Submitted:
26 March 2024
Posted:
27 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Results
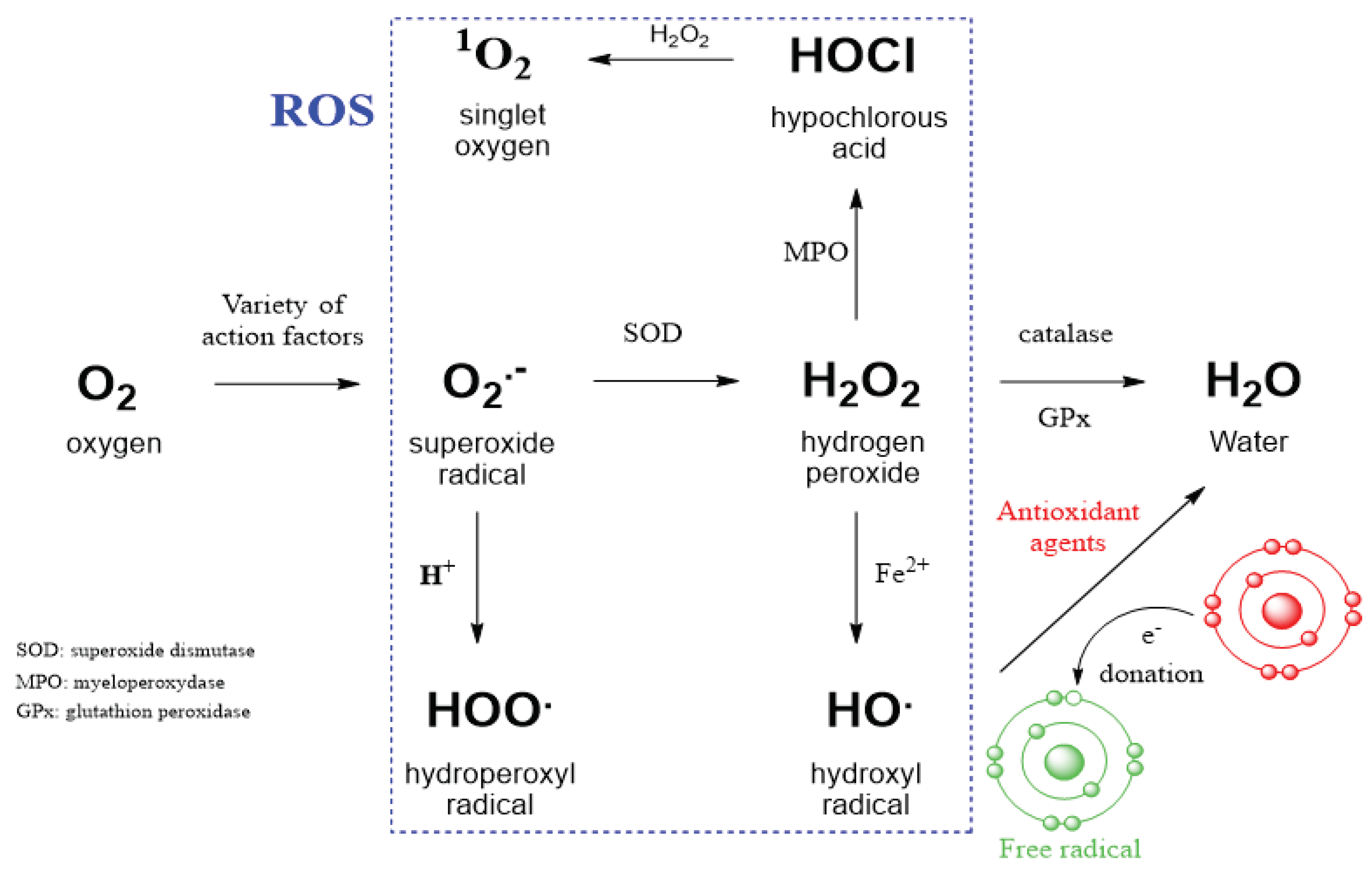
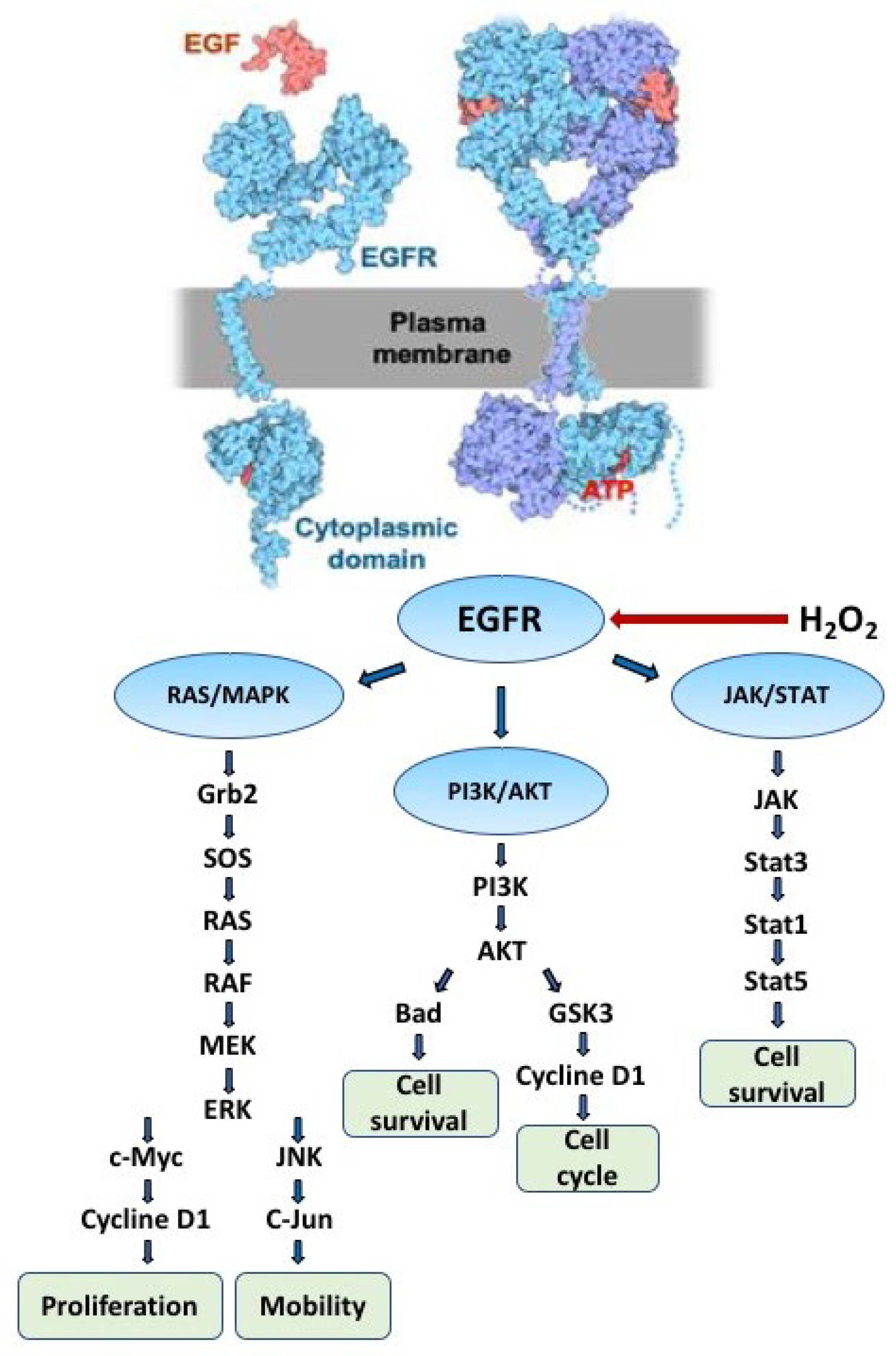
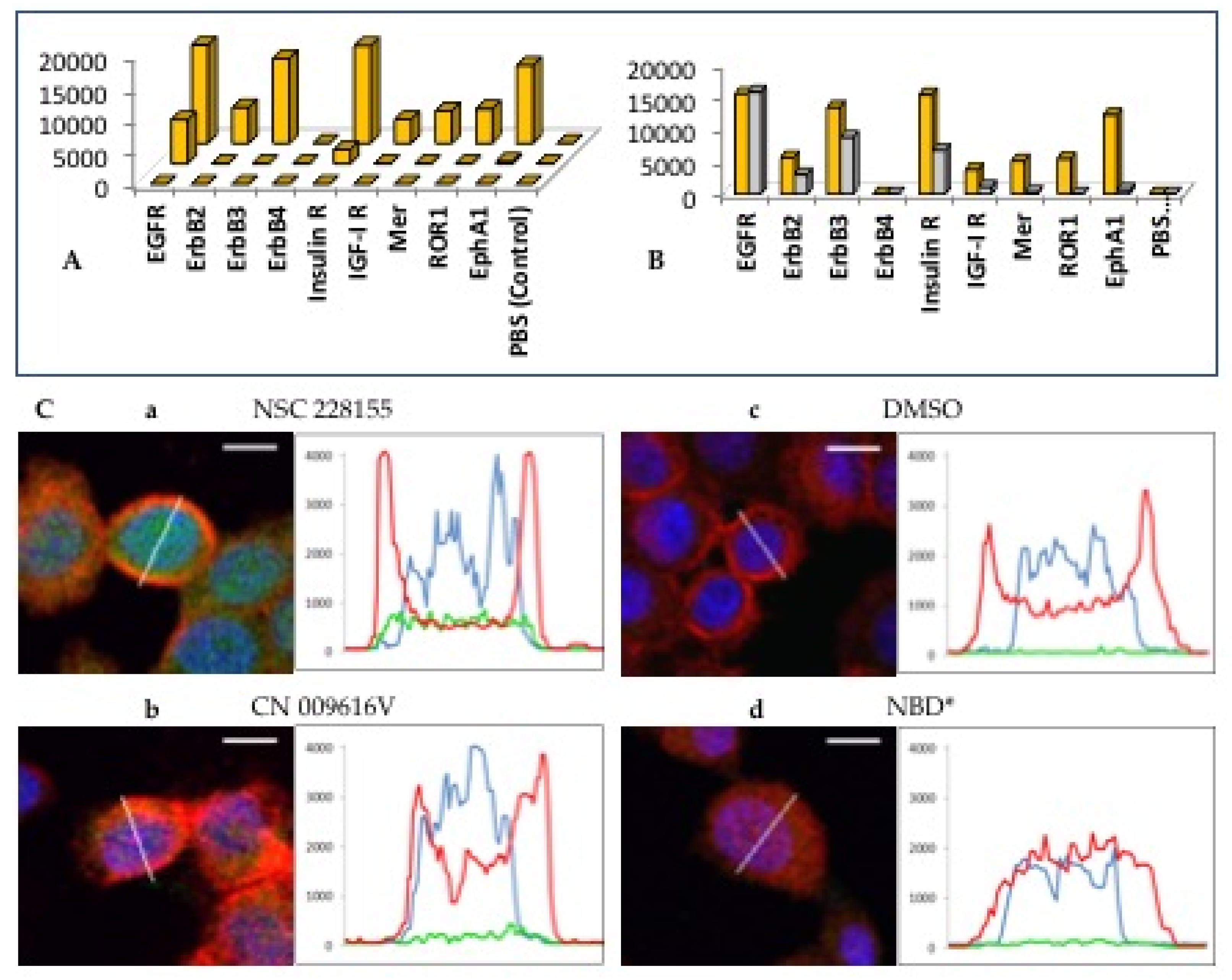
2.1. The Role of EGFR in Redox Processes
2.2. Straigthforward Detection of Hydrogen Peroxide Binding to Many Proteins
2.3. Nrf2-ARE Redox Pathway
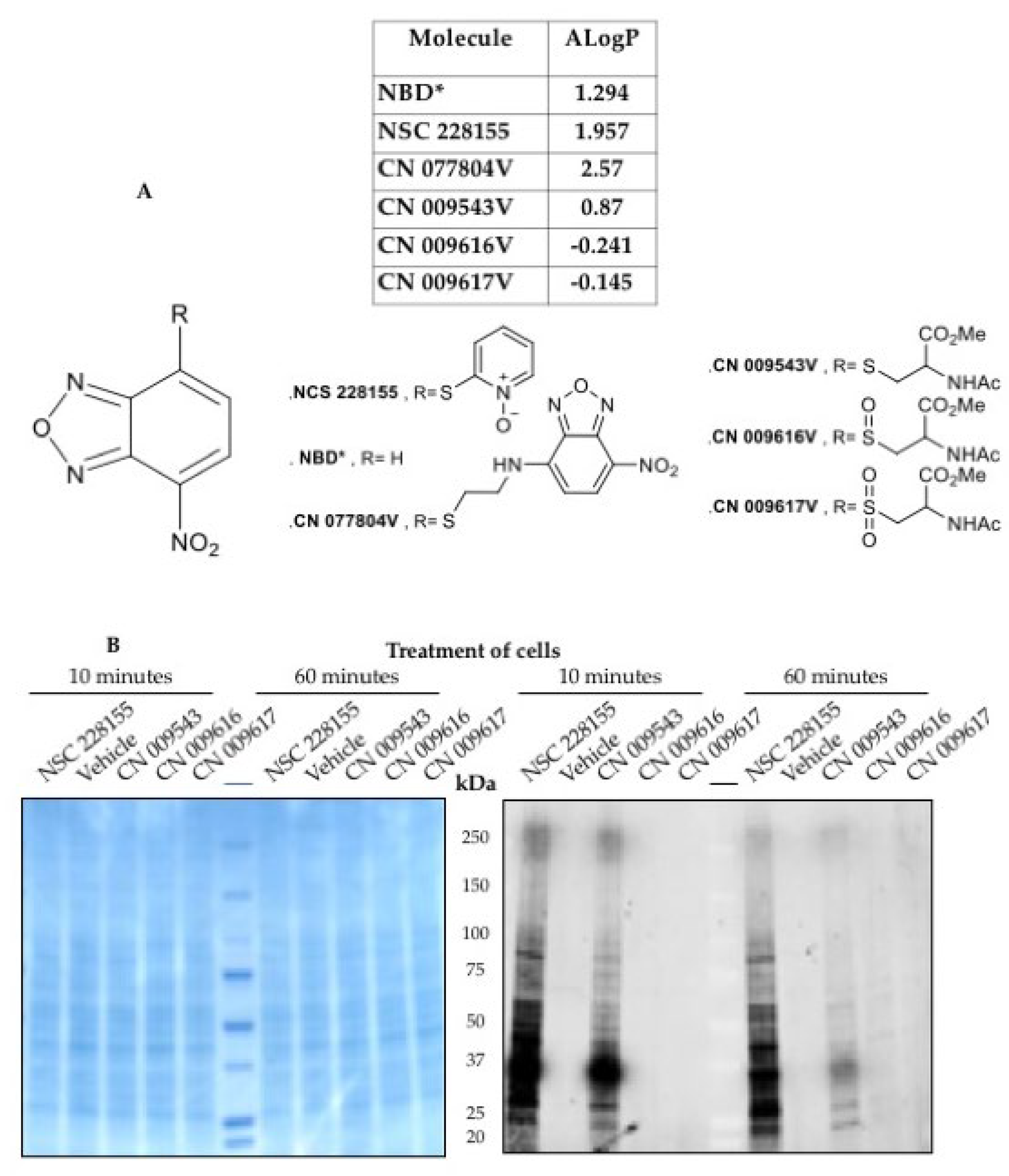
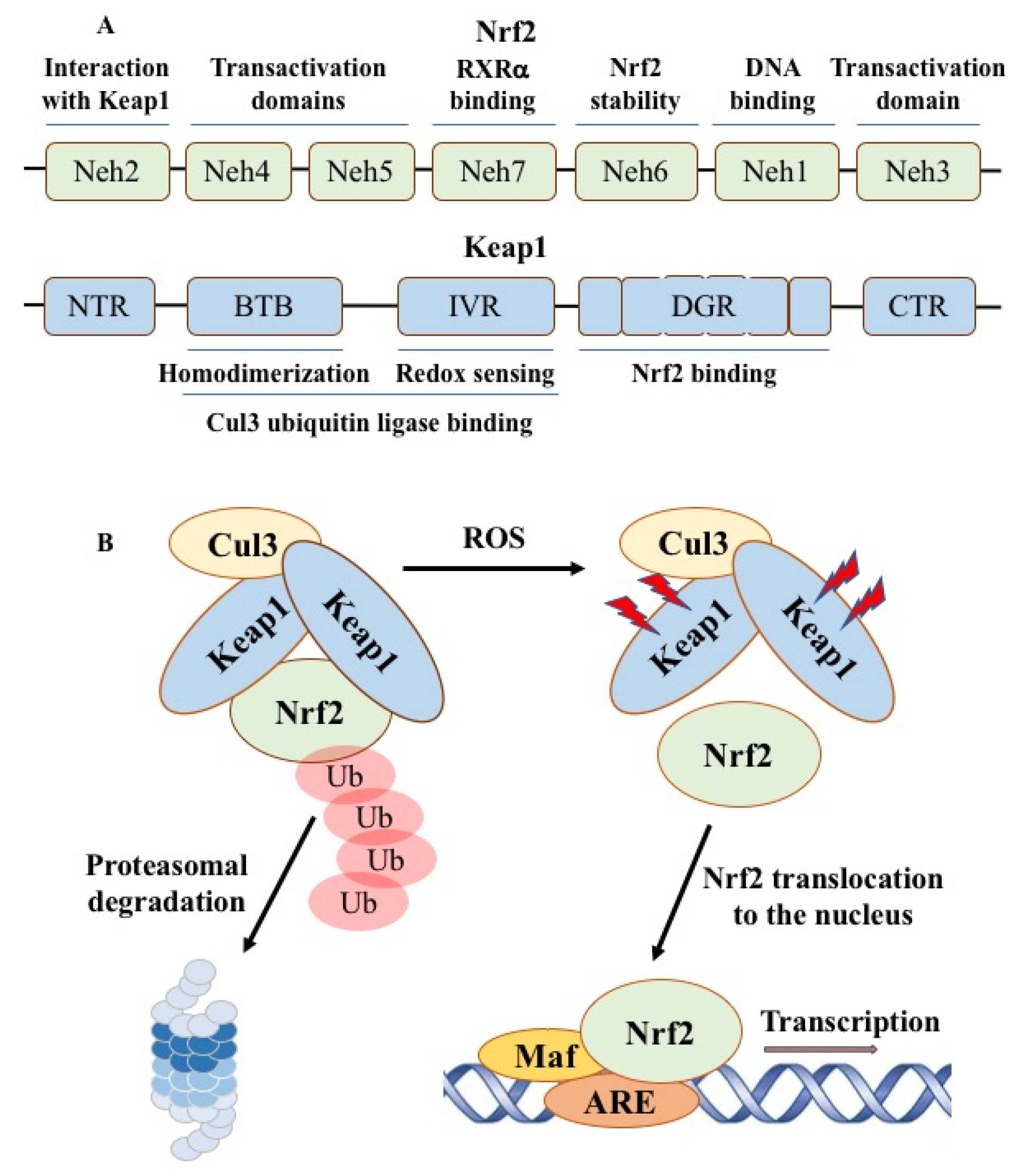
2.4. Therapeutic Opportunities by Targeting ROS-Producing Proteins
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auten, R.; Davis, J. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Miller, A.F. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide anion radical (O2-. ), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; Soubhye, J.; Deshwal, S.; Di Sante, M.; Kaludercic, N.; Di Lisa, F.; Schmidt, H.H.H.W. On the clinical pharmacology of reactive oxygen species. Pharmacol. Rev. 2020, 72(4), 801–828. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells 2018, 7(10), 156. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, D.; Abbruzzese, J.L.; Lu, W. Measurement of Reactive Oxygen Species by Fluorescent Probes in Pancreatic Cancer Cells. In: Su, G. (eds) Pancreatic Cancer. Methods in Molecular Biology. (2019), 1882. Humana Press, New York, NY.
