Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Characterisation
2.2. Amine Media
- 30 wt.% Monoethanolamine (MEA), CAS: 141-43-5, a primary amine extensively utilized for CO2 capture [2].
- 37 wt.% Methyldiethanolamine (MDEA) CAS: 105-59-9, a tertiary amine
- 30 wt.% MDEA + 21 wt.% piperazine (PZ), CAS: 110-85-0, generally used to improve capture kinetics [35].
- 30 wt.% 2-Amino-2-methyl-1-propanol (AMP), CAS: 124-68-5, a sterically hindered primary amine known for its elevated CO2 absorption capacity [36].
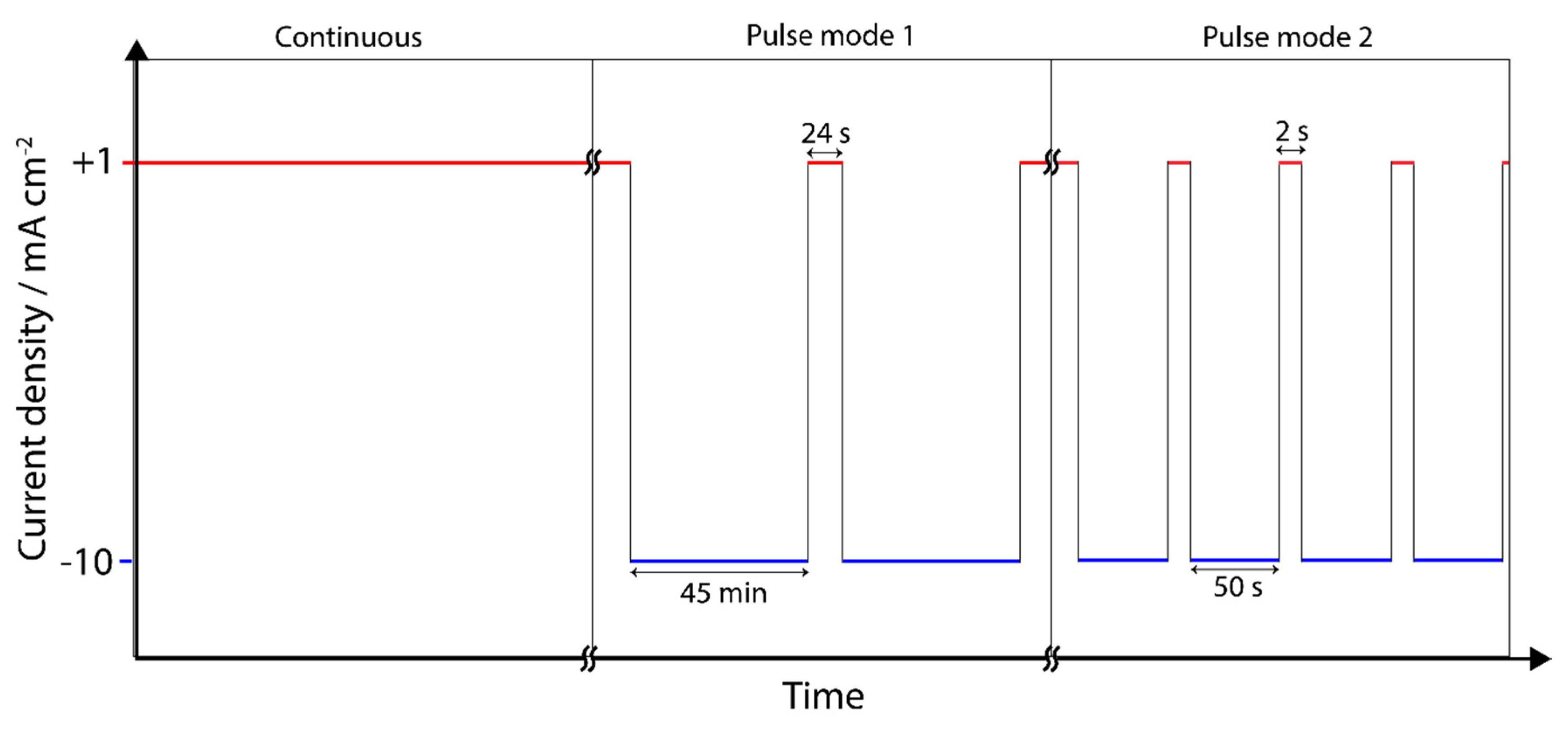
2.2. Electrochemical Tests
3. Results
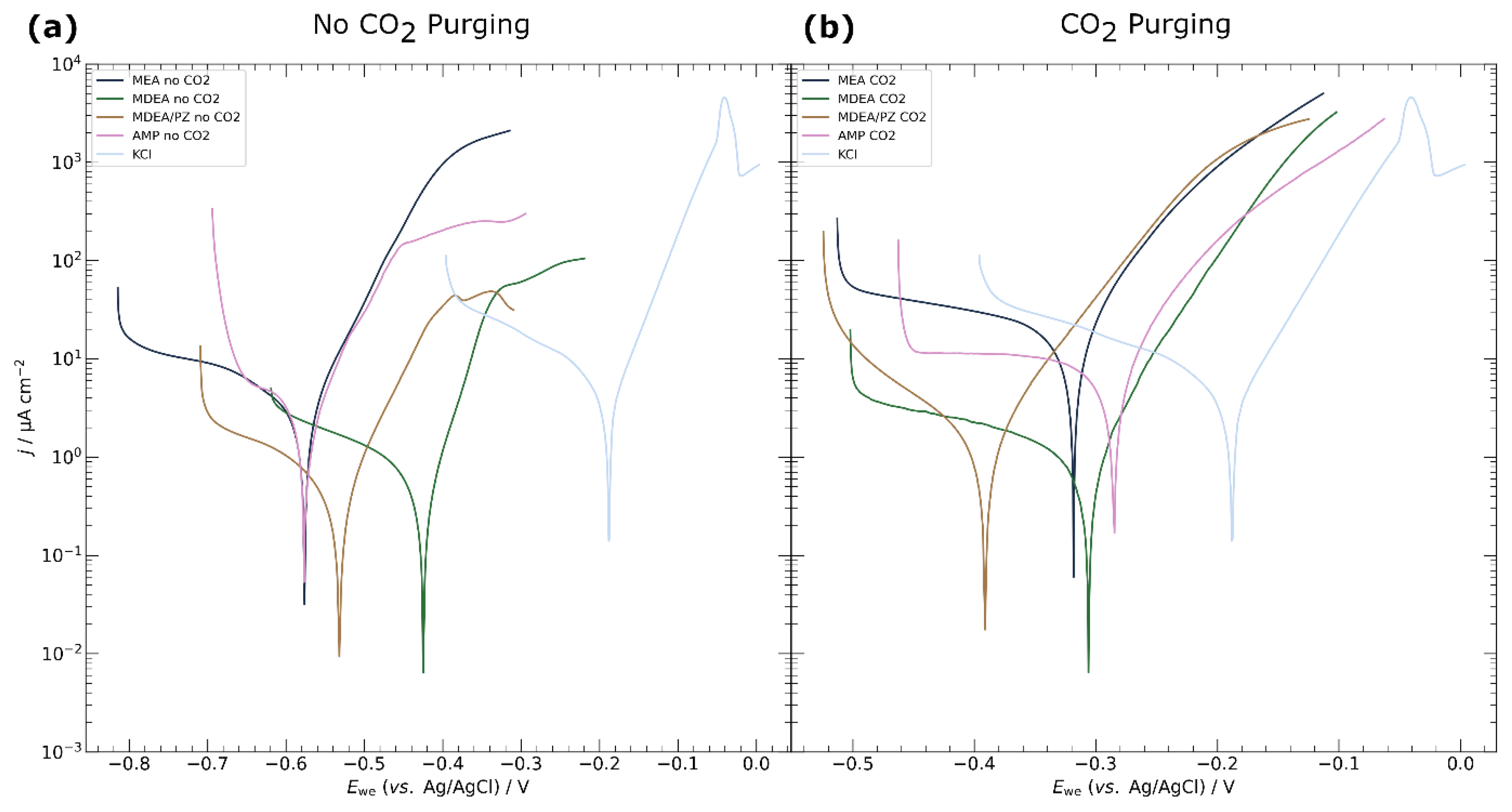
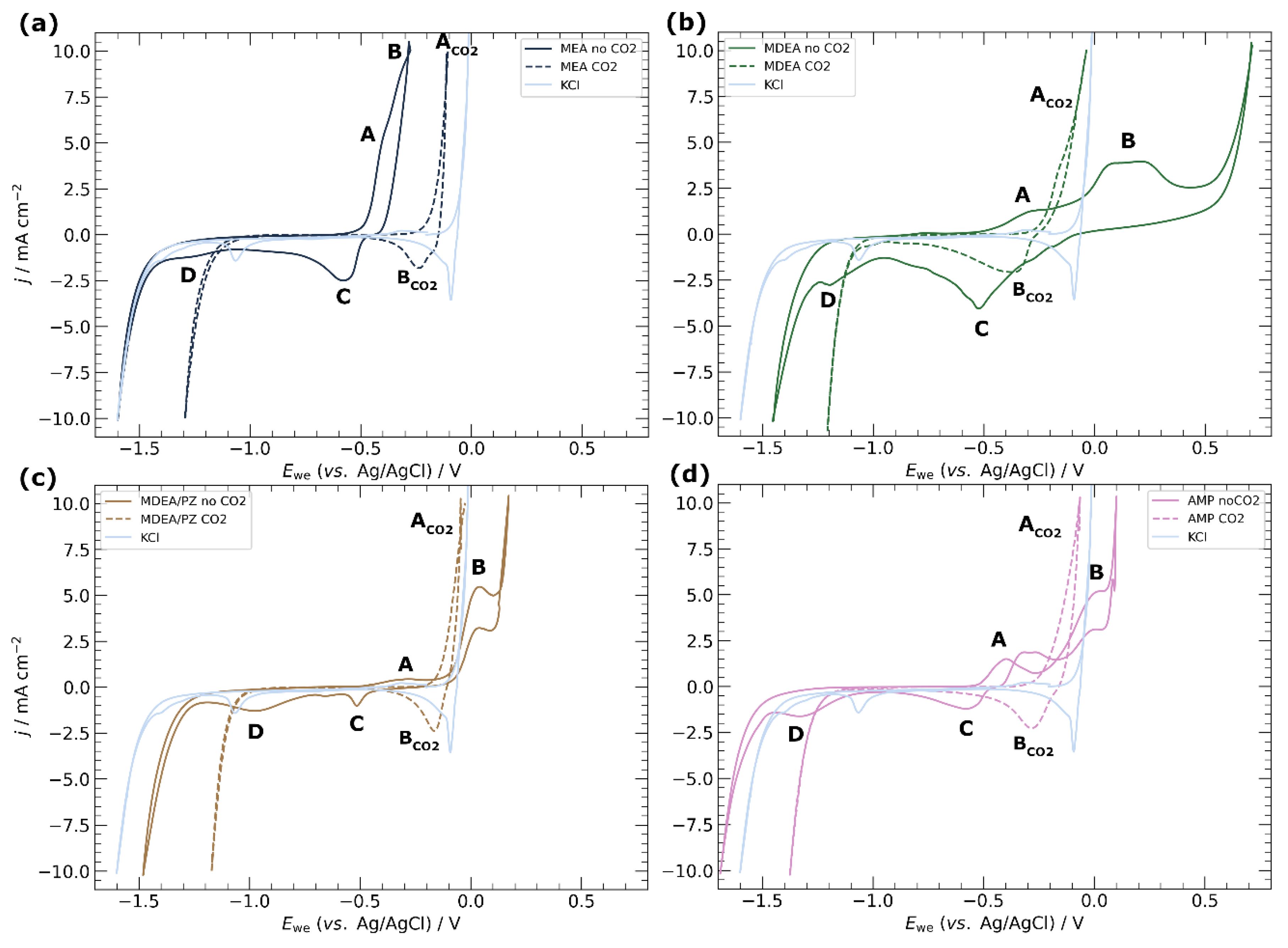
3.1. Electrochemical Characterisation of Electrodeposited Copper in Amine Media
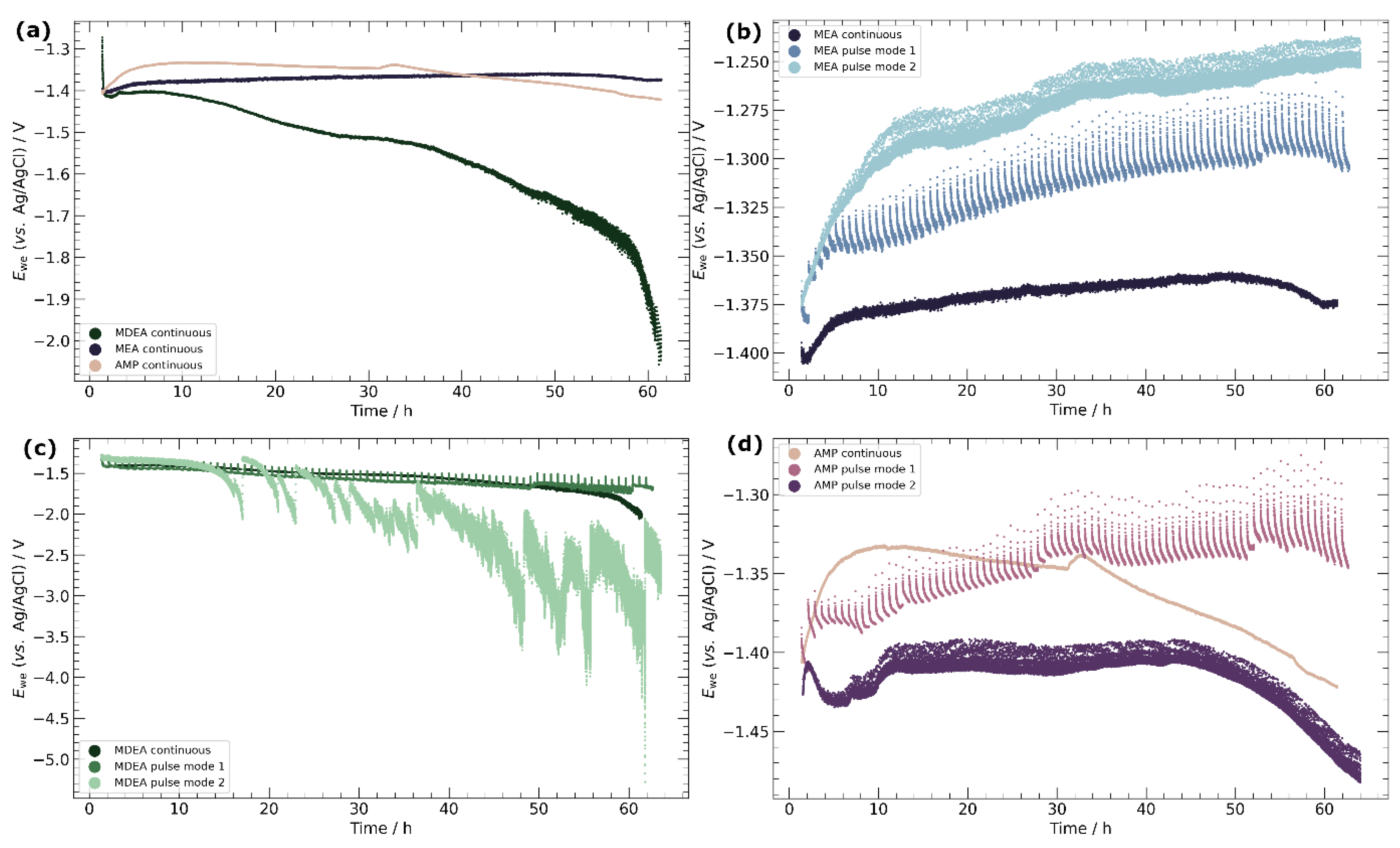
3.1. Chronopotentiometry
4. Discussion
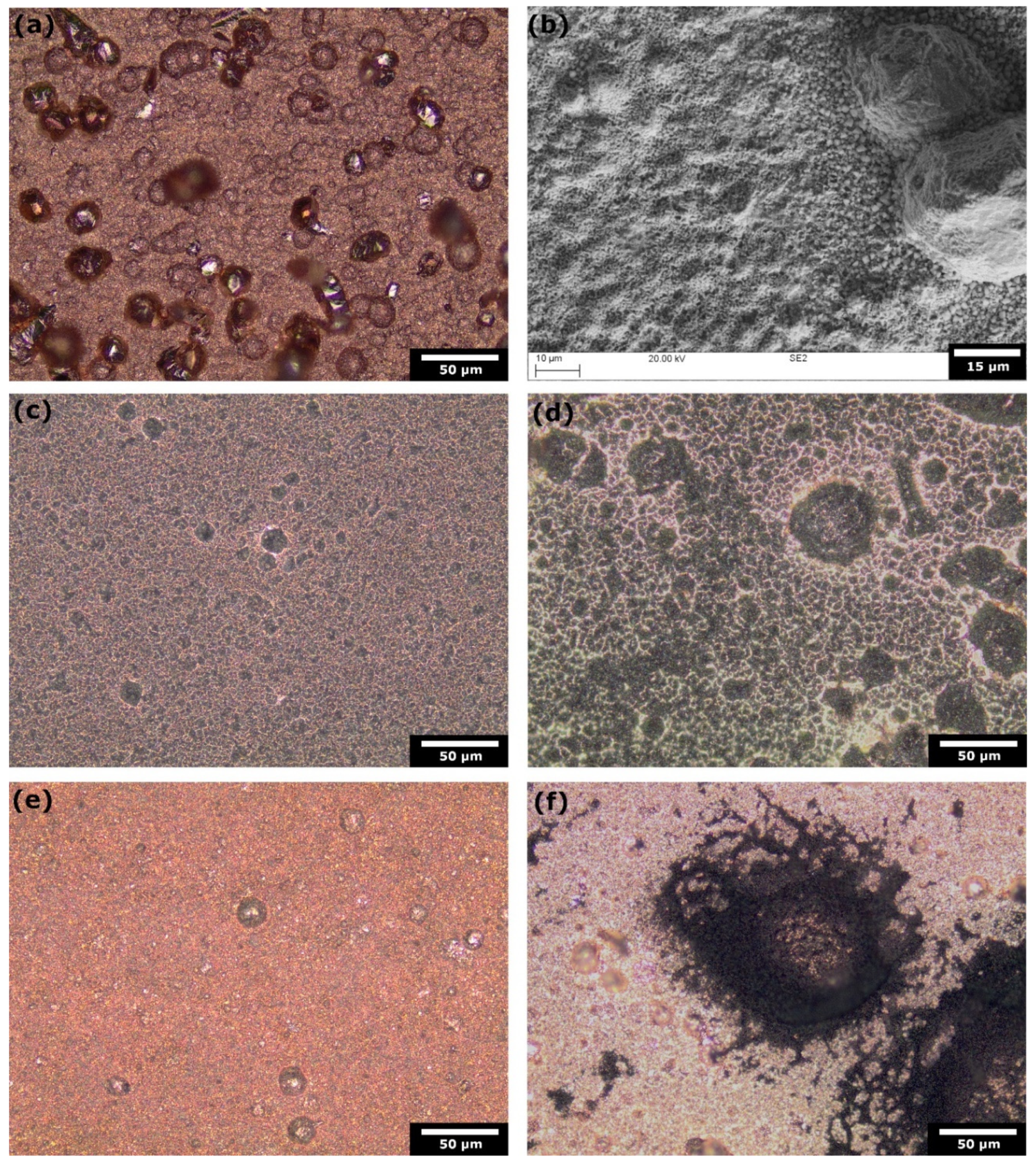
4.1. Corrosion of Copper Catalyst in Amine Media
3.3. Choice of Amine Capture Media for ECR
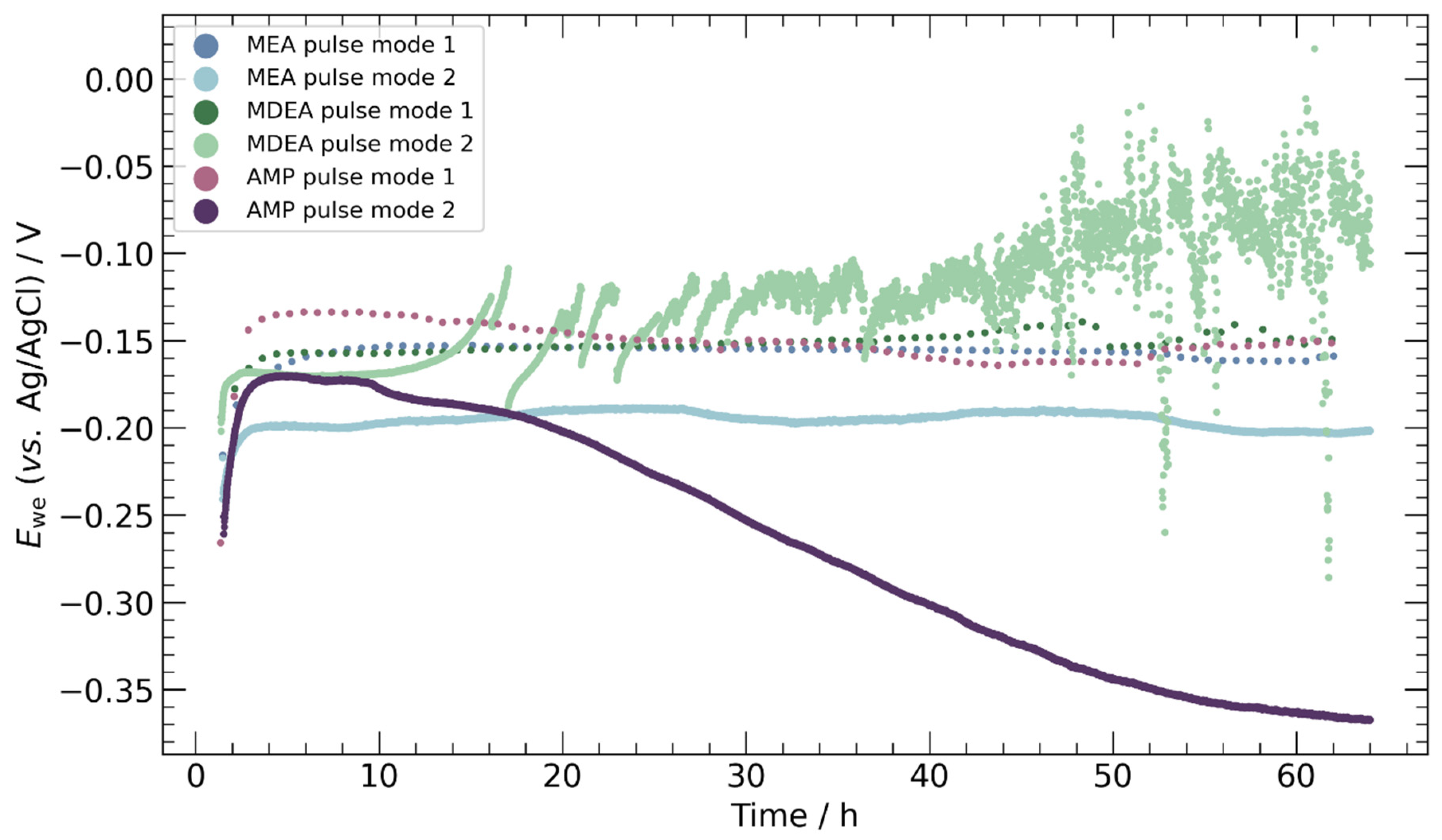
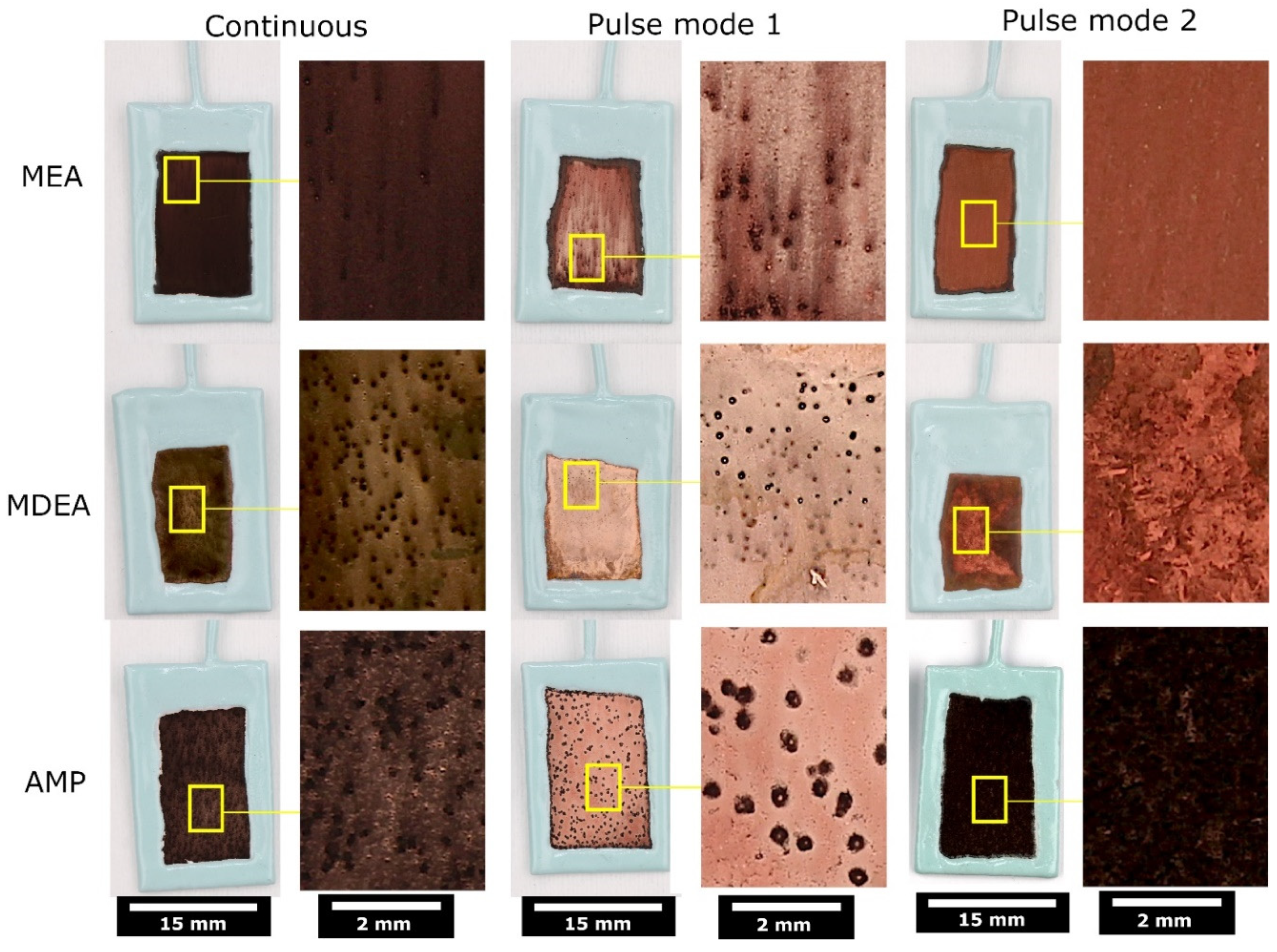
3.3. Pulse ECR in Amine Media
5. Conclusions
- Corrosion is not a significant impediment to the catalyst's longevity in amine media. Both computational modelling and experimental data corroborate that the inherent corrosion of copper in these conditions does not critically limit the operational lifespan of the catalyst. This insight alleviates concerns regarding the durability of copper catalysts in practical ECR applications.
