Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Design
2.2. Questionnaire Design and Content
2.3. Target Populations
2.4. Circulation of Questionnaire
2.5. Collation of Results
2.6. Statistical Analysis
2.7. Ethics
2.8. Role of the Manufacturer in the Study
3. Results
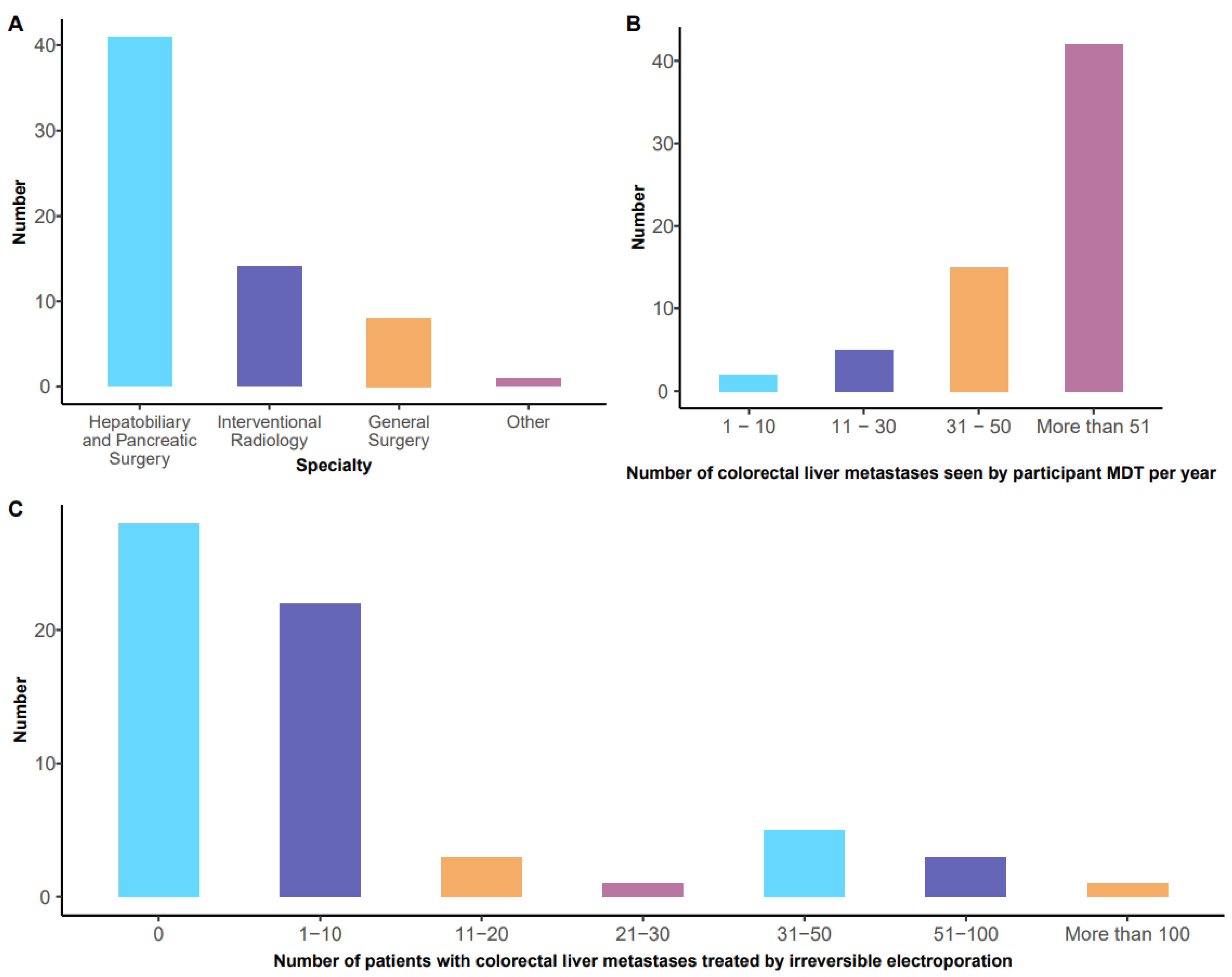
