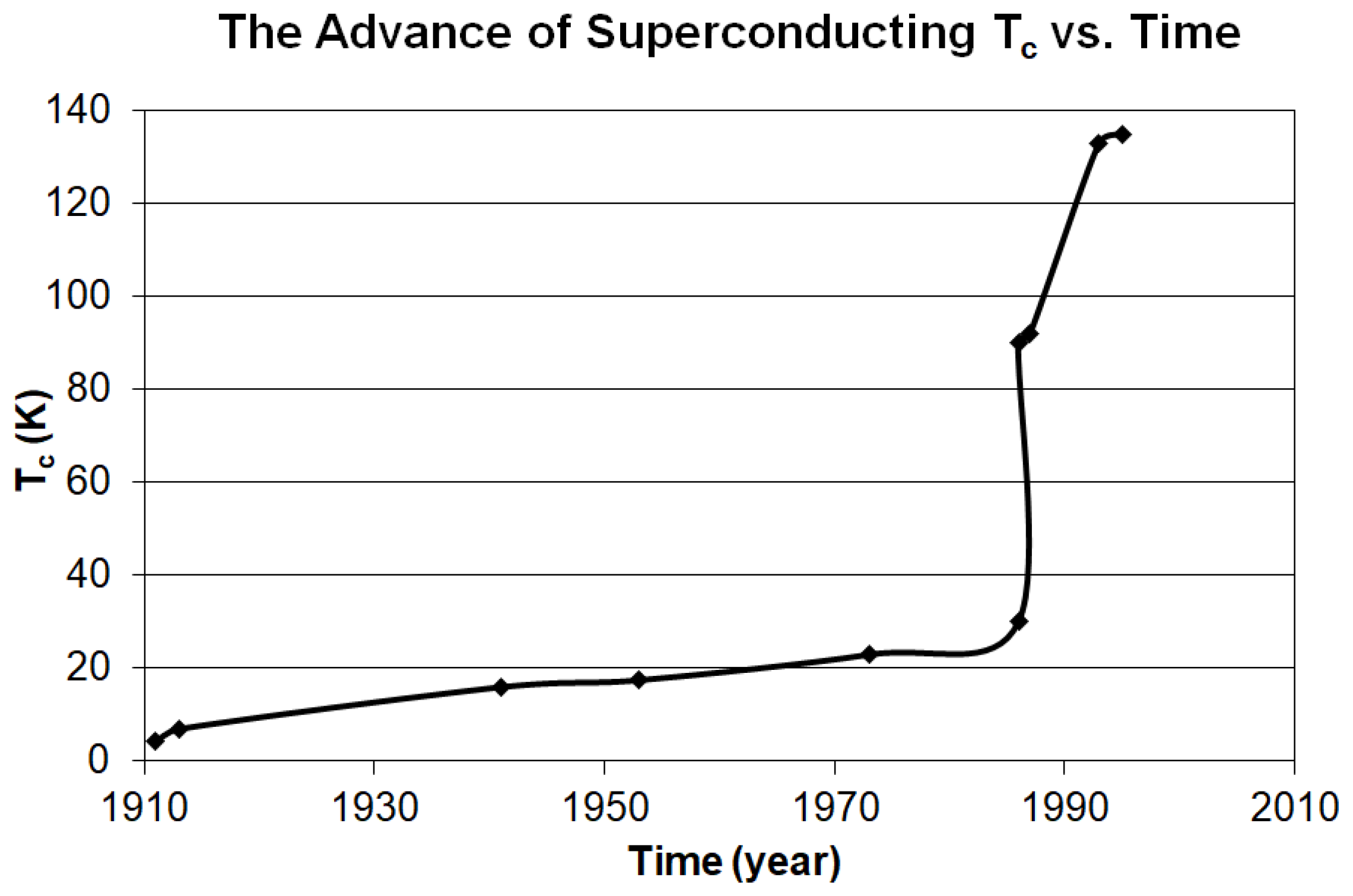
2.1. Search Motivation
2.2. Search Criterion and Experimental Methods
- Electrons should be paired.
- These paired elections should be on the material’s conduction band for superconductivity.
- The host material should provide a relevant packing environment or a crystallographic lattice arrangement for these electron pairs on the conduction band to flow over this lattice arrangement of the material.