- Primary amines, particularly MEA, demonstrate a higher compatibility with ECR processes, characterized by the absence of carbonate salt precipitation and more stable potentials over time. This observation emphasizes the importance of considering the amine type in optimizing ECR performance and underscores the potential for tailored catalyst-amine combinations to improve efficiency.
- Pulse ECR demonstrated significant potential in improving ECR stability, manifested by a shift in cathodic potential and effective mitigation of deposit on the catalyst surface through periodic oxidation. This highlights the importance of exploring innovative operational strategies to augment the stability and efficiency of ECR processes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- H. L. van Soest, M. G. J. den Elzen, and D. P. van Vuuren, “Net-zero emission targets for major emitting countries consistent with the Paris Agreement,” Nat Commun, vol. 12, no. 1, p. 2140, 2021. [CrossRef]
- G. T. Rochelle, “Amine Scrubbing for CO2 Capture,” Science (1979), vol. 325, no. 5948, pp. 1652–1654, 2009. [CrossRef]
- M. E. Boot-Handford et al., “Carbon capture and storage update,” Energy Environ Sci, vol. 7, no. 1, pp. 130–189, 2014. [CrossRef]
- J. B. Jakobsen, M. H. Rønne, K. Daasbjerg, and T. Skrydstrup, “Are Amines the Holy Grail for Facilitating CO2 Reduction?,” Angewandte Chemie International Edition, vol. 60, no. 17, pp. 9174–9179, 2021. [CrossRef]