- Loschen, G.; Azzi, A.; Richter, C.; Flohé, L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974, 42(1), 68–72. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. , Jaquet V., Marcucci F., Schmidt H.H.H.W. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017, 174(12), 1784-1796. [CrossRef]
- Lemmon M., A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Paul, M. D.; Grubb, H.N.; Hristova, K. Quantifying the strength of heterointeractions among receptor tyrosine kinases from different subfamilies: Implications for cell signaling. J. Biol. Chem. 2020, 295, 9917–9933. [Google Scholar] [CrossRef]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.J.; Ward, C.W.; Burgess, A.W. Epidermal Growth Factor Receptor: Mechanisms of Activation and Signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Cohen, S. Origins of growth factors: NGF and EGF. J. Biol. Chem. 2008, 283(49), 33793–33797. [Google Scholar] [CrossRef] [PubMed]
- Jura, N.; Zhang, X.; Endres, N.F.; Seeliger, M.A.; Schindler, T.; Kuriyan, J. Catalytic control in the EGF receptor and its connection to general kinase regulatory mechanisms. Mol. Cell. 2011, 42, 9–22. [Google Scholar] [CrossRef]
- Gamou, S.; Shimizu, N. Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett. 1995, 357, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S. , Kang, S. W.; Seo, M.S.; Baines, I.C.; Tekle, E.; Chock, P.B.; Rhee, S.G. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997, 272, 217–221. [Google Scholar]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9(1), 28–39. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry 2012, 51, 9954–9965. [Google Scholar] [CrossRef]
- Ferrari, E.; Tinti, M.; Costa, S.; Corallino, S.; Nardozza, A.P.; Chatraryamontri, A.; Ceol, A.; Cesareni, G.; Castagnoli, L. Identification of new substrates of the protein-tyrosine phosphatase PTP1B by Bayesian integration of proteome evidence. J. Biol. Chem. 2011, 286(6), 4173–4185. [Google Scholar] [CrossRef]
- Lee, S.R. , Kwon, K.S., Kim, S.R., Rhee, S.G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998, 273(25), 15366-15372. [CrossRef]
- Salmeen, A.; Andersen, J.N. , Myers, M.P., Meng, T.C.; Hinks, J.A.; Tonks, N.K.; Barford, D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003, 423(6941):769-773. [CrossRef]
