1. Introduction
Ejaculated mammalian sperm are morphologically mature but functionally unable to fertilize (Fujihara et al., 2018). Sperm must undergo biochemical and physiological modifications to acquire competency for acrosome reaction and interaction with the oocyte. These changes occur during their passage through the female reproductive tract in a process called sperm capacitation (Ickowicz et al., 2012; Stival et al., 2016). It is accomplished in vitro under controlled conditions by recreating the oviductal environment with defined media supplemented with essential ions such as bicarbonate, calcium, albumin, and energy substrates (Lemoine et al., 2008).
Sperm capacitation remains uncertain in lizards, despite sharing characteristics such as internal fertilization and possession of the epididymis, where sperm acquire motility (Dosemane & Bhagya, 2015). Females also have the ability to store sperm in the oviducts, allowing an asynchrony between mating and ovulation (Martínez-Torres, 2009; Ortega-León et al., 2009). These characteristics suggest that spermatozoa may require physiological changes after insemination to acquire fertilization ability. Thus far, only one study has conclusively shown sperm capacitation in crocodiles by noting increased intracellular levels of cyclic adenosine monophosphate (cAMP), which enhance motility and elevate protein phosphorylation levels (Nixon et al., 2016). Moreover, epididymal spermatozoa in Lacerta vivipara exhibit increased motility when incubated in a medium containing caffeine, a phosphodiesterase inhibitor known to elevate cAMP levels (Depeiges & Dacheux, 1985). These observations suggest that this mechanism may indeed occur within lizards.
Comprehension of reproductive biology is crucial for any assisted reproductive technologies (ARTs) development (Comizzoli & Holt, 2022), given the ongoing global decline in herpetofauna (Sinervo et al., 2010; Böhm et al., 2013). We selected Sceloporus torquatus as a model for advancing ARTs due to our understanding of its reproductive biology (Cruz-Cano et al., 2021; Sánchez-Rivera, 2017). We developed non-invasive semen collection methods by establishing the time to obtain greater volumes and generated sperm quality references (Martínez-Torres et al., 2019a; Martínez-Torres et al., 2019b). However, our attempts at sperm cryopreservation have yielded a low success rate in post-thawing recovery (Sánchez-Rivera et al., 2022). It is vital to grasp semen quality parameters, prevent spontaneous acrosome reactions (Lemoine et al., 2008), and enhance post-procedural recovery (Campbell et al., 2020). Besides, artificial insemination has been unsuccessful, possibly due to the use of freshly diluted sperm (unpublished data). These challenges underscore the importance of studying sperm physiology for successful ART implementation. We aimed to assess changes associated with sperm incubation using BWW medium and incubation parameters previously documented in crocodiles.
2. Materials and Methods
2.1. Animals
The capture of 15 adult males of Sceloporus torquatus (SVL > 70 mm) (Feria-Ortiz et al., 2001) was conducted in the Sierra de Guadalupe State Park, Coacalco, State of Mexico (19° 61’ N, 99° 11’ W, 2480 m altitude) under scientific collection licenses SPA/DGVD/086681/21 and SPARN/DGVD/12218/23 granted by the Secretaría del Medio Ambiente y Recursos Naturales. The collection occurred during the early stage of the reproductive season (October–November) in both 2021 and 2022. Morphometric data of the animals were recorded, including snout–vent length (using digital Vernier calipers to the nearest 0.01 mm) and body weight for each individual (measured using a digital scale with 0.1 g precision). The lizards were kept in outdoor enclosures measuring 3.0 × 5.0 × 2.0 m, with access to food and water, and then released into their natural habitat after completing experimental procedures.
2.2. Semen Collection and Incubation
Semen was collected by gently pressing the genital papillae, following the method described by Martínez-Torres et al. (2019b). Seminal volume and sperm concentration were measured. The ejaculates were washed with PBS and isolated from seminal plasma via double centrifugation at 2700 rpm for 10 minutes at room temperature. The sperm samples were diluted to a final volume of 150 μl using Biggers-Whitten-Whittingham (BWW) medium, with the following composition: 120 mM NaCl, 4.6 mM KCl, 1.7 mM CaCl2.2H2O, 1.2 mM KH2PO4, 1.2 mM MgSO4.7H2O, 5.6 mM D-glucose, 0.27 mM sodium pyruvate, 44 mM sodium lactate, 5 U/ml penicillin, 5 mg/ml streptomycin, 20 mM HEPES, 3 mg/ml BSA, 25 mM NaHCO3, pH 7.4, and an osmolarity of 300 mOsm (Nixon et al., 2016). All chemicals were purchased from Sigma. A portion of 30 μl was retrieved for assessment at 0 hours, while the remaining sample was incubated with 5% CO2 at 30°C for up to 3 hours. Additionally, a fraction was retrieved each hour for ongoing semen assessment.