- I. Sullivan et al., “Coupling electrochemical CO2 conversion with CO2 capture,” Nat Catal, vol. 4, no. 11, pp. 952–958, 2021. [CrossRef]
- D. Wakerley et al., “Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers,” Nat Energy, vol. 7, no. 2, pp. 130–143, 2022. [CrossRef]
- S. Nitopi et al., “Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte,” Chem Rev, vol. 119, no. 12, pp. 7610–7672, Jun. 2019. [CrossRef]
- M. G. Kibria et al., “Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design,” Advanced Materials, vol. 31, no. 31, 2019. [CrossRef]
- F. P. García de Arquer et al., “CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2,” Science (1979), vol. 367, no. 6478, pp. 661–666, 2020. [CrossRef]
- Y. Wang, J. Liu, and G. Zheng, “Designing Copper-Based Catalysts for Efficient Carbon Dioxide Electroreduction,” Advanced Materials, vol. 33, no. 46, p. 2005798, 2021. [CrossRef]
- C. Xiao and J. Zhang, “Architectural Design for Enhanced C2 Product Selectivity in Electrochemical CO2 Reduction Using Cu-Based Catalysts: A Review,” ACS Nano, vol. 15, no. 5, pp. 7975–8000, 2021. [CrossRef]
- J. Zhao, S. Xue, J. Barber, Y. Zhou, J. Meng, and X. Ke, “An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction,” J Mater Chem A Mater, vol. 8, no. 9, pp. 4700–4734, 2020. [CrossRef]
- S. Popović, M. Smiljanić, P. Jovanovič, J. Vavra, R. Buonsanti, and N. Hodnik, “Stability and Degradation Mechanisms of Copper-Based Catalysts for Electrochemical CO2 Reduction,” Angewandte Chemie International Edition, vol. 59, no. 35, pp. 14736–14746, 2020. [CrossRef]