- van Montfort, R.L.; Congreve, M.; Tisi, D.; Carr, R. ; Jhoti, H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 2003, 423(6941), 773-777. [CrossRef]
- Schwartz, P.A.; Kuzmic, P., Solowiej, J.; Bergqvist, S.; Bolanos, B.; Almaden, C.; Nagata, A.; Ryan, K.; Feng, J.; Dalvie, D.; Kath, J.C.; Xu, M.; Wani, R.; Murray, B.W. Covalent EGFR inhibitor analysis reveals importance of reversible interactions to potency and mechanisms of drug resistance. Proc. Natl. Acad. Sci. U S A 2014, 111(1), 173–178. [CrossRef]
- Sakanyan, V.; Angelini, M.; Le Béchec, M.; Lecocq, M.F.; Benaiteau, F.; Rousseau, B.; Gyulkhandanyan, A.; Gyulkhandanyan, L.; Logé, C.; Reiter, E.; Roussakis, C.; Fleury, F. Screening and discovery of nitro-benzoxadiazole compounds activating epidermal growth factor receptor (EGFR) in cancer cells. Sci. Rep. 2014, 4, 3977. [Google Scholar] [CrossRef]
- Toyo’oka, T. Development of Benzofurazan−bearing Fluorescence Labeling Reagents for separation and detection in high−performance liquid chromatography. Chromatography, 2012, 33, 1–17. [Google Scholar] [CrossRef]