2.3. Semen Assessment
We determined the total motility of spermatozoa using optical microscopy (Leica DM100) with a 40x objective and registered movement type when observed. We conducted the other tests at 100x magnification. Sperm viability was determined through eosin-nigrosin staining (Sánchez-Rivera et al., 2022). We established the sperm capacitation’s state based on the proportion of spermatozoa exhibiting chlortetracycline (CTC) assay patterns: Full/F (uniform fluorescence head), Band/B (post-acrosomal region without fluorescence), and Acrosome Reacted/AR (fluorescent-free head or a thin fluorescent band on the equatorial segment), following the method described by Naijian et al. (2013). Acrosome integrity was assessed using a lectin-binding assay with fluorescein-conjugated Pisum sativum agglutinin (PSA-FITC), following the protocol outlined by Sánchez-Rivera et al. (2022).
2.4. Statistical Analysis
The data are presented as mean ± standard deviation. Due to the non-normal distribution and lack of independence assumptions, we utilized the non-parametric Kruskal-Wallis test to establish significant differences among time incubation periods. Subsequently, we conducted Dunn’s post-hoc test for each sperm assessment. We assessed the effect of sperm incubation using the Wilcoxon test, with T0 as the control for each pair. A P-value of less than 0.05 was considered statistically significant. We carried out all the analyses and plots using the R (version 4.3.2) software on iOS.
3. Results
According to morphometric values, all males (n = 15) were considered adults. We obtained semen showing consistent characteristics typical of an ejaculate (Martínez-Torres et al., 2019b), including volume and sperm concentration (
Table 1).
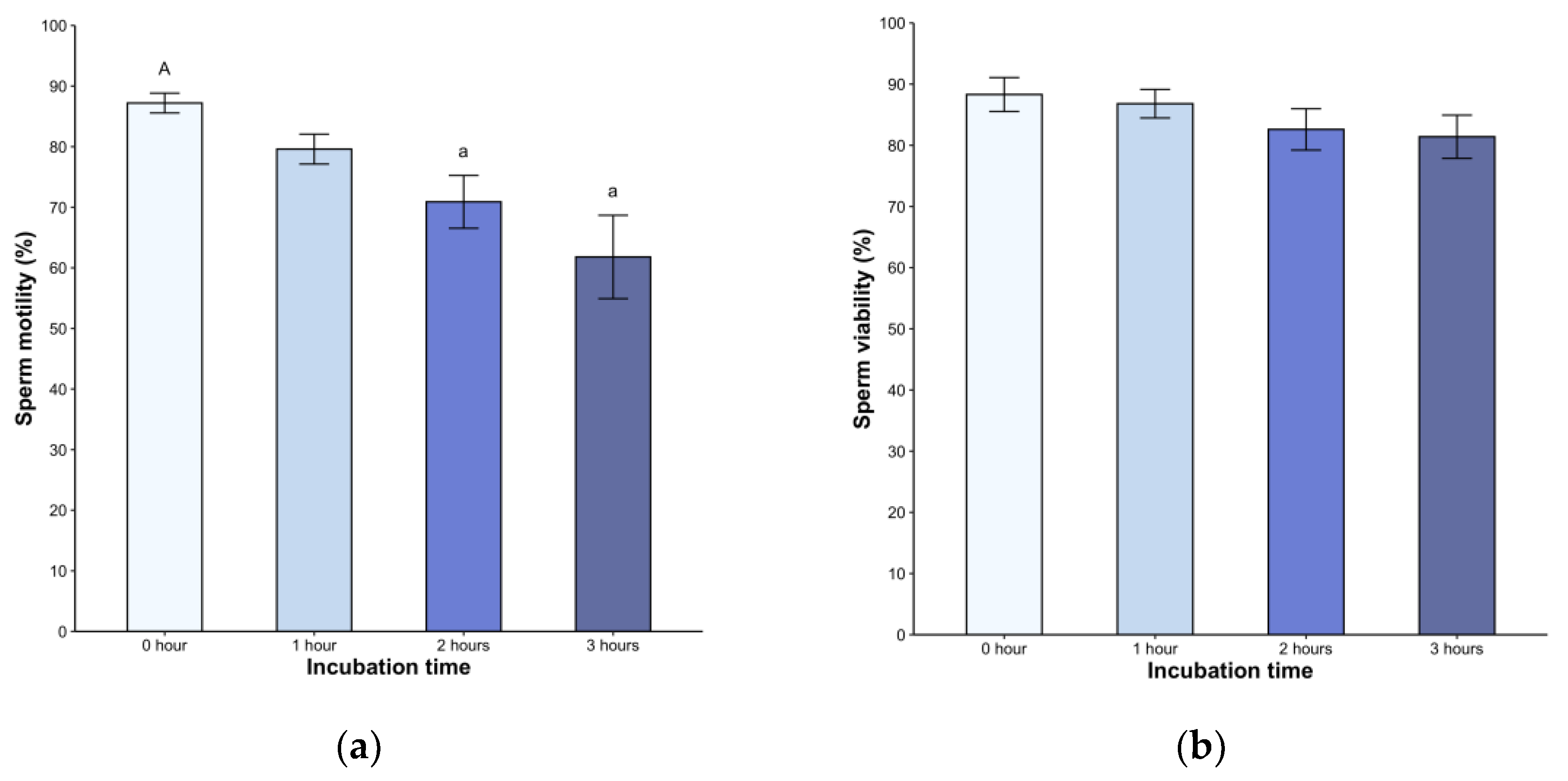
Following dilution, all samples exhibited high motilit
y, ranging from 79% to 98%. We found a significant decrease in the second
(82.6 ± 12.6%
) and third hour
s (81.4 ± 13.2%
, p < 0.05) post-incubation (H = 21.05, p < 0.05,
Figure 1a). A statistically significant difference was observed in the second and third hours post-treatment (p < 0.05) but without difference between the hours. Sperm viability remained above 80% throughout the entire 240-minute observation period (p < 0.05, Fig 1b). Of note