- J. Huang et al., “Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction,” Nat Commun, vol. 9, no. 1, 2018. [CrossRef]
- G. H. Simon, C. S. Kley, and B. Roldan Cuenya, “Potential-Dependent Morphology of Copper Catalysts During CO2 Electroreduction Revealed by In Situ Atomic Force Microscopy,” Angewandte Chemie International Edition, vol. 60, no. 5, pp. 2561–2568, 2021. [CrossRef]
- D. Gao, R. M. Arán-Ais, H. S. Jeon, and B. Roldan Cuenya, “Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products,” Nat Catal, vol. 2, no. 3, pp. 198–210, 2019. [CrossRef]
- P. Grosse, D. Gao, F. Scholten, I. Sinev, H. Mistry, and B. Roldan Cuenya, “Dynamic Changes in the Structure, Chemical State and Catalytic Selectivity of Cu Nanocubes during CO2 Electroreduction: Size and Support Effects,” Angewandte Chemie International Edition, vol. 57, no. 21, pp. 6192–6197, 2018. [CrossRef]
- H. Jung et al., “Electrochemical Fragmentation of Cu2O Nanoparticles Enhancing Selective C–C Coupling from CO2 Reduction Reaction,” J Am Chem Soc, vol. 141, no. 11, pp. 4624–4633, 2019. [CrossRef]
- S. Popovic, M. Bele, and N. Hodnik, “Reconstruction of Copper Nanoparticles at Electrochemical CO2 Reduction Reaction Conditions Occurs via Two-step Dissolution/Redeposition Mechanism,” ChemElectroChem, vol. 8, no. 14, pp. 2634–2639, 2021. [CrossRef]
- S. Garg et al., “How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers,” Energy Environ Sci, vol. 16, no. 4, pp. 1631–1643, 2023. [CrossRef]
