Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Recruitment of Participants
2.2. Sampling and Analysis
2.3. Data Management and Statistical Analysis
3. Result
3.1. Distribution of Socio-Demographic and Clinical Phenotypes of Patients
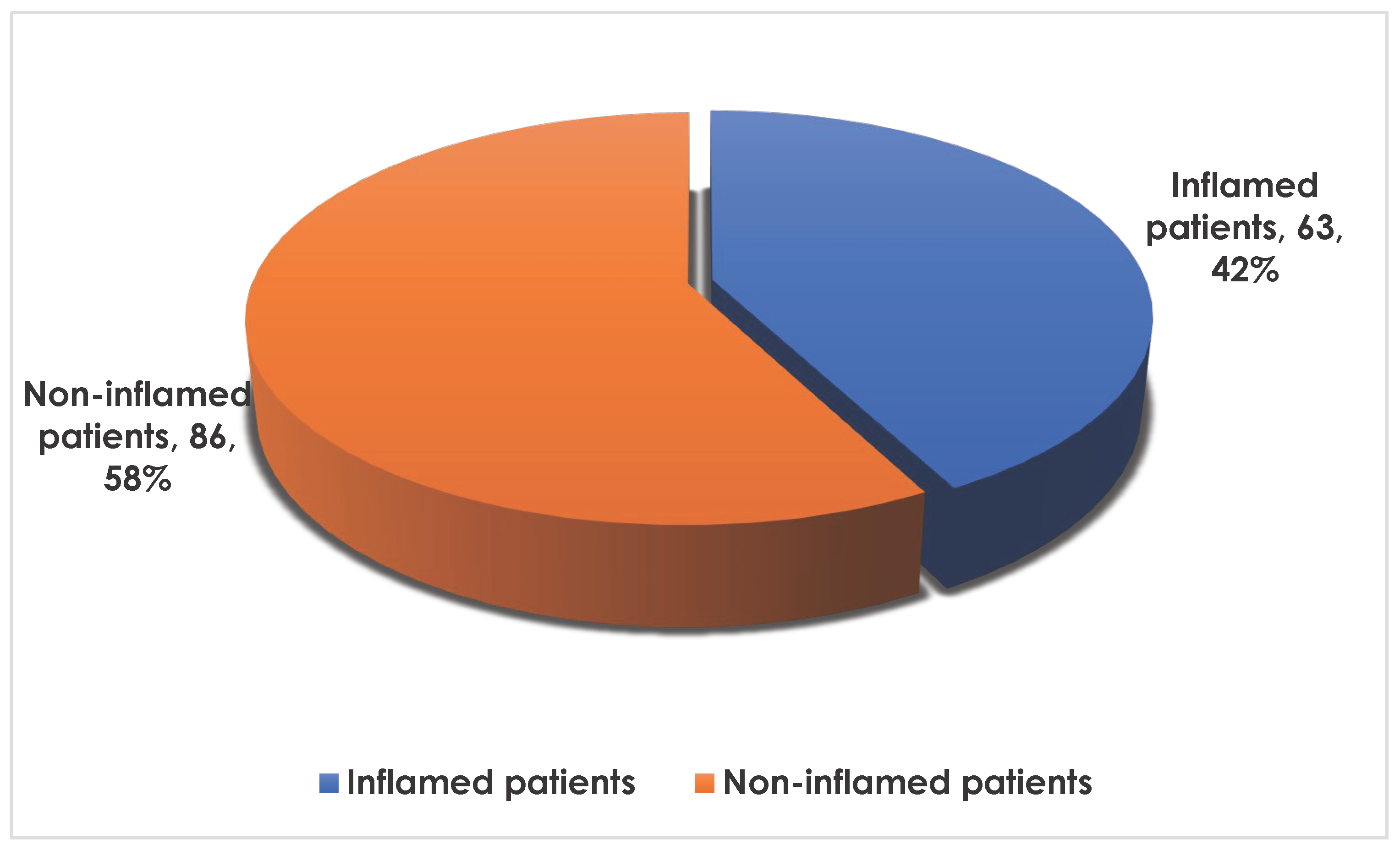
3.2. Frequency of Inflammation in the Population
3.3. Distribution of Socio-Demographic and Clinical Phenotypes of Patients Depending on Inflammation
3.4. Distribution of the Study Population According to Blood Count Profile
3.4.1. Frequency of Hemogram Disorders in the Population
3.4.2. Profile of the Complete Population Hemogram
3.5. Distribution of the Study Population by Serum Parameters
3.6. Factors Associated with Inflammation in Sickle Cell Patients: Multivariate Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meher S: Mohanty PK, Patel S, Das K, Sahoo S, Dehury S, et al. Haptoglobin Genotypes Associated with Vaso-Occlusive Crisis in Sickle Cell Anemia Patients of Eastern India. Hemoglobin. Taylor & Francis; 2020;0:1–7. [CrossRef]
- Nkashama GM, Wakamb GKA, Mulangu AM, Nkashama GM, Kupa BK, Numbi OL. De l’hémoglobine SS à SF: Intérêt de l’hydroxyurée dans la prise en charge de la drépanocytose chez 2 enfants congolais et revue de la literature. Pan Afr Med J. 2015;21:1317–22.
