Introduction
The newly claimed room temperature superconducting material, LK-99, revived the century’s hope for a superconductor to surpass the major milestone of superconducting transition (critical) temperature T
c over 298 K of room temperature [
1]. Unlike light element superconductors [
2], LK-99 is reported that it does not need an extraordinarily high pressure to maintain its superconducting state and thereby, meets the requirement for many applications. The whole world applauded this milestone achievement and celebrated the major landmark result in superconducting research history because it is a great accomplishment since the discovery of the superconducting phenomenon in 1911 [
3]. However, further study on the LK-99′s superconductivity sank this hope shortly after, and verified that the LK-99 is not a room temperature superconductor but rather an insulator [
4].
Even though several previous studies on successfully accomplishing high temperature superconductivity with the T
c of near or over the room temperature were confirmed on certain metal hydrides, especially the lanthanum hydride LaH
10, these approaches of their high T
c superconductivities required a high external pressure to metallize the relevant compounds [
5,
6]. In addition, the T
c of the dense metallic hydrogen was expected to exceed room temperature as predicted by Richardson [
7]. However, metallization of hydrogen needs an extremely high pressure of 500 GPa [
8]. This prerequisite condition of exerting high external pressure on the hydrides or the hydrogen to achieve these high T
c superconductivities prevents this sort of superconductors from being utilized for actual application and hence, this method of creating room temperature superconductors is not a desirable approach.
A major revolution in superconducting research history occurred in 1986 when the Ba-La-Cu-O system demonstrated an unusual high T
c [
9]. This discovery flagged the way of exploring high temperature superconductors in the cuprate system. Among these cuprate superconductors, HgBa
2Ca
2Cu
3O
8-δ revealed a T
c of 135 K at atmosphere pressure [
10]. Up to now this 135 K achievement is still recognized as the highest superconducting T
c under ambient pressure. However, it is still 163 K below the room temperature mark. More than three decades of search, research and synthesis after this work did not surpass this 135 K landmark, despite the fact that an inapplicable method of pressurizing this compound superconductor under 23.5 GPa boosted its T
c onset to about 155 K [
11]. Notice that an external radiation applying to the cuprate YBa
2Cu
3O
6.5 could induce room temperature superconductivity [
12,
13], but this superconducting state is only a metastable status with very short lifetime in picoseconds. Therefore, it is obvious that the method to achieve high T
c superconductivity through an external energy, such as pressure or radiation, is not technically appropriate.
More findings on other metal compounds, such as the iron based superconductor [
14] and the recently discovered nickelate superconductor [
15], were falling behind the T
c of the cuprate’s 135 K mark. A new report illustrated a relatively high T
c of 80 K for a superconducting nickelate La
3Ni
2O
7. Besides its relatively low T
c compared to the cuprate’s record, this accomplishment was also made through employing an external pressure of over 14 GPa on its single crystals [
16]. Consequently, a new search strategy is thought to be necessary to outshine the T
c of the cuprate’s 135 K mark and hopefully, this novel strategy can unearth certain materials in a manner to accomplish or surpass the superconducting T
c of 135 K milestone while a new superconducting mechanism can be developed to guide the research of future superconductors.
As an attempt to realize the dream of obtaining applicable and high temperature superconductors under atmosphere pressure, we used a different approach in our search effort. Here we would like to demonstrate our discoveries on certain thorium (Th) salts that, we believe, have undoubtedly exhibited their superconducting properties with high or room temperature Tcs.
Search Method and Results
The motivation of this searching work is stemmed from the lessons learned on the discovery of the superconductivity of the magnesium diboride (MgB
2). This is because when the MgB
2 was synthesized and structurally analyzed in 1954 [
17], no one noticed about its superconducting property till Nagamatsu repurposed it in 2001 [
18]. A similar example is the discovery of the superconductivity of La
3Ni
2O
7, which is 30 years after its synthesis [
19]. These unexpected long delays of discoveries are probably because the way to explore and confirm a superconductor involves a lot of effort along with relying on many special equipment and experimental conditions, and so on. These requirements greatly hindered the exploratory work on finding a new superconductor because it is hard to examine each compound at the time of its synthesis or discovery. Unfortunately, the mechanism for a material to turn its state from ordinary to superconducting still remains mysterious. Therefore, there is no theory to direct the prediction on which compound may hold superconducting property to date. This fact makes the exploration efforts on synthesizing a new superconducting material extremely hard. Consequently, the current work on searching for a new high T
c superconductor, such as the T
c of room temperature or even higher, is almost directionless.
Based on the current situation with no search direction through applying a mature theory, we decided to put several common properties of superconductors together into a search criterion and apply this criterion to our search efforts towards the previously synthesized compounds. The purpose of this search work is to catch the possible compounds with superconducting properties at high or room temperature that might have been ignored at the time of being made and characterized. We also hoped that a new superconducting mechanism can thus be emerged through this search effort.
We perceived that the transition for a material to turn its states from ordinary to superconducting is accompanied with the electron pairing under a sort of condition or external force, for instance, cooling or pressurizing, etc. This electron pairing needs to be in a crystallographic lattice arrangement that allows the paired electrons to flow on it in order to form the supercurrent. This means a superconductor should meet the following three important conditions that:
Electrons should be paired.
These paired elections should be on the material’s conduction band for superconductivity.
The host material should provide a relevant packing environment or a crystallographic lattice arrangement for these electron pairs on the conduction band to flow over this lattice arrangement of the material.
Two common experiments utilized for qualifying a material’s superconducting state and determining its T
c are to monitor the big drops of its electrical resistance and magnetic susceptibility upon cooling [
20,
21]. For a superconductor, its electrical resistance should suddenly decline toward zero when the temperature, in its cooling process, reaches its T
c while its magnetic susceptibility should start to turn from positive to negative. The polarity change of the material’s magnetic susceptibility represents the transition of the material from paramagnetic property to diamagnetism at T
c. This special nature of a superconductor means that if a material is at its superconducting state upon cooling to the temperature below its T
c, it should behave with high electrical conductivity, probably with a small resistance from the measurement system, and diamagnetism. Consequently, this coexistence of the material’s high electrical conductivity and diamagnetism became our search criterion for superconductors. In other words, our search effort is focusing on those materials that exhibit the coexistence of high electrical conductivity and diamagnetism at high or room temperature and ambient pressure.
Through our intensively searching efforts in using the above mentioned criterion, we uncovered a group of compounds that may have superconductivity at high or room temperatures and atmosphere pressure. This search work resulted in a granted patent recently [
21]. Among these claimed superconducting materials in that work, two Th salts, i.e., the thorium diiodide (ThI
2) and the thorium sulfide (ThS), were discussed in detail. The distinctive electrical and magnetic properties of these two Th compounds as well as their structural features at ambient condition strongly suggested that both ThI
2 and ThS were at their superconducting states at the experimental conditions being studied, apparently under high or room temperatures.
Detailed Description of the Th Salts
Most works on exploring the Th chemistry are concentrated in the time period before the 1970s. This means all the reported Th compounds are considered relatively old and received little or no attention for over fifty years. However, during the past fifty years, the understanding of superconductivity progressed extraordinarily, particularly after the revolutionary discovery of the high temperature compound superconductivity in 1986 [
9]. By the 1980s, the samples of the related Th compounds made before the 1970s might no longer be available for the study of their superconductive properties. It should be easy to imagine that this more than a decade gap between the synthetic works of the Th compounds and the discovery of the high T
c compound superconductors stripped the opportunity for these Th salts off being investigated about their properties of high or room temperature superconductivities.