, the spermatozoa displayed active linear movement, but a transition to deeper and less symmetrical flagellar bending, with non-linear movement in many spermatozoa, was observed starting at 2 hours post-incubation (not shown).
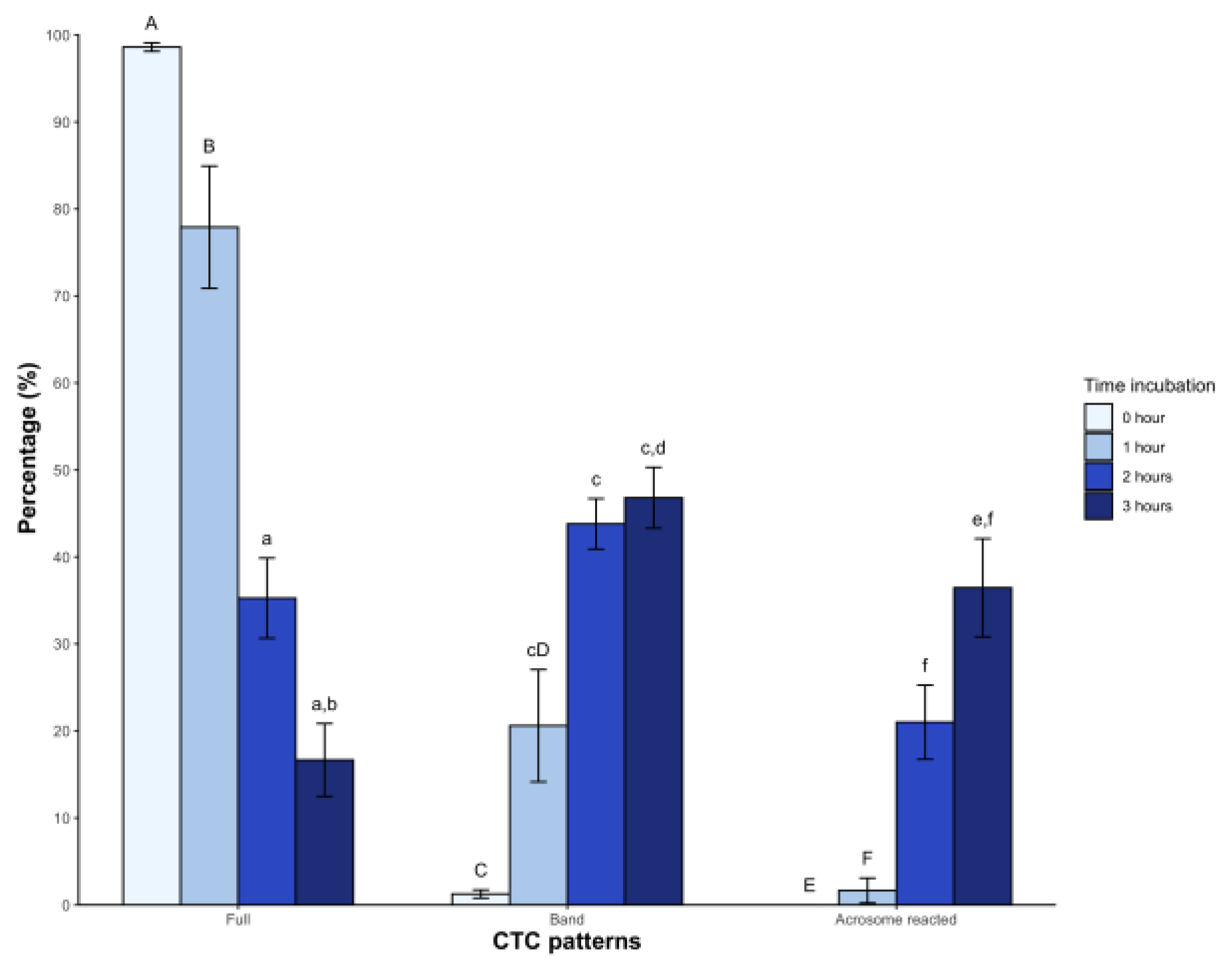
In the case of CTC patterns, a high percentage of sperm showed the Full pattern (98.6 ± 1.8%) at time 0, with a significant decrease starting from the second hour (35.2 ± 17.2%) and third hour (16.6 ± 15.6%, p < 0.05) post-incubation. The Band pattern showed low levels immediately after dilution with BWW medium (1.2 ± 1.7%), which gradually increased from the first hour and reached 46.8 ± 13.1% in the third hour. Regarding the acrosome-reacted pattern, it was initially absent but appeared from the second hour of incubation, reaching an average value of 36.4 ± 21.1% (p < 0.05) at 3 hours (
Figure 2).
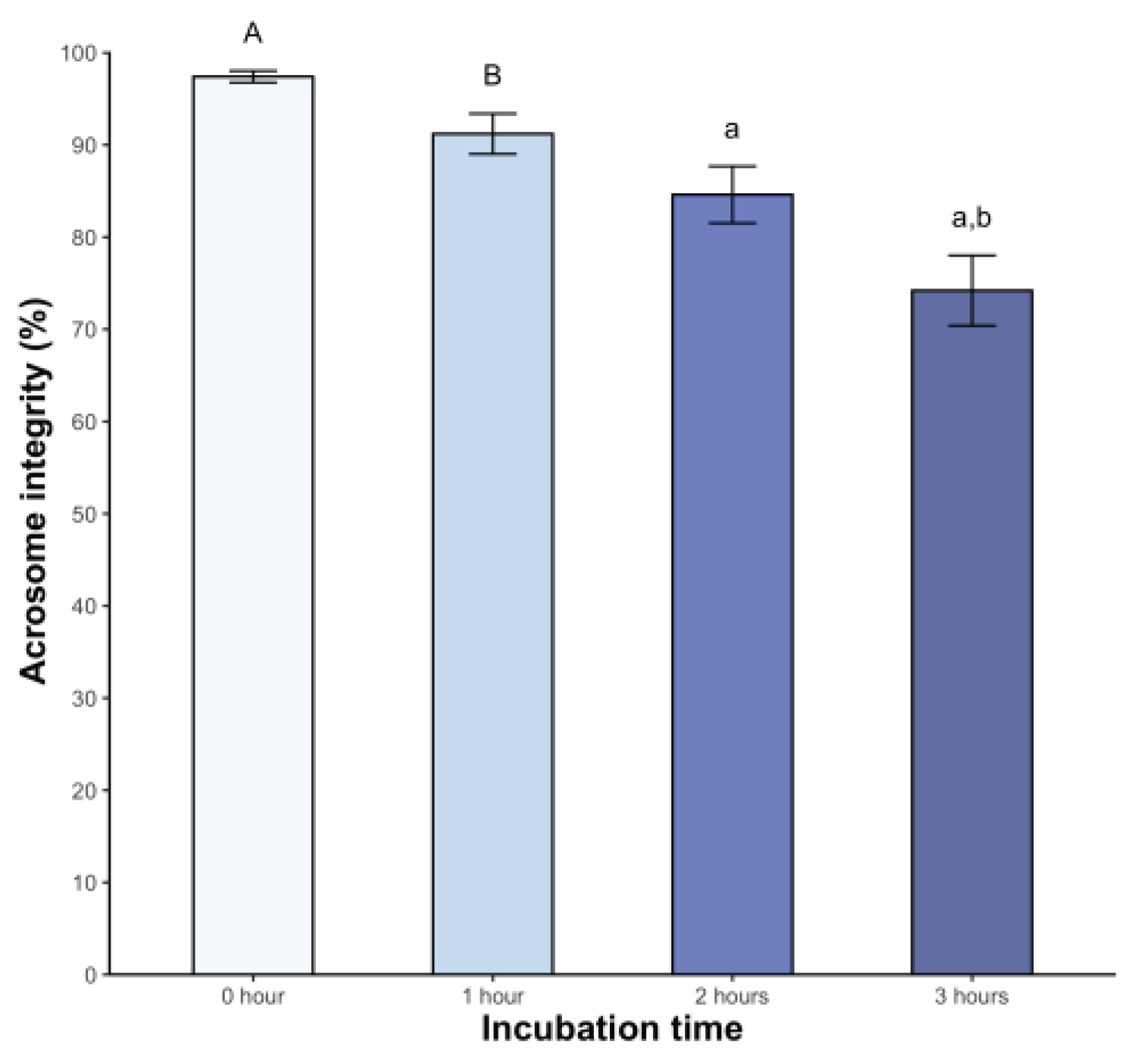
Significant changes in acrosome integrity were observed, with 15.4% of spermatozoa showing damage at 2 hours post-incubation (p < 0.05). This percentage increased to 25.8% at 3 hours post-incubation (p < 0.05) (
Figure 3).