- Cabannes R, Bonhomme J. Les hémoglobinopathies. Vie Med. Can. Fr. 1972. p. 458–67.
- Id HAL. Marqueurs du métabolisme du fer et dérivés de la L-arginine dans la cardiopathie ischémique : mise en évidence, intérêt de leur évaluation et rôle du stress oxydant en phase aiguë d ’ infarctus du myocarde Aurélie Gudjoncik To cite this version : HAL Id. 2018;
- Al-Saqladi AWM, Bin-Gadeem HA, Brabin BJ. Utility of plasma transferrin receptor, ferritin, and inflammatory markers in children with sickle cell disease. Paediatr Int Child Health. 2012;32:27–34. [CrossRef]
- Inati A, Musallam KM, Cappellini MD, Duca L, Taher AT. Nontransferrin-bound iron in transfused patients with sickle cell disease. Int J Lab Hematol. 2011;33:133–7. [CrossRef]
- Mangaonkar AA, Thawer F, Son J, Ajebo G, Xu H, Barrett NJ, et al. Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br J Haematol. 2020;189:1204–9. [CrossRef]
- Muazzam Nasrullah 2018. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2016;176:139–48.
- Bandeira ICJ, Rocha LBS, Barbosa MC, Elias DBD, Querioz JAN, Freitas MVC, et al. Chronic inflammatory state in sickle cell anemia patients is associated with HBB*S haplotype. Cytokine. Elsevier Ltd.; 2014;65:217–21. [CrossRef]
- Zahran AM, Nafady A, Saad K, Hetta HF, Abdallah AEM, Abdel-Aziz SM, et al. Effect of Hydroxyurea Treatment on the Inflammatory Markers Among Children With Sickle Cell Disease. Clin Appl Thromb. 2020;26. [CrossRef]
- Doupa D, Djite M, Gueye PM, Seck M, Faye BF, Seck SM, et al. Profil biochimique et hématologique des patients drépanocytaires homozygotes en phase stationnaire au centre National de Transfusion Sanguine de Dakar. Int J Biol Chem Sci. 2017;11:1706. [CrossRef]
- Teixeira J, Azevedo C De, Cristina K, Malmegrim R. Immune mechanisms involved in sickle cell disease pathogenesis : current knowledge and perspectives. Immunol Lett [Internet]. Elsevier; 2020;224:1–11. [CrossRef]
- Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. 2019; [CrossRef]
- Nader E, Romana M, Connes P. The Red Blood Cell — Inflammation Vicious Circle in Sickle Cell Disease. 2020;11:1–11. [CrossRef]
- Tshilolo L. abc. 2009;67:607–12.
- World Medical Association. Declaration of Helsinki, ethical principles for scientific requirements and research protocols. Bull World Health Organ [Internet]. 2013;1–4. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- Access O. Case series. 2016;8688:1–5.
