Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1.1. Catalyst Preparation
2.1.2. Characterization Methods
2.1.3. Catalytic Tests
2.1.1. Methane Reforming Reaction
2.1.2. Ethanol Steam Reforming (ESR)
3. Results and Discussion
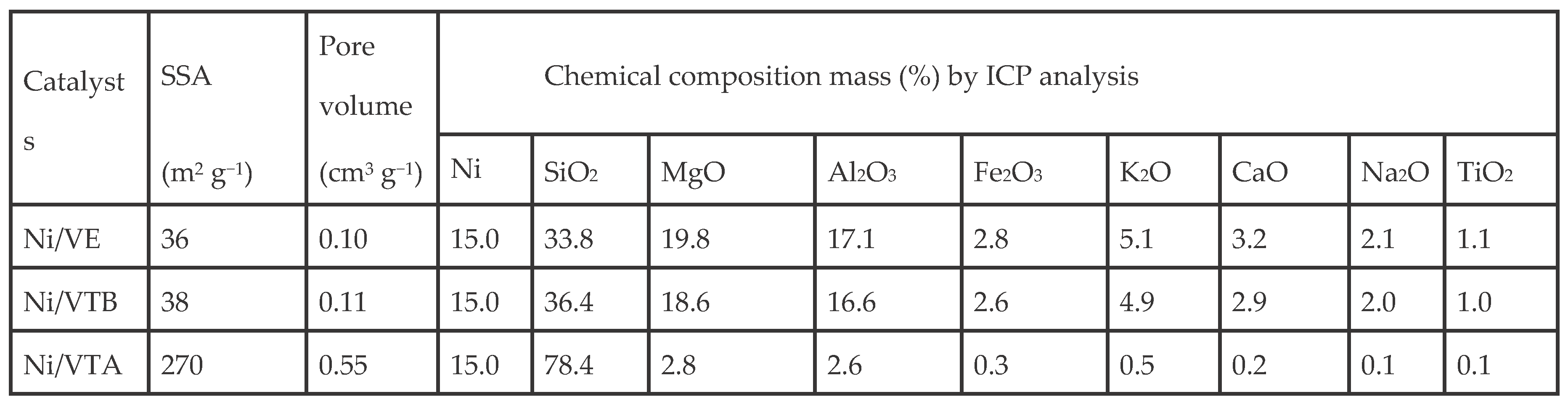
3.1. Characterization
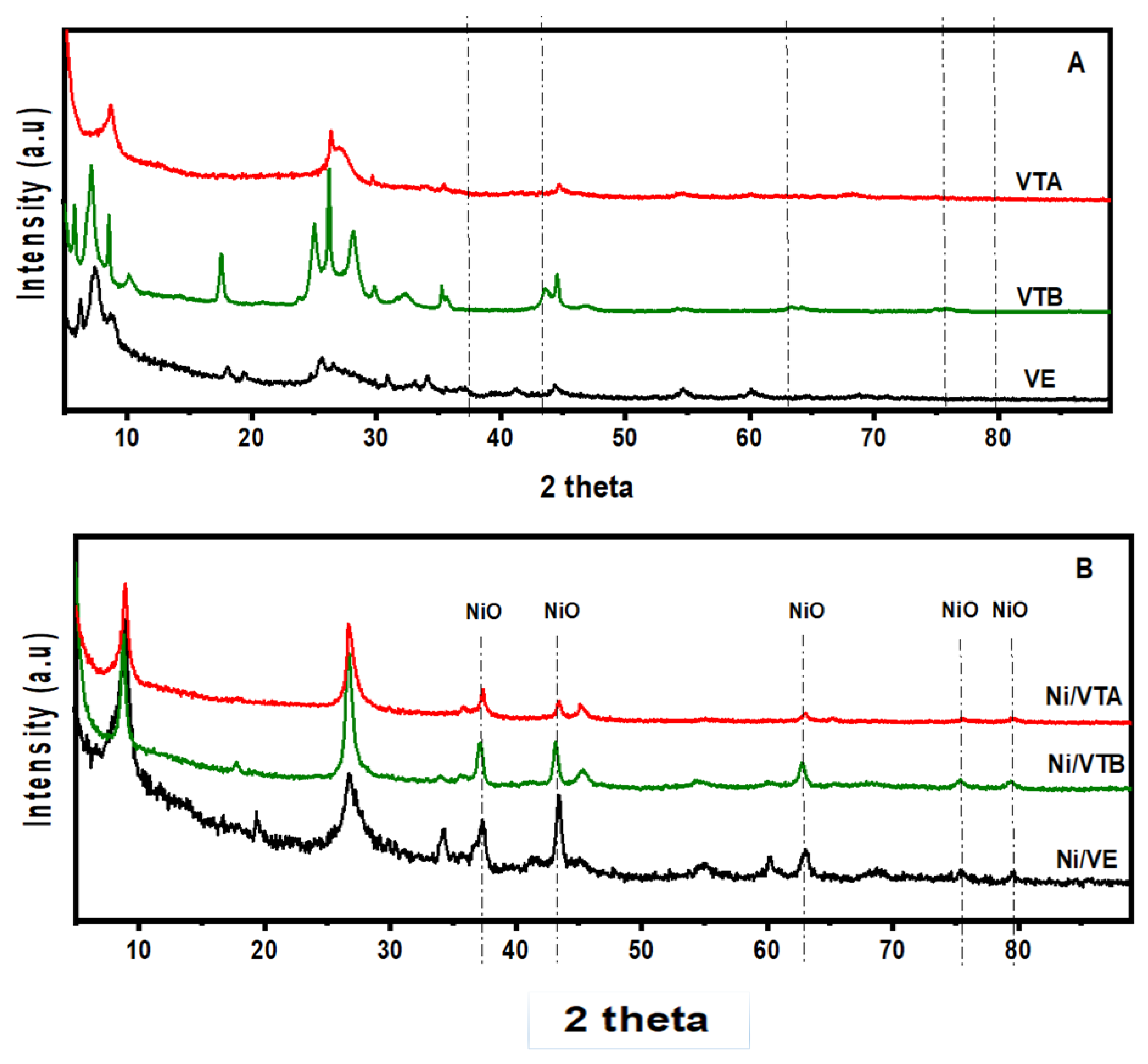
3.1.1. X-ray Diffraction
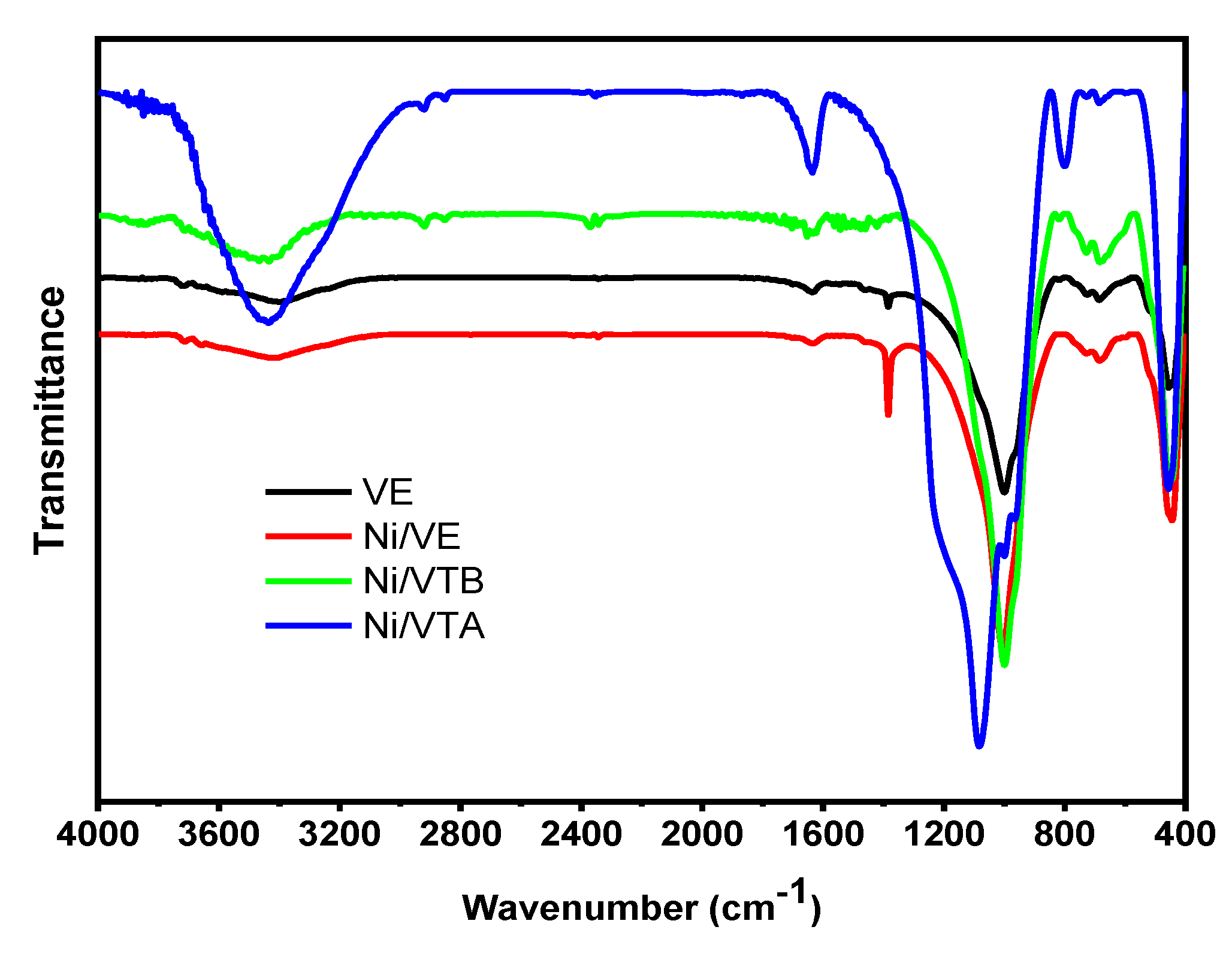
3.1.2. FTIR Spectroscopy
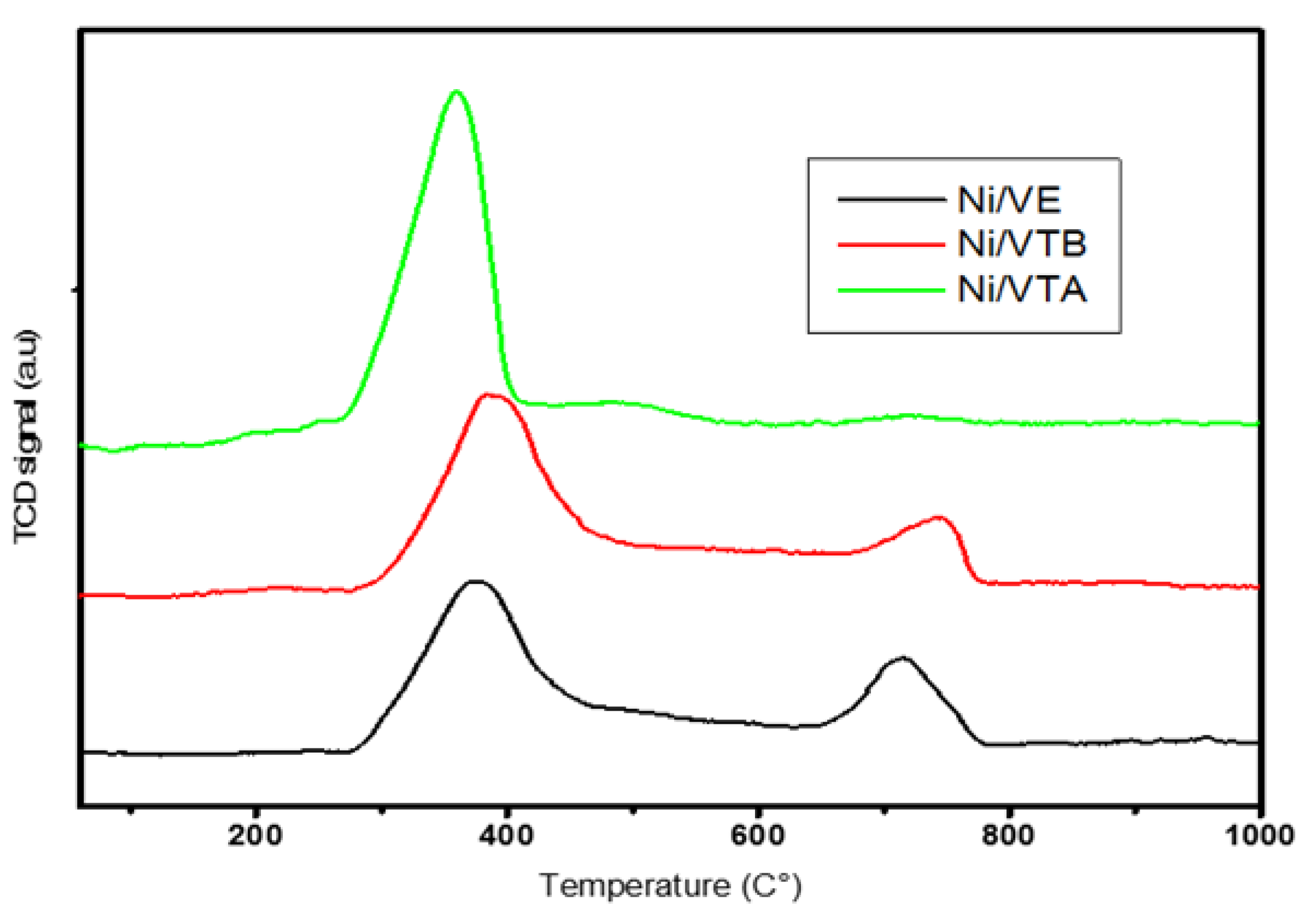
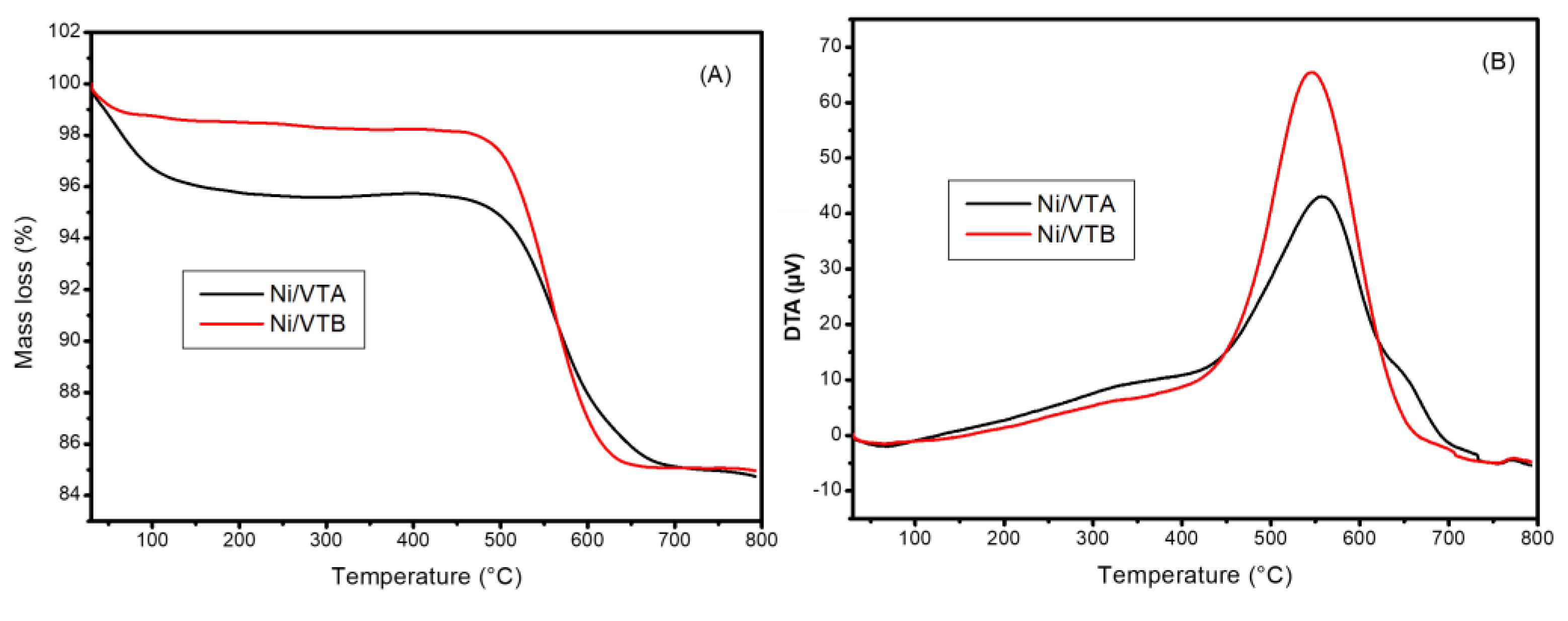
3.1.3. TPR Analyses
3.2. Catalytic Activity
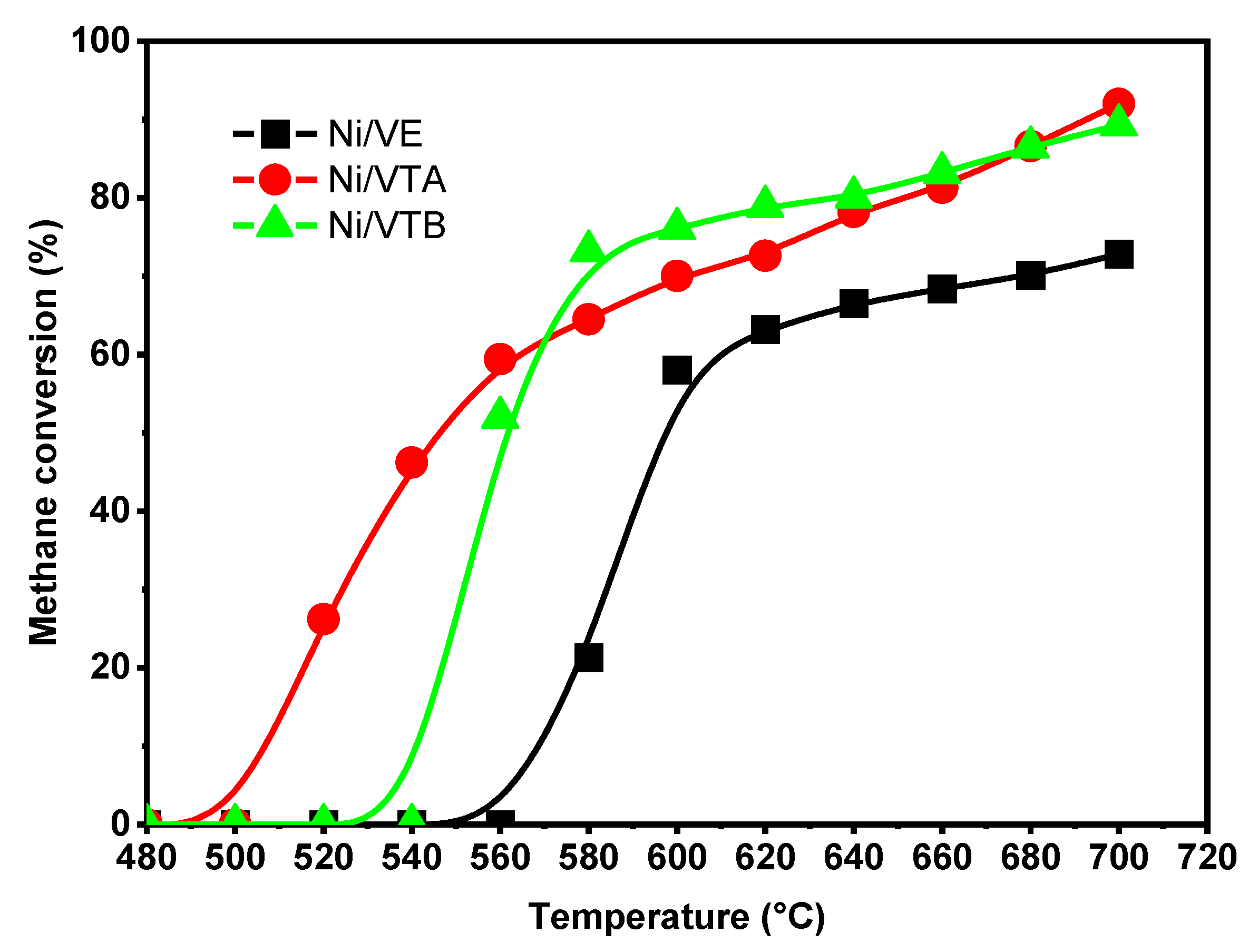
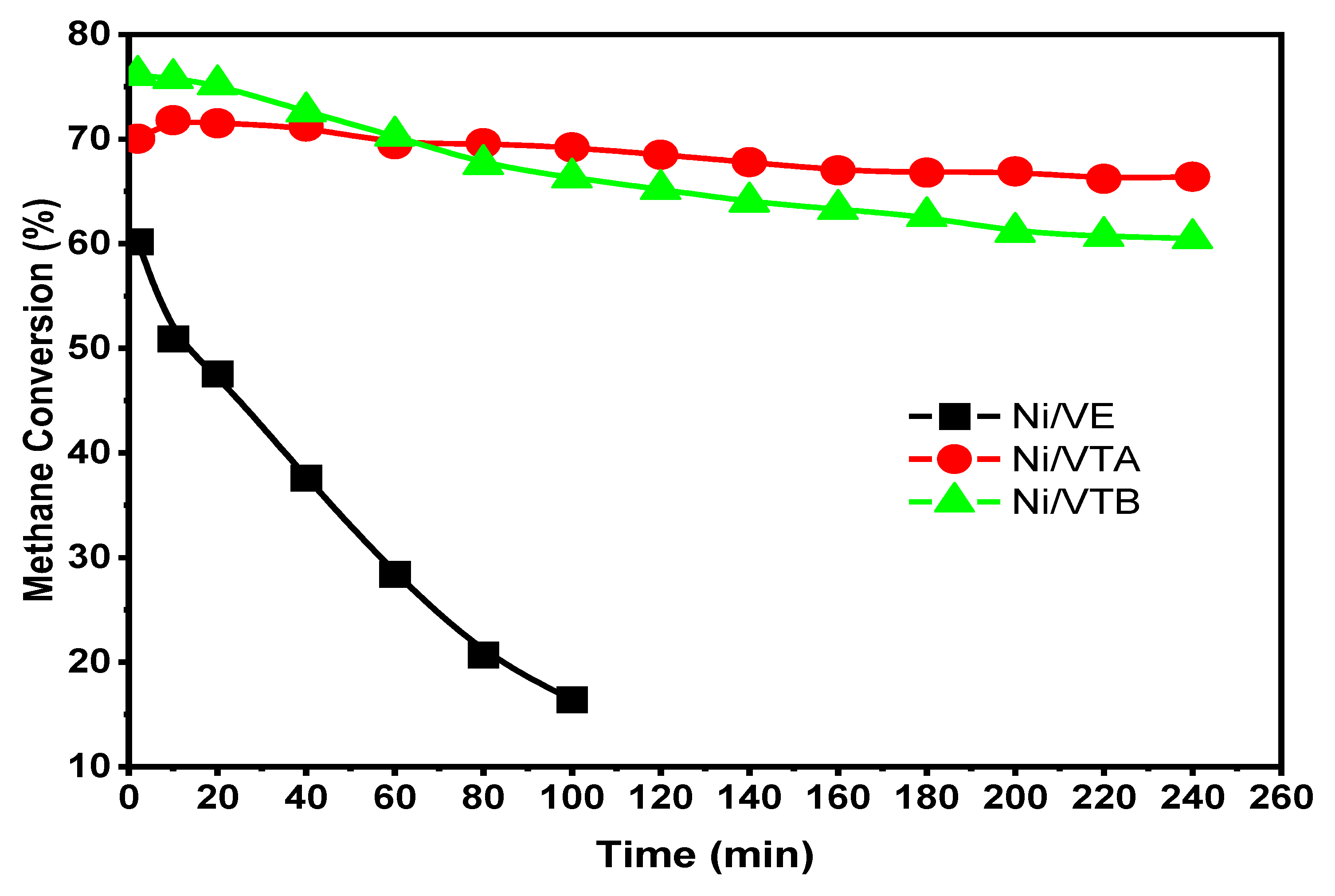
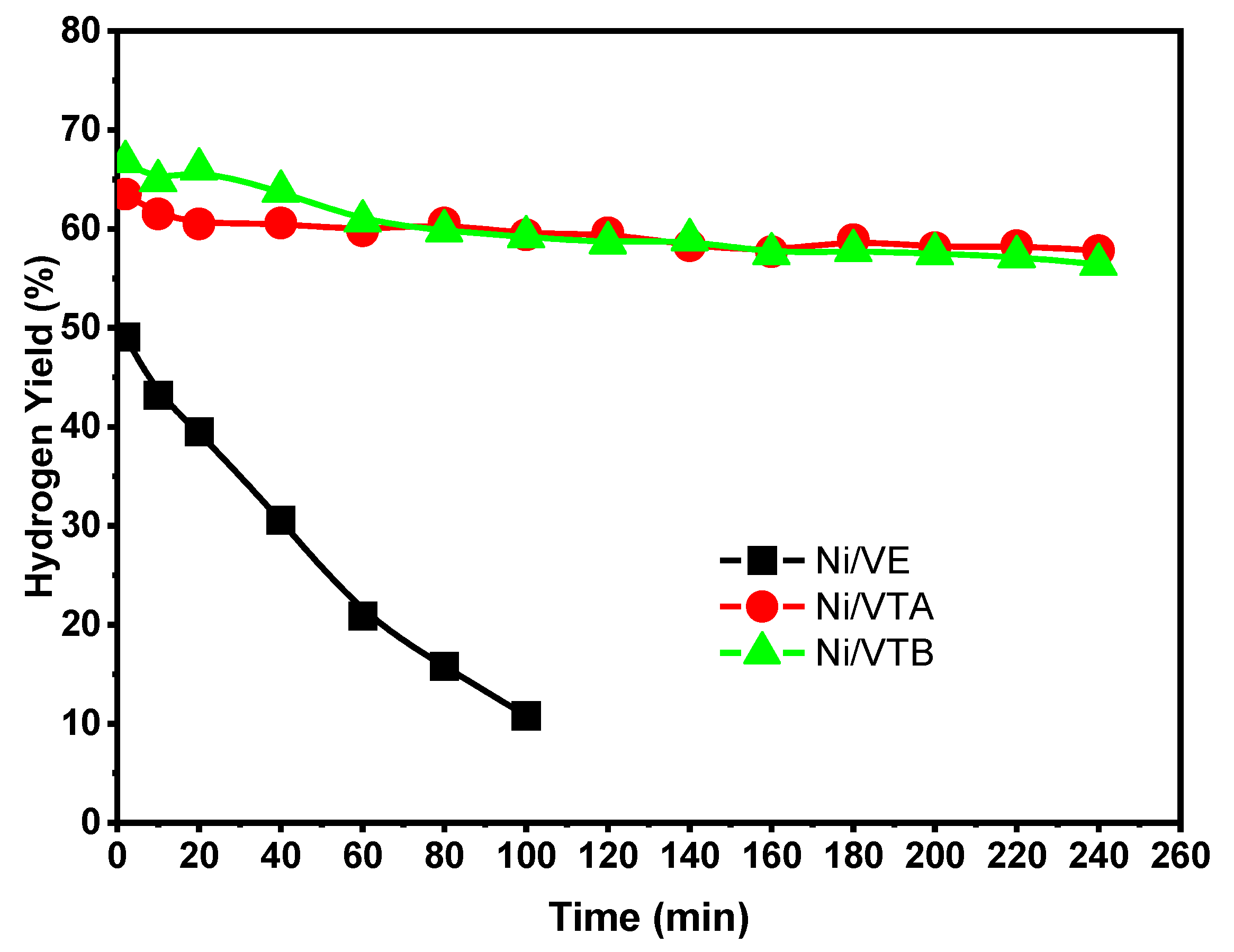
3.2.1. Dry Reforming of Methane
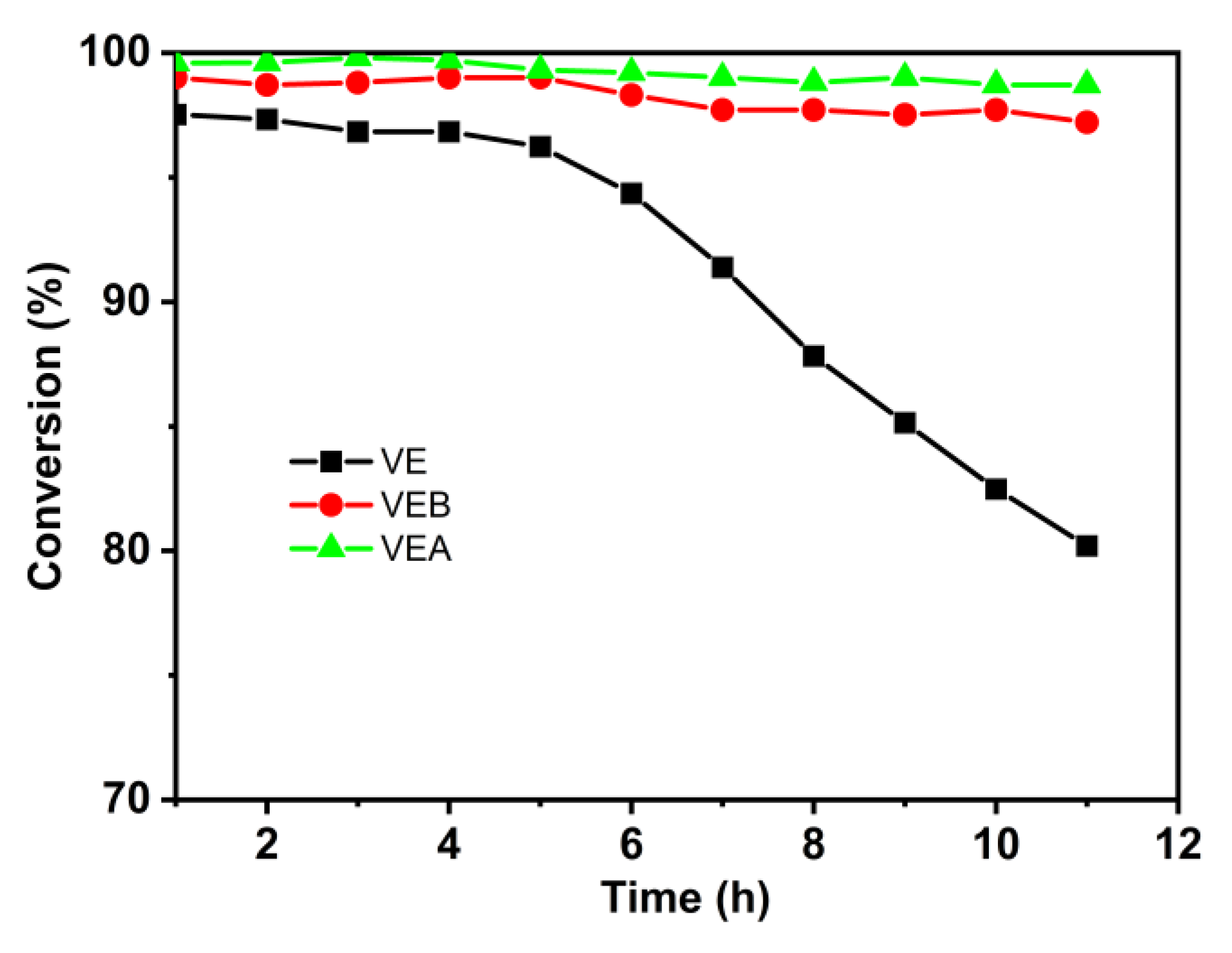
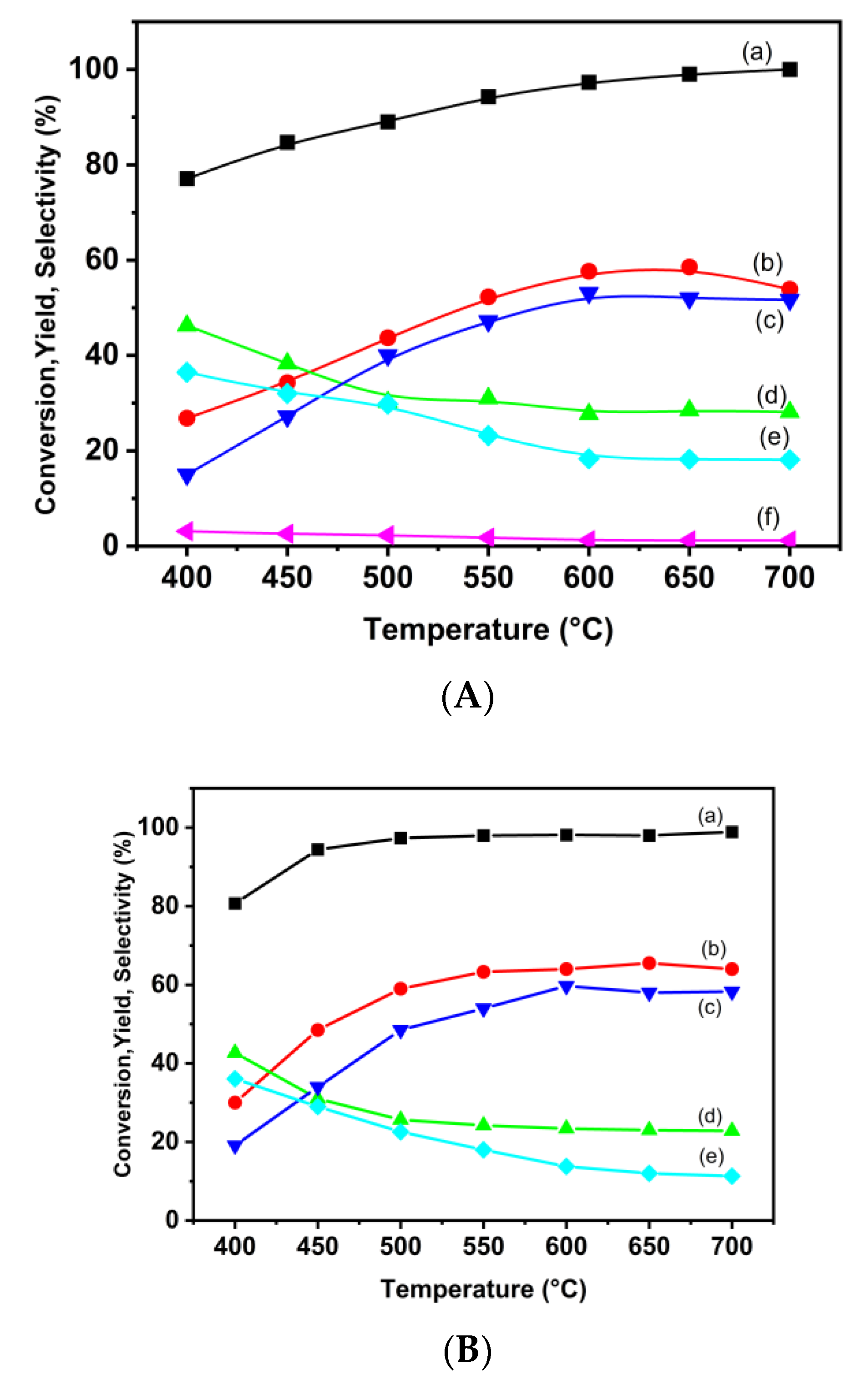
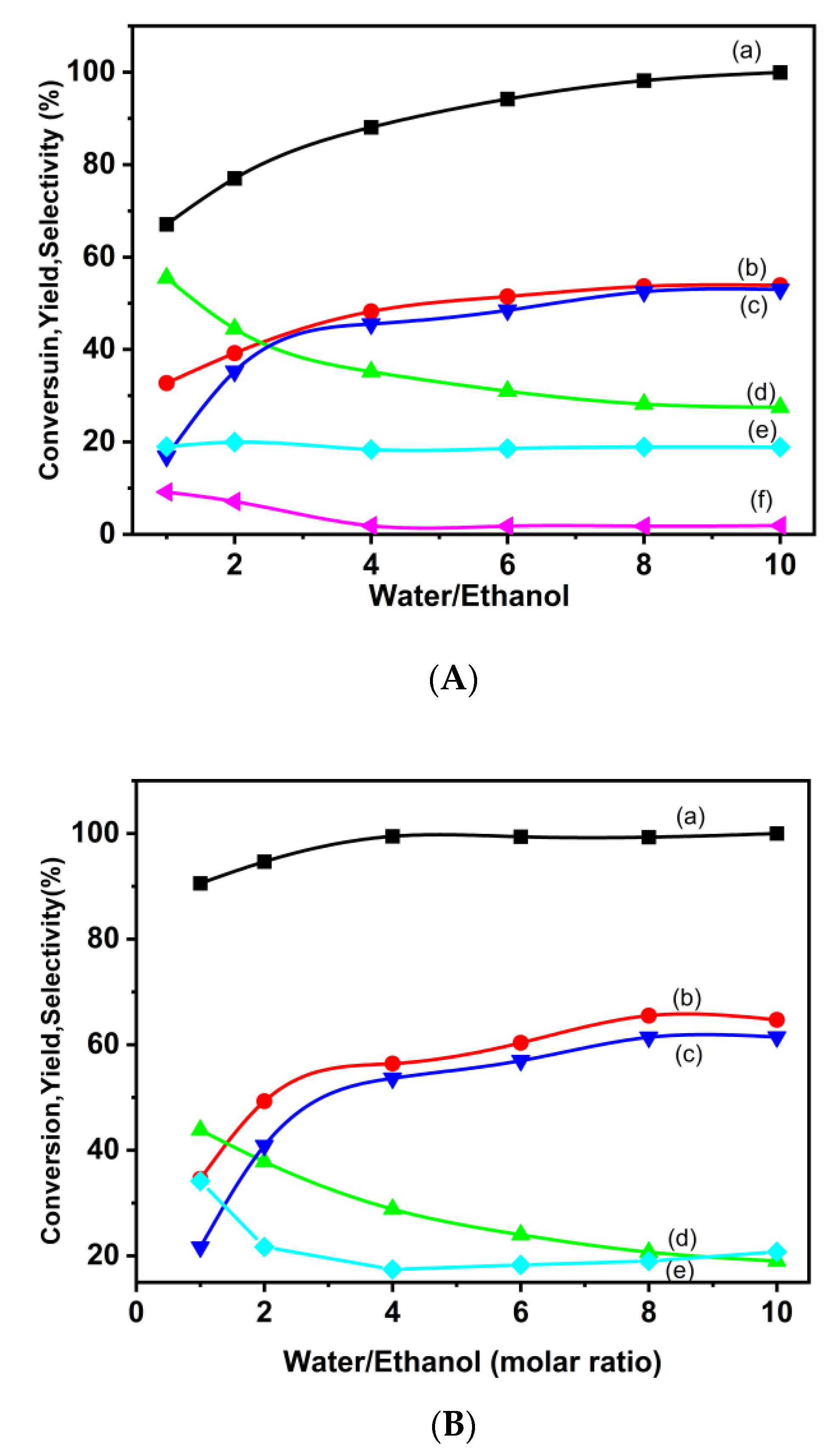
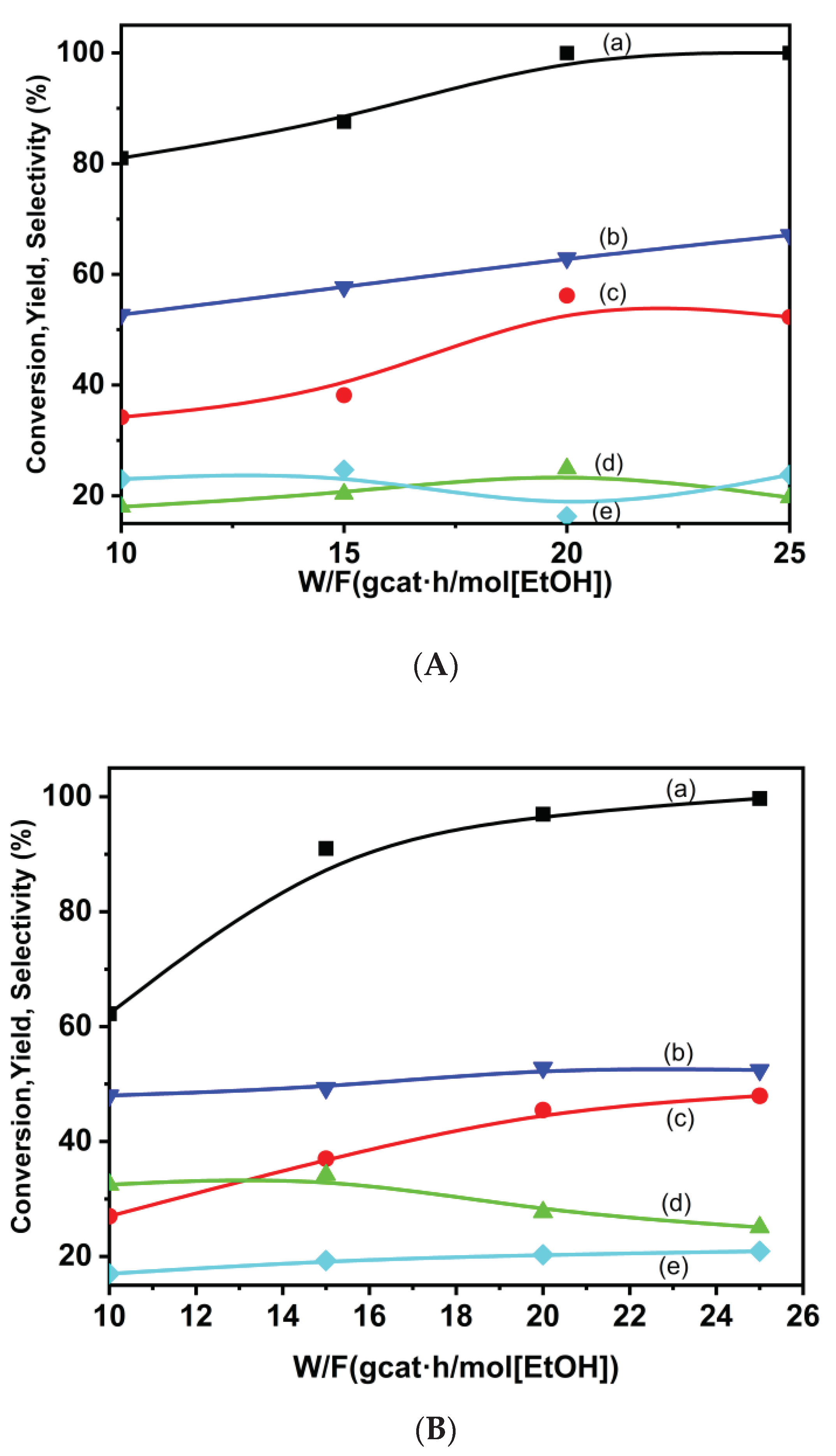
3.2.2. Ethanol Steam Reforming
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vermeiren, W.J.M.; Blomsma, E.; Jacobs, P.A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane. Catalysis Today 1992, 13, 427–436. [Google Scholar] [CrossRef]