- Blair, J. A.; Rauh, D.; Kung, C.; Yun, C.H.; Fan, Q.W.; Rode, H.; Zhang, C.; Eck, M.J.; Weiss, W.A.; Shokat, K. M. Nat. Chem. Biol. 2007, 3, 229–238. [Google Scholar]
- Heyne, B. ; Beddie, C; Scaiano, J. C. Synthesis and characterization of a new fluorescent probe for reactive oxygen species. Org. Biomol. Chem. 2007, 5, 1454–1458. [Google Scholar]
- Sakanyan, V.; Hulin, P.; Alves de Sousa, R.; Silva, V.; Hambardzumyan, A.; Nedellec, S.; Tomasoni, C.; Logé, C.; Pineau, C.; Roussakis, C.; Fleury, F.; Artaud, I. Activation of EGFR by small compounds through coupling the generation of hydrogen peroxide to stable dimerization of Cu/Zn SOD1. Sci. Rep. 2016, 6, 21088. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Lafont, F.; Benhelli-Mokrani, H.; LeBreton, M.; Hulin, P.; Chabot, T.; Paris, F.; Sakanyan, V.; Fleury, F. Rapid diminution in the level and activity of DNA-dependent protein kinase in cancer cells by a reactive nitro-benzoxadiazole compound. Int. J. Mol. Sci. 2016, 17(5), 703. [Google Scholar] [CrossRef] [PubMed]
