Most works on exploring the Th chemistry are concentrated in the time period before the 1970s. This means all the reported Th compounds are considered relatively old and received little or no attention for over fifty years. However, during the past fifty years, the understanding of superconductivity progressed extraordinarily, particularly after the revolutionary discovery of the high temperature compound superconductivity in 1986 [
9]. By the 1980s, the samples of the related Th compounds made before the 1970s might no longer be available for the study of their superconductive properties. It should be easy to imagine that this more than a decade gap between the synthetic works of the Th compounds and the discovery of the high T
c compound superconductors stripped the opportunity for these Th salts off being investigated about their properties of room temperature superconductivities.
As described above, these two conductive Th salts, ThI
2 and ThS, were synthesized a long time ago along with their analyses and characterizations [
22,
23,
24,
25,
26,
27]. The unusually high electrical conductivity and diamagnetism of these two salts were reported. Noticed that these electrical and magnetic properties or the coexistence of high electrical conductivity and diamagnetism are also owned by superconductors. In other words, these Th compounds are in their superconducting states at that experiment conditions. Surprisingly, that conditions were at ambient. Consequently, the room temperature superconductivity for these two Th compounds under atmosphere can be concluded.
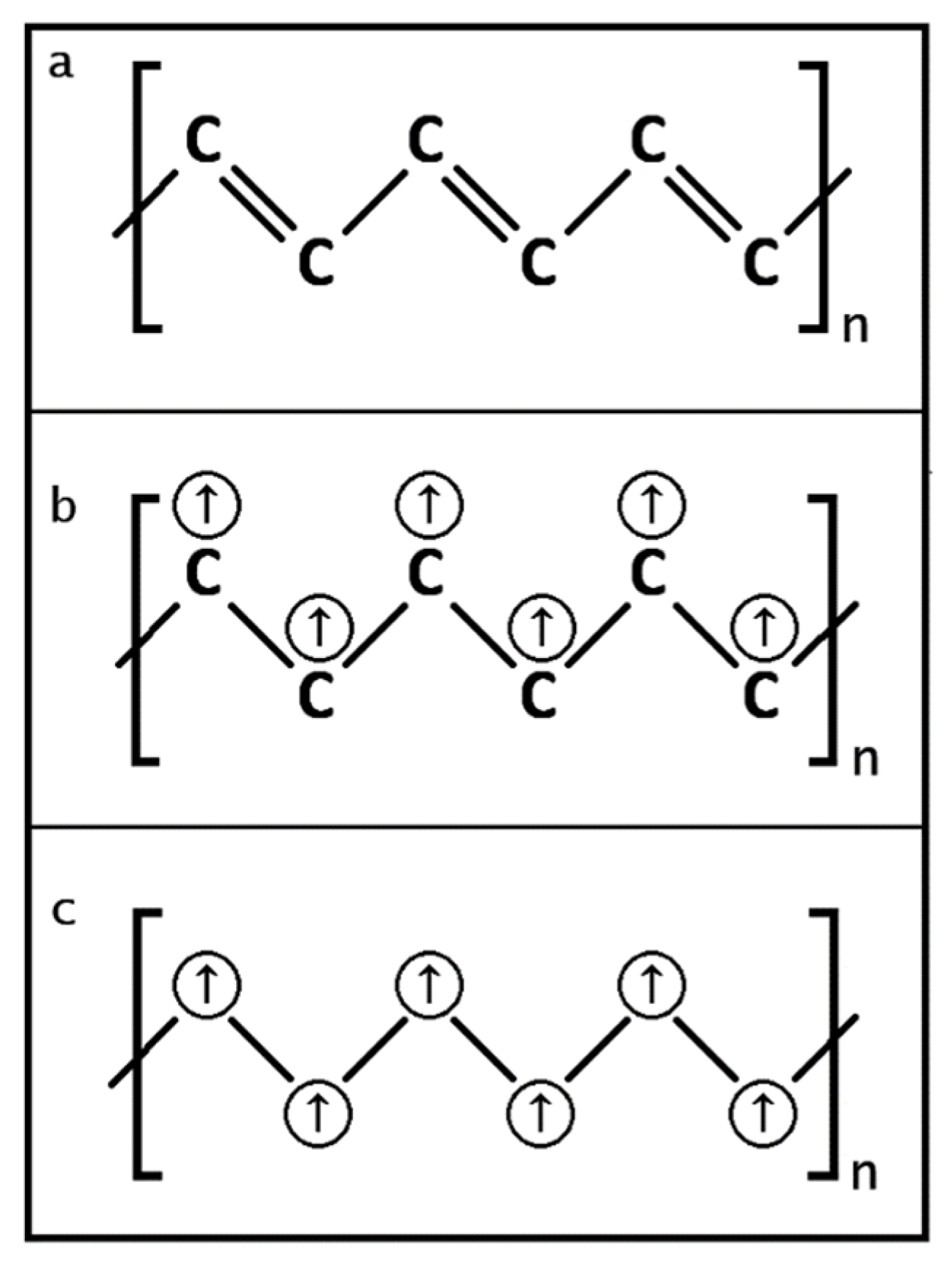
3.1.1. ThI2
The special diamagnetic property for ThI
2 was further deliberated by Clark [
22] and concluded to a construction with a unique molecular configuration. This configuration granted the ThI
2 a formula of [Th
4+(e
-)
2](I
-)
2. This means the Th’s oxidation state in the ThI
2 is not +2 as usual but having an exceptionally high value of +4 with a unique electron lone pair (e
-)
2 residing on the Th cation in a way to form an oxidation state +2 cation core of [Th
4+(e
-)
2]
2+. This [Th
4+(e
-)
2]
2+ cation core is coupled with two iodine anions or iodides, i.e., I
-s, to neutralize the whole molecule of ThI
2. In other words, Clark deduced the formula for the whole ThI
2 salts as constructed by [Th
4+(e
-)
2](I
-)
2 to attain the compound’s electrical neutrality. We browsed the chemistry of other elements and found that this type of cation core for a Th cation, coupled with a conductive electron lone pair, i.e. (e
-)
2, is really unique. We believe that the conductivity of the Th compounds by the electron lone pair (e
-)
2 is the key for the Th salts to behave superconductors.