4. Discussion
Mammalian sperm incubation in defined media reveals biochemical and physiological changes during capacitation (Puga Molina et al., 2018; Sáez-Espinosa et al., 2020). Considering the lack of studies about sperm physiology in lizards, it is essential to determine if their sperm undergoes capacitation (as reported in crocodiles) (Nixon et al., 2016) to advance the implementation of ARTs in this group. To address this, we incubated Sceloporus torquatus spermatozoa in BWW medium at 30 ºC with 5% CO2 for up to 3 hours to assess functional changes in sperm quality.
We observed a consistently high percentage of motility (above 79 %), which decreased over time starting at 2 hours. This trend suggests that the medium may favor the metabolic processes of sperm (Bustani & Baiee, 2021), potentially because its composition improves cell longevity (Witte & Schäfer-Somi, 2007). Considering the specific variations of each species, the choice of medium is crucial for adequate manipulation of gametes (Kito & Ohta, 2005). BWW medium promotes sperm motility and consistently induces increased cAMP levels and protein tyrosine phosphorylation in mammals (McPartlin et al., 2008). Additionally, it enhanced motility in Crocodylus porosus in a 120-minute incubation (Nixon et al., 2016). In light of the observed effect of phosphodiesterase inhibitor (which increases cAMP levels) on Lacerta vivipara sperm, resulting in increased motility (Depeiges & Dacheux, 1985), we hypothesize that this mechanism is conserved in this group and S. torquatus would react similarly under capacitation conditions.
We also noted modifications in motility patterns in many sperm, with increased and deeper flagellar beat amplitude, less symmetrical bending, and nonlinear movement. These observations suggest the hyperactive movement, which may facilitate the zona pellucida penetration during fertilization, as observed in mammals (Ho & Suarez, 2001). However, we did not quantify the proportion of spermatozoa undergoing these changes. A comprehensive assessment of motility using computer-assisted sperm analysis (CASA) is essential to detect movement types and evaluate the proportion of hyperactivated sperm in a sample (Suarez, 2008).
On the other hand, the CTC assay monitors changes in the sperm head plasma membrane of bicarbonate-responsive cells by labeling sperm and detecting fluidity changes, which correspond with increased tyrosine phosphorylation, indicative of hyperactivated motility (Gadella & Boerke, 2016). This assay is applied for the first time in any non-avian reptile. Incubation induced significant changes, with a decrease in the F pattern (non-capacitated sperm) over time and a higher percentage of the B pattern (capacitated sperm) after 2 hours. Similar observations in dogs (Rota et al., 1999), mice (Kito & Ohta, 2005), and boars (Ded et al., 2019) have been reported. The AR pattern also increased from the second hour onwards. The above coincides with the findings of the acrosomal integrity assay. Similar results were found in chickens under mammalian capacitating conditions (Priyadarshana et al., 2020). Based on these findings, we inferred that sperm incubated under the described conditions underwent molecular changes consistent with capacitation, attributable to medium composition.
The constituents of the BWW medium induce diverse changes in the sperm. Albumin, for instance, modifies lipid composition and membrane fluidity by reducing plasma membrane cholesterol content (McPartlin et al., 2009; Witte & Schäfer-Somi, 2007). Moreover, the presence of bicarbonate (above 15mM) in the medium initiates early changes that promote sperm capacitation by activating adenylate cyclase and elevating intracellular cAMP levels, resulting in hyperpolarization of the plasma membrane and increased intracellular pH (Ickowicz et al., 2012; Soriano-Úbeda et al., 2019). Calcium ions play a crucial role in this process by activating protein kinase A (PKA), which phosphorylates proteins involved in sperm functions like hyperactivation and the acrosome reaction (Nixon et al., 2020; Vyklicka & Lishko, 2020). Although the effects of glucose are unknown, it is essential for capacitation in mice, while pyruvate and lactate may inhibit it (Kito & Ohta, 2005).