- Burma, S.; Chen, B.P.; Chen, D.J. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 2006, 5, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Groeger, A.L.; Freeman, B.A. Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Mol. Interv. 2010, 10(1), 39–50. [Google Scholar] [CrossRef] [PubMed]
- Schülke, K.H.; Ospina, F.; Hörnschemeyer, K.; Gergel, S.; Hammer, S.C. Substrate profiling of anion methyltransferases for promiscuous synthesis of S-Adenosylmethionine analogs from haloalkanes. Chembiochem. 2022, 23(4), e202100632. [Google Scholar] [CrossRef]
- Edwards, D.R.; Lohman, D.C.; Wolfenden, R. Catalytic proficiency: the extreme case of S-O cleaving sulfatases. J. Am. Chem. Soc. 2012, 134(1), 525–531. [Google Scholar] [CrossRef]
- Kenakin, T.; Miller, L.J. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010, 62, 265–304. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H. M.; Sokoloski, E. A.; Levine, R. L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U S A 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R; Takakura, H. Kamiya, M.; Kobayashi, E.; Komatsu, T.; Ueno, T.; Terai, T.: Hanaoka, K.; Nagano, T.; Urano, Y. Development of a sensitive bioluminogenic probe for imaging highly reactive oxygen species in living rats. Angew. Chem. Int. Ed. Engl. 2015, 54, 14768–14771.