- Corthals, S.; Van Nederkassel, J.; Geboers, J.; De Winne, H.; Van Noyen, J.; Moens, B.; Sels, B.; Jacobs, P. Influence of composition of MgAl2O4 supported NiCeO2ZrO2 catalysts on coke formation and catalyst stability for dry reforming of methane. Catalysis Today 2008, 138, 28–32. [Google Scholar] [CrossRef]
- Barroso-Quiroga, M.M.; Castro-Luna, A.E. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. International Journal of Hydrogen Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Asami, K.; Li, X.; Fujimoto, K.; Koyama, Y.; Sakurama, A.; Kometani, N.; Yonezawa, Y. CO2 reforming of CH4 over ceria-supported metal catalysts. Catalysis Today 2003, 84, 27–31. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catalysis Reviews 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. Journal of Catalysis 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Selvarajah, K.; Phuc, N.H.H.; Abdullah, B.; Alenazey, F.; Vo, D.-V.N. Syngas production from methane dry reforming over Ni/Al2O3 catalyst. Research on Chemical Intermediates 2016, 42, 269–288. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane: Effect of pretreatments. Applied Catalysis A: General 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Boukha, Z.; Kacimi, M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Ziyad, M. Methane dry reforming on Ni loaded hydroxyapatite and fluoroapatite. Applied Catalysis A: General 2007, 317, 299–309. [Google Scholar] [CrossRef]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Hydrogen production on Ni loaded apatite-like oxide synthesized by dissolution-precipitation of natural phosphate. International Journal of Hydrogen Energy 2017, 42, 19458–19466. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Chen, R.; Liu, X. Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light. RSC Advances 2014, 4, 406–408. [Google Scholar] [CrossRef]
- Gharin Nashtifan, S.; Azadmehr, A.; Maghsoudi, A. Comparative and competitive adsorptive removal of Ni2+ and Cu2+ from aqueous solution using iron oxide-vermiculite composite. Applied Clay Science 2017, 140, 38–49. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhu, X.-H. Competitive Adsorption of Ag+, Pb2+, Ni2+, and Cd2+ Ions on Vermiculite. Separation Science and Technology 2010, 45, 277–287. [Google Scholar] [CrossRef]
- Chmielarz, L.; Rutkowska, M.; Jabłońska, M.; Węgrzyn, A.; Kowalczyk, A.; Boroń, P.; Piwowarska, Z.; Matusiewicz, A. Acid-treated vermiculites as effective catalysts of high-temperature N2O decomposition. Applied Clay Science 2014, 101, 237–245. [Google Scholar] [CrossRef]
