3.2.1. Diagrams of Electron Lone Pair Network
We further investigated the nature of the electron pairings for these two Th salts. We plotted the crystal lattice of [Th
4+(e
-)
2](I
-)
2 by removing all its elements while only displaying the compound’s electron lone pairs in its crystal lattice. We further expanded the plotting to display 16 unit cells.
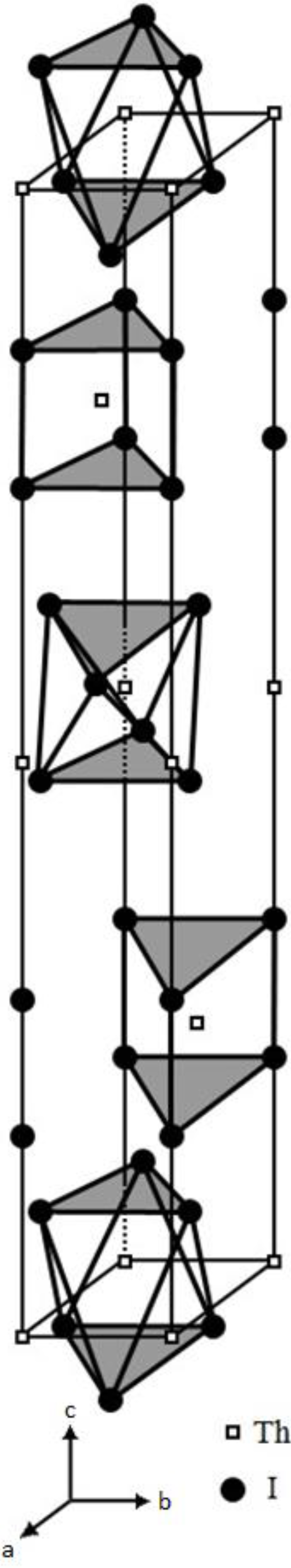
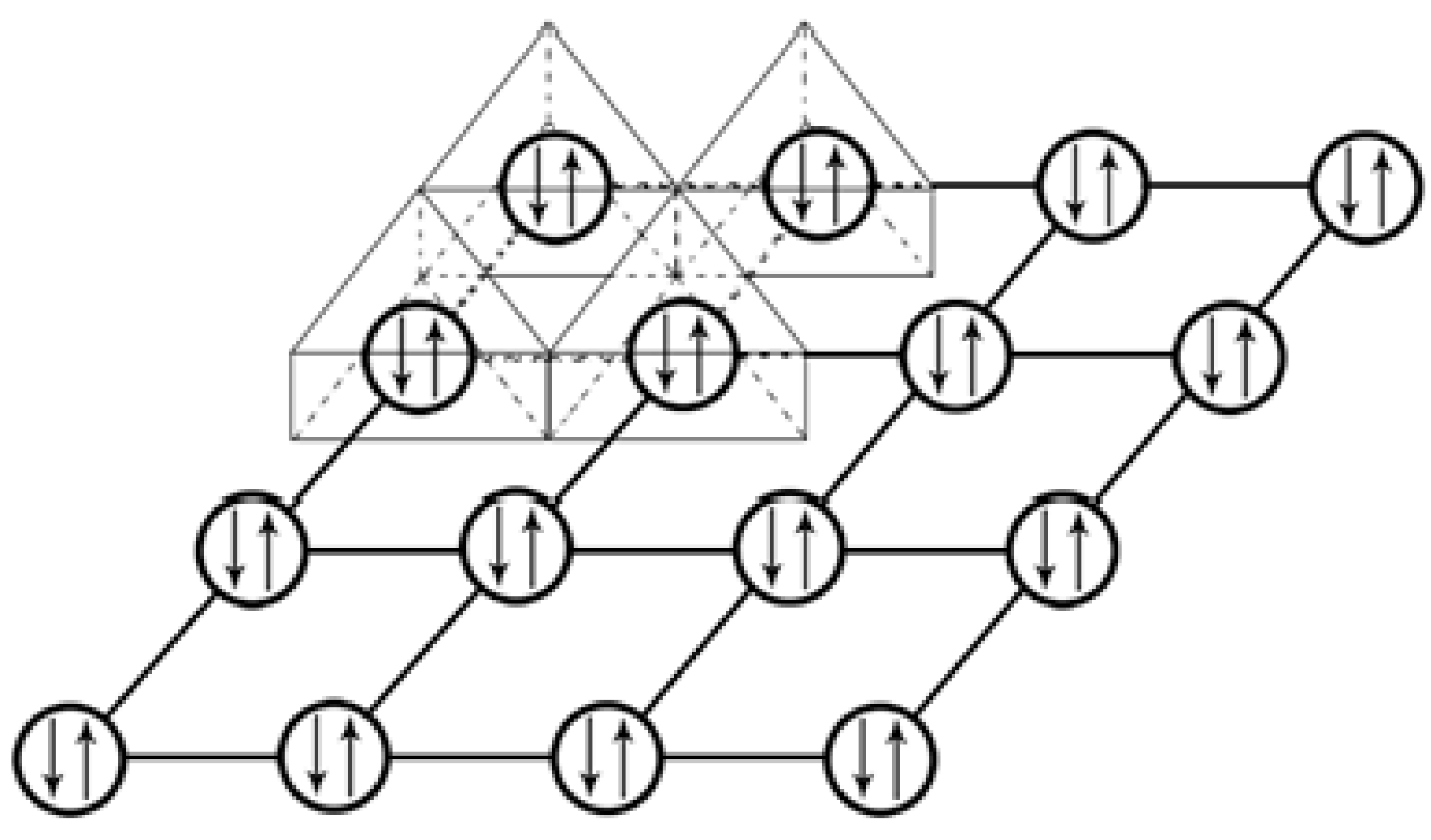
Figure 6 is the diagram that exhibits the (0, 0,
) plane of the expanded 16 unit cells with only displaying the [Th
4+(e
-)
2](I
-)
2′s electron lone pairs for better visualizing the 2-D relationship of these electron lone pairs [
30].