The time in incubation has recently been recognized as a significant factor affecting sperm quality. In vitro studies on human sperm have shown a wide range from 1 to 24 hours to capacitation induction, leading to sperm subpopulations with varying degrees of functionality (Sáez-Espinosa et al., 2020). We found the changes in sperm assessments starting at two hours, which accentuated at the third hour accompanied by a significant decrease in total motility. However, the limited sperm volumes in lizards hamper our ability to incubate longer periods or devise protocols to select the sperm with capacitation-associated effects.
Further research on changes in oviductal content and functional assays in both mated and unmated females would offer valuable insights (Friesen et al., 2020) for optimizing the formulation of a more suitable medium. Incubation with calcium or progesterone ionophores can also be investigated, as both activate PKA-mediated signaling pathways, potentially improving efficiency in capacitation induction (Nixon et al., 2020).
5. Conclusions
Our study revealed some suggestive changes associated with sperm capacitation, such as a change in the type of movement characterized by increased and deeper flagellar beat amplitude, increased occurrence of capacitation patterns, and damaged sperm in the acrosome after two hours of incubation. These observations support the idea that this process also occurs in saurian. Establishing if sperm capacitation is a prerequisite for acquiring fertilization competence is crucial to improving the success of the implementation of any ART in this group of animals.
Author Contributions
Conceptualization, MMT and AM; investigation, UÁSR, NBCC, and CAR; data curation, formal analysis and visualization, UÁSR, NBCC; writing—original draft preparation, MMT and UÁSR; writing—review and editing, AM, NBCC, and CAR. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Nacional Autónoma de México through the PAPIIT projects awarded to MMT and AM: IN217722 (FESI) and IN205421 (FESC), respectively. Additionally, support was provided by the Consejo Nacional de Ciencia y Tecnología scholarship (CVU 893879) granted to UÁSR.
Institutional Review Board Statement
All procedures were carried out with the approval of the Institutional Subcommittee for the Care and Use of Experimental Animals at the Faculty of Veterinary Medicine and Zootechnics (UNAM) under the protocol DC-2018/2-16.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors would like to extend their gratitude to the General Coordination of Ecological Conservation of the State of Mexico for providing facilities for lizard collection and special thanks to Enrique González-Hernández for his invaluable comments on the project and to Alicia Alcántar for laboratory support. This paper is part of the requirements for obtaining a Doctoral degree at the Posgrado en Ciencias de la Producción y de la Salud Animal, UNAM of U.Á. Sánchez Rivera.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Böhm, M., Collen, B., Baillie, J. E. M., Bowles, P., Chanson, J., Cox, N., Hammerson, G., Hoffmann, M., Livingstone, S. R., Ram, M., Rhodin, A. G. J., Stuart, S. N., Van Dijk, P. P., Young, B. E., Afuang, L. E., Aghasyan, A., García, A., Aguilar, C., Ajtic, R., … Zug, G. (2013). The conservation status of the world’s reptiles. Biological Conservation, 157, 372-385. [CrossRef]
- Bustani, G. S., & Baiee, F. H. (2021). Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Veterinary World, 1220-1233. [CrossRef]
- Campbell, L., Cafe, S. l., Upton, R., Sean Doody, J., Nixon, B., Clulow, J., & Clulow, S. (2020). A model protocol for the cryopreservation and recovery of motile lizard sperm using the phosphodiesterase inhibitor caffeine. Conservation Physiology, 8(1). [CrossRef]
- Comizzoli, P., & Holt, W. V. (2022). Recent Progress in Spermatology Contributing to the Knowledge and Conservation of Rare and Endangered Species. Annual Review of Animal Biosciences, 10(1), 469-490. [CrossRef]