- Y. Xu et al., “Self-Cleaning CO2 Reduction Systems: Unsteady Electrochemical Forcing Enables Stability,” ACS Energy Lett, vol. 6, no. 2, pp. 809–815, 2021. [CrossRef]
- M. Sassenburg, M. Kelly, S. Subramanian, W. A. Smith, and T. Burdyny, “Zero-Gap Electrochemical CO2 Reduction Cells: Challenges and Operational Strategies for Prevention of Salt Precipitation,” ACS Energy Lett, vol. 8, no. 1, pp. 321–331, 2023. [CrossRef]
- C. A. Obasanjo, G. Gao, B. N. Khiarak, T. H. Pham, J. Crane, and C.-T. Dinh, “Progress and Perspectives of Pulse Electrolysis for Stable Electrochemical Carbon Dioxide Reduction,” Energy and Fuels, vol. 37, no. 18, pp. 13601–13623, 2023. [CrossRef]
- C. A. Obasanjo et al., “In situ regeneration of copper catalysts for long-term electrochemical CO<inf>2</inf> reduction to multiple carbon products,” J Mater Chem A Mater, vol. 10, no. 37, pp. 20059–20070, 2022. [CrossRef]
- X.-D. Zhang et al., “Asymmetric Low-Frequency Pulsed Strategy Enables Ultralong CO2 Reduction Stability and Controllable Product Selectivity,” J Am Chem Soc, vol. 145, no. 4, pp. 2195–2206, 2023. [CrossRef]
- H. S. Jeon et al., “Selectivity Control of Cu Nanocrystals in a Gas-Fed Flow Cell through CO2 Pulsed Electroreduction,” J Am Chem Soc, vol. 143, no. 19, pp. 7578–7587, 2021. [CrossRef]
- T. N. Nguyen et al., “Catalyst Regeneration via Chemical Oxidation Enables Long-Term Electrochemical Carbon Dioxide Reduction,” J Am Chem Soc, vol. 144, no. 29, pp. 13254–13265, 2022. [CrossRef]