This network linkage in
Figure 6 offers the interactions among the adjacent electron lone pairs on the crystallographic (0, 0,
) plane. Similar 2-D features of the electron lone pairs can also be plotted for other crystallographic planes of the [Th
4+(e
-)
2](I
-)
2, such as for (0, 0,
), (0, 0,
) and (0, 0, 0) planes, but not included here to avoid redundancy. It is believed that the supercurrent should flow through these planes carried by these electron lone pairs. Consequently, we can anticipate that this 2-D layer structure would enable ThI
2 to only have the 2-D anisotropic superconductivity as many other compound superconductors.
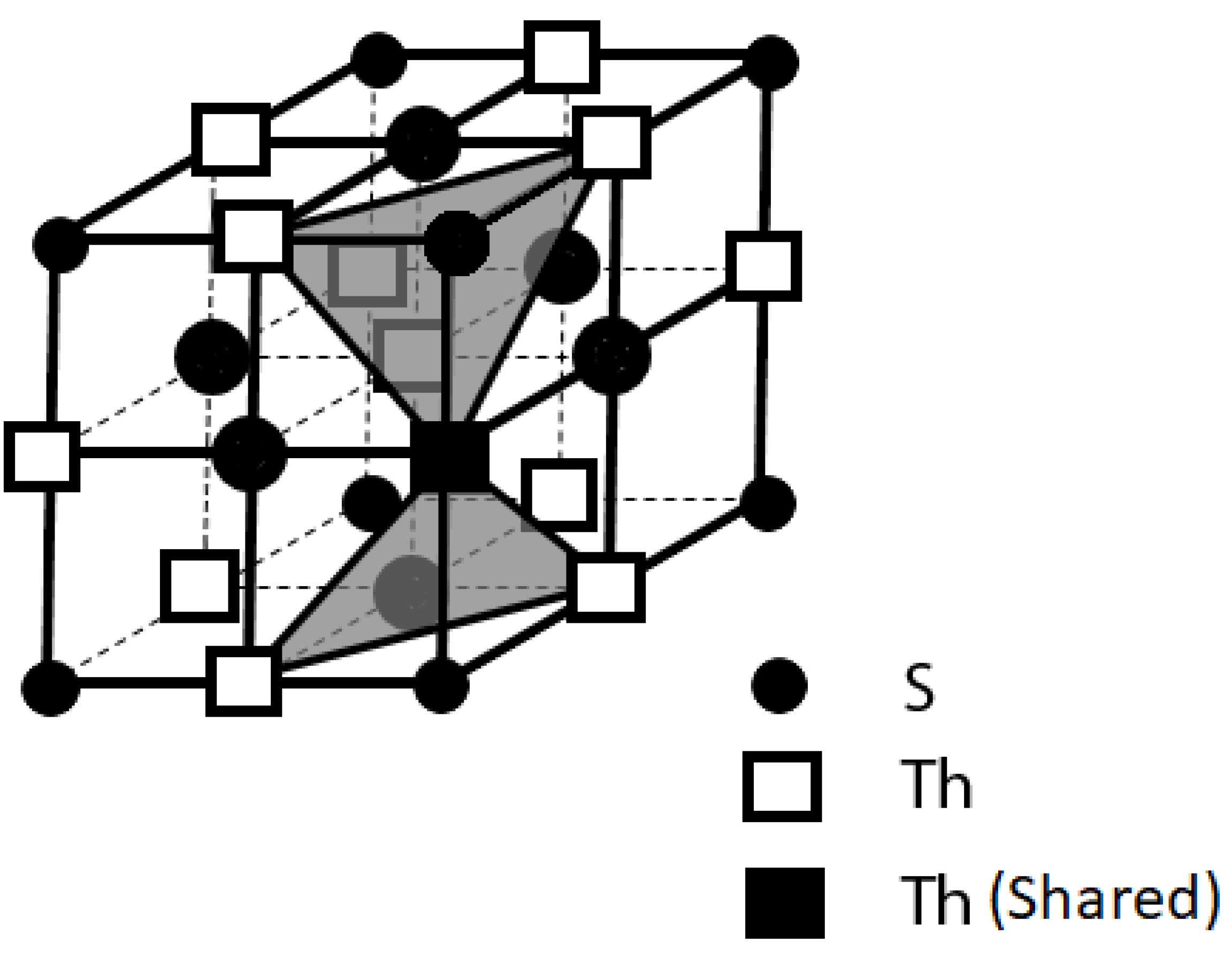
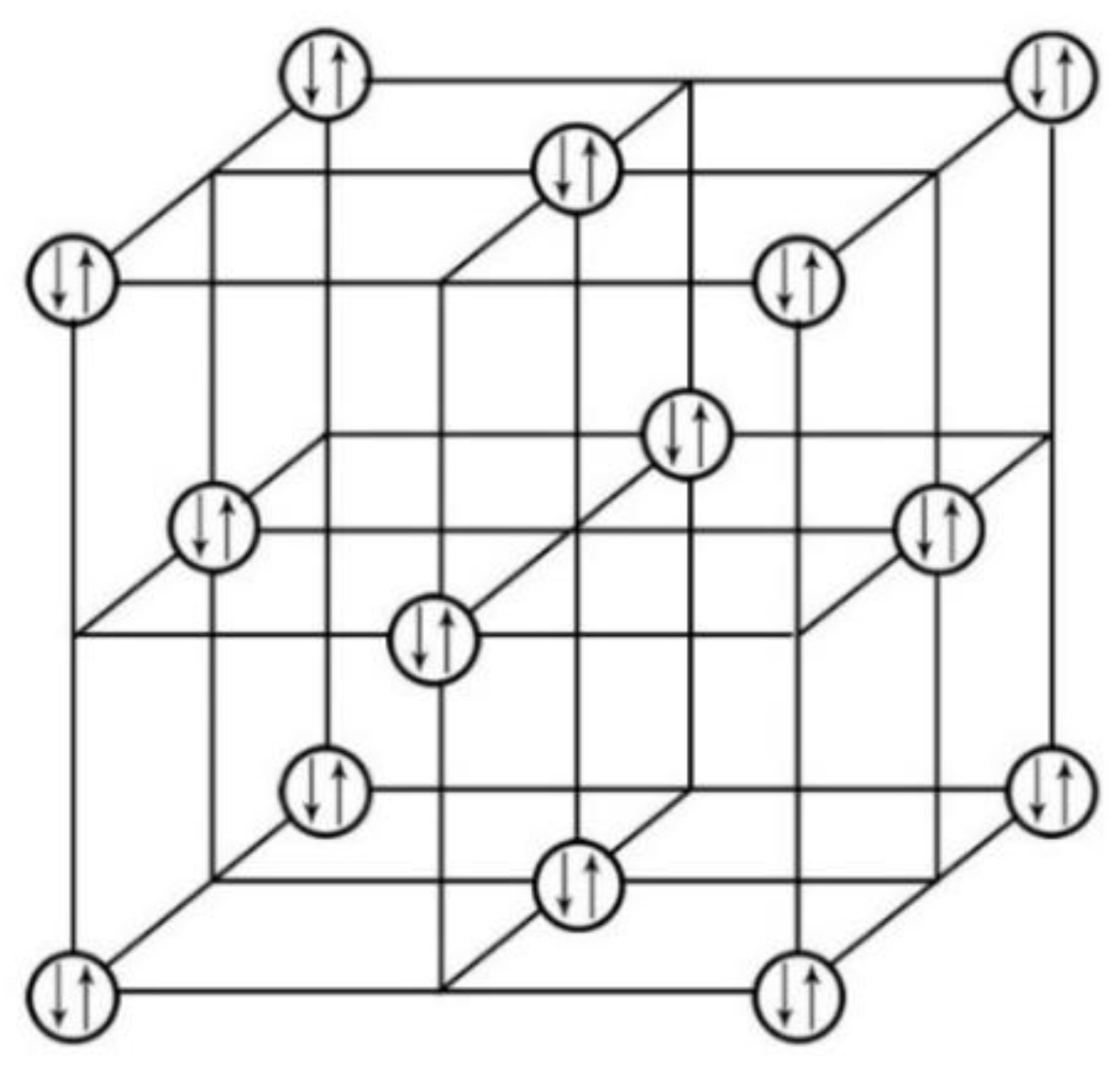
We did similar plotting for ThS by using the same method as for ThI
2 through removing all the elements and keeping only the electron lone pairs on its crystal lattice.
Figure 7 displays the layout of the ThS’s electron lone pairs on its crystal lattice [
31].