- Cruz-Cano, N. B., Sánchez-Rivera, U. Á., Álvarez-Rodríguez, C., Dávila-Govantes, R., Cárdenas-León, M., & Martínez-Torres, M. (2021). Sex steroids are correlated with environmental factors and body condition during the reproductive cycle in females of the lizard Sceloporus torquatus. General and Comparative Endocrinology, 314, 113921. [CrossRef]
- Ded, L., Dostalova, P., Zatecka, E., Dorosh, A., Komrskova, K., & Peknicova, J. (2019). Fluorescent analysis of boar sperm capacitation process in vitro. Reproductive Biology and Endocrinology, 17(1), 109. [CrossRef]
- Depeiges, A., & Dacheux, J. L. (1985). Acquisition of sperm motility and its maintenance during storage in the lizard, Lacerta vivipara. Reproduction, 74(1), 23-27. [CrossRef]
- Dosemane, D., & Bhagya, M. (2015). In vitro Study of the Spermatozoa Motility in the Lizard Eutropis carinata. International Journal of Zoological Research, 11(3), 89-95. [CrossRef]
- Feria-Ortiz, M., de Oca, A. N.-M., Ugarte, I. H. S., de Oca, A. N.-M., & Ugarte, I. H. S. (2001). Diet and Reproductive Biology of the Viviparous Lizard Sceloporus torquatus torquatus (Squamata: Phrynosomatidae). Journal of Herpetology, 35(1), 104. [CrossRef]
- Friesen, C. R., Kahrl, A. F., & Olsson, M. (2020). Sperm competition in squamate reptiles. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 375(1813), 20200079. [CrossRef]
- Fujihara, Y., Miyata, H., & Ikawa, M. (2018). Factors controlling sperm migration through the oviduct revealed by gene-modified mouse models. Experimental Animals, 67(2), 91-104. [CrossRef]
- Gadella, B. M., & Boerke, A. (2016). An update on post-ejaculatory remodeling of the sperm surface before mammalian fertilization. Theriogenology, 85(1), 113-124. [CrossRef]
- Ho, H., & Suarez, S. (2001). Hyperactivation of mammalian spermatozoa: Function and regulation. Reproduction, 122(4), 519-526. [CrossRef]
- Ickowicz, D., Finkelstein, M., & Breitbart, H. (2012). Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian Journal of Andrology, 14(6), 816-821. [CrossRef]
- Kito, S., & Ohta, Y. (2005). Medium effects on capacitation and sperm penetration through the zona pellucida in inbred BALB/c spermatozoa. Zygote, 13(2), 145-153. [CrossRef]
- Lemoine, M., Grasseau, I., Brillard, J. P., & Blesbois, E. (2008). A reappraisal of the factors involved in in vitro initiation of the acrosome reaction in chicken spermatozoa. Reproduction, 136(4), 391-399. [CrossRef]
- Martínez-Torres, M. (2009). Almacenamiento de espermatozoides en la vagina de la lagartija vivípara Sceloporus torquatus (Sauria: Prhynosomatidae). Acta zoológica mexicana, 25(3), 497-506.
- Martínez-Torres, M., Álvarez-Rodríguez, C., Luis, J., & Sánchez-Rivera, U. Á. (2019). Electroejaculation and semen evaluation of the viviparous lizard Sceloporus torquatus (Squamata: Phrynosomatidae). Zoo Biology, 38(4), 393-396. [CrossRef]
- Martínez-Torres, M., Sánchez-Rivera, U. Á., Cruz-Cano, N. B., Castro-Camacho, Y. J., Luis, J., & Medrano, A. (2019). A non-invasive method for semen collection and evaluation in small and median size lizards. Reproduction in Domestic Animals, 54, 54-58. [CrossRef]
- McPartlin, L. A., Littell, J., Mark, E., Nelson, J. L., Travis, A. J., & Bedford-Guaus, S. J. (2008). A defined medium supports changes consistent with capacitation in stallion sperm, as evidenced by increases in protein tyrosine phosphorylation and high rates of acrosomal exocytosis. Theriogenology, 69(5), 639-650. [CrossRef]
- McPartlin, L. A., Suarez, S. S., Czaya, C. A., Hinrichs, K., & Bedford-Guaus, S. J. (2009). Hyperactivation of Stallion Sperm Is Required for Successful In Vitro Fertilization of Equine Oocytes1. Biology of Reproduction, 81(1), 199-206. [CrossRef]
- Naijian, H. R., Kohram, H., Shahneh, A. Z., Sharafi, M., & Bucak, M. N. (2013). Effects of different concentrations of BHT on microscopic and oxidative parameters of Mahabadi goat semen following the freeze–thaw process. Cryobiology, 66(2), 151-155. [CrossRef]
- Nixon, B., Anderson, A. L., Smith, N. D., McLeod, R., & Johnston, S. D. (2016). The Australian saltwater crocodile ( Crocodylus porosus ) provides evidence that the capacitation of spermatozoa may extend beyond the mammalian lineage. Proceedings of the Royal Society B: Biological Sciences, 283(1830), 20160495. [CrossRef]