2.3. Search Results
3. Discussion
3.1. Detailed Description of the Th Salts
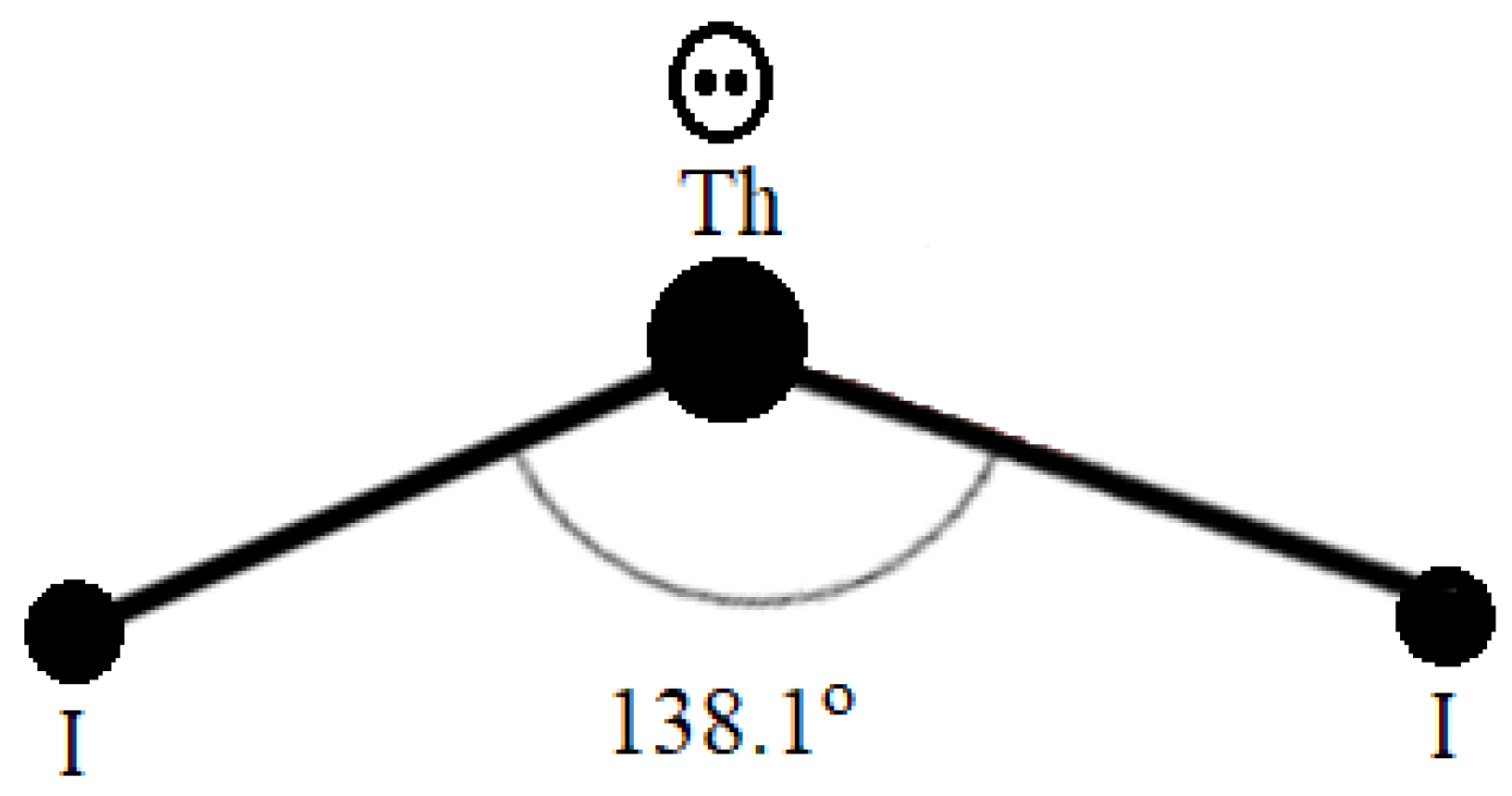
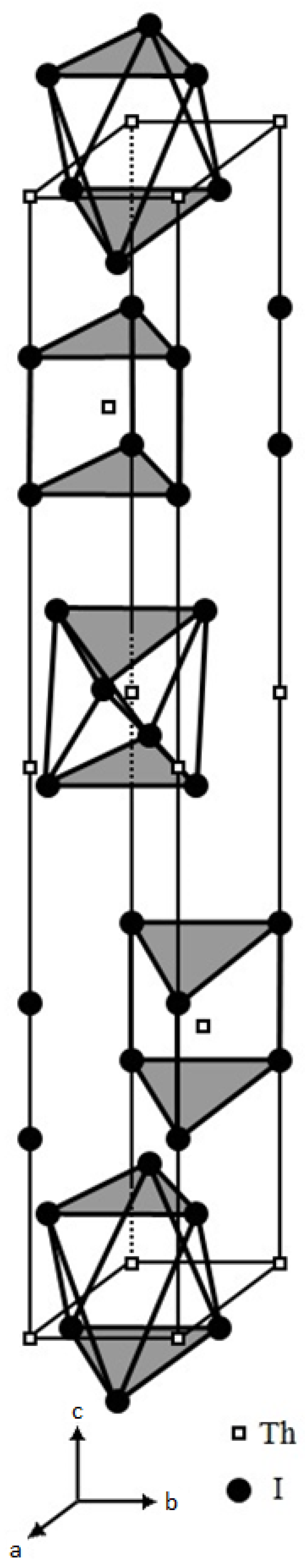
3.1.1. ThI2
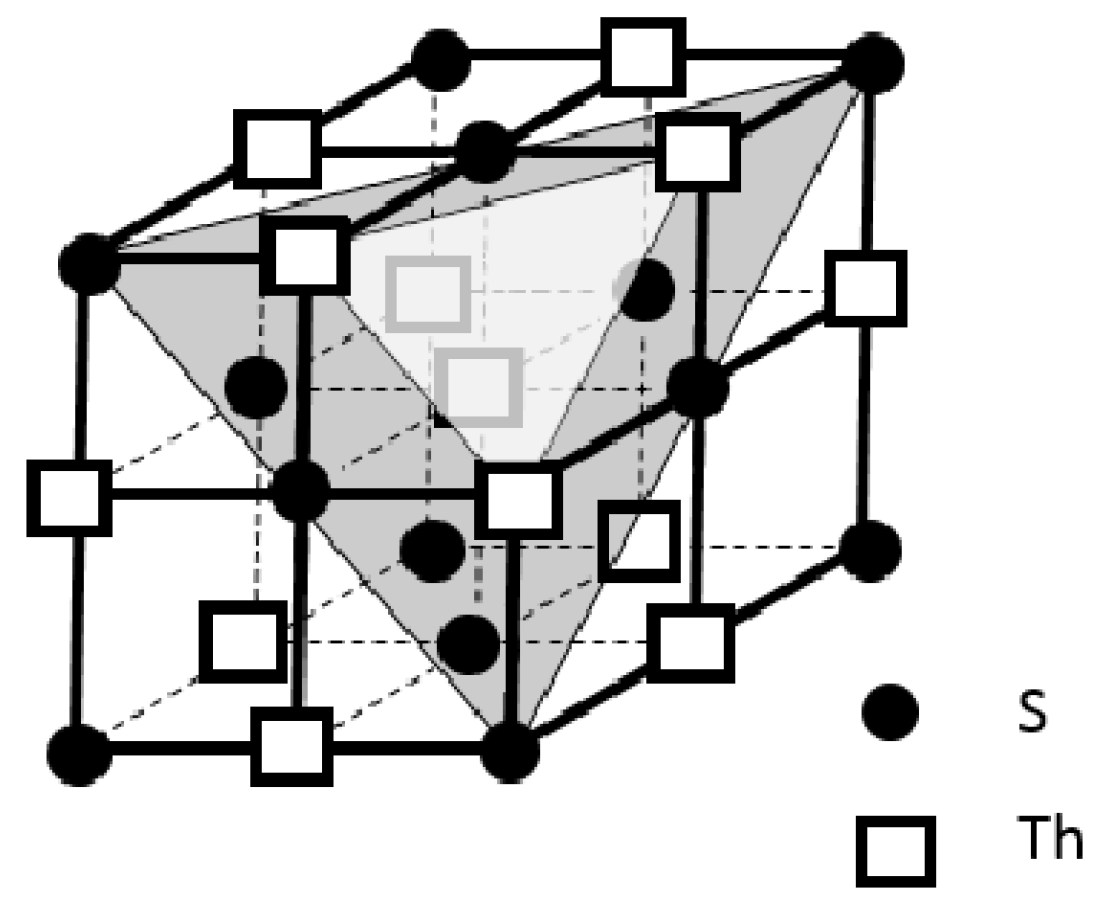