Utilizing the same method as above for ThI
2, the electron lone pairs in the network were plotted by displaying only the electron lone pairs in the crystal lattice of ThS through stripping off all the elements in its unit cell. It is clear from
Figure 7 that the electron lone pairs in the crystal lattice of the ThS is oriented in a 3-D manner. Consequently, the 3-D superconducting feature for ThS can be established.
3.2.2. Electron Parings and our New Superconducting Mechanism
We also explored the electron’s pairing nature of these Th compounds as compared to the pairing style in the Cooper pairs. We found that these two pairing styles are not the same. We believe that the pairing in these Th compounds is a “two particle” pure electron-electron pairing in a localized manner while the Cooper’s pairing is a “three particle” electron-electron mediated by a phonon in a manner over a long distance.
Figure 1 clearly demonstrated a uniquely localized and closely correlated electron pairing feature on the Th cation for these Th salts as [Th(e
-)
2]
2+ cation core. This means these two electrons are paired on the same atomic orbital of the same Th cation in a way that they neither exist on two paralleled degenerate atomic orbitals as many ordinary compounds or metals, nor reside far apart separately in a long distance as the pairing of the Cooper pair. We, therefore, concluded that this special electron pairing configuration for these Th compounds is truly unique and totally different from the phonon mediated long distance Cooper style pairing.
Based on the pairing nature we discovered for the Th salts, we developed our description about the superconducting mechanism for this special electron configuration of the Th salts, i.e., a coupled electron pair residing on each of the Th cation [
22,
28]. We named this sort of electron pair as the “Zhao pair” for our mechanism description [
31]. In this mechanism we developed, each “Zhao pair” is located on each metal (Th) cation in a crystallographic lattice arrangement to form a cation network. This network is constructed by many Th cations as shown in
Figure 6 and
Figure 7. We named this cation network as the “Zhao pair network”. The “Zhao pairs”, on the “Zhao pair network”, can interact with each other, “in pair”, because of the special network structure. This “Zhao pair” interaction should lead to the delocalization of the “Zhao pairs”, over the “Zhao pair network”, and thus, the supercurrent would flow through the carriers of the delocalized “Zhao pairs”. When a supercurrent generated under an external force, such as an electrical field, the “Zhao pairs” can hop, “in pairs”, over the “Zhao pair network”, such as over the (0, 0,
) plane, for the [Th
4+(e
-)
2](I
-)
2 in a 2-D anisotropic manner. This means the superconductivity or the supercurrent is stemmed from the delocalized “Zhao pairs” that can move at a long distance, “in pairs”, by external force over its crystallographic lattice arrangement or the “Zhao pair network”. On the contrary, the Cooper pair as described in the BCS theory is a long distance pairing by two individual electrons separated by a space range from 10s of angstroms to nanometer scales [
32,
33]. These two electrons, mediated by a phonon, keep apart in space in a long distance range but migrating in a cooperative manner to carry the supercurrent.
We also noticed that there is a similarity between the “Zhao pair” and the Cooper pair in that both of them allow the presence of the electron pairs in a long distance fashion. The long range presence of the “Zhao pairs” is through the delocalization of the “Zhao pairs” over the “Zhao pair network”. During the flow of the supercurrent, the “Zhao pairs” are always kept intact in a closely paired manner. The presence of the “Zhao pair” in a large range over the “Zhao pair network” is the migration of the “Zhao pairs” or flowing the supercurrent. This means the two electrons in the “Zhao pairs” are neither being separated on their site with its Th cation nor during hopping. Consequently, the migration of the “Zhao pairs” is essentially the replacement of the “Zhao pairs” from one Th cation site to another on the “Zhao pair network” through transferring the “Zhao pairs”, as long as the compound’s superconducting state is maintained. On the other hand, the Cooper pair is also present in a long distance but the two electrons in the Cooper pair are separated in space and can be a long distance apart individually.