This distinctive formulation of the [Th
4+(e
-)
2](I
-)
2 along with its ligand coordination style was further confirmed through a single crystal X-ray diffraction work by Guggenberger [
28].
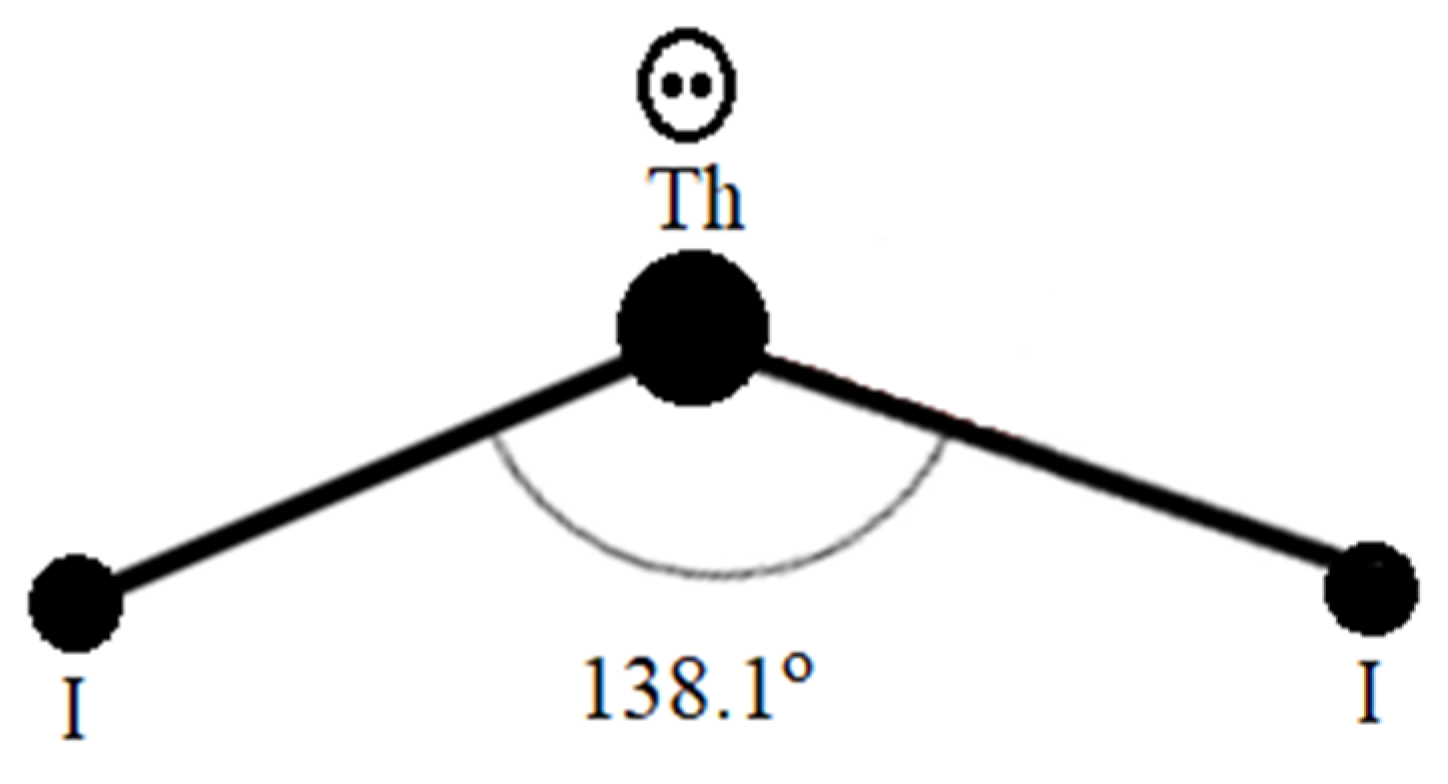
Figure 1 demonstrates the [Th
4+(e
-)
2](I
-)
2′s molecular configuration from this structural analysis.
