Submitted:
25 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
In Vitro Experiments
In Vivo Experiments
Diets
3. Results
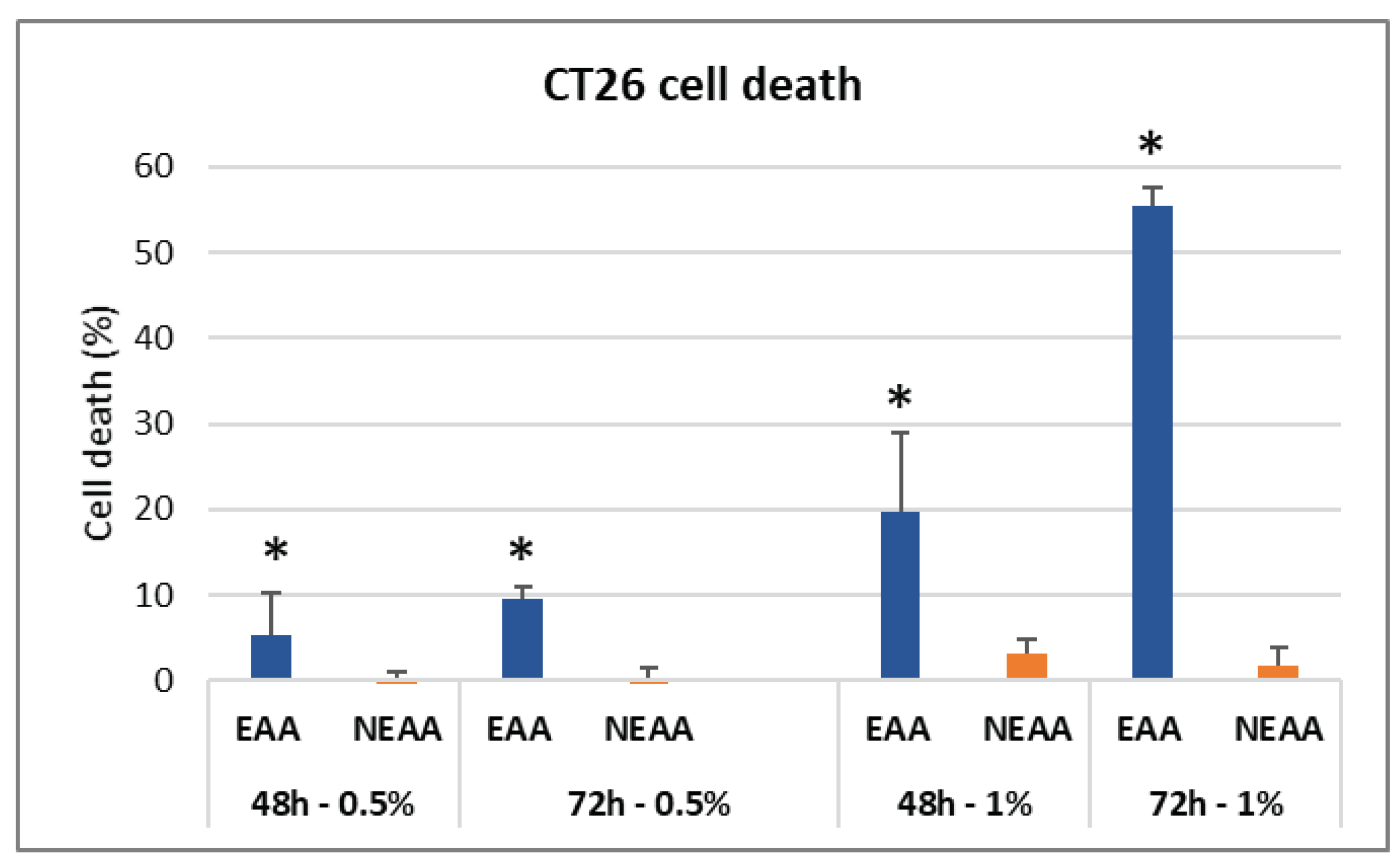
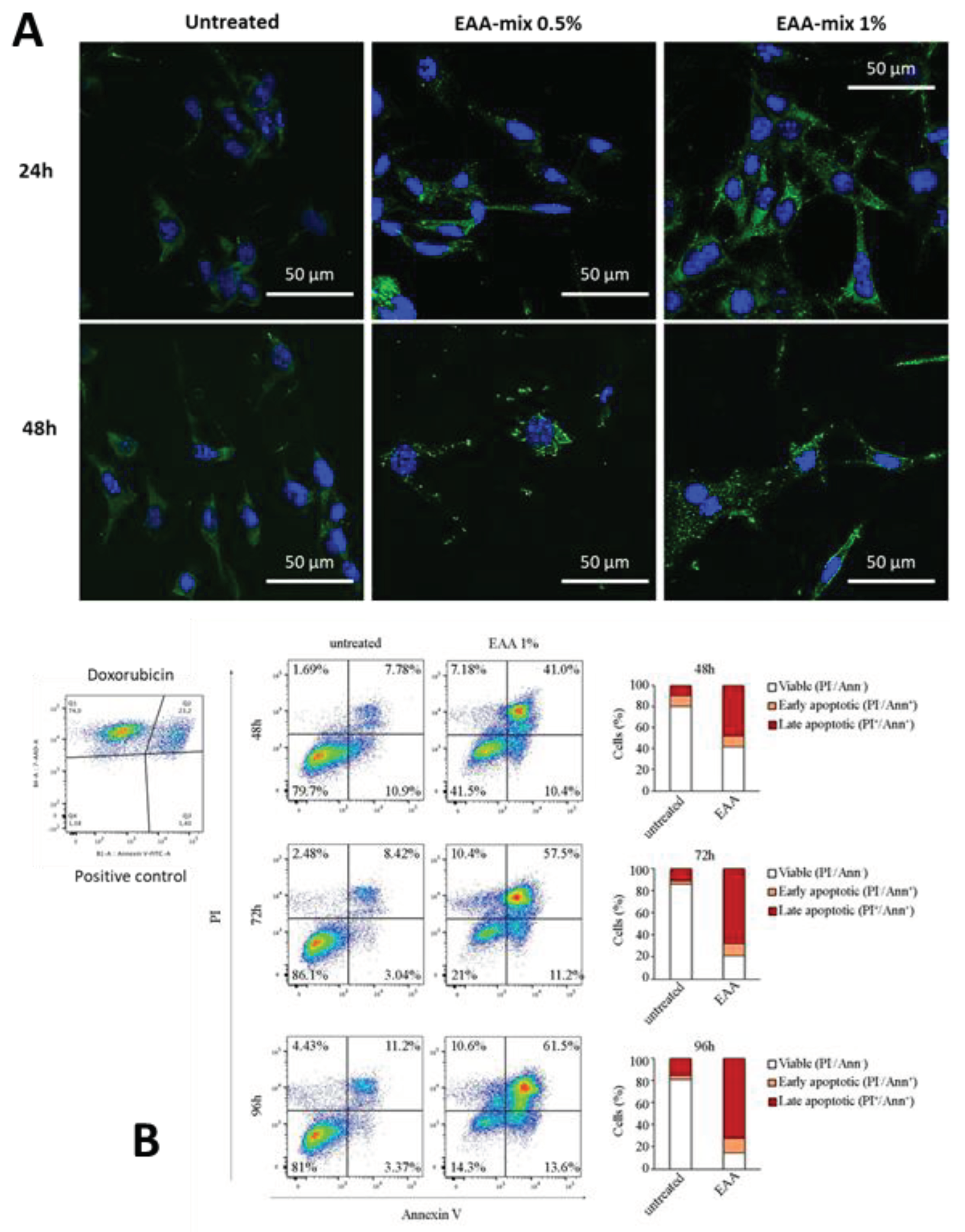
3.1. In Vitro Experiments
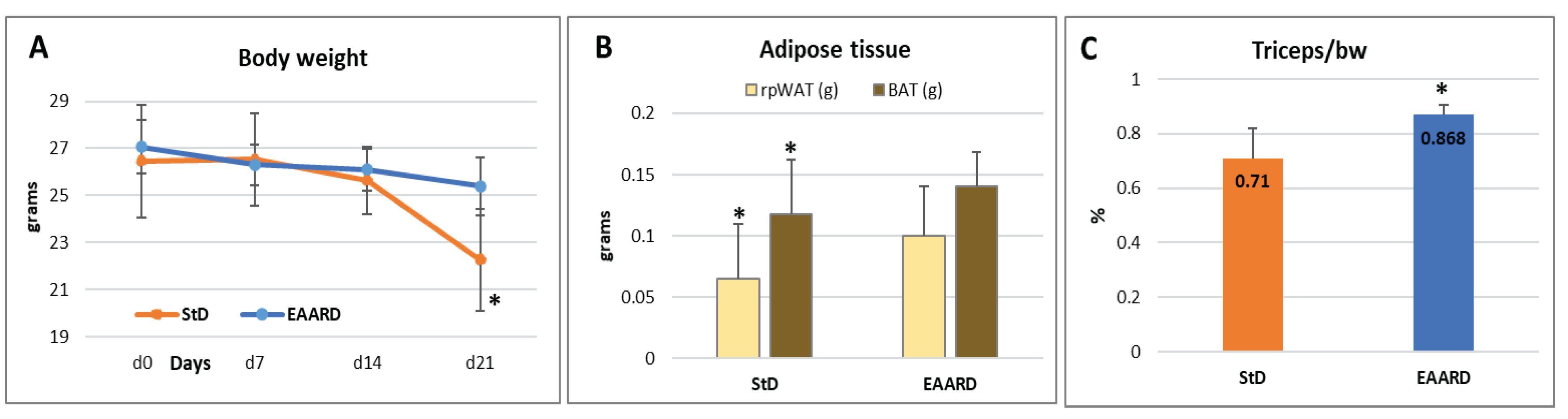