3.2.4. Networks for Ordinary Conductor and Superconductor
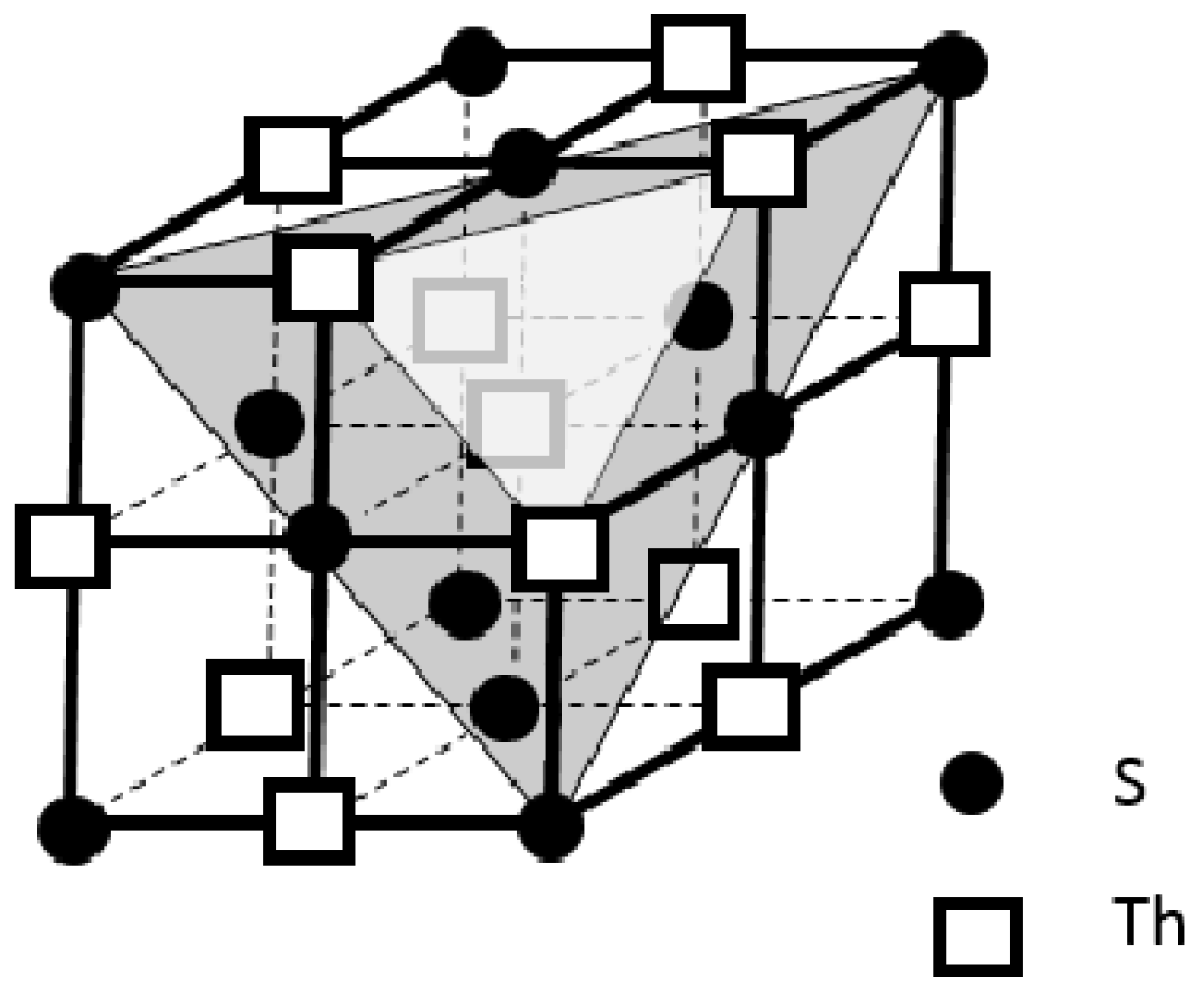
In our superconducting mechanism, the way to construct a superconductor is by putting the “Zhao pairs” in a “Zhao pair network”. Generally speaking, a compound with a “Zhao pair” on its cation, such as [Th
4+(e
-)
2]
2+I
2, can be chemically designed and made through synthetic chemistry. The “Zhao pair network” of this compound can be constructed by crystal growth through stacking its structural unit or structural building block, such as [ThI
6] in
Figure 3a. The periodic nature of crystal structure by the structural building blocks would result in a “Zhao pair network” as sketched in
Figure 6. For other possible superconductors, the synthetic work should encompass an appropriate choice of different elements to compose a molecule with an electron lone pair on the cation. The crystal growth of this compound would reveal the compound’s packing style by its relevant structural building blocks. The structural information of this compound can be obtained by single crystal X-ray diffraction analysis. This structural information can be utilized for subsequent redesign of the compound for optimizing the compound’s superconducting property. At any rate, the only way to turn the electron lone pair on the compound’s cation to the “Zhao pair” is through modulating the compound’s crystal structure. The essential idea of this work is to pack the compound’s structural building blocks in a way to form a “Zhao pair network”, i.e., to boost the electron lone pairs on the cations into the conduction band. The crystal structure of the compound can always be modified, via the changes of compound’s chemical compositions, processing and crystal growth conditions, etc., to achieve the best setting of a “Zhao pair network”. The final superconductor should have its chemical compositions and lattice parameters arranged for the optimized property of superconductivity.