- Chmielarz, L.; Wojciechowska, M.; Rutkowska, M.; Adamski, A.; Węgrzyn, A.; Kowalczyk, A.; Dudek, B.; Boroń, P.; Michalik, M.; Matusiewicz, A. Acid-activated vermiculites as catalysts of the DeNOx process. Catalysis Today 2012, 191, 25–31. [Google Scholar] [CrossRef]
- Xin, H.; Yu, F.; Zhu, M.-Y.; Ouyang, F.-H.; Dai, B.; Dan, J.-M. Hydrochlorination of acetylene using expanded multilayered vermiculite (EML-VMT)-supported catalysts. Chinese Chemical Letters 2015, 26, 1101–1104. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Applied Clay Science 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Chmielarz, L.; Zjeżdżałka, P.; Jabłońska, M.; Kowalczyk, A.; Żelazny, A.; Vázquez Sulleiro, M.; Michalik, M. Vermiculite-based catalysts for oxidation of organic pollutants in water and wastewater. Acta Geodynamica et Geomaterialia 2013, 10, 341–352. [Google Scholar] [CrossRef]
- Maqueda, C.; Perez-Rodriguez, J.L.; Šubrt, J.; Murafa, N. Study of ground and unground leached vermiculite. Applied Clay Science 2009, 44, 178–184. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Freitas, O.; Chmielarz, L.; Mordarski, G.; Figueiredo, S. Simultaneous removal of dyes and metal cations using an acid, acid-base and base modified vermiculite as a sustainable and recyclable adsorbent. Science of The Total Environment 2017, 576, 398–408. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, C.L.; Shen, S.L.; Zhang, K.; Meng, H.; Guo, K.; Chen, J.F. Effects of silica sources on the properties of magnetic hollow silica. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2009, 334, 131–136. [Google Scholar] [CrossRef]
- Kang, K.-M.; Kim, H.-W.; Shim, I.-W.; Kwak, H.-Y. Catalytic test of supported Ni catalysts with core/shell structure for dry reforming of methane. Fuel Processing Technology 2011, 92, 1236–1243. [Google Scholar] [CrossRef]
- Daza, C.E.; Kiennemann, A.; Moreno, S.; Molina, R. Dry reforming of methane using Ni–Ce catalysts supported on a modified mineral clay. Applied Catalysis A: General 2009, 364, 65–74. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno; Rashid, U. ; Zainal, Z. CeO2–SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Applied Catalysis A: General 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. Journal of CO2 Utilization 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Seo, Y.T.; Seo, D.J.; Yoon, W.L.; Park, S.B. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Applied Catalysis A: General 2008, 340, 183–190. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Sc promoted and aerogel confined Ni catalysts for coking-resistant dry reforming of methane. RSC Advances 2017, 7, 4735–4745. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Coupled reforming of methane to syngas (2H2-CO) over Mg-Al oxide supported Ni catalyst. Applied Catalysis A: General 2018, 550, 176–183. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G.; Sener, C.; Dogu, T. MCM-41 supported PdNi catalysts for dry reforming of methane. Applied Catalysis B: Environmental 2009, 92, 250–261. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, B.S.; Tian, Y.L. CO2 reforming of methane over Ni/Sm2O3–CaO catalyst prepared by a sol–gel technique. Catalysis Communications 2007, 8, 661–667. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamaoka, R.; Arai, M. Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst. International Journal of Molecular Sciences 2015, 16, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Trevisanut, C.; Mari, M.; Millet, J.-M.M.; Cavani, F. Chemical-loop reforming of ethanol over metal ferrites: An analysis of structural features affecting reactivity. International Journal of Hydrogen Energy 2015, 40, 5264–5271. [Google Scholar] [CrossRef]