- Tsamesidis, I.; Egwu, C.O.; Pério, P.; Augereau, J.M.; Benoit-Vical, F.; Reybier, K. An LC-MS Assay to measure superoxide radicals and hydrogen peroxide in the blood system. Metabolites 2020, 10(5), 175. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.T.; Miziorko, H.; Epstein, C.J.; Wallace, D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U S A 1999, 96, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ge, W.; Martínez-Jarquín, S.; He, Y.; Wu, R.; Stoffel, M.; Zenobi, R. Mass spectrometry reveals high levels of hydrogen peroxide in pancreatic cancer cells. Angew. Chem. Int. Ed. Engl. 2023, 62(19), e202213703. [Google Scholar] [CrossRef] [PubMed]
- Beissenhirtz, M.K.; Scheller, F.W.; Lisdat, F. A superoxide sensor based on a multilayer cytochrome c electrode. Anal. Chem. 2004, 76, 4665–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Qiu, T.; Yang-Ping, L.; Yang, L. Superoxide anion radical generation in the NaOH/H(2)O(2)/Fe(III) system: a spin trapping ESR study. Magn. Reson. Chem. 2006, 44(1), 38–44. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U S A 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M. The Molecular probes handbook: A guide to fluorescent probes and labeling technologies. Life Technologies Corporation. 2010.
- Sakanyan, V.; Benaiteau, F.; Alves de Sousa, R.; Pineau, C.; Artaud, I. Straightforward detection of reactive compound binding to multiple proteins in cancer cells: Towards a better understanding of electrophilic stress. Ann. Clin. Exp. Metabol. 2016, 1, 1006. [Google Scholar]
- Marinho, H.S.; Real, C.; Zirn, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U S A 1994, 91(21), 9926–9930. [Google Scholar] [CrossRef]
- Ohta, T.; Iijima, K.; Miyamoto, M.; Nakahara, I.; Tanaka, H.; Ohtsuji, M.; Suzuki, T.; Kobayashi, A.; Yokota, J.; Sakiyama, T.; Shibata, T.; Yamamoto, M.; Hirohashi, S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008, 68, 1303−1309. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D. D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21−43. [Google Scholar] [CrossRef]
- Joshi, G.; Johnson, J.A. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2012, 7(3), 218–229. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018, 29(17), 1727–1745. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Bustamante Munguira, E.; Juan, C.A.; Plou, F.J.; Pérez, L.E. Electrophilic compounds in the human diet and their role in the induction of the transcription factor NRF2. Int. J. Mol. Sci. 2024, 25, 3521. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U S A 2018, 115(23), 5839–5848. [Google Scholar] [CrossRef]
- Unoki, T.; Akiyama, M.; Kumagai, Y. Nrf2 activation and its coordination with the protective defense systems in response to electrophilic stress. Int. J. Mol. Sci. 2020, 21, 545. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Reck, M. KEAP1/NRF2 (NFE2L2) mutations in NSCLC - Fuel for a superresistant phenotype? Lung Cancer 2021, 159, 10−17. [Google Scholar] [CrossRef]
- Cheraghi, O.; Dabirmanesh, B.; Ghazi, F.; Amanlou, M.; Atabakhshi-Kashi, M.; Fathollahi, Y.; Khajeh, K. The effect of Nrf2 deletion on the proteomic signature in a human colorectal cancer cell line. BMC Cancer 2022, 22(1), 979. [Google Scholar] [CrossRef]
- Bono, S.; Feligioni, M.; Corbo, M. Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Mol. Neurodegener. 2021, 16(1), 71. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-responsive trio raising cellular defenses and engaging immune system. Chem. Res. Toxicol. 2022, 35(10), 1690–1700. [Google Scholar] [CrossRef]
- Ahn, S.G; Thiele, D.J. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003, 17(4), 516–528. [Google Scholar] [CrossRef]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996, 271(50), 32253–32259. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38(2), 167–197. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2(5), e1600200. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020, 52(2), 192–203. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nishigori, C.; Tanaka, T.; Uchida, K.; Nikaido, O.; Osawa, T.; Hiai, H.; Imamura, S.; Toyokuni, S. 8-hydroxy-2'-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J. Invest. Dermatol. 1996, 107(5), 733–737. [Google Scholar] [CrossRef] [PubMed]
- Lonkar, P.; Dedon, P.C. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int. J. Cancer 2011, 128, 1999−2009. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4(8), 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48 (2), 158−167. [CrossRef]
- Niki, E.; Tsuchiya, J.; Tanimura, R.; Kamiya, Y. Regeneration of vitamin E from a chromanoxyl radical by glutathione and vitamin C. Chem. Lett. Jpn. 1982, 11 (6), 789−792. [CrossRef]
- Gropper, S.S.; Smith, J.L.; Carr, T.P. Advanced nutrition and human metabolism, 7th Ed, Cengage Learning Inc., Wadsworth, 2017.