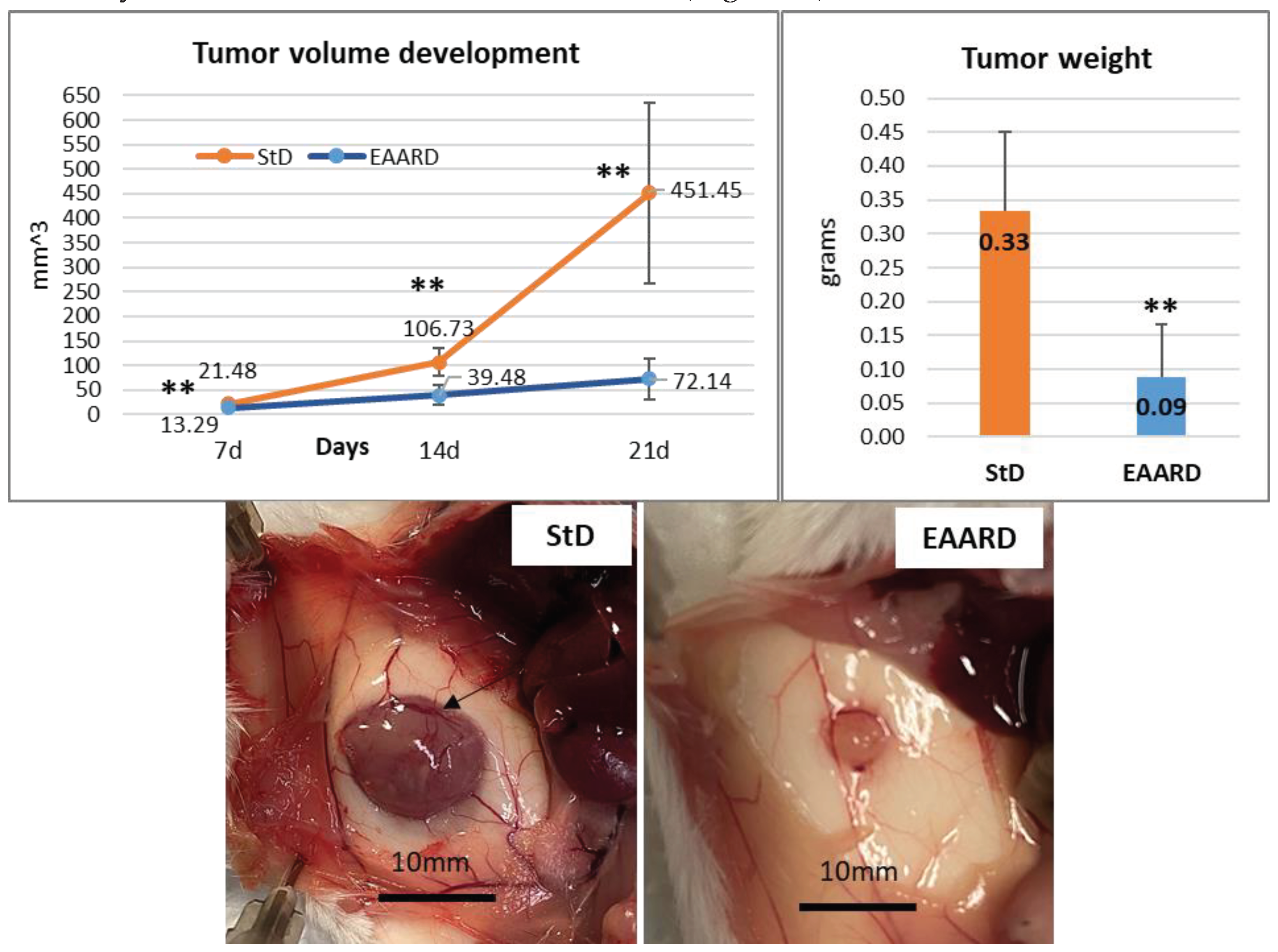
3.2. In Vivo Experiments
3.2.1. Sub-Cutaneous (s.c.) CT26 Injection
3.2.2. Subcutaneous Tumor Development