From the structural information elucidated by Guggenberger [
28], we can tell that the two iodides in [Th
4+(e
-)
2](I
-)
2 are not linearly located around the Th cation as for most bi-coordinated molecules, such as for carbon dioxide (CO
2), calcium chloride (CaCl
2), etc., but are 138.1
o apart. This bent coordination style by ligands occurs to other bi-coordinated molecules only with the presence of electron lone pairs, i.e., (e
-)
2, on the cations or the positively charged atoms, such as water (H
2O), hydrogen sulfide (H
2S), etc. H
2O’s configuration is that the two hydrogen atoms form a 104.5
o angle around the oxygen atom because the hydrogen atoms are pushed by two electron lone pairs residing on the oxygen atom. Moreover, the two hydrogen atoms in H
2S reveal an angle of only 92.1
o, again, pushed by the two electron lone pairs on the sulfur atom.
It is obvious that the electron lone pairs (e-)2 on the cations of the Th compounds as compared to the electron lone pairs (e-)2 on H2O or H2S are different because the electron lone pairs (e-)2 on the Th compounds are located on the conduction band to carry the supercurrent while electron lone pairs (e-)2 for H2O or H2S are isolated residing on the valence band to make them insulators.
Clark also carried out the necessary measurements and calculations for the ThI
2‘s magnetic susceptibility and concluded that the two electrons on the Th cation of the ThI
2 are indeed paired, other than two individually unpaired electrons residing on two different degenerate atomic orbitals of the Th cation [
22].
With the confirmation of the ThI
2‘s molecular configuration to be [Th
4+(e
-)
2](I
-)
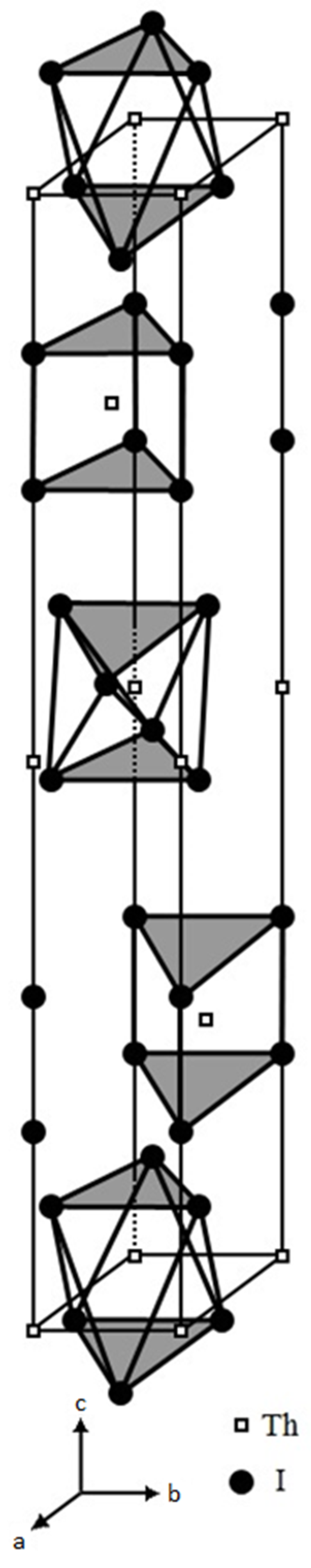
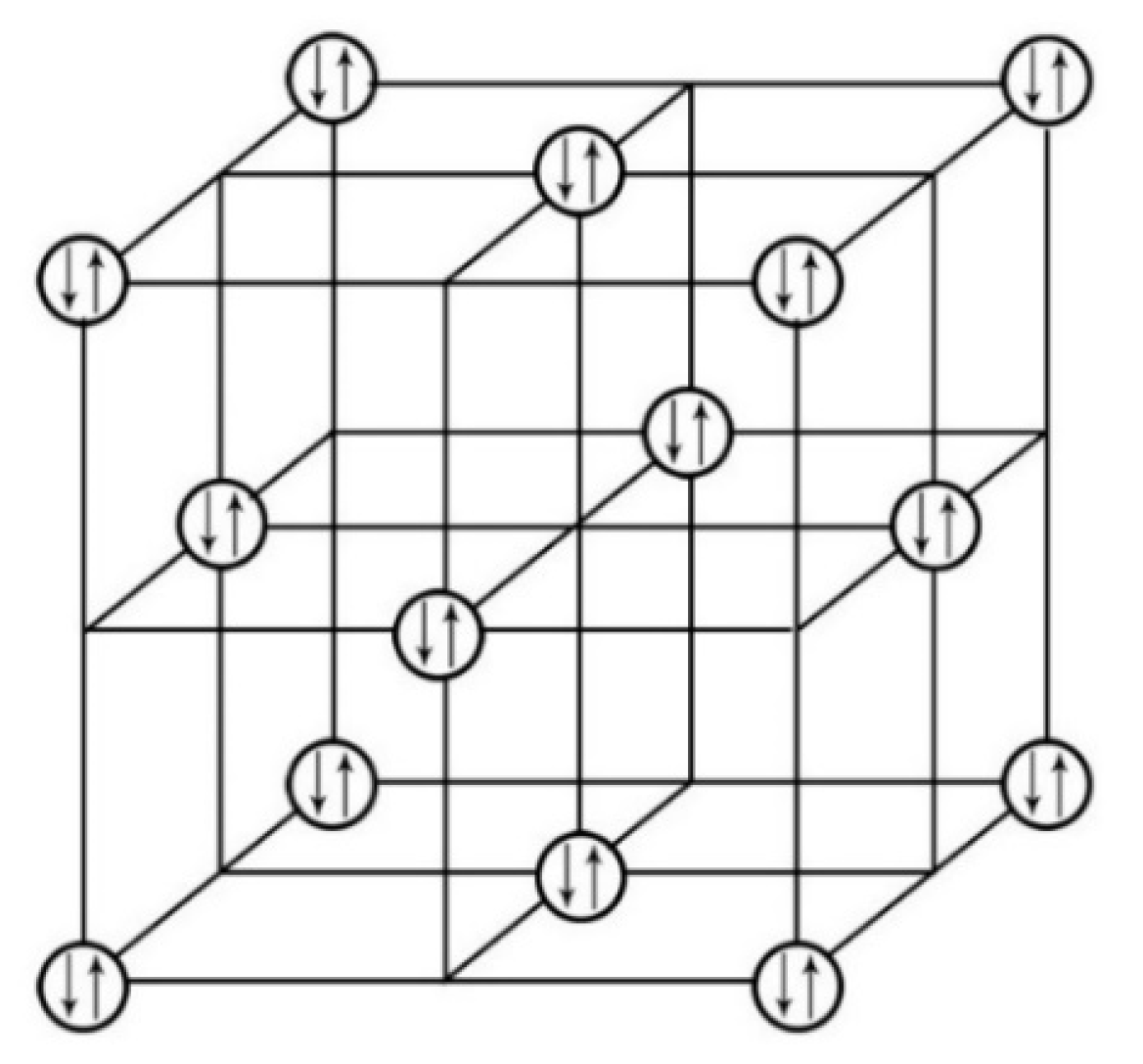
2, we further scrutinized its structural features by replotting its unit cell as illustrated in
Figure 2. In this unit cell, two geometries of the structural [ThI
6] units, i.e., the trigonal-antiprismatic and the trigonal-prismatic, are stacked alternatively along its c-axis.
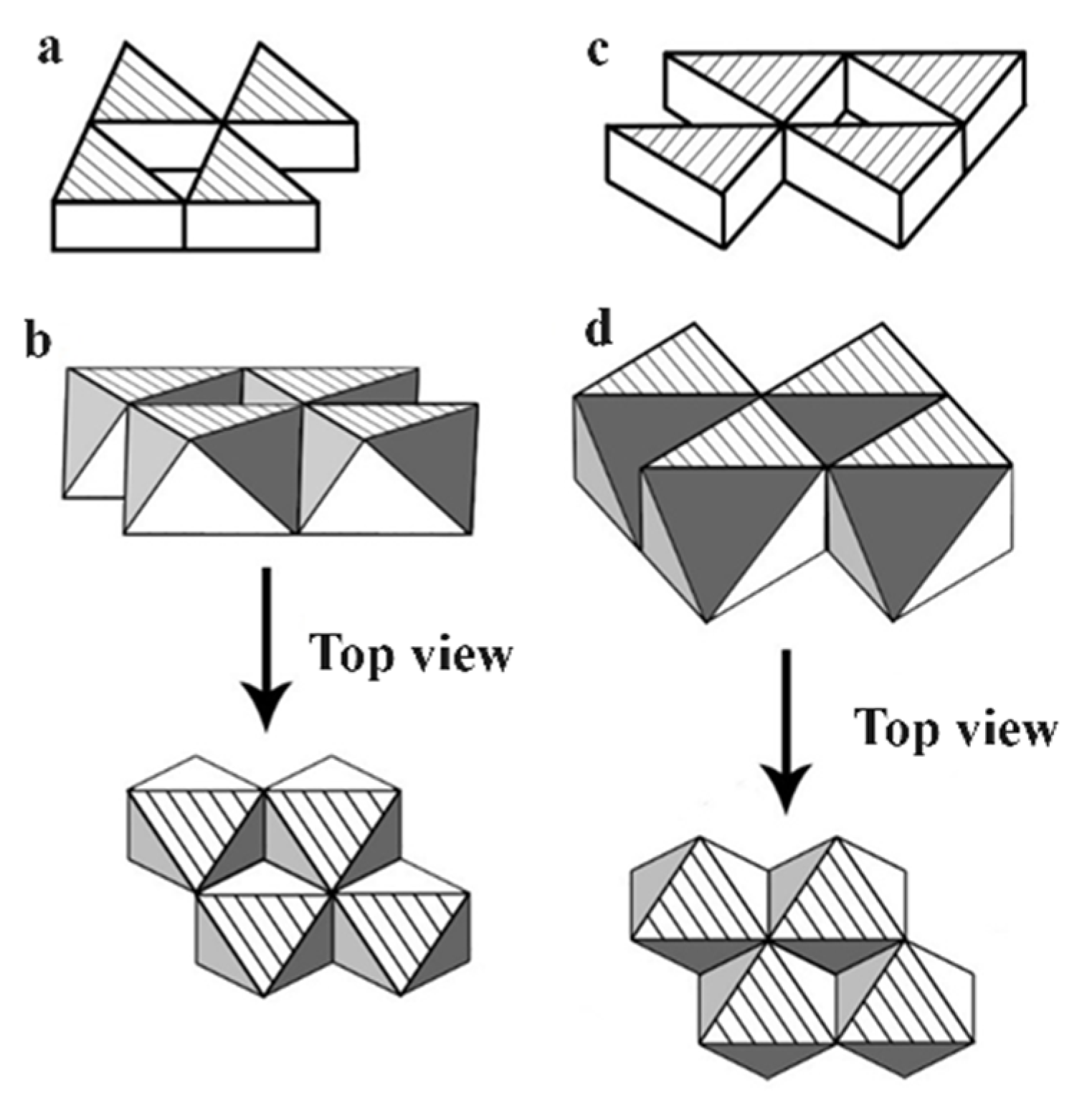
We also expanded the plotting through sketching each of the [ThI
6] structural units in its crystal lattice, either the trigonal-antiprismatic or the trigonal-prismatic, into four crystal unit cells [
21]. This plotting demonstrated a two-dimensional (2-D) edge sharing of the layered linkage in ThI
2 as shown in
Figure 3. This layer structure ensures the electrical paths for the supercurrent to flow through these layers because electron lone pairs (e
-)