3.1. Specialty of Respondents and Exposure to Patients with Colorectal Liver Metastases (Figure 1A,B)
3.2. Reported Experience with Irreversible Electroporation for Colorectal Hepatic Metastases (Figure 1C)
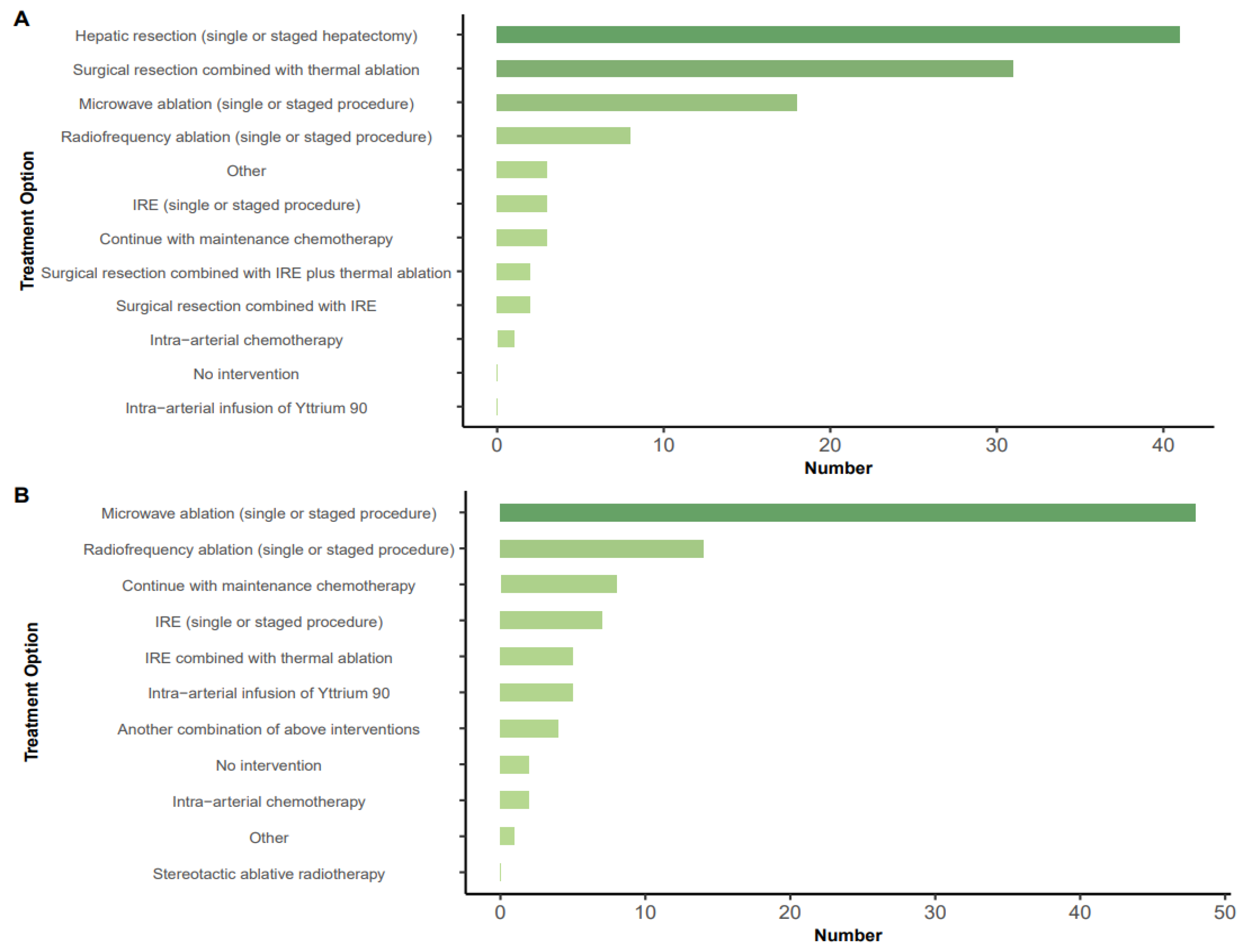
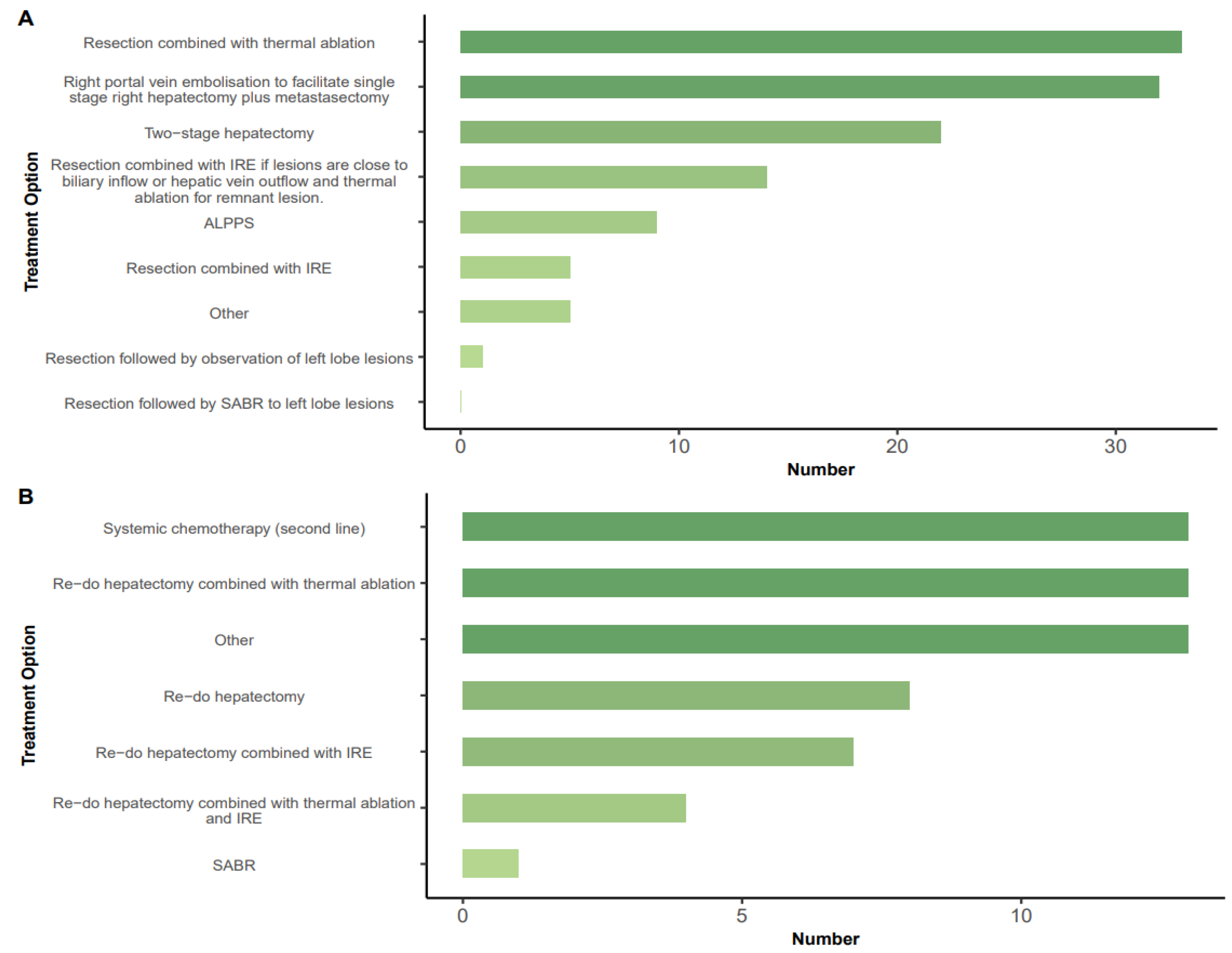
3.3. Treatment Options in Specific Scenarios
3.4. Mode of Delivery of IRE (Table 1)