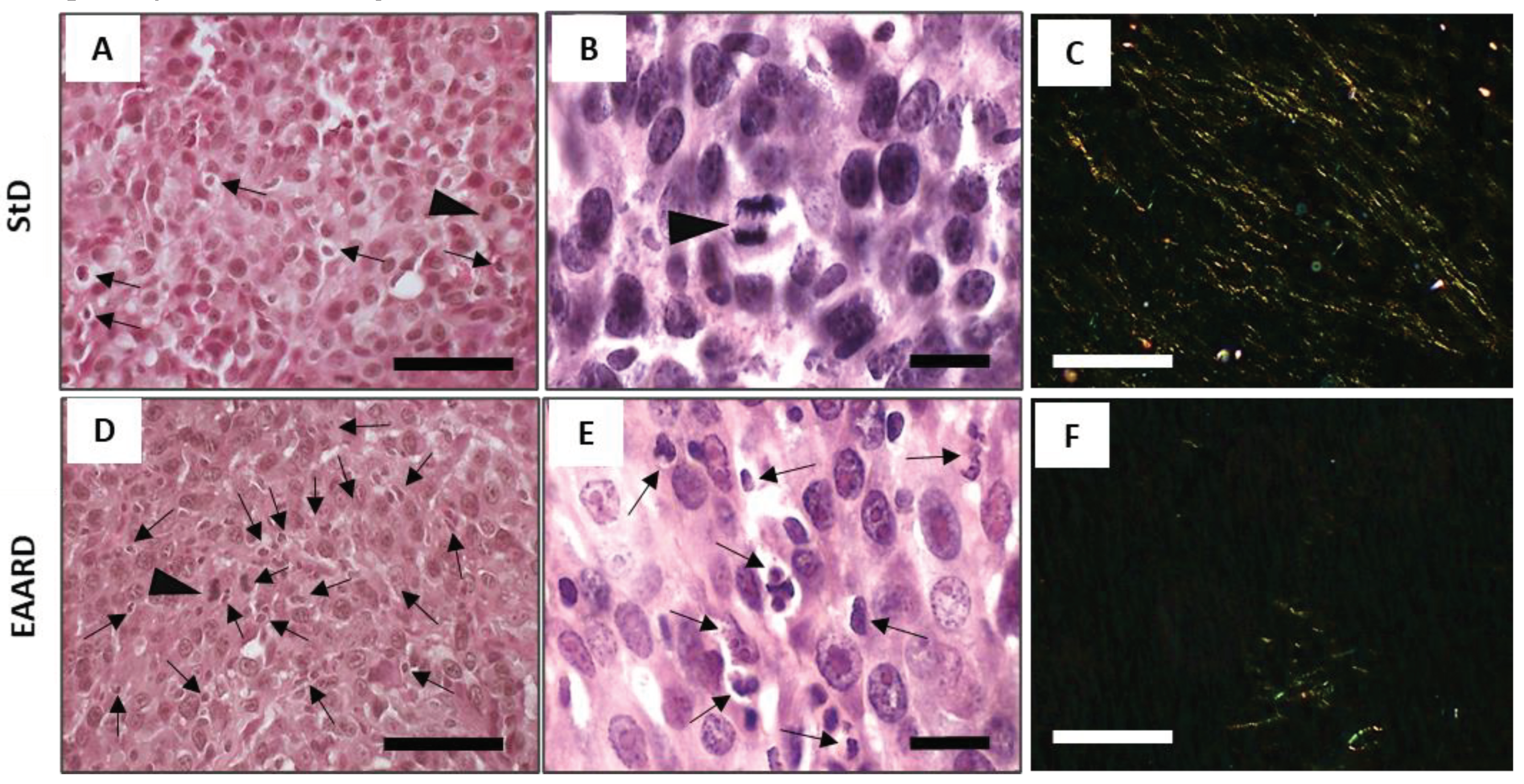
3.3. Histopathological Examination
3.3.1. Eosin/Hematoxylin (E/H) Staining
3.3.2. Sirius-Red Staining