- Ashrafizadeh, M. ; Ahmadi, Z;; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 2020, 20(2), 116-133. [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician's expectation be matched by the reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007, 26(1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yang, L.; Huang, Y.; Tao, L.; Chen, D. ′′Novel′′ synthetic antioxidants in house dust from multiple locations in the Asia-Pacific region and the United States. Environ. Sci. Technol. 2021, 55 (13), 8675−8682.
- Gong, X.; Zhang, W.; Zhang, S.; Wang, Y.; Zhang, X.; Lu, Y.; Sun, H.; Wang, L. Organophosphite antioxidants in mulch films are important sources of organophosphate pollutants in farmlands. Environ. Sci. Technol., 2021, 55(11), 7398-7406. [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54 (19), 11706−11719. [CrossRef]
- Liang, B.; Li, J.; Du, B.; Pan, Z.; Liu, L.-Y.; Zeng, L. E-Waste recycling emits large quantities of emerging aromatic amines and organophosphites: A poorly recognized source for another two classes of synthetic antioxidants. Environ. Sci. Technol. Lett. 2022, 9, 625−631. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; Cortina, A.E.; Biswas, R.G.; Kock, F.V.C.; Soong, R.; Jenne, A.; Du, B.; Hou, F.; He, H.; Lundeen, R.; Gilbreath, A.; Sutton, R.; Scholz, N.L.; Davis, J.W.; Dodd, M.C.; Simpson, A.; McIntyre, J.K.; Kolodziej, E.P. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371 (6525), 185−189. [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Alack, K.; Richter, M.J.; Kruger, K. Immune-regulating effects of exercise on cigarette smoke-induced inflammation. J. Inflamm. Res. 2018, 11, 155−167. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants (Basel) 2021, 10(2), 277. [Google Scholar] [CrossRef]
- Yeretssian, G.; Lecocq, M.; Lebon, G.; Hurst, H.C.; Sakanyan, V. Competition on nitrocellulose-immobilized antibody arrays: from bacterial protein binding assay to protein profiling in breast cancer cells. Mol. Cell. Proteomics 2005, 4(5), 605–617. [Google Scholar] [CrossRef]
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S; Prochownik, E. V. Small-molecule inhibitors of the Myc oncoprotein. Biochim. Biophys. Acta 2014, 1849, 525–543.
- Korolev, S.P.; Kondrashina, O.V.; Druzhilovsky, D.S.; Starosotnikov, A.M.; Dutov, M.D.; Bastrakov, M.A.; Dalinger, I.L.; Filimonov, D.A.; Shevelev, S.A.; Poroikov, V.V.; Agapkina, Y.Y.; Gottikh, M.B. Structural-functional analysis of 2,1,3-benzoxadiazoles and their N-oxides as HIV-1 integrase inhibitors. Acta Naturae. 2013, 5(1), 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sakanyan, V. Reactive chemicals and electrophilic stress in cancer: A minireview. High Throughput. 2018, 7(2), 12. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Aoki, M.; Tsuji, S.; Itoyama, Y.; Sobue, G.; Togo, M.; Hamada, C.; Tanaka, M.; Akimoto, M., Nakamura, K.; et al.; Writing Group; Edaravone. (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well-defined patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16(7), 505–512. [CrossRef]
- Yamashita, T.; Abe, K. Update on antioxidant therapy with edaravone: expanding applications in neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25(5), 2945. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers 2019, 11(8), 1191. [CrossRef]
- Brauer, G. Handbook of preparative inorganic chemistry. Vol. 1. Translation editing by Reed F. (2nd ed.). New York: Academic Press. p. 140., 1963, ISBN 978-0-12-126601-1.
- Iradyan, M. , Iradyan, N., Hulin, P.; Hambardzumyan, A.; Gyulkhandanyan, A., Alves de Sousa, R., Hessani, A., Roussakis, C., Bollot, G., Bauvais, C.; Sakanyan, V. Targeting degradation of EGFR through the allosteric site leads to cancer cell detachment-promoted death. Cancers (Basel) 2019, 11(8), 1094. [CrossRef]
- Sakanyan, V.; Iradyan, N.; Alves de Sousa, R. Targeted strategies for degradation of key transmembrane proteins in cancer. BioTech (Basel) 2023, 12(3), 57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