As described above, these two conductive Th salts, ThI
2 and ThS, were synthesized a long time ago along with their analyses and characterizations [
22,
23,
24,
25,
26,
27]. The unusually high electrical conductivity and diamagnetism of these two salts were reported. Noticed that these electrical and magnetic properties or the coexistence of high electrical conductivity and diamagnetism are also owned by superconductors. In other words, these Th compounds are in their superconducting states at that experiment conditions. Surprisingly, that conditions were at ambient. Consequently, the high or room temperature superconductivity for these two Th compounds under atmosphere can be concluded.
The special diamagnetic property for ThI
2 was further deliberated by Clark [
22] and concluded to a construction with a unique molecular configuration. This configuration granted the ThI
2 a formula of [Th
4+(e
-)
2](I
-)
2. This means the Th’s oxidation state in the ThI
2 is not +2 as usual, but having an exceptionally high value of +4 with a unique electron lone pair (e
-)
2 residing on the Th cation in a way to form an oxidation state +2 cation core of [Th
4+(e
-)
2]
2+. This [Th
4+(e
-)
2]
2+ cation core is coupled with two iodine anions or iodides, i.e., I
-s, to neutralize the whole molecule of ThI
2. In other words, Clark deduced the formula for the whole ThI
2 salts as constructed by [Th
4+(e
-)
2](I
-)
2 to attain the compound’s electrical neutrality. We browsed the chemistry of other elements and found that this type of cation core for a Th cation, coupled with a conductive electron lone pair, i.e., is really unique. We believe that the conductivity of the Th compounds by the electron lone pair (e
-)
2 is the key for the Th salts to behave superconductors.
This distinctive formulation of the [Th
4+(e
-)
2](I
-)
2 along with its ligand coordination style was further confirmed through a single crystal X-ray diffraction work by Guggenberger [
28].
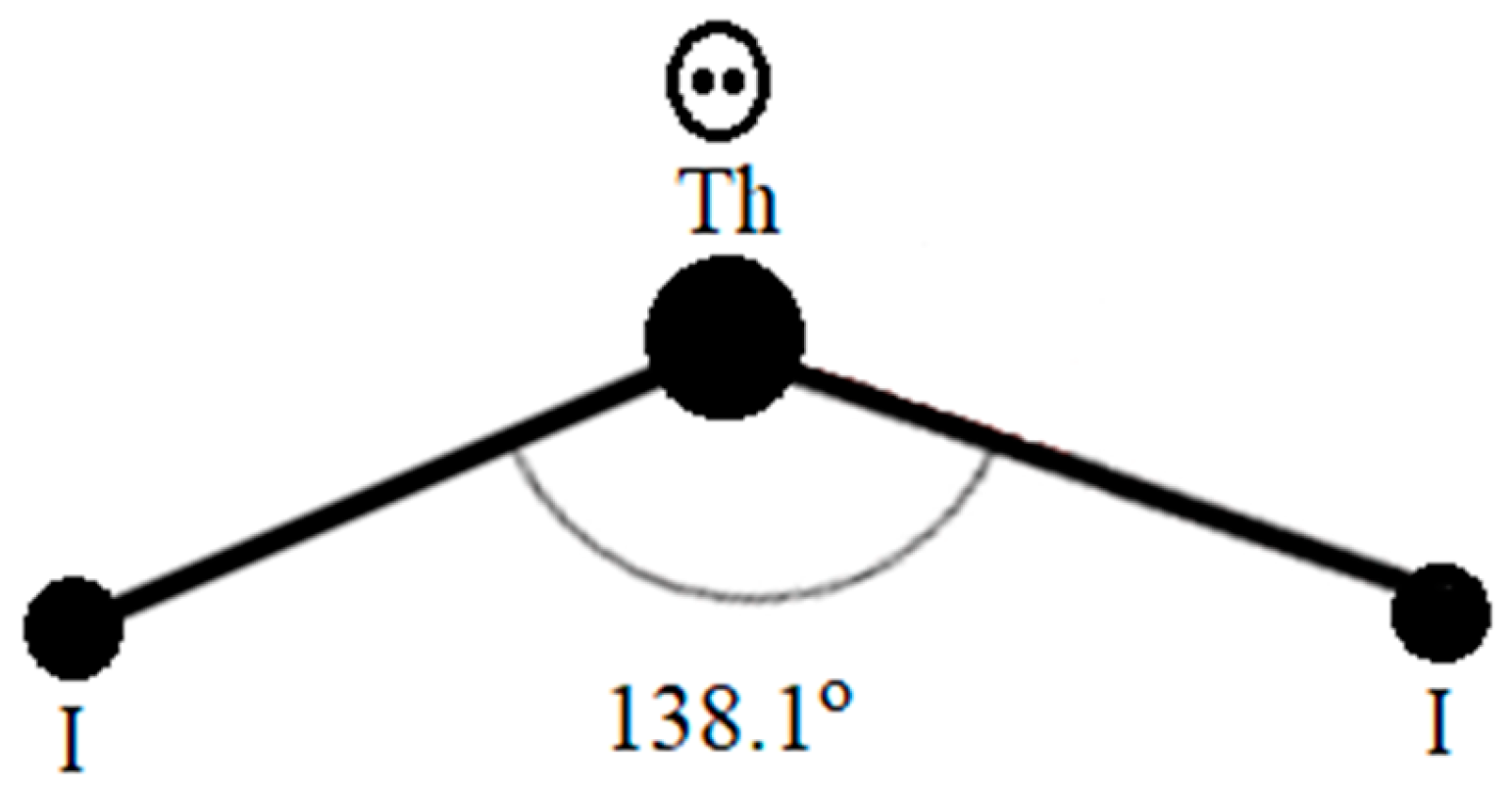
Figure 1 demonstrates the [Th
4+(e
-)
2](I
-)
2′s molecular configuration from this structural analysis.
From the structural information elucidated by Guggenberger [
28], we can tell that the two iodides in [Th
4+(e
-)
2](I
-)
2 are not linearly located around the Th cation as for most bi-coordinated molecules, such as for carbon dioxide (CO
2), calcium chloride (CaCl
2), etc., but are 138.1
o apart. This bent coordination style by ligands occurs to other bi-coordinated molecules only with the presence of electron lone pairs, i.e., (e
-)
2, on the cations or the positively charged atoms, such as water (H
2O), hydrogen sulfide (H
2S), etc. H
2O’s configuration is that the two hydrogen atoms form a 104.5
o angle around the oxygen atom because they are pushed by two electron lone pairs residing on the oxygen atom. Moreover, the two hydrogen atoms in H
2S reveal an angle of only 92.1
o, again, pushed by the two electron lone pairs on the sulfur atom.
It is obvious that the electron lone pairs (e-)2 on the cations of the Th compounds as compared to the electron lone pairs (e-)2 on H2O or H2S are different because the electron lone pairs (e-)2 on the Th compounds are located on the conduction band to carry the supercurrent while electron lone pairs (e-)2 for H2O or H2S are isolated residing on the valence band to make them insulators.