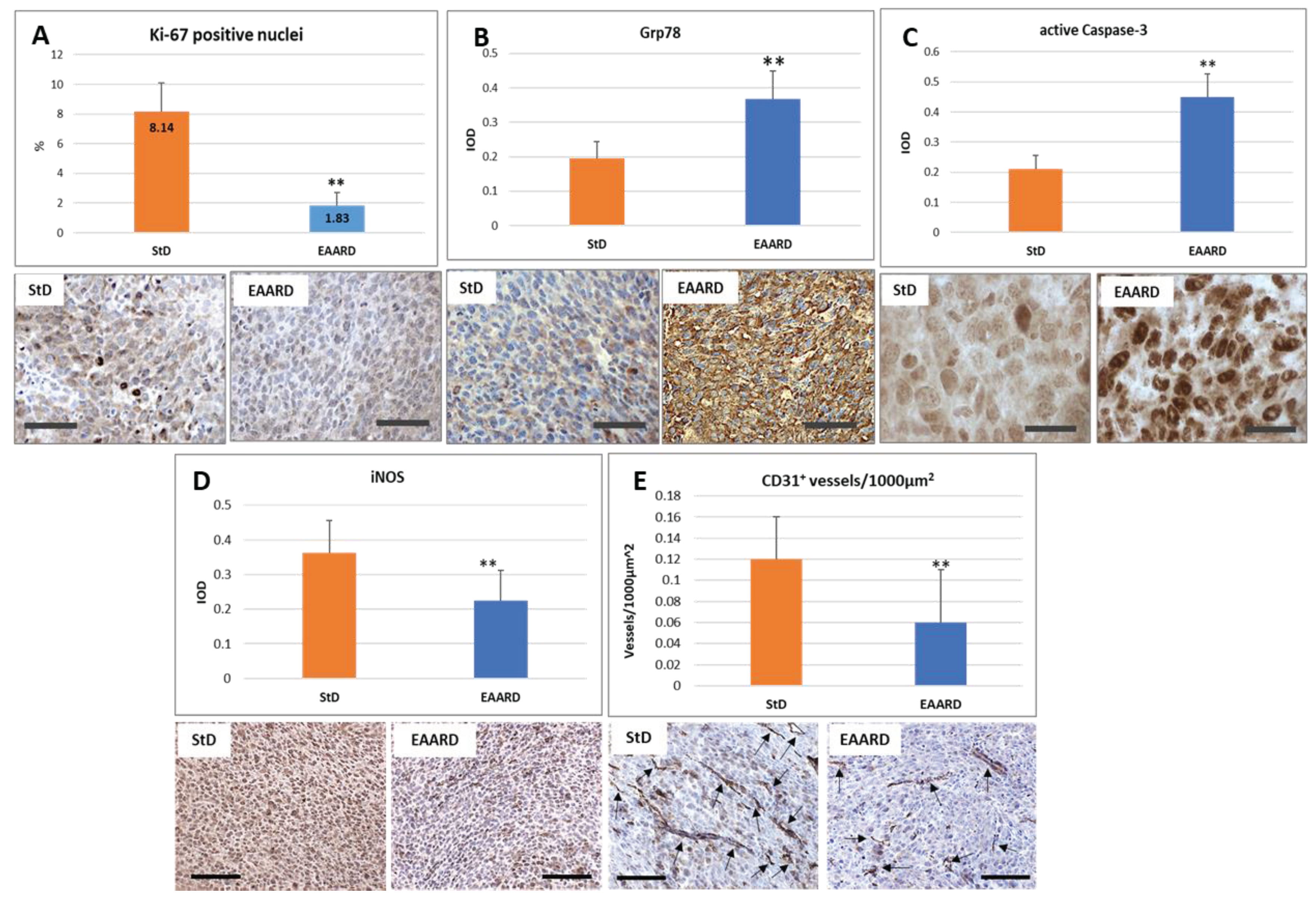
3.4. Immunohistochemical Analysis
3.4.1. Anti-Ki-67 Staining
3.4.2. Anti-GRP78 Staining
3.4.3. Anti-Caspase-3 Staining