- Nixon, B., Cafe, S. L., Eamens, A. L., De Iuliis, G. N., Bromfield, E. G., Martin, J. H., Skerrett-Byrne, D. A., & Dun, M. D. (2020). Molecular insights into the divergence and diversity of post-testicular maturation strategies. Molecular and Cellular Endocrinology, 517, 110955. [CrossRef]
- Nixon, B., Johnston, S. D., Skerrett-Byrne, D. A., Anderson, A. L., Stanger, S. J., Bromfield, E. G., Martin, J. H., Hansbro, P. M., & Dun, M. D. (2019). Modification of Crocodile Spermatozoa Refutes the Tenet That Post-testicular Sperm Maturation Is Restricted To Mammals*. Molecular & Cellular Proteomics, 18, S58-S76. [CrossRef]
- Ortega-León, A. M., Cruz, M. V.-S., Zúñiga-Vega, J. J., Castillo, R. C., & Cruz, F. R. M. la. (2009). Sperm Viability in the Reproductive Tract of Females in a Population of Sceloporus mucronatus Exhibiting Asynchronous Reproduction. Western North American Naturalist, 69(1), 96-104. [CrossRef]
- Priyadarshana, C., Setiawan, R., Tajima, A., & Asano, A. (2020). Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus). PLOS ONE, 15(11), e0241181. [CrossRef]
- Puga Molina, L. C., Luque, G. M., Balestrini, P. A., Marín-Briggiler, C. I., Romarowski, A., & Buffone, M. G. (2018). Molecular Basis of Human Sperm Capacitation. Frontiers in Cell and Developmental Biology, 6, 72. [CrossRef]
- Rota, A., Peña, A. I., Linde-Forsberg, C., & Rodriguez-Martinez, H. (1999). In vitro capacitation of fresh, chilled and frozen–thawed dog spermatozoa assessed by the chlortetracycline assay and changes in motility patterns. Animal Reproduction Science, 57(3-4), 199-215. [CrossRef]
- Sáez-Espinosa, P., Huerta-Retamal, N., Robles-Gómez, L., Avilés, M., Aizpurua, J., Velasco, I., Romero, A., & Gómez-Torres, M. (2020). Influence of in vitro capacitation time on structural and functional human sperm parameters. Asian Journal of Andrology, 22(5), 447. [CrossRef]
- Sanchez-Rivera, U. Á. (2017). Análisis hormonal y citológico de la espermatogénesis mediante biopsia testicular en Sceloporus torquatus (Sauria: Phrynosomatidae) [Tesis para obtener el título de Licenciatura en Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México]. http://132.248.9.195/ptd2017/mayo/0759253/Index.html.
- Sanchez-Rivera, U. A., Medrano, A., Cruz-Cano, N. B., Alcantar-Rodriguez, A., Davila-Govantes, R., Castro-Camacho, Y. J., & Martinez-Torres, M. (2022). Implementation of a method for sperm cryopreservation in sceloporine lizards. CONSERVATION PHYSIOLOGY, 10(1), coac068. [CrossRef]
- Sinervo, B., Méndez-de-la-Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., Villagrán-Santa Cruz, M., Lara-Resendiz, R., Martínez-Méndez, N., Calderón-Espinosa, M. L., Meza-Lázaro, R. N., Gadsden, H., Avila, L. J., Morando, M., De la Riva, I. J., Sepulveda, P. V., Rocha, C. F. D., Ibargüengoytía, N., Puntriano, C. A., Massot, M., … Sites, J. W. (2010). Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science, 328(5980), 894-899. [CrossRef]
- Soriano-Úbeda, C., Romero-Aguirregomezcorta, J., Matás, C., Visconti, P. E., & García-Vázquez, F. A. (2019). Manipulation of bicarbonate concentration in sperm capacitation media improves in vitro fertilisation output in porcine species. Journal of Animal Science and Biotechnology, 10(1), 19. [CrossRef]
- Stival, C., Puga Molina, L. D. C., Paudel, B., Buffone, M. G., Visconti, P. E., & Krapf, D. (2016). Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. En M. G. Buffone (Ed.), Sperm Acrosome Biogenesis and Function During Fertilization (Vol. 220, pp. 93-106). Springer International Publishing. [CrossRef]
- Suarez, S. S. (2008). Control of hyperactivation in sperm. Human Reproduction Update, 14(6), 647-657. [CrossRef]
- Vyklicka, L., & Lishko, P. V. (2020). Dissecting the signaling pathways involved in the function of sperm flagellum. Current Opinion in Cell Biology, 63, 154-161. [CrossRef]
- Witte, T. S., & Schäfer-Somi, S. (2007). Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Animal Reproduction Science, 102(3-4), 181-193. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).