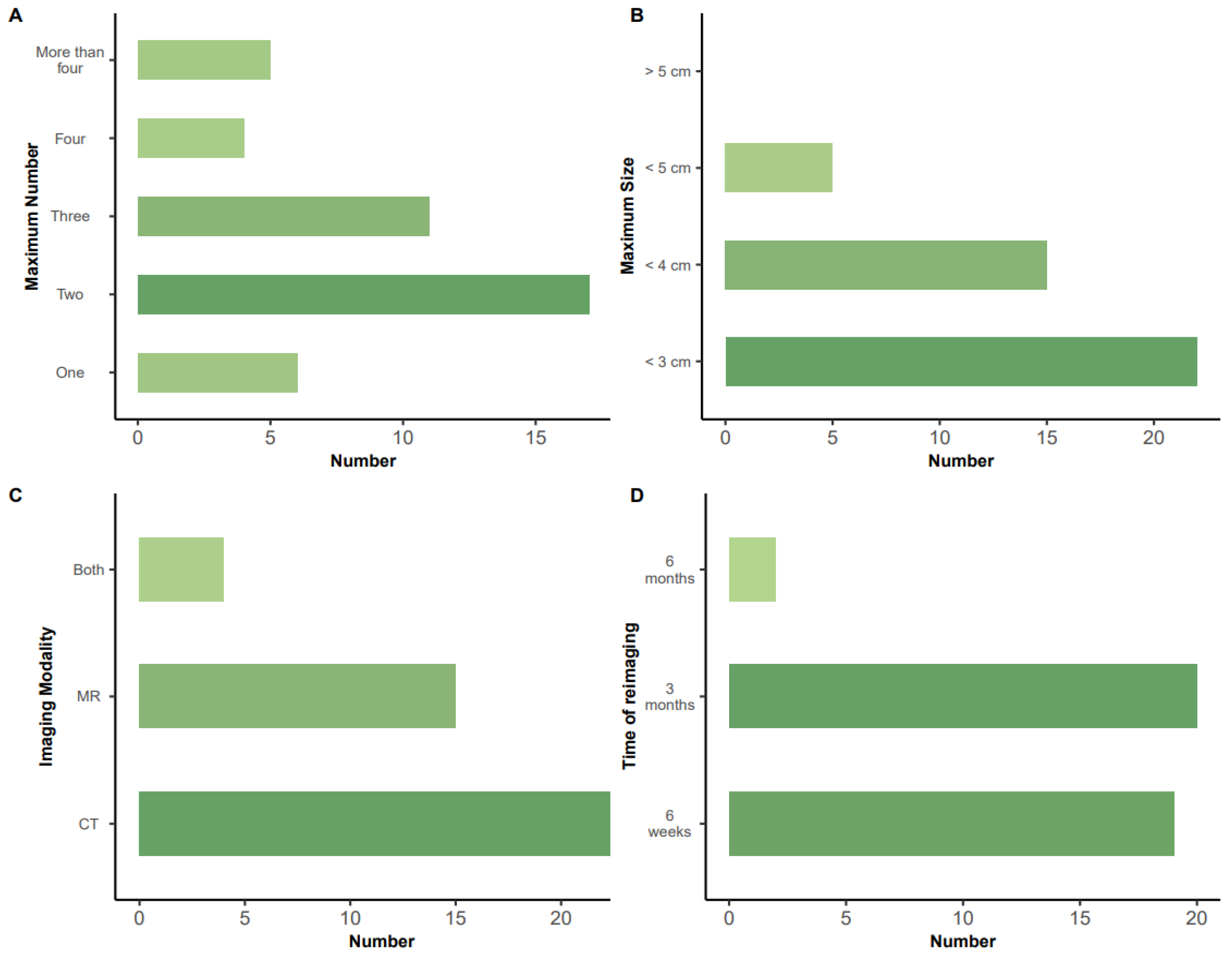
3.5. Number and Size of Lesions Treatable by IRE (Figure 4A,B)
3.6. Mode and Timing of Assessment of Response to IRE (Figure 4C,D):
3.7. Feasibility and Design of Future Randomized Controlled Trial of IRE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- a)
- Interventional radiology
- b)
- General Radiology
- c)
- Hepatobiliary and Pancreatic Surgery
- d)
- Pancreatic Surgery
- e)
- Oncology
- f)
- Other (Please specify)
- g)
- None of the above.
- a)
- Do you treat patients with liver metastases from colorectal cancer?
- b)
- Are you a member of a multidisciplinary team/tumour board for patients with this condition?