- Carrasco-Ruiz, S.; Zhang, Q.; Gándara-Loe, J.; Pastor-Pérez, L.; Odriozola, J.A.; Reina, T.R.; Bobadilla, L.F. H2-rich syngas production from biogas reforming: Overcoming coking and sintering using bimetallic Ni-based catalysts. International Journal of Hydrogen Energy 2023, 48, 27907–27917. [Google Scholar] [CrossRef]
- Yang, E.; Nam, E.; Jo, Y.; An, K. Coke resistant NiCo/CeO2 catalysts for dry reforming of methane derived from core@shell Ni@Co nanoparticles. Applied Catalysis B: Environmental 2023, 339, 123152. [Google Scholar] [CrossRef]
- Wang, M.; Kim, S.Y.; Jamsaz, A.; Pham-Ngoc, N.; Men, Y.; Jeong, D.H.; Shin, E.W. Effect of active sites distributions on temperature dependent–coke formation over Ni/CexZr1−xO2–Al2O3 catalysts for ethanol steam reforming: Coke precursor gasification. Applied Surface Science 2024, 644, 158746. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Zhao, Y.; Wang, H.; Liu, H.; Zhang, Q. A mini review on recent progress of steam reforming of ethanol. RSC Advances 2023, 13, 23991–24002. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Bioalcohol Reforming: An Overview of the Recent Advances for the Enhancement of Catalyst Stability. Catalysts 2020, 10. [Google Scholar] [CrossRef]
- Abdelkader, A.; Daly, H.; Saih, Y.; Morgan, K.; Mohamed, M.A.; Halawy, S.A.; Hardacre, C. Steam reforming of ethanol over Co3O4–Fe2O3 mixed oxides. International Journal of Hydrogen Energy 2013, 38, 8263–8275. [Google Scholar] [CrossRef]
- Braga, A.; dos Santos, J.; Bueno, J.M.C.; Damyanova, S. Hydrogen Production by Ethanol Steam Reforming. Athens Journal of Sciences 3, 7-16. [CrossRef]
- Ob-eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts 2019, 9. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, J.; Yang, Y. Recent Status in Catalyst Modification Strategies for Hydrogen Production from Ethanol Steam Reforming. ChemCatChem 2023, 15, e202300733. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukiewicz, W.; Czylkowska, A.; Maniecki, T. Correction to: Steam reforming of ethanol for hydrogen production: influence of catalyst composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and process conditions. Reaction Kinetics, Mechanisms and Catalysis 2021, 134, 1089–1089. [Google Scholar] [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