2 on the Th cations are on the conduction band. Additionally, this layered structural feature also appears in many compound superconductors and therefore, further warrants the structural demand for ThI
2 to be a superconductor. Furthermore, this 2-D layered structural feature was believed to grant the [Th
4+(e
-)
2](I
-)
2 as a 2-D superconductor and may be the structural reason to have the 2-D anisotropic conducting property for many other compound superconductors.
) plane; (b) The [ThI
6] on (0, 0,
) plane; (c) The [ThI
6] on (0, 0,
) plane and (d) The [ThI
6] on (0, 0, 0) plane. The four planes shown in 4a-4d all illustrated the layered edge sharing property of the [ThI
6] units. The connections in
Figure 3a and 3c are easy to be seen and only the side views are given while the extra top views in 3b and 3d are included for better visualizing the edge sharing features of the 4-cell connections of the [ThI
6] units.
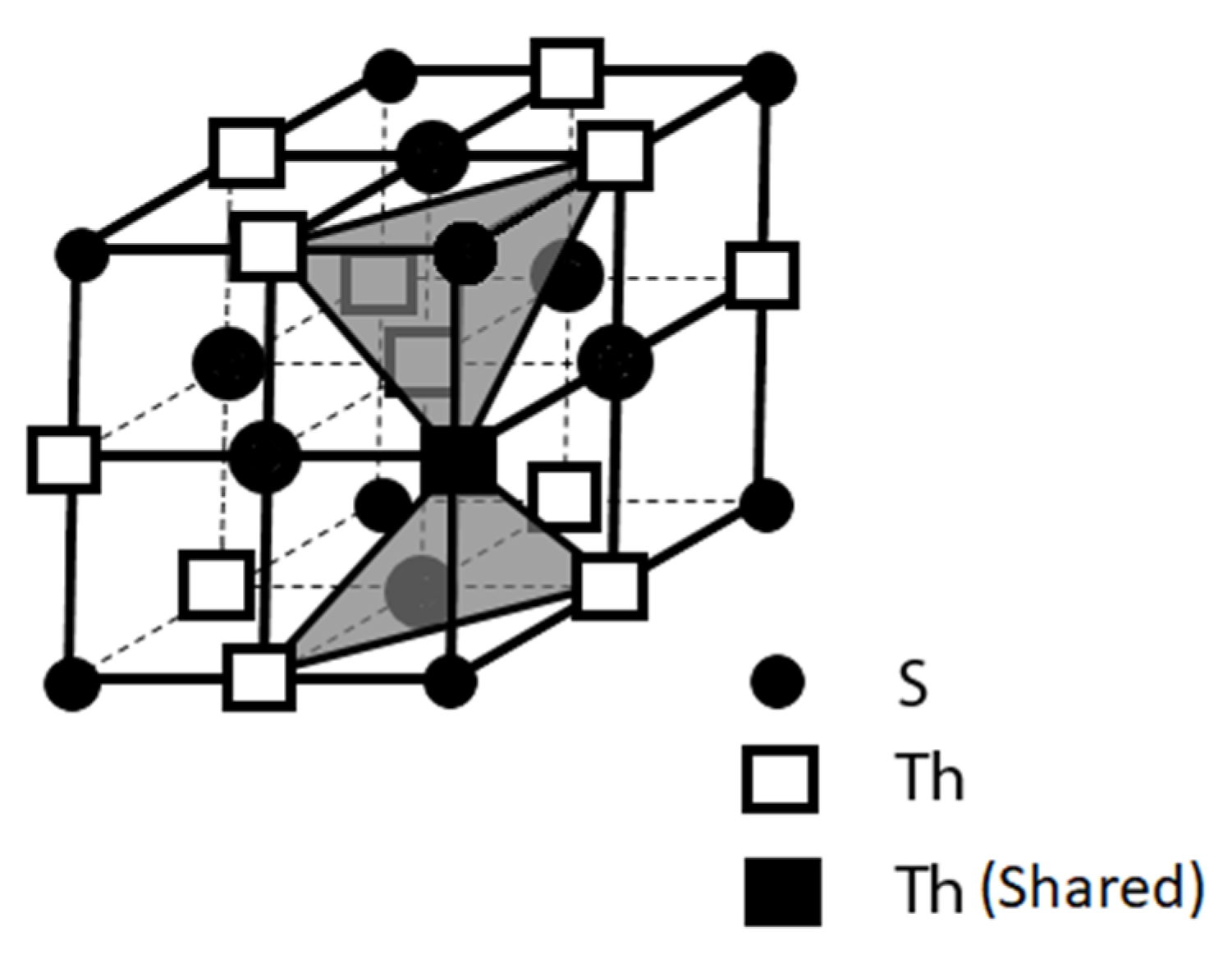
3.1.2. ThS
Another successful finding in our search effort for room temperature superconductors is ThS because it also demonstrates the property of the coexistence of high electrical conductivity and diamagnetism [
21]. We also studied the ThS’s atomic linkage through replotting its crystal structure, and the layered structural feature is also delineated for the Th cations and the S anions along [111] direction as shown in
Figure 4.