- Houwing ME, de Pagter PJ, van Beers EJ, Biemond BJ, Rettenbacher E, Rijneveld AW, et al. Sickle cell disease: Clinical presentation and management of a global health challenge. Blood Rev [Internet]. Elsevier; 2019;37:100580. [CrossRef]
- Sombodi U, Wembonyama SO, Luboya O. Profil hématologique et nutritionnel du drépanocytaire homozygote SS âgé de 6 à 59 mois à Lubumbashi, République Démocratique du Congo. 2015;8688:1–6.
- Wonkam A, Josiane V, Bitoungui N, Ngogang J. Africa : beyond the preliminary data from Cameroon. 2016;18:237–41.
- Rees DC, Gibson JS. Biomarkers in sickle cell disease. Br J Haematol. 2012;156:433–45. [CrossRef]
- Cople-rodrigues CS, Omena J, Fleury MK, Bacelo AC, Koury JC, Citelli M. Selenium status and hemolysis in SCD patients. Nutrients. 2019;11:1–11.
- Ofori DA, Anjarwalla P, Mwaura L, Jamnadass R, Stevenson PC, Smith P, et al. No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共分散構造分析Title. Molecules. 2020;2:1–12.
- A TB, Agbetiafa K, M SYAG. Rev. CAMES-Série A, Sciences et Médecine Profil lipidoprotéinique, risque athérogène et état inflammatoire chez les porteurs du trait drépanocytaire au Togo LIPID, LIPOPROTEIN PROFILE, ATHEROGENIC RISK AND INFLAMMATORY STATUS IN SICKLE-CELL TRAIT CAR. 2011;12:209–15.
- Omena J, Cople-Rodrigues C dos S, Cardoso JD do A, Soares AR, Fleury MK, Brito F dos SB, et al. Serum hepcidin concentration in individuals with sickle cell anemia: Basis for the dietary recommendation of Iron. Nutrients. 2018;10.
- Sarray S, Saleh LR, Saldanha FL, Al-habboubi HH, Mahdi N, Almawi WY. Cytokine Serum IL-6, IL-10, and TNF levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady-state condition. Cytokine [Internet]. Elsevier Ltd.; 2015;72:43–7. [CrossRef]
- Makani J, Ofori-Acquah SF, Nnodu O, Wonkam A, Ohene-Frempong K. Sickle cell disease: New opportunities and challenges in Africa. Sci World J. 2013;2013. [CrossRef]
- Yahouédéhou SCMA, da Guarda CC, Figueiredo CVB, Santiago RP, Carvalho SP, Fiuza LM, et al. Hydroxyurea alters hematological, biochemical and inflammatory biomarkers in Brazilian children with SCA: Investigating associations with βS haplotype and α-thalassemia. PLoS One. 2019;14:1–13.
- Makulo JR, Itokua KE, Lepira RK, Bundutidi GM, Aloni MN, Ngiyulu RM, et al. The magnitude of elevated iron stores and risk associated in steady state sickle cell anemia Congolese children: A cross-sectional study. BMC Hematol. BMC Hematology; 2019;19:1–6.
- Um SSN, Seungue J, Alima AY, Mbono R, Mbassi H, Chelo D, et al. A cross-sectional study of the growth of children with sickle cell disease, aged 2 to 5 years in Yaoundé, Cameroon. Pan Afr Med J. 2019;34:1–10.
- Lee N, Makani J, Tluway F, Makubi A, Armitage AE, Pasricha S, et al. EBioMedicine Decreased Hepcidin Levels Are Associated with Low Steady-state Hemoglobin in Children With Sickle Cell Disease in Tanzania. EBioMedicine [Internet]. The Authors; 2019;34:158–64. [CrossRef]
- Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–13. [CrossRef]
- Tshilolo L, Ngole ZM, Ngiyulu R, D KN. Le statut martial chez soixante-douze drépanocytaires homozygotes congolais. 2016;83–7.