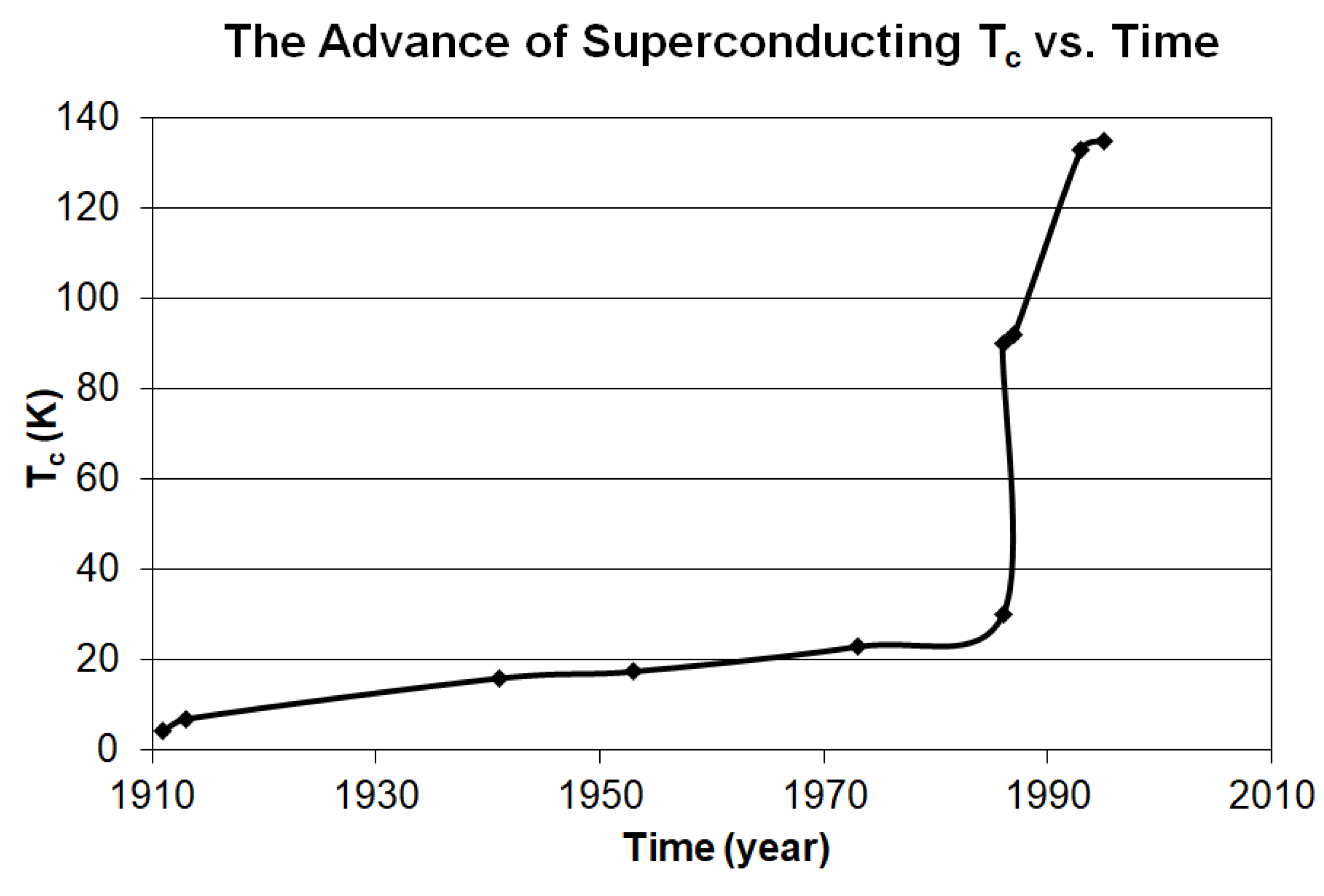
It is noted that our superconducting mechanism does not require a phonon to mediate the 2 electrons into an electron pair like the Cooper pair. This means that our mechanism will not have the 30 K limitation for the superconducting Tc. Consequently, it will not be necessary to limit the search for the new high or room temperature Tc superconductors in the compounds that must contain a light element.
In addition, our superconducting mechanism without phonon mediation may be suitable for explaining the unconventional superconducting phenomenon illustrated by compounds like cuprates.
The mechanism of H2S’s superconductivity is not clear yet. We can attempt to use our novel mechanism to describe it as follows. Under 150 GPa pressure and 203.5 K temperature, H2S is metallized to H3S. We think that at least one hydrogen atom in H3S becomes hydride, H-, which is a hydrogen atom with an electron lone pair on it. During the pressurization/cooling process, the hydrides H-s align into a lattice structure, i.e., the “Zhao pair network”, which enable the electron lone pairs on H-’s to interact from each other. This interaction would boost the electron lone pairs to their conduction band and convert the electron lone pairs into the “Zhao pairs” with delocalization capability. Consequently, H2S becomes a superconductor.