- J. Yano and S. Yamasaki, “Pulse-mode electrochemical reduction of carbon dioxide using copper and copper oxide electrodes for selective ethylene formation,” J Appl Electrochem, vol. 38, no. 12, pp. 1721–1726, 2008. [CrossRef]
- E. R. Cofell et al., “Potential Cycling of Silver Cathodes in an Alkaline CO2 Flow Electrolyzer for Accelerated Stress Testing and Carbonate Inhibition,” ACS Appl Energy Mater, vol. 5, no. 10, pp. 12013–12021, 2022. [CrossRef]
- M. Abdinejad, Z. Mirza, X. Zhang, and H.-B. Kraatz, “Enhanced Electrocatalytic Activity of Primary Amines for CO2 Reduction Using Copper Electrodes in Aqueous Solution,” ACS Sustain Chem Eng, vol. 8, no. 4, pp. 1715–1720, 2020. [CrossRef]
- B. Bohlen, N. Daems, and T. Breugelmans, “Electrochemical Production of Formate Directly from Amine-Based CO2 Capture Media,” ECS Meeting Abstracts, vol. MA2023-01, no. 26, p. 1722, 2023. [CrossRef]
- M. N. Hossain, S. Ahmad, I. S. da Silva, and H.-B. Kraatz, “Electrochemical Reduction of CO2 at Coinage Metal Nanodendrites in Aqueous Ethanolamine,” Chemistry – A European Journal, vol. 27, no. 4, pp. 1346–1355, 2021. [CrossRef]
- L. Chen et al., “Electrochemical Reduction of Carbon Dioxide in a Monoethanolamine Capture Medium,” ChemSusChem, vol. 10, no. 20, pp. 4109–4118, 2017. [CrossRef]
- D. Filotás, T. Nagy, L. Nagy, P. Mizsey, and G. Nagy, “Extended Investigation of Electrochemical CO2 Reduction in Ethanolamine Solutions by SECM,” Electroanalysis, vol. 30, no. 4, pp. 690–697, 2018. [CrossRef]