3.1.2. ThS
3.2. Electron Pairing and Our Novel Superconducting Mechanism
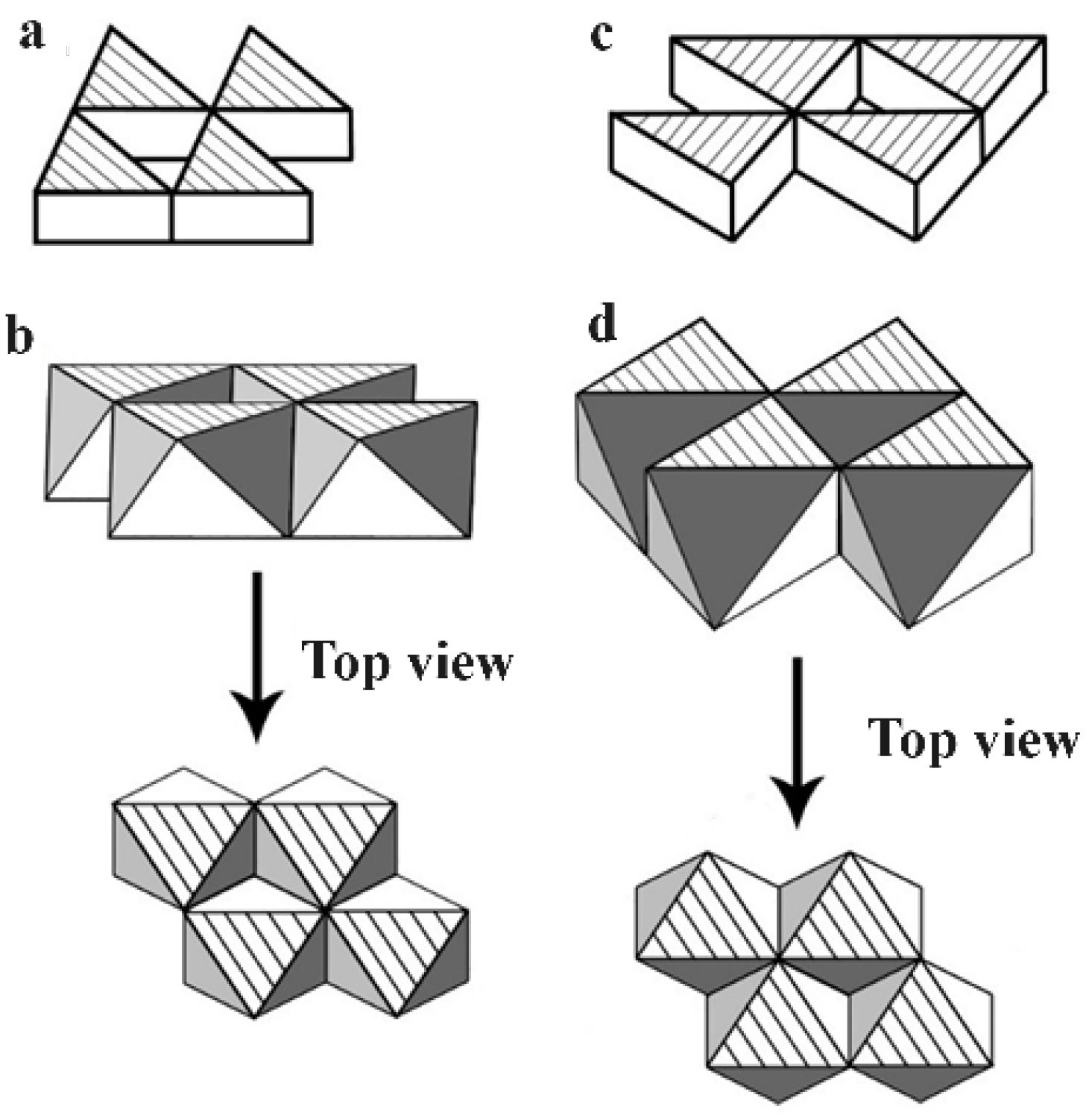
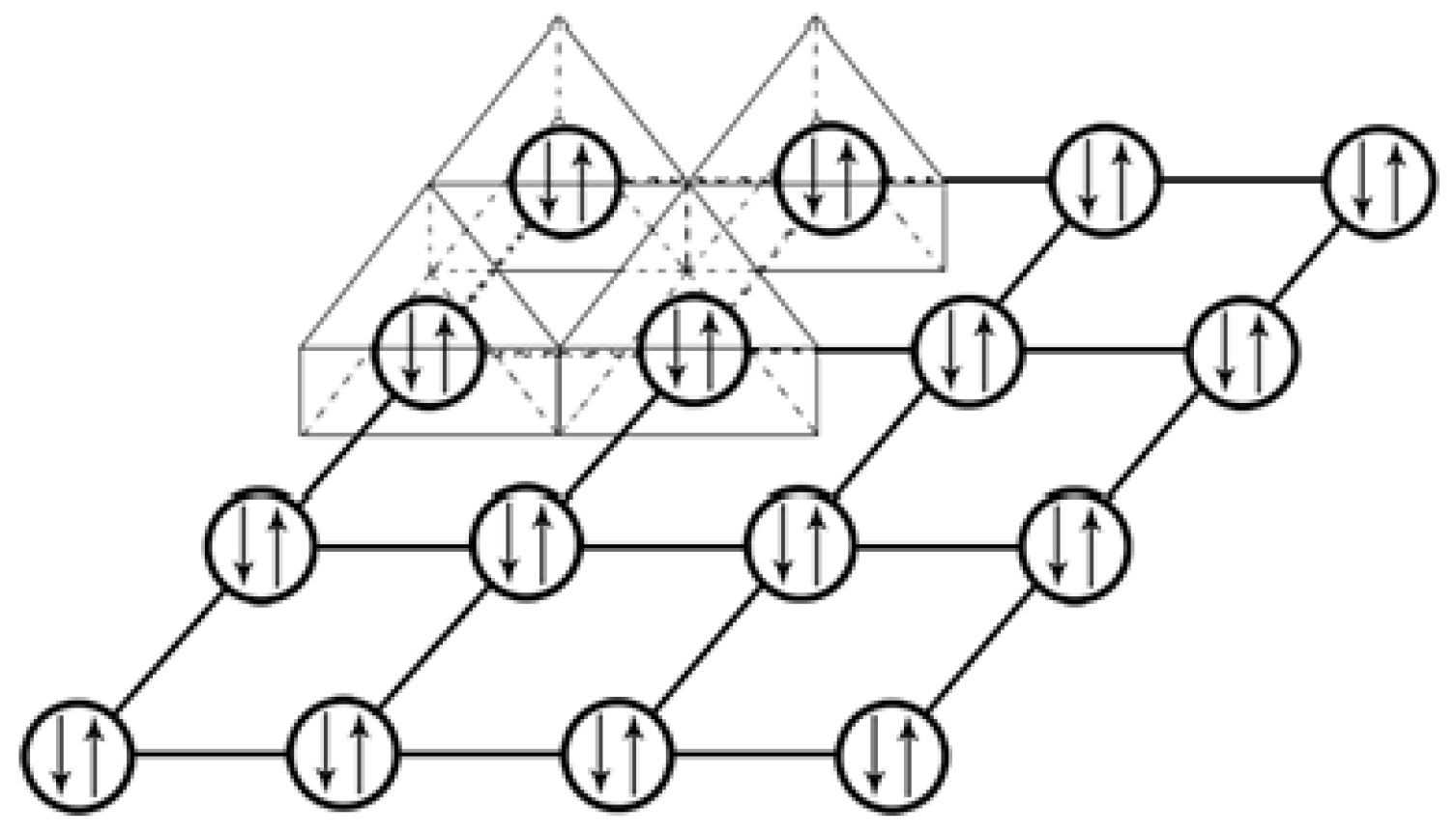
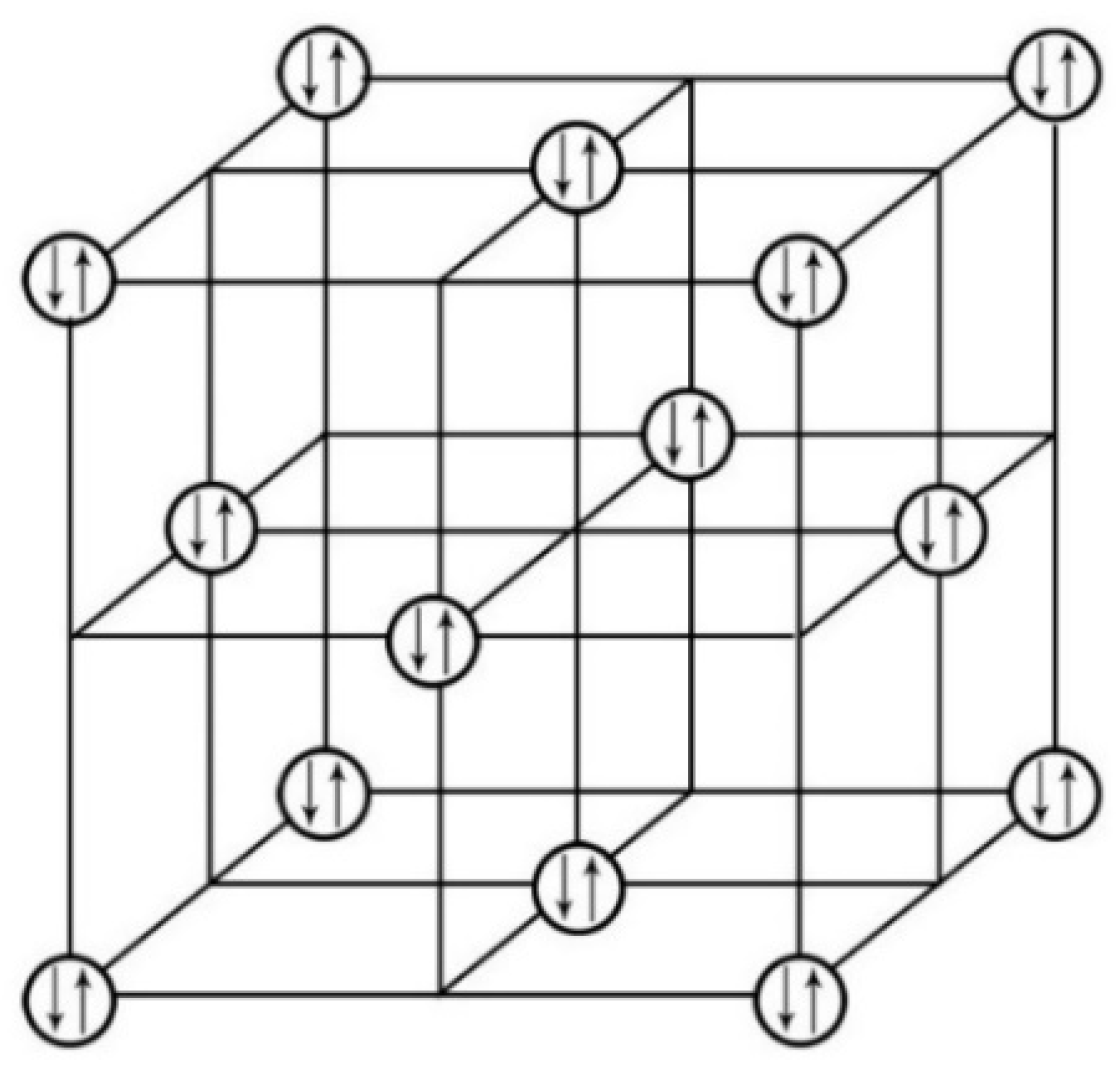