Clark also carried out the necessary measurements and calculations for the ThI
2‘s magnetic susceptibility and concluded that the two electrons on the Th cation of the ThI
2 are indeed paired, other than two individually unpaired electrons residing on two different degenerate atomic orbitals of the Th cation [
22].
With the confirmation of the ThI
2‘s molecular configuration to be [Th
4+(e
-)
2](I
-)
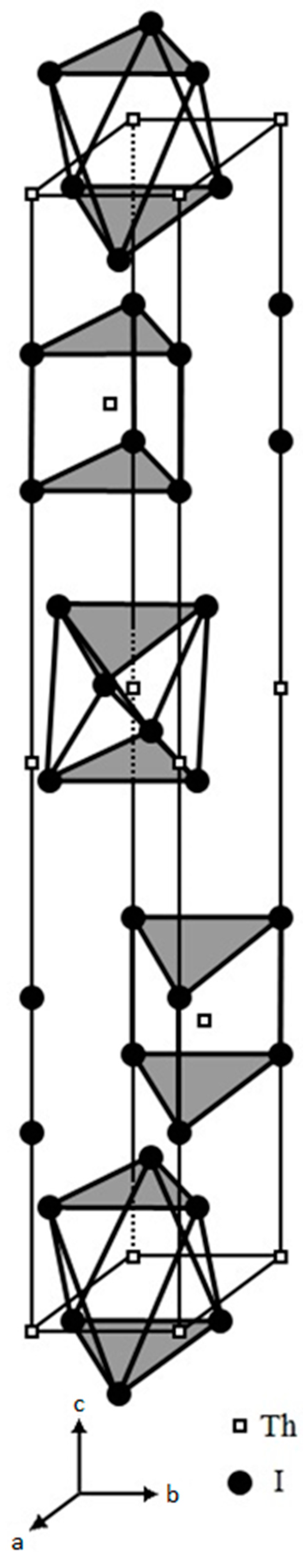
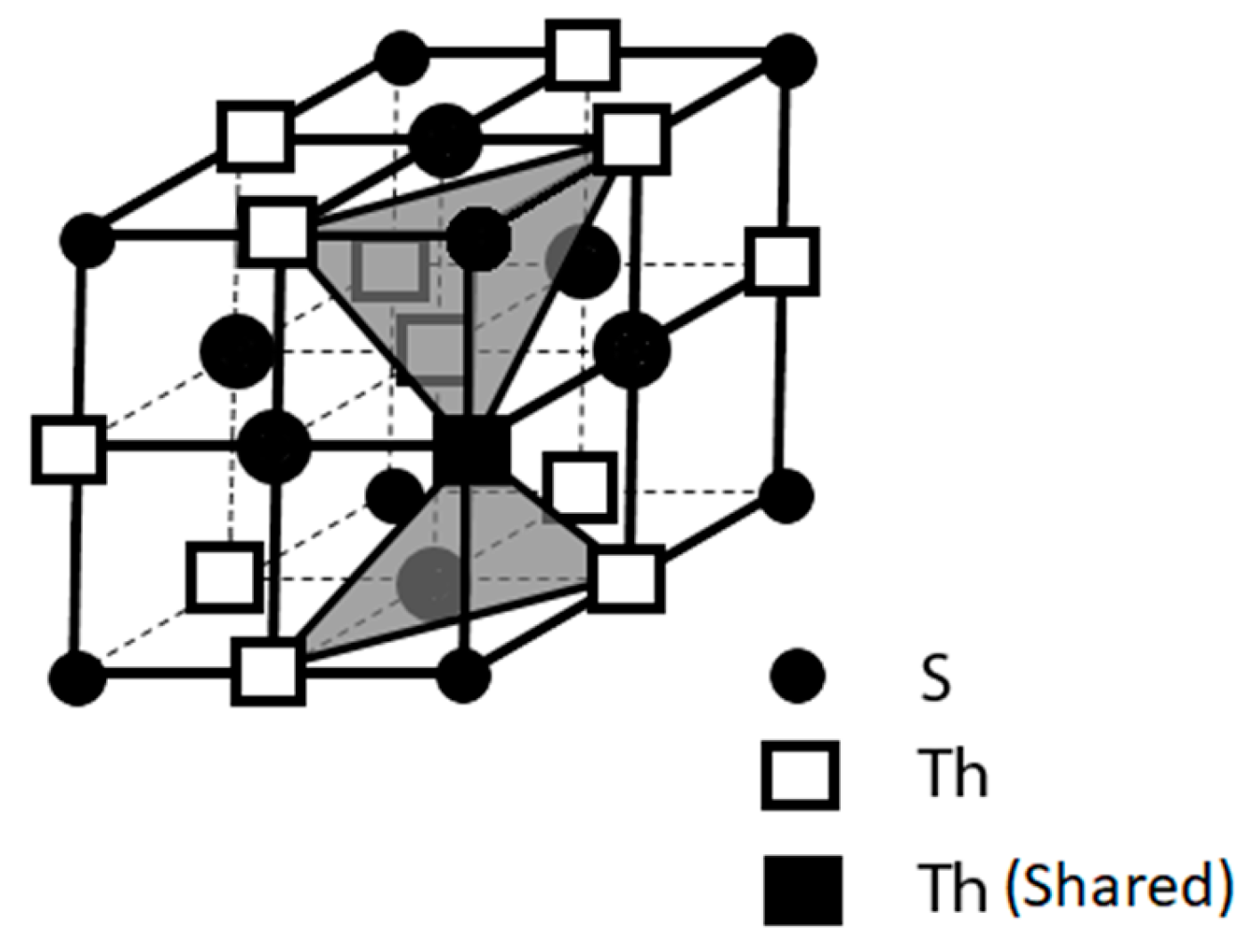
2, we further scrutinized its structural features by re-plotting its unit cell as illustrated in
Figure 2. In this unit cell, two geometries of the structural [ThI
6] units, i.e., the trigonal-antiprismatic and the trigonal-prismatic, are stacked alternatively along its c-axis.
We also expanded the plotting through sketching each of the [ThI
6] structural units in its crystal lattice, either the trigonal-antiprismatic or the trigonal-prismatic, into four crystal unit cells [
21]. This plotting demonstrated a two-dimensional (2-D) edge-sharing of the layered linkage in ThI
2 as shown in
Figure 3. This layer structure ensures the electrical paths for the supercurrent to flow through these layers because electron lone pairs (e
-)
2 on the Th cations are on the conduction band. Additionally, this layered structural feature also appears in many compound superconductors and therefore, further warrants the structural demand for ThI
2 to be a superconductor. Furthermore, this 2-D layered structural feature was believed to grant the [Th
4+(e
-)
2](I
-)
2 as a 2-D superconductor and may be the structural reason to have the 2-D anisotropic conducting property for many other compound superconductors.