- P. T. Frailie and G. T. Rochelle, “Kinetics of Aqueous Methyldiethanolamine/Piperazine for CO2 Capture,” in Process Systems and Materials for CO2 Capture, 2017, pp. 137–152. [CrossRef]
- G. Sartori and D. W. Savage, “Sterically hindered amines for carbon dioxide removal from gases,” Industrial & Engineering Chemistry Fundamentals, vol. 22, no. 2, pp. 239–249, 1983. [CrossRef]
- K. R. Trethewey and J. Chamberlain, Corrosion for science and engineering, second edition. United States: NACE International, Houston, TX (United States), 1995. [Online]. Available: https://www.osti.gov/biblio/378108.
- B. Beverskog and I. Puigdomenech, “Revised Pourbaix Diagrams for Copper at 25 to 300°C,” J Electrochem Soc, vol. 144, no. 10, p. 3476, 1997. [CrossRef]
- S. M. Abd el Haleem and B. G. Ateya, “Cyclic voltammetry of copper in sodium hydroxide solutions,” J Electroanal Chem Interfacial Electrochem, vol. 117, no. 2, pp. 309–319, 1981. [CrossRef]
- G. Astm, “Standard practice for calculation of corrosion rates and related information from electrochemical measurements,” G102-89, ASTM International, West Conshohocken, USA, 2004.
- C. W. Li and M. W. Kanan, “CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films,” J Am Chem Soc, vol. 134, no. 17, pp. 7231–7234, 2012. [CrossRef]
- N. MacDowell et al., “An overview of CO2 capture technologies,” Energy Environ Sci, vol. 3, no. 11, pp. 1645–1669, 2010. [CrossRef]