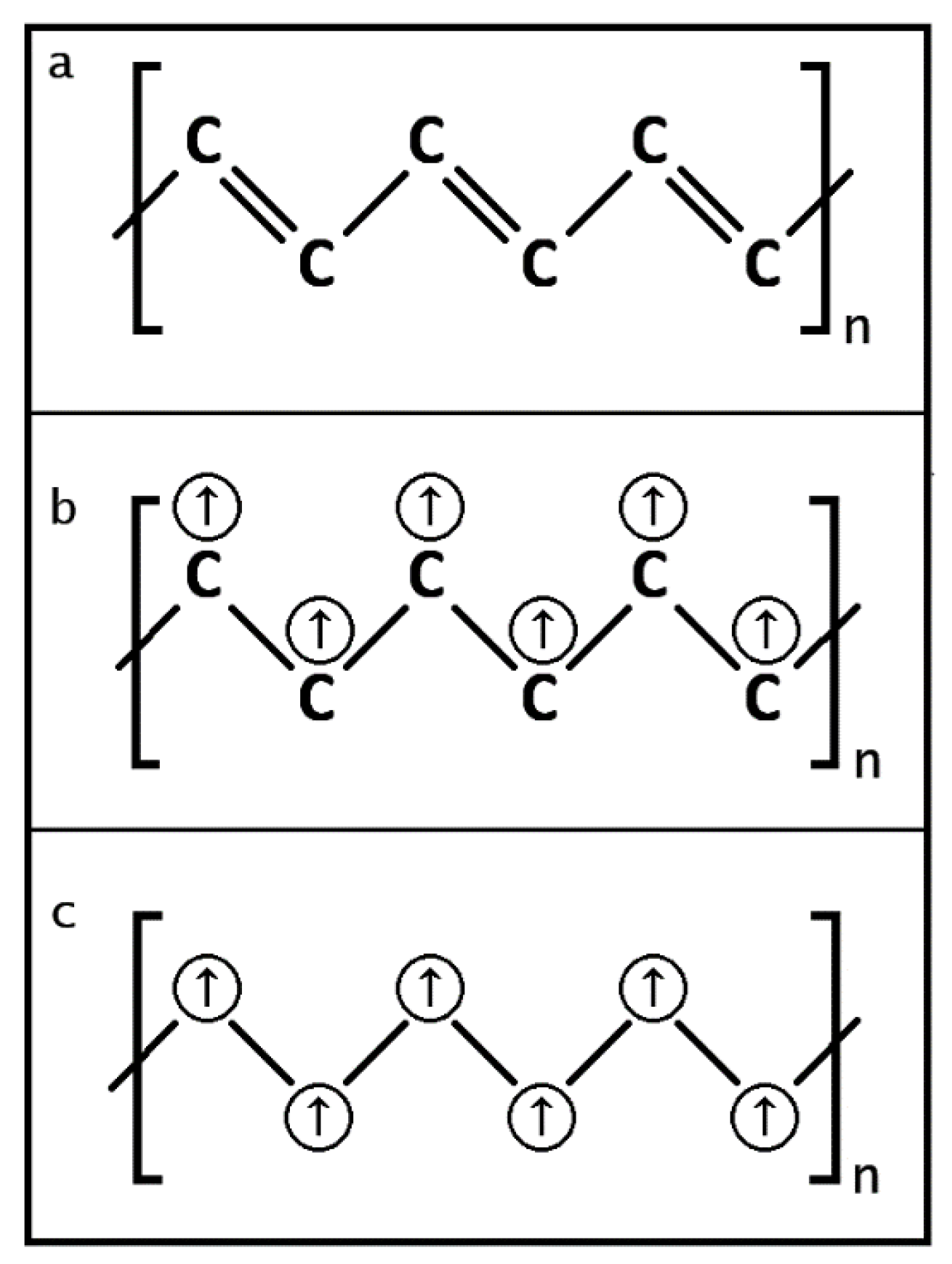
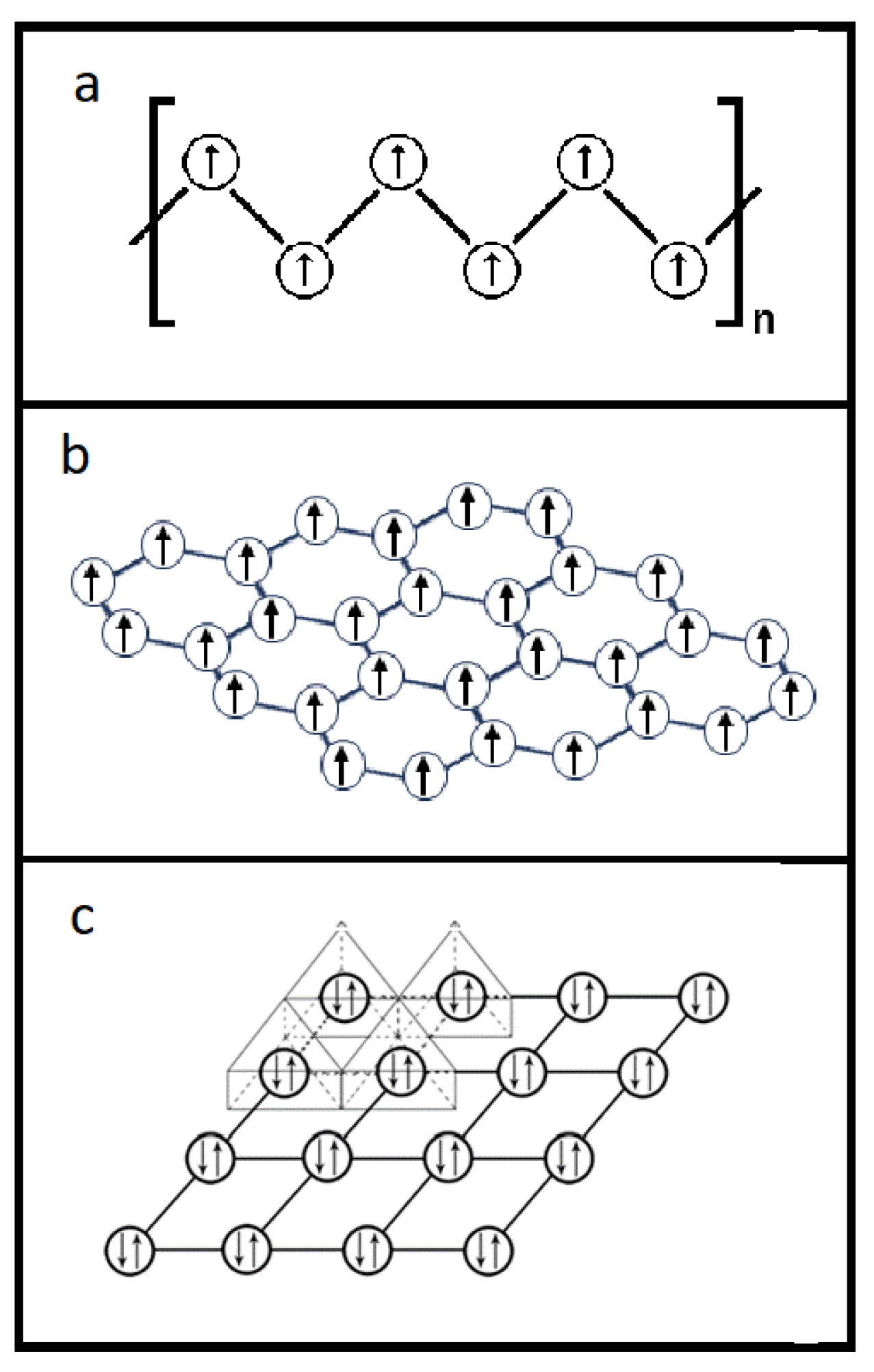
3.2.1. Diagrams of Electron Lone Pair Network
3.2.2. Electron Parings and our New Superconducting Mechanism
3.3.3. Limitation of BCS Theory
3.3.4. Networks for Ordinary Conductor and Superconductor
4. Future Directions
5. Conclusions
References
- S. Lee, J.-H. Kim, Y.-W. Kwon, Preprint arXiv:2307.12008 (2023).
- P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov, S. I. Shylin, Nature 525, 73 (2015).
- K. Onnes, KAWA, 1479 (1911).
- D. Garisto, Nature 620, 705 (2023).
- P. Drozdov, P. P. Kong, V. S. Minkov, S. P. Besedin, M. A. Kuzovnikov, S. Mozaffari, L. Balicas, E. F. Balakirev, D. E. Graf, V. B. Prakapenka, E. Greenberg, D. A. Knyazev, M. Tkacz, M. I. Eremets, Nature 569, 528 (2019).