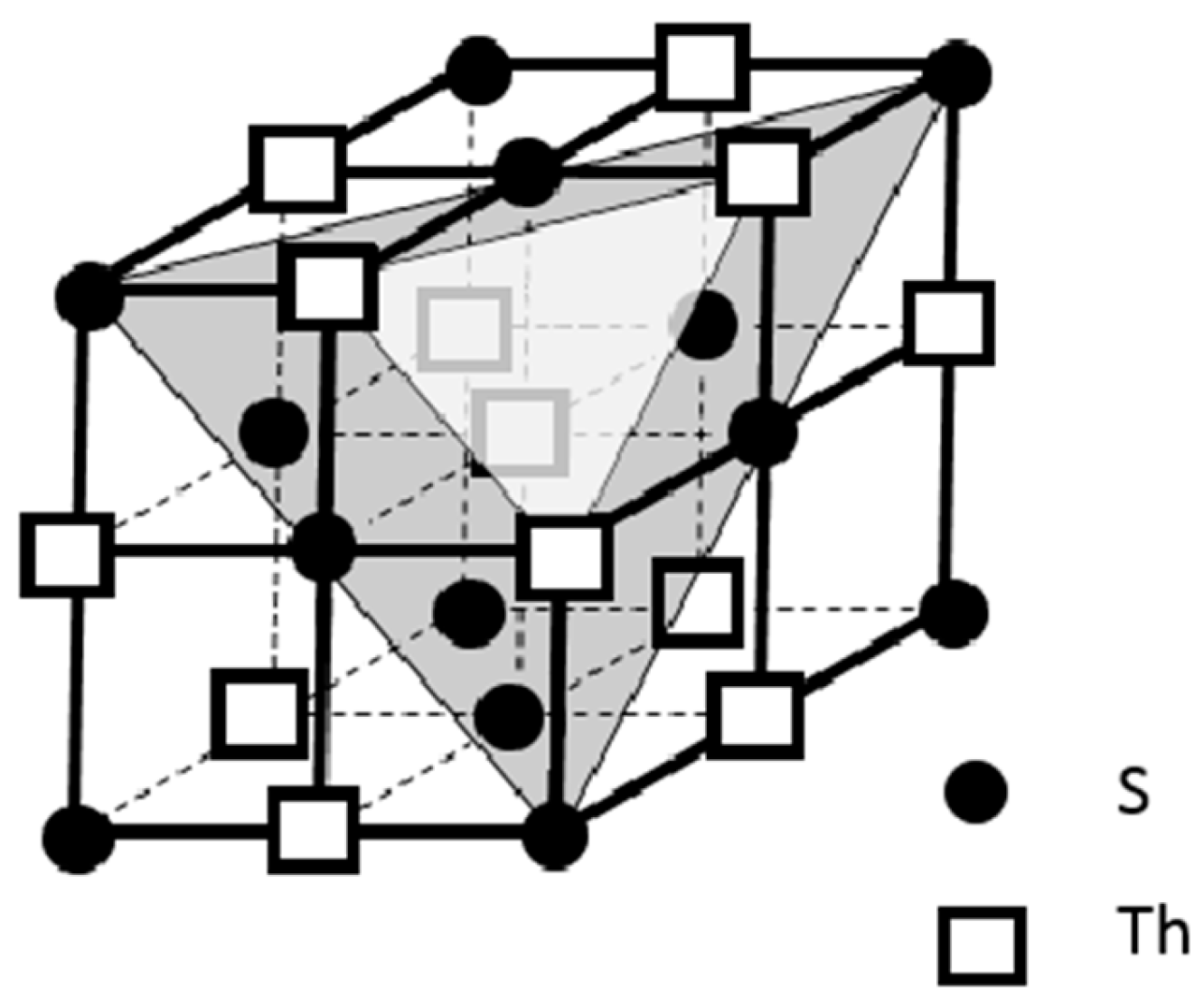
Another successful finding in our search effort for high or room temperature superconductors is ThS because it also demonstrates the property of the coexistence of high electrical conductivity and diamagnetism [
21]. We also studied the ThS’s atomic linkage through replotting its crystal structure, and the layered structural feature is also delineated for the Th cations and the S anions along [111] direction as shown in
Figure 4.
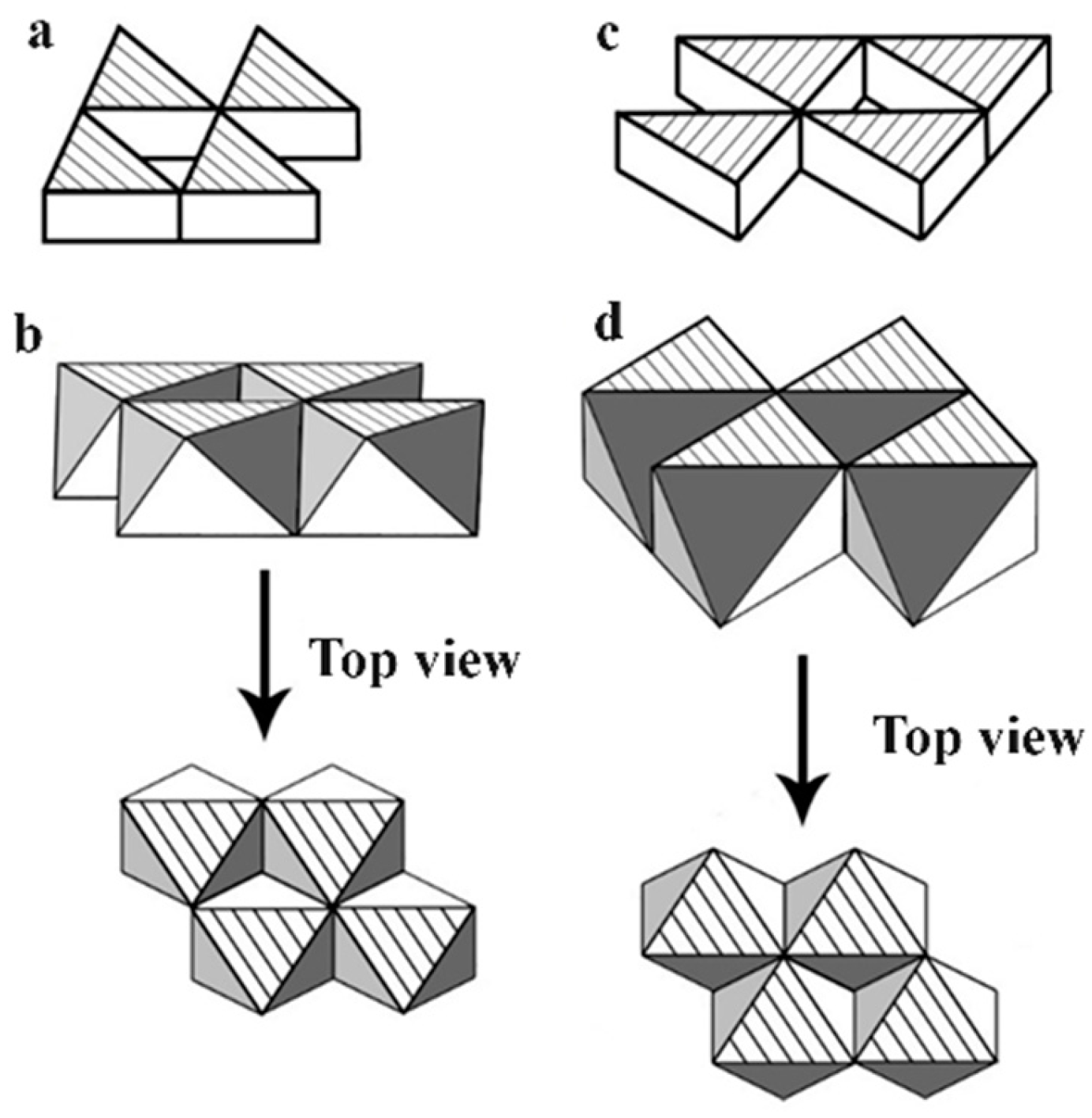
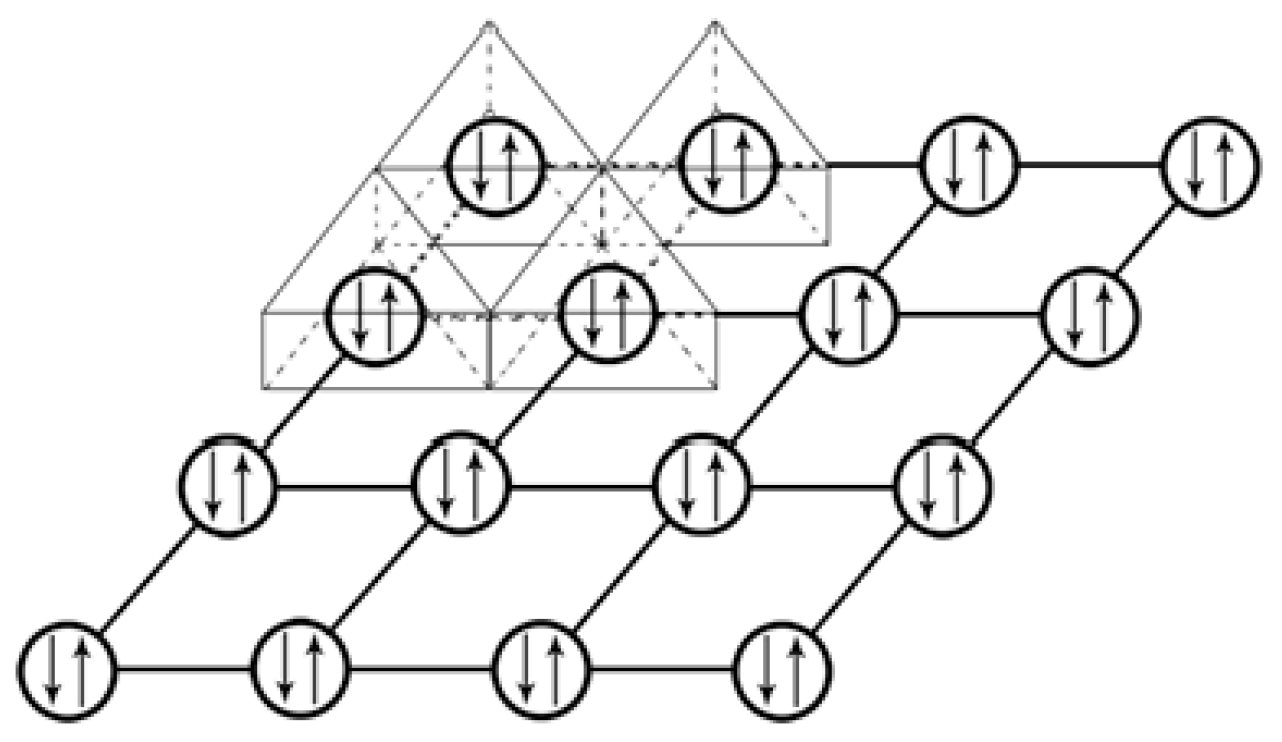
We further explored the linkage of the Th cations in the crystal lattice of ThS as sketched in
Figure 5, in which two small triangles are chosen to present two exemplified Th cation planes. One plane is along [111] direction, i.e., the upper triangle in
Figure 5, and another in [11
] direction, the lower triangle. Notice that a Th cation, indicated as a solid filled rectangle in
Figure 5, is landed on both of these two crystallographic planes to conclude a plane sharing situation of this Th cation by two planes. This plane sharing results in the formation of an intersection between these two planes. It should be easy to envisage that other Th cations in the crystal lattice of ThS should be located in their relative intersections of their {111} planes in the same way as this solid filled rectangle. This means every Th cations in the crystal lattice of ThS should sit along the <111> family to make the Th cations in a 3-D network fashion. Consequently, all the Th cations in
Figure 5 should be marked as solid filled rectangles. In other words, all the Th cations in the unit cell of ThS should be organized in a 3-D manner or a 3-D network.
It is noted that ThS might be an ideal compound superconductor for high power and relatively high current density applications because of the aforementioned 3-D networking structure with its comparatively high packing density by the Th cations. ThS has the sodium chloride (NaCl) crystal structure with the face centered cubic (FCC) packing style. Each Th cation is surrounded by 6 I