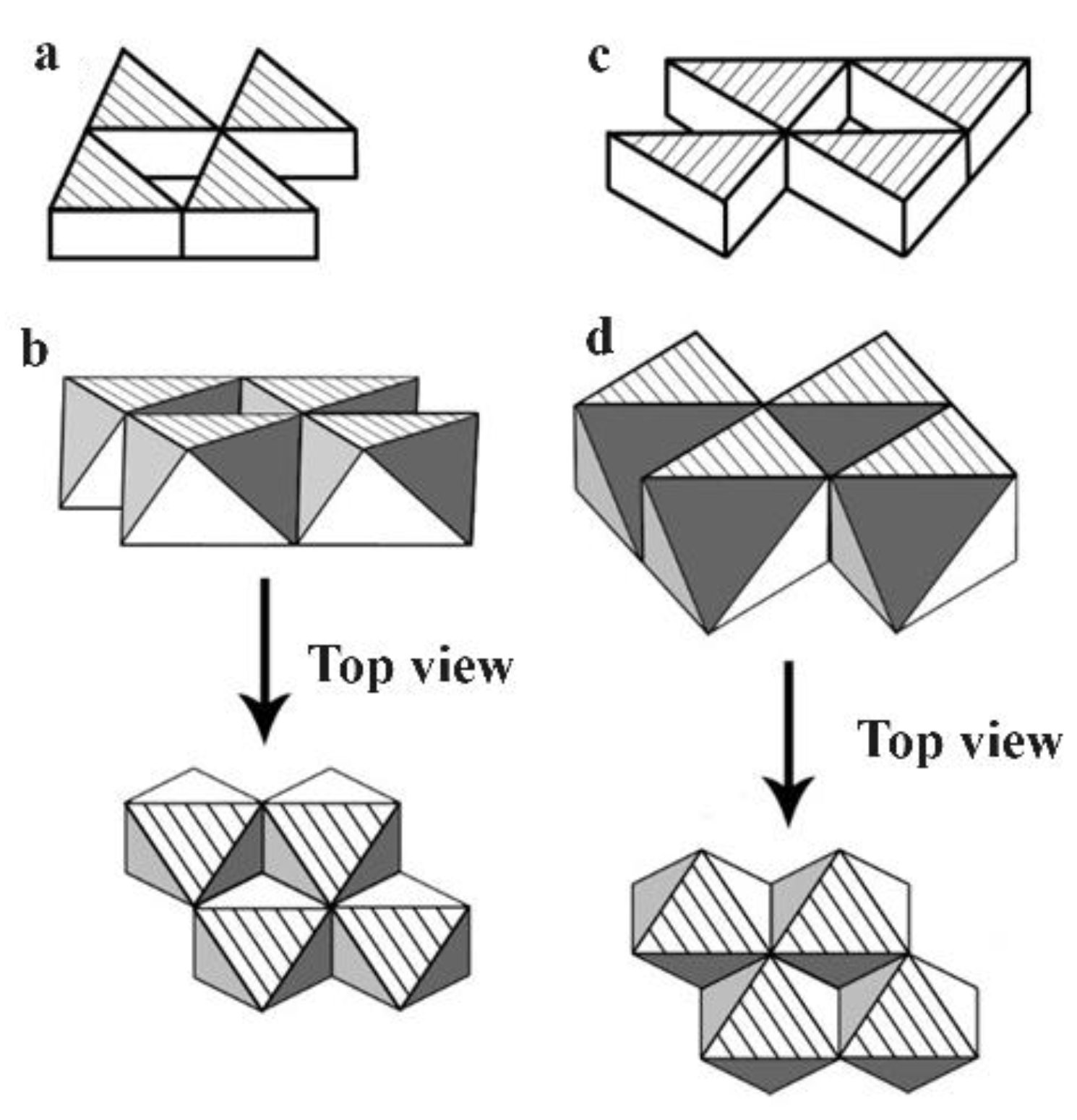
The superconductive mechanism in using the “Zhao pair” flowing over the “Zhao pair network” for supercurrent is very similar to the π electron network of a conductive polymer or a graphene. The difference between the superconductor and the conductive polymer or the graphene with the π electron network is that the superconductor has a network with electron pairs on the conduction band in its network while the network of conductive polymer or graphene is composed by many single π electrons, also on their conduction band, that each carbon atom owns only one of the π electrons.