- c)
- Please select the approximate number of patients with colorectal hepatic metastases that your MDT reviews per year:
- d)
- How many patients with colorectal hepatic metastases have you treated with liver IRE to date
- a)
- Hepatic resection (single or staged hepatectomy).
- b)
- Radiofrequency ablation (single or staged procedure)
- c)
- Microwave ablation (single or staged procedure)
- d)
- Irreversible electroporation (single or staged procedure)
- e)
- Irreversible electroporation combined with surgery
- f)
- Irreversible electroporation combined with thermal ablation
- g)
- Intra-arterial infusion of 90Yttrium
- h)
- Intra-arterial chemotherapy
- i)
- Maintenance chemotherapy
- j)
- No intervention
- k)
- Other (Please specify)
- a)
- Radiofrequency ablation (single or staged procedure)
- b)
- Microwave ablation (single or staged procedure)
- c)
- Irreversible electroporation
- d)
- Irreversible electroporation combined with thermal ablation
- e)
- Stereotactic ablative radiotherapy (SABR)
- f)
- Intra-arterial infusion of 90Yttrium
- g)
- Intra-arterial chemotherapy
- h)
- Maintenance chemotherapy
- i)
- Another combination of above interventions
- j)
- No intervention
- k)
- Other (Please specify)
- a)
- Right portal vein embolisation to facilitate right hepatectomy plus metastasectomy.
- b)
- Two-stage hepatectomy.
- c)
- ALPPS.
- d)
- Resection combined with thermal ablation
- e)
- Resection combined with Irreversible electroporation
- f)
- Resection combined with irreversible electroporation if lesion close to biliary structure or remnant outflow and thermal ablation for remnant lesion.
- g)
- Resection followed by SABR to left lobe remnant lesions.
- h)
- Resection followed by observation of left lobe lesions.
- i)
- Other (please specify).
- a)
- Systemic chemotherapy.
- b)
- Re-do hepatectomy
- c)
- Re-do hepatectomy combined with thermal ablation
- d)
- Re-do hepatectomy combined with irreversible electroporation
- e)
- SABR
- f)
- Other (please specify)
- a)
- Percutaneous with ultrasound guidance.
- b)
- Open surgery with ultrasound guidance.
- c)
- Percutaneous with CT guidance.
- d)
- Open surgery with CT guidance
- a)
- Do you use IRE solely for lesions close to biliary inflow/vascular outflow structures?
- b)
- Do you use a combination of IRE and thermal ablation?
- c)
- Could you use IRE as sole ablative treatment?
- d)
- What is the maximal number of lesions that you can treat with IRE at one sitting?
- e)
- What is the maximal size of hepatic lesion that you can treat with IRE (in cm)?
- a)
- Do you routinely undertake a CT scan 24h after IRE and prior to discharge from hospital?
- b)
- What is the optimum imaging modality for assessment of response to IRE? (Text)
- c)
- What is the optimum time after IRE for assessment of ablation?
- d)
- Is RECIST 1.1 the optimum method for assessment of response?
- e)
- If the answer to d is NO, how do you assess response?
- a)
- Do you think that there should be a randomized trial to evaluate the role of IRE?
- b)
- If NO, is that because IRE is an established treatment?
- c)
- If NO, is that because IRE has no role as an ablative treatment for colorectal hepatic metastases?
- d)
- If YES – would you compare IRE to liver resection?
- e)
- If YES – would you compare IRE to thermal ablation?
- f)
- If YES – would you compare IRE to resection & ablation (in different settings)?
Appendix B. PubMed Citable Co-Authors under the LIVERMET-IRE Collaborative
References
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021, 19, 329–359. [CrossRef] [PubMed]
- Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK et al. World J Clin Oncol 2020, 11, 761–808.