- Itokua KE, Makulo JR, Lepira FB, Aloni MN, Ekulu PM, Sumaili EK, et al. Albuminuria, serum antioxidant enzyme levels and markers of hemolysis and inflammation in steady state children with sickle cell anemia. BMC Nephrol [Internet]. BMC Nephrology; 2016;17:1–6. [CrossRef]
- Sanogo I, Belinga S, Guifo O, Wamba G. Degree of Anemia, Indirect Markers of Hemolysis, and Vascular Complications of Sickle Cell Disease in Africa. Short title: Anemia, Hemolysis Markers and sickle cell disease complications. 2017;1–24. [CrossRef]
| Variables | Effective | Frequency (%) | |
|---|---|---|---|
| Sex: | F | 74 | (49.7) |
| M | 75 | (50.3) | |
| BMI | Thin | 98 | (65.77) |
| Normal | 51 | (34.23) | |
| History of infectious crises: | No | 101 | (67.8) |
| Yes | 48 | (32.2) | |
| History of anemic crises: | No | 71 | (47.7) |
| Yes | 78 | (52.3) | |
| History of VOS: | No | 68 | (45.6) |
| Yes | 81 | (54.4) | |
| History of CVD and stroke | No | 146 | (98.0) |
| Yes | 3 | (2.01) | |
| Mean VOS/month, Med (IQR) | 1.00 [0.00-2.00] | ||
| Age, Med[q1-q3] | 9.00 [4.00-13.0] | ||
| BMI, Mean±sd | 17.2±3.07 | ||
| Variables |
No N=86 n(%) |
Yes N=63 n(%) |
p-value | |
|---|---|---|---|---|
| Sex: | F | 35 (40.7) | 39 (61.9) | 0.017* |
| M | 51 (59.3) | 24 (38.1) | ||
| Age, Med[q1-q3] | 8.00 [5.00-12.0] | 10.0 [4.00-14.0] | 0.461 | |
| BMI, Mean±sd | 17.0±3.15 | 17.4±2.96 | 0.482 | |
| BMI | Thin | 58(67.44) | 40(63.49) | 0.864 |
| Normal | 28(32.56) | 23(36.51) | ||
| History of infectious crises: | No | 59 (68.6) | 42 (66.7) | 0.942 |
| Yes | 27 (31.4) | 21 (33.3) | ||
| History of anemic crises: | No | 43 (50.0) | 28 (44.4) | 0.614 |
| Yes | 43 (50.0) | 35 (55.6) | ||
| History of VOS: | No | 43 (50.0) | 25 (39.7) | 0.279 |
| Yes | 43 (50.0) | 38 (60.3) | ||
| History of CVD and stroke | No | 86 (100) | 60 (95.2) | 0.074 |
| Yes | 0 (0.00) | 3 (4.76) | ||
| Mean VOS/month, Med (IQR) | 1.00 [0.00-1.00] | 1.00 [1.00-2.00] | 0.072 | |
| Variables | Non -inflamed N=86n(%) | Inflamed N=63n(%) |
Total N=149 n(%) |
p-value | OR [IC 95%] | p-value OR | |
|---|---|---|---|---|---|---|---|
| RBC | Low | 83 (96.5) | 52 (82.5) | 135 (90.6) | 0.009 | - | 0.154 |
| High | 0 (0.00) | 2 (3.17) | 2 (1.34) | Ref. | Ref. | ||
| Normal | 3 (3.49) | 9 (14.3) | 12 (8.05) | - | 0.604 | ||
| Reticulocyte | High | 84 (97.7) | 58 (92.1) | 142 (95.3) | 0.134 | 0.28 [0.05;1.47] | 0.137 |
| Normal | 2 (2.33) | 5 (7.94) | 7 (4.70) | Ref. | Ref. | ||