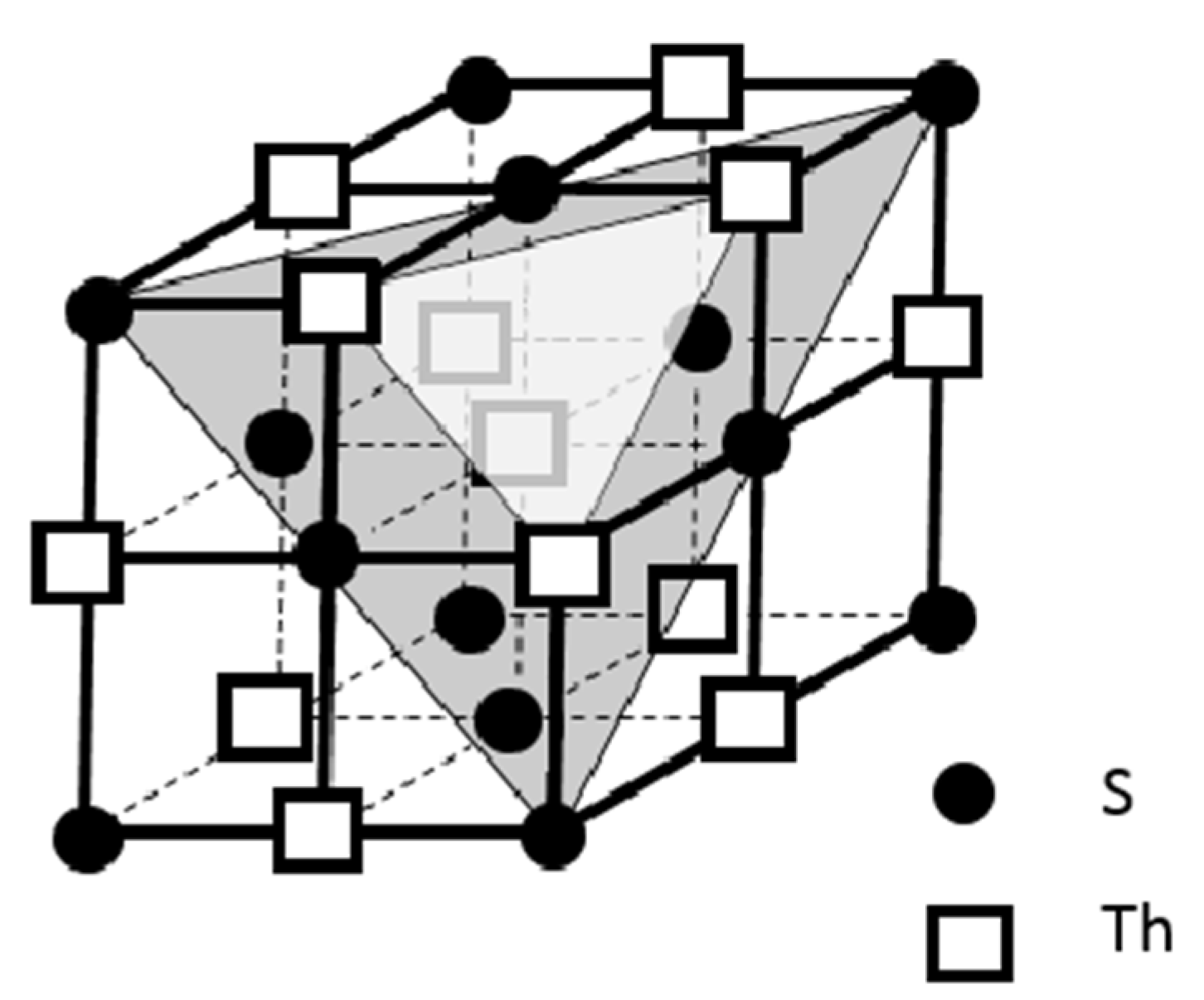
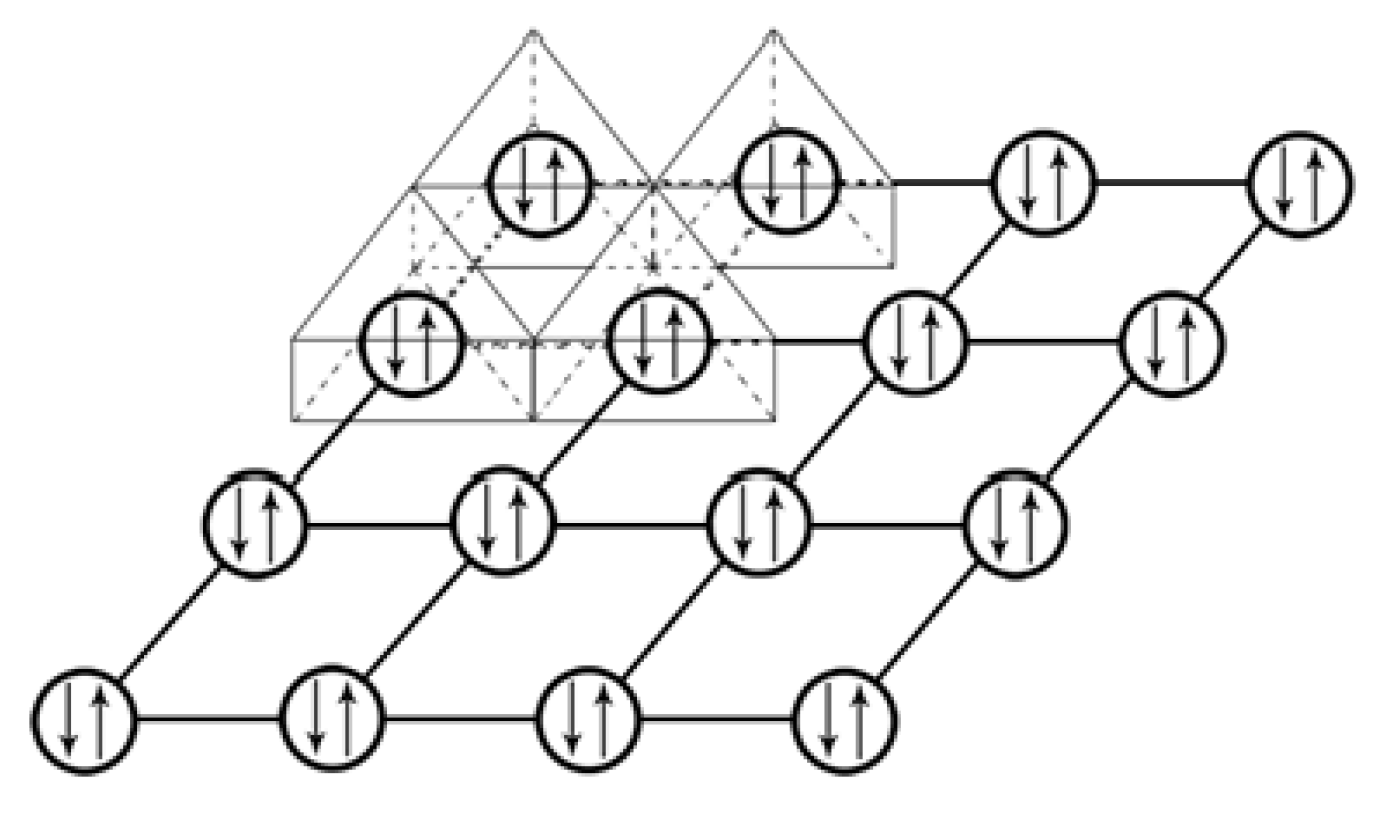
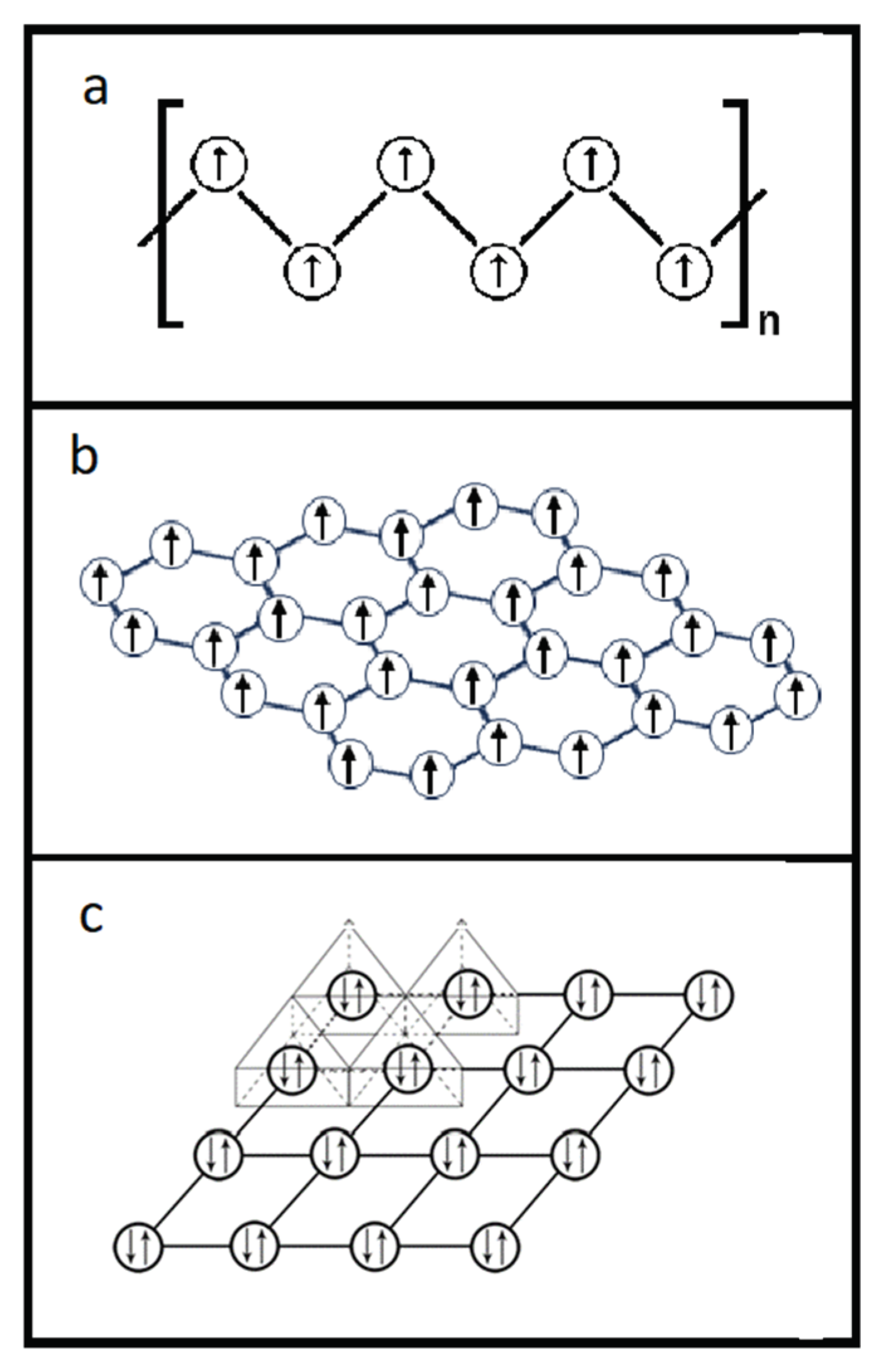
We further explored the linkage of the Th cations in the crystal lattice of ThS as sketched in
Figure 5, in which two small triangles are chosen to present two exemplified Th cation planes. One plane is along [111] direction, i.e., the upper triangle in
Figure 5, and another in [11
] direction, the lower triangle. Notice that a Th cation, indicated as a solid filled rectangle in
Figure 5, is landed on both of these two crystallographic planes to conclude a plane sharing situation of this Th cation by two planes. This plane sharing results in the formation of an intersection between these two planes. It should be easy to envisage that other Th cations in the crystal lattice of ThS should be located in their relative intersections of their {111} planes in the same way as this solid filled rectangle. This means every Th cations in the crystal lattice of ThS should sit along the <111> family to make the Th cations in a 3-D network fashion. Consequently, all the Th cations in
Figure 5 should be marked as solid filled rectangles. In other words, all the Th cations in the unit cell of ThS should be organized in a 3-D manner or a 3-D network.