- D. Grockowiak, M. Ahart, T. Helm, Coniglio, R. Kumar, K. Glazyrin, G. Garbarino, Y. Meng, M. Oliff, V. Williams, N. W. Ashcroft, R. J. Hemley, M. Somayazulu, S. W. Tozer, Front. Elec. Mat. 2, 837651 (2022).
- F. Richardson, N. W. Ashcroft, Phys. Rev. Lett. 78, 118 (1997).
- R. F. Service, Science 355, 332 (2017).
- J. G. Bednorz, K. A. Muller, Z. Physik B Condensed Matter 64, 189 (1986).
- R. L. Meng, L. Beauvais, X. N. Zhang, Z. J. Huang, Y. Y. Sun, Y. Y. Xue, C. W. Chu, Phys. C 216, 21 (1993).
- M. Nunez-Regueiro, J.-L. Tholence, E. V. Antipov, J.-J. Capponi, M. Marezio, Science 262, 97 (1993).
- R. Mankowsky, A. Subedi, M. Forst, S. O. Mariager, M. Chollet, H. T. Lemke, J. S. Robinson, J. M. Glownia, M. P. Minitti, A. Frano, M. Fechner, N. A. Spaldin, T. Loew, B. Keimei, A. Georges, A. Cavalleri, Nature 516, 71 (2014).
- W. Hu, S. Kaiser, D. Nicoletti, C. R. Hunt, I. Gierz, M. C. Hoffmann, M. Le Tacon, T. Loew, B. Keimer, A. Cavalleri, Nature Mater. 13, 705 (2014).
- Y. Kamihara, T. Watanabe, M. Hirano, H. Hosono, J. Am. Chem. Soc. 130, 3296 (2008).
- Li, K. Lee, B. Y. Wang, M. Osada, S. Crossley, H. R. Lee, Y. Cui, Y. Hikita, H. Y. Hwang, Nature 572, 624 (2019).
- H. Sun, M. Huo, X. Hu, J. Li, Z. Liu, Y. Han, L. Tang, Z. Mao, P. Yang, B. Wang, J. Cheng, D.-X. Yao, G.-M. Zhang, M. Wang, Nature 621, 493 (2023).
- M. E. Jones, R. E. Marsh, J. Am. Chem. Soc. 76, 1434 (1954).
- J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani, J. Akimitsu, Nature 410, 63 (2001).
- M. Hepting, Nature 621, 475 (2023).
- M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang, C. W. Chu, Phys. Rev. Lett. 58 (9) 908-910 (1987).
- Zhao, G. J. Zhao, JP 6710314 B2.
- R. J. Clark, J. D. Corbett, Inorg. Chem. 2, 460 (1963).
- D. Eastman, L. Brewer, L. A. Bromley, P. W. Gilles,N. L. Lofgren, J. Am. Chem. Soc. 72, 4019 (1950).
- R. Didchenko, F. P. Gortsema, Inorg. Chem. 2, 1079 (1963).
- P. D. Shalek, J. Am. Ceram. Soc. 46, 155 (1963).
- V. Samsonov, G. N. Dubrovskaya, Atomnaya Energiya 15, 428 (1963).
- E. D. Eastman, L. Brewer, L. A. Bromley, P. W. Gilles, N. L. Lofgren, J. Am. Chem. Soc. 73, 3896 (1950).
- L. J. Guggenberger, R. A. Jacobson, Inorg. Chem. 7, 2257 (1968).
- M. Tetenbaum, J. Appl. Phys. 35, 2468 (1964).
- D. Zhao, 6th Inter. Conf. Superconductivity and Magnetism, 372 (2018).
- D. Zhao, 13th Inter. Conf. Solid State Chem. 125 (2018).
- R. Abd-Shukor, Results in Phys. 25, 104219 (2021).
- E. F. Talantsev, Materials 16, 4367 (2023).
- Y. Ye, Nature Mater. 22, 671 (2023).
- P. Puphal, M. Y. P. Akbar, M. Hepting, E. Goering, M. Isobe, A. A. Nugroho, B. Keimer, Preprint, arXiv:2308.06256 (2023).
- Rey-Garcia, R. Ibanez, L. A. Angurel, F. M. Costa, G. F. de la Fuente, Crystals 11, 38 (2021).
- V. C. C. Hatnean, A. Pui, A. Simonov, M. C. Hatnean, Crystals 13, 1687 (2023).
- P. Telang, A. Maljuk, D. Rout, R. Hu, M. Skoulatos, K. Karmakar, S. Seiro, B. Roessli, U. Stuhr, B. Buchner, S.-W. Cheong, S. Singh, J. Cryst. Growth 507, 406 (2019).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