| HB | Low | 84 (97.7) | 58 (92.1) | 142 (95.3) | 0.134 | 0.28 [0.05;1.47] | 0.137 |
| Normal | 2 (2.33) | 5 (7.94) | 7 (4.70) | Ref. | Ref. | ||
| anemia | Slight | 22(55.6) | 24(38.1) | 46(32.39) | 0.046 * | 1.78 [0.87;3.65] | 0.118 |
| Moderate | 57(67.28) | 28(44.44) | 85(59.86) | Ref. | Ref. | ||
| Severe | 5(5.81) | 6(9.52) | 11 (7.75) | 2.31 [0.65;8.27] | 0.215 | ||
| Hematocrit | Low | 84 (97.7) | 58 (92.1) | 142 (95.3) | 0.249 | - | 0.172 |
| High | 0 (0.00) | 2 (3.17) | 2 (1.34) | Ref. | Ref. | ||
| Normal | 2 (2.33) | 3 (4.76) | 5 (3.36) | - | 0.476 | ||
| MCV | Microcytosis | 33 (38.4) | 24 (38.1) | 57 (38.3) | 1.000 | 0.99 [0.51;1.93] | 0.975 |
| Normocytosis | 53 (61.6) | 39 (61.9) | 92 (61.7) | Ref. | Ref. | ||
| MCH | Low | 29 (33.7) | 21 (33.3) | 50 (33.6) | 1.000 | 0.98 [0.49;1.96] | 0.963 |
| Normal | 57 (66.3) | 42 (66.7) | 99 (66.4) | Ref. | Ref. | ||
| MCHC | Hypochromia | 5 (5.81) | 3 (4.76) | 8 (5.37) | 1.000 | 0.81 [0.19;3.52] | 0.804 |
| Normal | 81 (94.2) | 60 (95.2) | 141 (94.6) | Ref. | Ref. | ||
| WBC | Leucocytosis | 73 (84.9) | 58 (92.1) | 131 (87.9) | 0.283 | 2.07 [0.70;6.13] | 0.195 |
| Normal | 13 (15.1) | 5 (7.94) | 18 (12.1) | Ref. | Ref. | ||
| Granulocytes | Low | 1 (1.16) | 0 (0.00) | 1 (0.67) | <0.001 * | - | 0.393 |
| High | 23 (26.7) | 37 (58.7) | 60 (40.3) | Ref. | Ref. | ||
| Normal | 62 (72.1) | 26 (41.3) | 88 (59.1) | 0.26 [0.13;0.52] | <0.001 | ||
| Lymphocyte | Lymphocytosis | 66 (76.7) | 53 (84.1) | 119 (79.9) | 0.366 | 1.61 [0.69;3.72] | 0.276 |
| Normal | 20 (23.3) | 10 (15.9) | 30 (20.1) | Ref. | Ref. | ||
| Monocyte | Monocytosis | 84 (97.7) | 60 (95.2) | 144 (96.6) | 0.651 | 0.48 [0.08;2.94] | 0.458 |
| Normal | 2 (2.33) | 3 (4.76) | 5 (3.36) | Ref. | Ref. | ||
| Neutrophil | Neutropenia | 2 (2.33) | 0 (0.00) | 2 (1.34) | <0.001 * | - | 0.164 |
| Neutrophilia | 25 (29.1) | 39 (61.9) | 64 (43.0) | Ref. | Ref. | ||
| Normal | 59 (68.6) | 24 (38.1) | 83 (55.7) | 0.26 [0.13;0.52] | <0.001 | ||
| Eosinophil | Eosinophilia | 34 (39.5) | 39 (61.9) | 73 (49.0) | 0.011 * | 2.49 [1.28;4.84] | 0.008 |
| Normal | 52 (60.5) | 24 (38.1) | 76 (51.0) | Ref. | Ref. | ||
| Basophil | Basophilia | 0 (0.00) | 11 (17.5) | 11 (7.38) | <0.001 * | - | <0.001 |
| Normal | 86 (100) | 52 (82.5) | 138 (92.6) | Ref. | Ref. | ||
| Thrombocyte | Thrombocytopenia | 2 (2.33) | 3 (4.76) | 5 (3.36) | 0.079 | 1.34[0.21;8.6] | 0.789 |
| Thrombocytosis | 59 (68.6) | 32 (50.8) | 91 (61.1) | 0.48 [0.24;0.97] | 0.041 | ||