] direction (lower triangle), demonstrates that the Th cation (solid filled rectangle) can sit on both of these planes.
It is noted that ThS might be an ideal compound superconductor for high power and relatively high current density applications because of the aforementioned 3-D networking structure with its comparatively high packing density by the Th cations. ThS has the sodium chloride (NaCl) crystal structure with the face centered cubic (FCC) packing style. Each Th cation is surrounded by 6 I
-’s in an octahedral manner. As depicted above in
Figure 4, its packing featured that the layers of the Th cations and the S anions are stacked in layer-by-layer manner along its crystallographic <111> family directions. In the meantime, the conductive layers made by the Th cations on the {111} family planes intersect from each other to form an efficient 3-D network of Th cations for electrical conduction and therefore, the flow of the electrical supercurrent on this 3-D network would not be confined in a 2-D anisotropic manner as for ThI
2 and many other compound superconductors.
The discovery of superconductivity for ThS might have been happened 60 years ago when Tetenbaum carried out a study on its electrical property under variable temperatures from nearly 0 K to almost 1000 K in 1964 [
29]. Unfortunately, he did not use the Kelvin connection to lead the sample and thus, concluded that ThS is only a highly conductive (metallic) material, instead of a superconductor. The electrical behavior of ThS under variable temperature in his test probably reflected the metallic behavior of ThS without superconductivity along with the contact resistance between the platinum leads and the ThS sample in his experiment. In addition, the current applied over the ThS sample in Tetenbaum’s experiment was relatively high at 50-100 mA for a sample size with dimensions of several millimeters. Such a high measurement current might exceed the ThS’s superconducting critical current and thus, compromise the ThS’s superconducting state. In other words, under such an experimental condition, the paired electrons on the conduction band of the ThS sample were forced being separated into an unpaired state, i.e., two unpaired electrons. Therefore, the ThS sample in Tetenbaum’s experiment had completely lost its property of superconductivity and been converted into an ordinary metallic material with only the unpaired electrons on its conduction band.