-’s in an octahedral manner. As depicted above in
Figure 4, its packing featured that the layers of the Th cations and the S anions are stacked in layer-by-layer manner along its crystallographic <111> family directions. In the meantime, the conductive layers made by the Th cations on the {111} family planes intersect from each other to form an efficient 3-D network of Th cations for electrical conduction and therefore, the flow of the electrical supercurrent on this 3-D network would not be confined in a 2-D anisotropic manner as for ThI
2 and many other compound superconductors.
The discovery of superconductivity for ThS might have been happened 60 years ago when Tetenbaum carried out a study on its electrical property under variable temperatures from nearly 0 K to almost 1000 K in 1964 [
29]. Unfortunately, he did not use the Kelvin connection to lead the sample and thus, concluded that ThS is only a highly conductive (metallic) material, instead of a superconductor. The electrical behavior of ThS under variable temperature in his test probably reflected the metallic behavior of ThS without superconductivity along with the contact resistance between the platinum leads and the ThS sample in his experiment. In addition, the current applied over the ThS sample in Tetenbaum’s experiment was relatively high at 50-100 mA for a sample size with dimensions of several millimeters. Such a high measurement current might exceed the ThS’s superconducting critical current and thus, compromise the ThS’s superconducting state. In other words, under such an experimental condition, the paired electrons on the conduction band of the ThS sample were forced being separated into an unpaired state, i.e., two unpaired electrons. Therefore, the ThS sample in Tetenbaum’s experiment had completely lost its property of superconductivity and been converted into an ordinary metallic material with only the unpaired electrons on its conduction band.