| Normal | 25 (29.1) | 28 (44.4) | 53 (35.6) | Ref. | Ref. | ||
| Variables | Non-inflamed N=86 axxx | Inflamed N=63, axxx |
Total N=149 axxx |
P-value | Or IC 95% | P-Or |
|---|---|---|---|---|---|---|
| RBC (T/L) | 2.72 [2.34-3.03] | 2.70 [2.51-3.09] | 2.72 [2.39-3.09] | 0.400 | 1.28 [1.80;0.91] | 0.149 |
| Reticulocytes (G/L) | 355 [286-368] | 345 [250-378] | 350[275-375] | 0.242 | 1.00 [1.00;1.00] | 0.060 |
| HB (g/dL) | 7.50 [7.00-8.17] | 8.10 [7.55-9.30] | 7.80 [7.10-8.40] | 0.008 | 1.27 [1.59;1.02] | 0.036* |
| HTE (%) | 21.6 [19.5-23.0] | 22.6 [21.0-24.9] | 21.9 [20.1-24.4] | 0.031* | 1.05 [1.11;0.99] | 0.080 |
| MCV (fL) | 80.5 [74.2-85.0] | 82.0 [75.0-90.5] | 81.0 [74.0-87.0] | 0.127 | 1.02 [1.06;0.98] | 0.257 |
| MCHC (pg/c) | 28.9 [25.7-31.2] | 30.0 [25.4-32.5] | 29.2 [25.5-31.6] | 0.208 | 1.03 [1.11;0.95] | 0.495 |
| MCH (g/L) | 35.7 [34.5-37.0] | 35.8 [34.3-37.4] | 35.7 [34.3-37.1] | 0.963 | 0.98 [1.11;0.87] | 0.751 |
| WBC (G/L) | 15,6 [12.5-18.2] | 17.7 [13.4-23.5] | 15.8 [13-20.4] | 0.004* | 1.00 [1.00;1.00] | 0.001* |
| Granulocytes (G/L) | 6.1 [4.7-8.4] | 8.5 [5.9-10.3] | 6.6 [4.9-8.9] | <0.001* | 1.00 [1.00;1.00] | <0.001* |
| Lymphocytes (G/L) | 6.3 [4.2-8.8] | 6.1 [5.4-8.7] | 6230 [4.5-8.8] | 0.417 | 1.00 [1.00;1.00] | 0.140 |
| Monocytes (G/L) | 2140 [1.5-2.8] | 2.4 [1.6-3.3] | 2.1 [1.5-3] | 0.237 | 1.00 [1.00;1.00] | 0.080 |
| Neutrophils (G/L) | 5.7 [4.4-7.9] | 7.8 [5.5-9.6] | 6.2 [4.6-8.3] | <0.001* | 1.00 [1.00;1.00] | <0.001* |
| Eosinophils (/mL) | 356 [275-491] | 498 [346-599] | 388 [288-518] | <0.001* | 1.00 [1.01;1.00] | <0.001* |
| Basophils (/mL) | 98.1 [75.8-135] | 137 [95.5-165] | 107 [79.4-143] | <0.001* | 1.02 [1.02;1.01] | <0.001* |
| Thrombocytes (G/mL) | 468 [386-598] | 408 [268-542] | 460 [323-573] | 0.005* | 1.00 [1.00;1.00] | 0.016* |
| Variables |
Inflamed N=63n(%) |
Non inflamed N=86n(%) | Total N=149 n(%) | P value | OR[IC 95%] | P value Or | |
|---|---|---|---|---|---|---|---|
| Iron, axxx | 1.91 [1.52-2.48] | 1.84 [1.50-2.31] | 1.86 [1.51-2.46] | 0.616 | 1.06 [1.44;0.78] | 0.694 | |
| Iron | Low | 0 (0.00) | 1 (1.16) | 1 (0.67) | 0.774 | - | 0.568 |
| High | 57 (90.5) | 74 (86.0) | 131 (87.9) | Ref. | Ref. | ||
| Normal | 6 (9.52) | 11 (12.8) | 17 (11.4) | 0.71 [0.25;2.03] | 0.538 | ||
| Ferritin, axxx | 669 [429-835] | 426 [234-660] | 555 [317-749] | <0.001* | 1.00 [1.00;1.00] | <0.001* | |
| Ferri | High | 61 (96.8) | 66 (76.7) | 127 (85.2) | 0.001* | 9.24 [2.07;41.2] | <0.001* |