- F. Bougie and M. C. Iliuta, “Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams,” J Chem Eng Data, vol. 57, no. 3, pp. 635–669, 2012. [CrossRef]
- M. Edali, A. Aboudheir, and R. Idem, “Kinetics of carbon dioxide absorption into mixed aqueous solutions of MDEA and MEA using a laminar jet apparatus and a numerically solved 2D absorption rate/kinetics model,” International Journal of Greenhouse Gas Control, vol. 3, no. 5, pp. 550–560, 2009. [CrossRef]
| Media | CO2 | Ecorr / V | jcorr / µA cm-2 | Rp / kΩ cm2 |
| MEA | no | -0.58 ± 0.01 | 2.4 ± 0.1 | 3.8 ± 0.7 |
| yes | -0.29 ± 0.02 | 14.1 ± 6.9 | 0.7 ± 0.2 | |
| MDEA | no | -0.42 ± 0.01 | 0.3 ± 0.1 | 19.0 ± 4.4 |
| yes | -0.33 ± 0.03 | 1.0 ± 0.1 | 10.4 ± 0.5 | |
| MDEA/PZ | no | -0.53 ± 0.01 | 0.4 ± 0.1 | 18.3 ± 6.3 |
| yes | -0.39 ± 0.01 | 0.9 ± 0.6 | 12.6 ± 11.3 | |
| AMP | no | -0.57 ± 0.01 | 1.5 ± 0.3 | 5.6 ± 1.1 |
| yes | -0.29 ± 0.02 | 7.7 ± 1.3 | 1.0 ± 0.6 | |
| KCl | no | -0.20 ± 0.03 | 2.4 ± 0.6 | 3.7 ± 1.1 |
| Dissolution into Cu(I) | Dissolution into Cu(II) | ||||
| Media | CO2 | CR / mm y-1 | Time / days | CR / mm y-1 | Time / days |
| MEA | no | 0.056 | 328 | 0.028 | 655 |
| yes | 0.327 | 56 | 0.164 | 112 | |
| MDEA | no | 0.007 | 2623 | 0.003 | 5242 |
| yes | 0.023 | 787 | 0.012 | 1573 | |
| MDEA/PZ | no | 0.009 | 1967 | 0.005 | 3931 |
| yes | 0.021 | 874 | 0.010 | 1747 | |
| AMP | no | 0.035 | 525 | 0.017 | 1048 |
| yes | 0.179 | 102 | 0.089 | 204 | |
| KCl | no | 0.056 | 328 | 0.028 | 655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