Electron Pairing and Our Novel Superconducting Mechanism
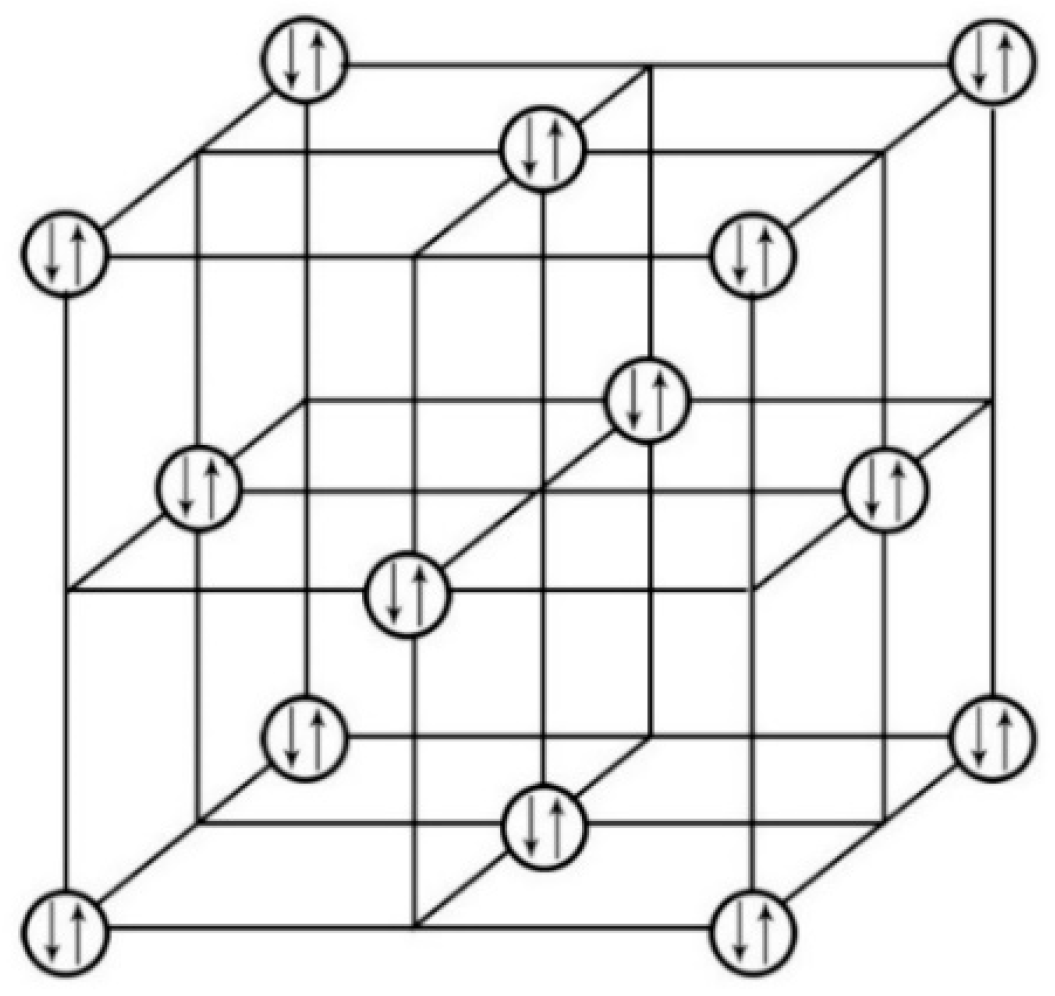
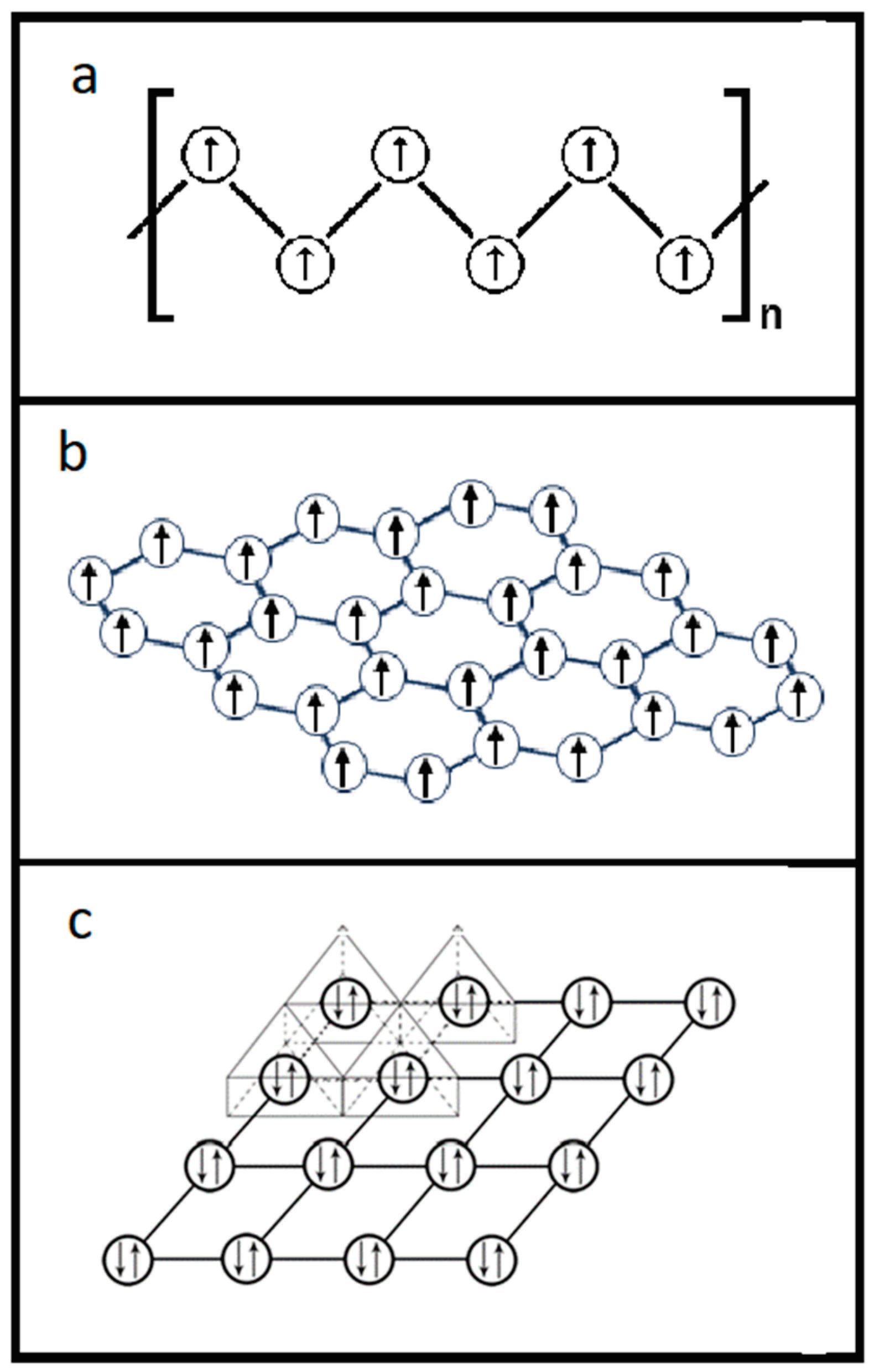
We further investigated the nature of the electron pairings for these two Th salts. We plotted the crystal lattice of [Th
4+(e
-)
2](I
-)
2 by removing all its elements while only displaying the compound’s electron lone pairs in its crystal lattice. We further expanded the plotting to display 16 unit cells.
Figure 6 is the diagram that exhibits the (0, 0,
) plane of the expanded 16 unit cells with only displaying the [Th
4+(e
-)
2](I
-)
2′s electron lone pairs for better visualizing the 2-D relationship of these electron lone pairs [
30].