- Siriwardena AK, Mason JM, Mullamitha S, Hancock HC, Jegatheeswaran S. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol 2014, 11, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018, 16, 874–901. [CrossRef]
- Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994, 343, 1405–10. [Google Scholar]
- Chan AKC, Mason JM, Baltatzis M, Siriwardena AK; CoSMIC Collaborators. Management of Colorectal Cancer with Synchronous Liver Metastases: An Inception Cohort Study (CoSMIC). Ann Surg Oncol 2022, 29, 1939–1951. [CrossRef] [PubMed]
- Nieuwenhuizen S, Dijkstra M, Puijk RS, Geboers B, Ruarus AH, Schouten EA, Nielsen K, de Vries JJJ, Bruynzeel AME, Scheffer HJ et al. Microwave Ablation, Radiofrequency Ablation, Irreversible Electroporation, and Stereotactic Ablative Body Radiotherapy for Intermediate Size (3-5 cm) Unresectable Colorectal Liver Metastases: a Systematic Review and Meta-analysis. Curr Oncol Rep 2022, 24, 793–808.
- Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol 2004, 5, 550–560. [CrossRef] [PubMed]
- Tago T, Katsumata K, Udou R, Kasahara K, Mazaki J, Kuwabara H et al. Significance of radiofrequency ablation for unresectable colorectal cancer with liver metastases. Anticancer Research. 2021, 41, 5539–5547. [Google Scholar]
- Pillai K, Akhter J, Chua TC, Shehata M, Alzahrani N, Al-Alem I, Morris DL. Heat sink effect on tumour ablation characteristics as observed in monopolar radiofrequency, bipolar, radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015, 94, e580.
- Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford). 2011, 13, 168–173. [Google Scholar]
- Scheffer HJ, Nielsen K, van Tilborg AA, Vieveen JM, Bouwman RA, Kazemier G, Niessen HW, Meijer S, van Kuijk C, van den Tol MP, Meijerink MR. Ablation of colorectal liver metastases by irreversible electroporation: results of the COLDFIRE-I ablate-and-resect study. Eur Radiol 2014, 24, 2467–2475.
- Meijerink MR, Ruarus AH, Vroomen LGPH, Puijk RS, Geboers B, Nieuwenhuizen S, van den Bemd BAT, Nielsen K, de Vries JJJ, van Lienden KP et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology 2021, 299, 470–480.
- Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J,De Baere T, Osterlund P, Yoshino T, Martinell E. ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023, 34, 10–32.
- Spiers HVM, Lancellotti F, de Liguori Carino N, Pandanaboyana S, Frampton AE, Jegatheeswaran S et al. Irreversible Electroporation for Liver Metastases from Colorectal Cancer: A Systematic Review. Cancers (Basel) 2023, 15, 2428.
- https://handbook-5-1.cochrane.org/chapter_8/8_4_introduction_to_sources_of_bias_in_clinical_trials.htm (accessed 16th August 2023).
- Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F,et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012, 17, 1225–1239. [CrossRef] [PubMed]
- Rocha FG and Helton, WS. Resectability of colorectal liver metastases: an evolving definition. HPB (Oxford). 2012, 14, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Imai K, Adam R, Baba H. How to increase the resectability of initially unresectable colorectal liver metastases: A surgical perspective. Ann Gastroenterol Surg, 2019; 3, 476–486.
- Elias D, Lasser P, Hoang JM, Leclere J, Debaene B, Bognel C et al. Repeat hepatectomy for cancer. Br J Surg 1993, 80, 1557–1562. [Google Scholar]
- Ruarus AH, Barabasch A, Catalano O, Leen E, Narayanan G, Nilsson A et al. Irreversible Electroporation for Hepatic Tumors: Protocol Standardization Using the Modified Delphi Technique. J Vasc Interv Radiol 2020, 31, 1765–1771. [CrossRef] [PubMed]
- Ruers T, Punt C, Van Coevorden F, Pierie JPEN, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012, 23, 2619–2626.
- Tinguely P, Ruiter SJS, Engstrand J, de Haas RJ, Nilsson H, Candinas D et al. A prospective multicentre trial on survival after Microwave Ablation VErsus Resection for Resectable Colorectal liver metastases (MAVERRIC). Eur J Cancer 2023, 187, 65–76. [CrossRef]
| Method of treatment | Number (%) (47 respondents) |
|---|---|
| Percutaneous with CT guidance | 33 (70) |
| Percutaneous with ultrasound guidance | 16 (34) |
| Open surgery with ultrasound guidance | 11 (23) |
| Open surgery with CT guidance | 1 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