| Normal | 2 (3.17) | 20 (23.3) | 22 (14.8) | Ref. | Ref. | ||
| Tranferrin, axxx | 2.08 [1.59-2.74] | 2.89 [1.91-3.51] | 2.55 [1.72-3.27] | 0.003* | 0.70 [0.92;0.53] | 0.011* | |
| TF: | Low | 29 (46.0) | 24 (27.9) | 53 (35.6) | 0.007 | 9.06 [1.88;43.6] | 0.002* |
| High | 2 (3.17) | 15 (17.4) | 17 (11.4) | Ref. | Ref. | ||
| Normal | 32 (50.8) | 47 (54.7) | 79 (53.0) | 5.11 [1.09;23.9] | 0.023 | ||
| CTF, axxx | 51.9 [39.8-68.5] | 72.2 [47.7-87.8] | 63.8 [43.0-81.7] | 0.003* | 0.99 [1.00;0.97] | 0.011* | |
| CST, axxx | 65.6 [45.9-96.0] | 54.7 [34.5-94.0] | 63.6 [36.0-95.1] | 0.092 | 1.00 [1.00;1.00] | 0.614 | |
| CST | High | 60 (95.2) | 66 (76.7) | 126 (84.6) | 0.004* | 6.06 [1.71;21.4] | 0.002 |
| Normal | 3 (4.76) | 20 (23.3) | 23 (15.4) | Ref. | Ref. | ||
| CTF | Low | 22 (34.9) | 21 (24.4) | 43 (28.9) | 3.47 [1.46;8.20] | 0.005 * | |
| High | 13 (20.6) | 43 (50.0) | 56 (37.6) | 0.001* | Ref. | Ref. | |
| Normal | 28 (44.4) | 22 (25.6) | 50 (33.6) | 4.21 [1.83;9.70] | 0.001* | ||
| CRP, axxx | 11.7 [8.72-22.5] | 3.00 [2.00-4.50] | 5.00 [3.00-10.0] | 0.001* | - | 0.940 | |
| CRP | High | 63 (100) | 0 (0.00) | 63 (42.3) | 0.001* | - | - |
| Normal | 0 (0.00) | 86 (100) | 86 (57.7) | Ref. | Ref. | ||
| IL6, axxx | 5.01 [2.87-8.59] | 2.83 [1.19-4.53] | 3.97 [1.41-6.54] | <0.001* | 1.01 [1.02;1.01] | 0.002* | |
| IL6 | High | 61 (96.8) | 73 (84.9) | 134 (89.9) | 0.034* | 5.43 [1.18;25.0] | 0.016* |
| Normal | 2 (3.17) | 13 (15.1) | 15 (10.1) | Ref. | Ref. | ||
| Dependent: inflammation | No | Yes | OR (multivariable), IC 95%, p value |
|
|---|---|---|---|---|
| Ferritin | Normal | 20 (90.9) | 2 (9.1) | - |
| High | 66 (52.0) | 61 (48.0) | 4.96 (1.15-36.42, p=0.056) | |
| Transferrin | Normal | 47 (59.5) | 32 (40.5) | - |
| High | 15 (88.2) | 2 (11.8) | 0.67 (0.08-4.28, p=0.685) | |
| Low | 24 (45.3) | 29 (54.7) | 1.73 (0.41-9.02, p=0.476) | |
| TIBC | Normal | 22 (44.0) | 28 (56.0) | - |
| High | 43 (76.8) | 13 (23.2) | 0.49 (0.17-1.38, p=0.181) | |
| Low | 21 (48.8) | 22 (51.2) | 0.49 (0.09-2.08, p=0.350) | |
| TSC | Normal | 20 (87.0) | 3 (13.0) | - |
| High | 66 (52.4) | 60 (47.6) | 2.30 (0.50-13.36, p=0.308) | |
| IL6 | Normal | 13 (86.7) | 2 (13.3) | - |
| High | 73 (54.5) | 61 (45.5) | 6.23 (1.43-45.96, p=0.030*) | |
| Hemoglobin | Normal | 2 (28.6) | 5 (71.4) | - |
| Low | 84 (59.2) | 58 (40.8) | 0.08 (0.00-0.84, p=0.081) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