This network linkage in
Figure 6 offers the interactions among the adjacent electron lone pairs on the crystallographic (0, 0,
) plane. Similar 2-D features of the electron lone pairs can also be plotted for other crystallographic planes of the [Th
4+(e
-)
2](I
-)
2, such as for (0, 0,
), (0, 0,
) and (0, 0, 0) planes, but not included here to avoid redundancy. It is believed that the supercurrent should flow through these planes carried by these electron lone pairs. Consequently, we can anticipate that this 2-D layer structure would enable ThI
2 to only have the 2-D anisotropic superconductivity as many other compound superconductors.
We did similar plotting for ThS by using the same method as for ThI
2 through removing all the elements and keeping only the electron lone pairs on its crystal lattice.
Figure 7 displays the layout of the ThS’s electron lone pairs on its crystal lattice [
31].
Utilizing the same method as above for ThI
2, the electron lone pairs in the network were plotted by displaying only the electron lone pairs in the crystal lattice of ThS through stripping off all the elements in its unit cell. It is clear from
Figure 7 that the electron lone pairs in the crystal lattice of the ThS is oriented in a 3-D manner. Consequently, the 3-D superconducting feature for ThS can be established.
We also explored the electron’s pairing nature of these Th compounds as compared to the pairing style in the Cooper pairs. We found that these two pairing styles are not the same. We believe that the pairing in these Th compounds is a “two particle” pure electron-electron pairing in a localized manner while the Cooper’s pairing is a “three particle” electron-electron mediated by a phonon in a manner over a long distance.
Figure 1 clearly demonstrated a uniquely localized and closely correlated electron pairing feature on the Th cation for these Th salts as [Th(e
-)
2]
2+ cation core. This means these two electrons are paired on the same atomic orbital of the same Th cation in a way that they neither exist on two paralleled degenerate atomic orbitals as many ordinary compounds or metals, nor reside far apart separately in a long distance as the pairing of the Cooper pair. We, therefore, concluded that this special electron pairing configuration for these Th compounds is truly unique and totally different from the phonon mediated long distance Cooper style pairing.
Based on the pairing nature we discovered for the Th salts, we developed our description about the superconducting mechanism for this special electron configuration of the Th salts, i.e., a coupled electron pair residing on each of the Th cation [
22,
28]. We named this sort of electron pair as the “Zhao pair” for our mechanism description [
31]. In this mechanism we developed, each “Zhao pair” is located on each metal (Th) cation in a crystallographic lattice arrangement to form a cation network. This network is constructed by many Th cations as shown in
Figure 6 and
Figure 7. We named this cation network as the “Zhao pair network”. The “Zhao pairs”, on the “Zhao pair network”, can interact with each other because of the special network structure. This “Zhao pair” interaction should lead to the delocalization of the “Zhao pairs”, over the “Zhao pair network”, and thus, the supercurrent would flow through the carriers of the delocalized “Zhao pairs”. When a supercurrent generated under an external force, such as an electrical field, the “Zhao pairs” can hop, “in pairs”, over the “Zhao pair network”, such as over the (0, 0,
) plane, for the [Th
4+(e
-)
2](I
-)
2 in a 2-D anisotropic manner. This means the superconductivity or the supercurrent is stemmed from the delocalized “Zhao pairs” that can move at a long distance, “in pairs”, by external force over its crystallographic lattice arrangement or the “Zhao pair network”. On the contrary, the Cooper pair as described in the BCS theory is a long distance pairing by two individual electrons separated by a space range from 10s of angstroms to nanometer scales [
32,
33]. These two electrons, mediated by a phonon, keep apart in space in a long distance range but migrating in a cooperative manner to carry the supercurrent.
We also noticed that there is a similarity between the “Zhao pair” and the Cooper pair in that both of them allow the presence of the electron pairs in a long distance fashion. The long range presence of the “Zhao pairs” is through the delocalization of the “Zhao pairs” over the “Zhao pair network”. During the flow of the supercurrent, the “Zhao pairs” are always kept intact in a closely paired manner. The presence of the “Zhao pair” in a large range over the “Zhao pair network” is the migration of the “Zhao pairs” or flowing the supercurrent. This means the two electrons in the “Zhao pairs” are neither being separated on their site with its Th cation nor during hopping. Consequently, the migration of the “Zhao pairs” is essentially the replacement of the “Zhao pairs” from one Th cation site to another on the “Zhao pair network” through transferring the “Zhao pairs”, as long as the compound’s superconducting state is maintained. On the other hand, the Cooper pair is also present in a long distance but the two electrons in the Cooper pair are separated in space and can be a long distance apart individually.
It is well known that the BCS theory with the Cooper pairing scheme should be only suitable to be applied to explain the superconducting phenomenon for the conventional superconductors because of its 30 K limitation for T
c and therefore, it is inappropriate to utilize it to the high temperature unconventional superconductors [
34]. Unfortunately, all the attractive high T
c superconductors are unconventional with their T
cs much higher than the 30 K limit.
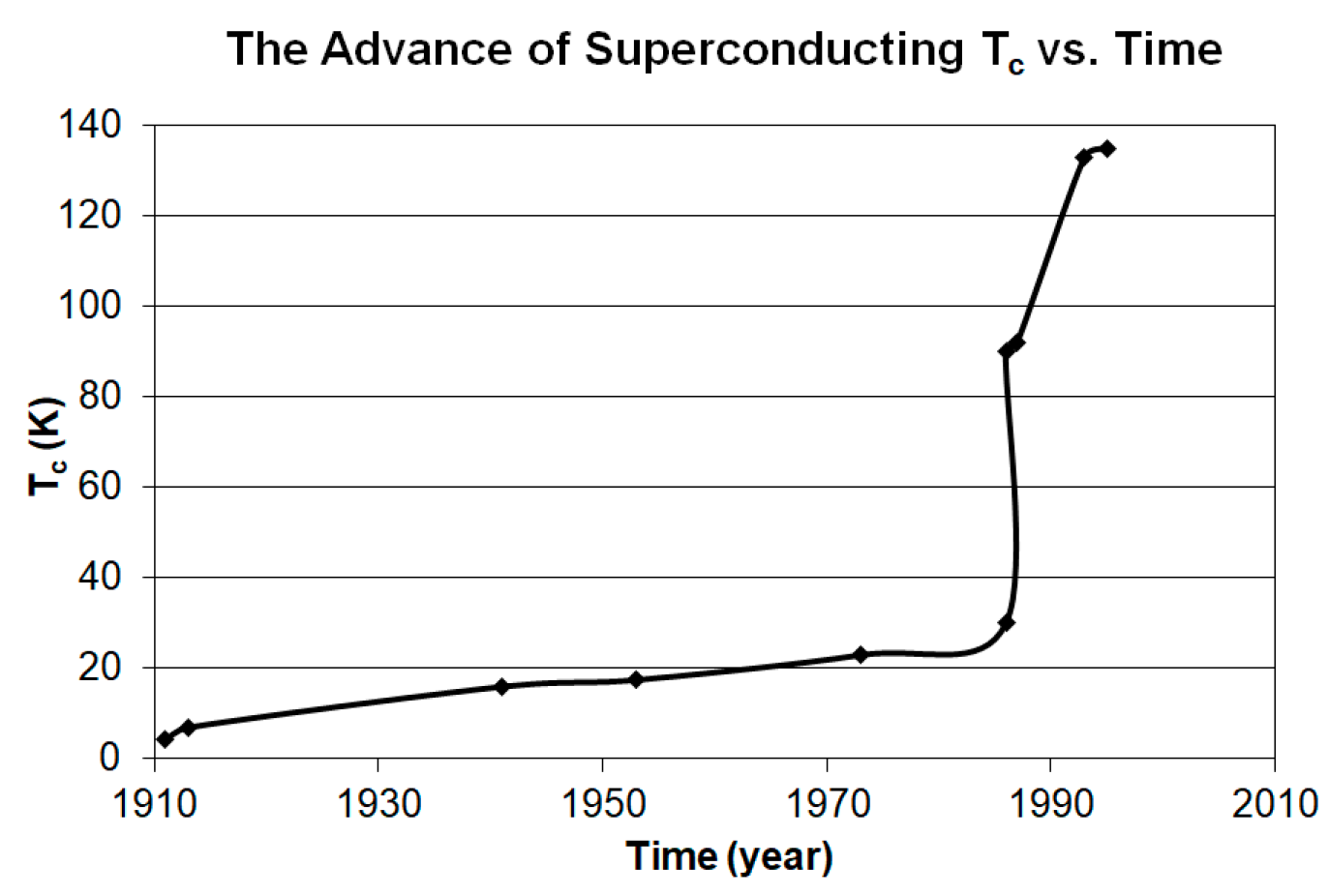
Figure 8 elucidates the history of the advances of superconducting T
c, from which we can tell that all the landmark unconventional superconductors discovered after 1986 have fallen outside of the coverage by the BCS theory. This means there should be an urgent need for a replacement of the BCS theory with a different superconducting mechanism to cover the explanations for the superconducting phenomenon for these high temperature unconventional superconductors.