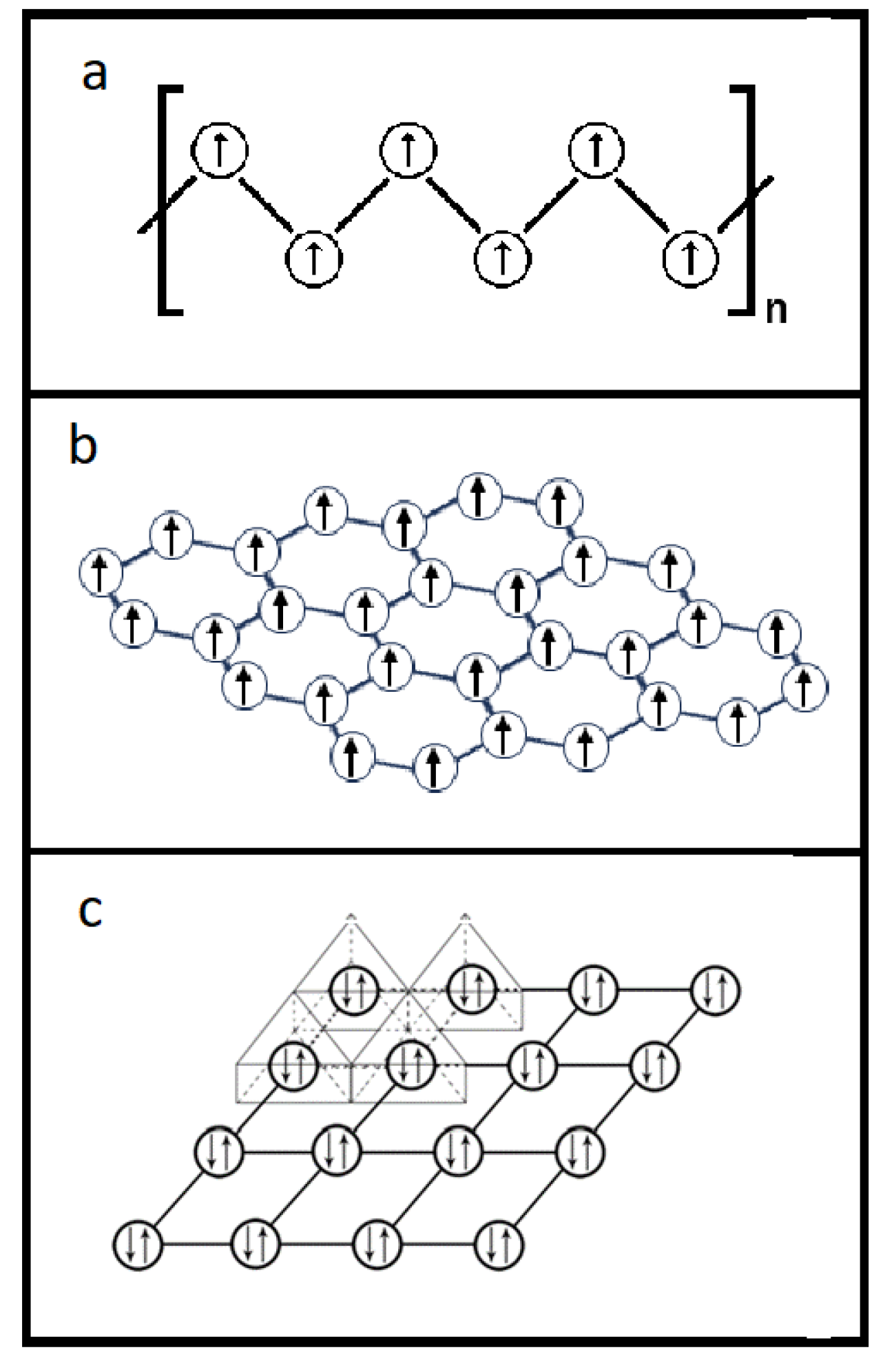
Figure 9 exemplifies a so-called conjugated π bonding system for a conductive polymer where
Figure 9a is the common graphic illustration of the conjugated carbon-carbon double bonds;
Figure 9b demonstrates the real bonding situation that the carbons on the backbone of the conductive polymer are actually linked through a single σ carbon-carbon bond while the nature of the second bond, i.e., the π bond, between two carbons is essentially the delocalized π electrons in the bonding system. This means each carbon atom on the conductive backbone holds one electron equally, other than the style of conjugating in
Figure 9a.
Figure 9c is the replotting of the conductive polymer’s bonding system as in
Figure 9b through removing all the carbon atoms in a manner to display only the delocalized π electrons in the π electron network. The migration of the unpaired π electrons on the π electron network generates the ordinary electrical current.
The π bonding system for graphene can also be drawn in the same way as for the conductive polymer and a 2-D network for conducting ordinary current can be revealed because, again, the π electrons on the network are not paired.
As described above,
Figure 9c sketched the π electron network for a conductive polymer while
Figure 6 and
Figure 7 display the electron pair networks or the “Zhao pair networks”. Again, the difference between the π electron network and the “Zhao pair network” is that the π electron network is formed by single electrons to carry the ordinary current and the “Zhao pair network” uses the “Zhao pairs” to conduct the supercurrent.
Figure 10 demonstrates the similarities and differences of these two types of electron networks by combining the network structures from
Figure 6 and
Figure 9 as well as the graphene’s network. Both networks for the ordinary current and for the supercurrent allow electron delocalization, where the ordinary networks in
Figure 10a and
Figure 10b grant single electron’s delocalization and the supercurrent network in
Figure 10c enables the delocalization of electron pairs.
Though the superconducting mechanism and the superconducting network we have developed looks pretty suited to be applied to explain the superconductivity of Th compounds, we anticipate that this mechanism about the “Zhao pairs” and the “Zhao pair network” can also be utilized to describe the superconducting phenomenon of other superconductive materials including both conventional and unconventional cases.