In our superconducting mechanism, the way to construct a superconductor is by putting the “Zhao pairs” in a “Zhao pair network”, which is a scenario very similar to the π electron network of a conductive polymer or a graphene. The difference between the superconductor and the conductive polymer or the graphene with the π electron network is that the superconductor has a network with electron pairs on the conduction band in its network while the network of conductive polymer or graphene is composed by many single π electrons, also on their conduction band, that each carbon atom owns only one of the π electrons.
Figure 9 exemplifies a so-called conjugated π bonding system for a conductive polymer where
Figure 9a is the common graphic illustration of the conjugated carbon-carbon double bonds;
Figure 9b demonstrates the real bonding situation that the carbons on the backbone of the conductive polymer are actually linked through a single σ carbon-carbon bond while the nature of the second bond, i.e., the π bond, between two carbons is essentially the delocalized π electrons in the bonding system. This means each carbon atom on the conductive backbone holds one electron equally, other than the style of conjugating in
Figure 9a.
Figure 9c is the replotting of the conductive polymer’s bonding system as in
Figure 9b through removing all the carbon atoms in a manner to display only the delocalized π electrons in the π electron network. The migration of the unpaired π electrons on the π electron network generates the ordinary electrical current.
The π bonding system for graphene can also be drawn in the same way as for the conductive polymer and a 2-D network for conducting ordinary current can be revealed because, again, the π electrons on the network are not paired.
As described above,
Figure 9c sketched the π electron network for a conductive polymer while
Figure 6 and
Figure 7 display the electron pair networks or the “Zhao pair networks”. Again, the difference between the π electron network and the “Zhao pair network” is that the π electron network is formed by single electrons to carry the ordinary current and the “Zhao pair network” uses the “Zhao pairs” to conduct the supercurrent.
Figure 10 demonstrates the similarities and differences of these two types of electron networks by combining the network structures from
Figure 6 and
Figure 9 as well as the graphene’s network. Both networks for the ordinary current and for the supercurrent allow electron delocalization, where the ordinary networks in
Figure 10a and
Figure 10b grant single electron’s delocalization and the supercurrent network in
Figure 10c enables the delocalization of electron pairs.
Though the superconducting mechanism we have developed looks pretty suited to be applied to explain the superconductivity of Th compounds, we anticipate that this mechanism about the “Zhao pairs” and the “Zhao pair network” can also be utilized to describe the superconducting phenomenon of other superconductive materials.
Future Work
Future work to re-verify our new discovery of the high or room temperature superconductivity for these Th salts requires the re-synthesis of these old Th compounds, even though the previous experiments had confirmed their nature of superconductivity. Regrettably, because of the radioactive nature of thorium-232 (Th-232) element with its half-life of 14 billion years, the Th related materials such as chemical reagents including metal and metal compounds are regulated worldwide by the government authorities. This means the access to the Th related materials becomes hard because of the regulation. Therefore, the re-confirmation of their high Tc superconductivity through experiments becomes impossible unless the use of the corresponding Th metal and the related Th reagents can be deregulated. Because the radioactive level of the Th-232 is very low and its radiation is through a relatively weak α-decay, it is anticipated that this deregulation would be reasonably safe, especially the deregulation of the Th-232 usage can be applied first for the research purpose. The usage of the Th’s reagents would grant scientists and professionals to obtain necessary starting materials to carry out the re-synthesis of the relevant Th compounds, including ThS and ThI2 aforementioned above.
Because of the refractory properties of these Th compounds, the synthetic work on preparing high purity of the relevant Th salts may be challenging as the works made at the middle of last century revealed about 5% of impurities [
23,
25]. We hope that the new technology utilizing advanced facilities and new procedures of chemical syntheses can ultimately resolve the synthetic problems and obtain much purer Th compounds than those made many years ago. The growth of the single crystals for the Th salts can be attempted, on the purpose to prepare the highly qualified testing samples for the superconducting assessments, such as the growth through utilizing the traveling solvent floating zone (TSFZ) method. Crystallization would not only further purify the materials but also prepare more suitable single crystals for the sample’s research. As an example, the TSFZ was successfully employed for the single crystal growth of the LK-99 samples [
35], which provided the excellent LK-99 crystals for its study. As for the refractory compounds with high melting points such as the Th salts, the relevant crystal growth can be done, for instance, through utilizing either the laser floating zone (LFZ) [
36] or the laser-diode-heated floating zone (LDFZ) [
37,
38] methods. The laser sources in the LFZ or the LDFZ can provide thousands of kilowatt power to furnace in a way to push the temperature of furnace to surpass 2000
o C with ease and therefore, to establish the environment for the crystal growths of these Th salts. The success of synthesizing pure Th salts and growing their single crystalline samples will be a significant step in enabling the research about ThI
2 and ThS as well as other Th salts for their high temperature superconducting properties in the future. It is also hoped that the future work will eventually furnish enough information for fully and scientifically understanding the superconducting property. Theoretically, the understanding of the superconducting phenomenon is of great importance to develop an appropriate superconducting mechanism that not only explains the cause for superconducting but also resolves various problems in the scientific field. Practically, the correct superconducting mechanism would be great helpful for directing the synthetic work on preparing various superconductors and empowering the realization of many applications that need zero electrical resistance, such as for power transmission, energy storage, super computer, maglev transportation system, fusion energy, etc. The reconfirmation of the Th compounds’ high or room temperature superconductivity would be one of the efforts to make the intriguing superconducting candidates for achieving a big advance in superconducting science and technology.