3.4.4. Anti-iNOS Staining
3.4.5. Anti-CD31 Vascular Staining
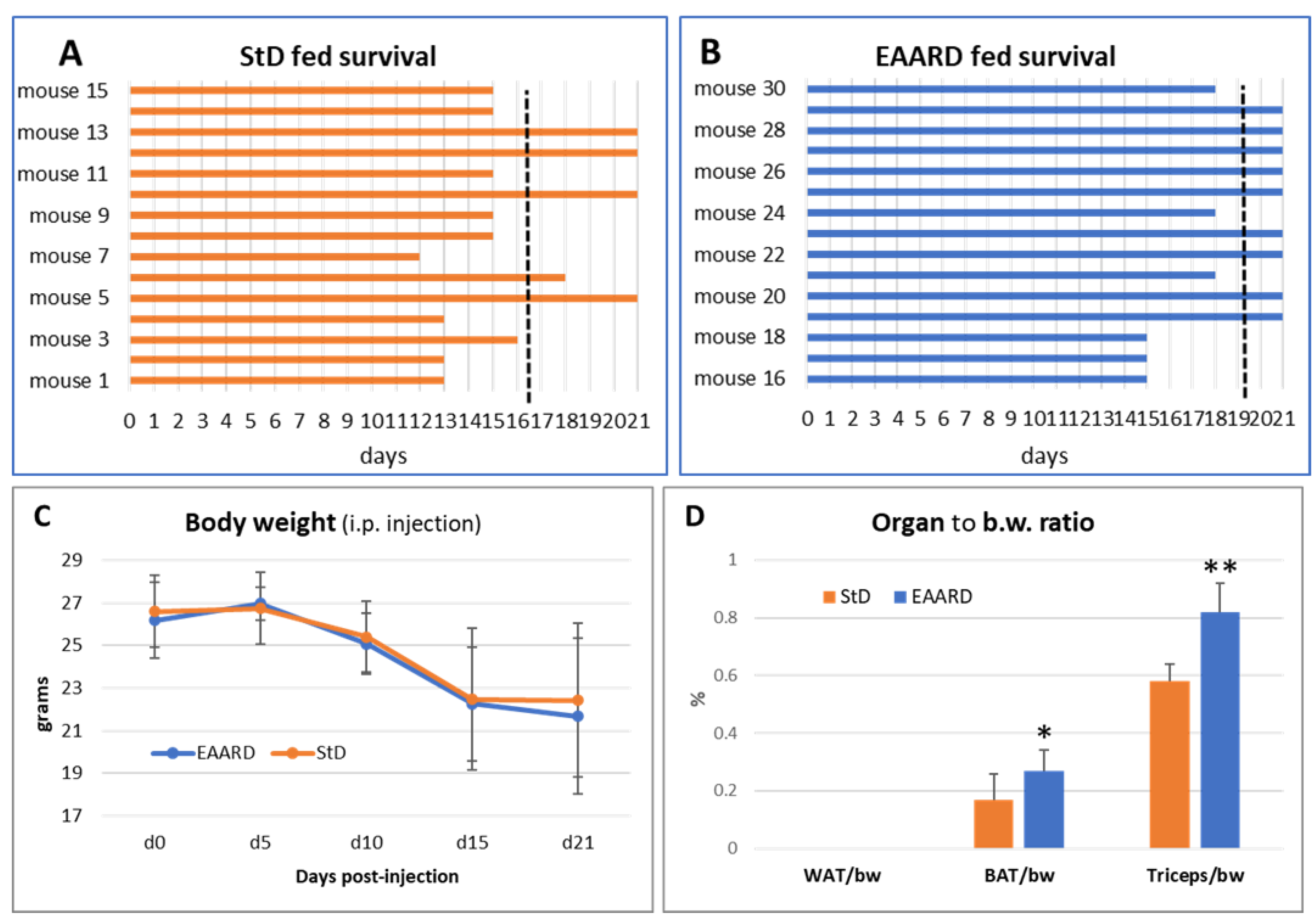
3.5. Intraperitoneal (i.p.) CT26 Injection
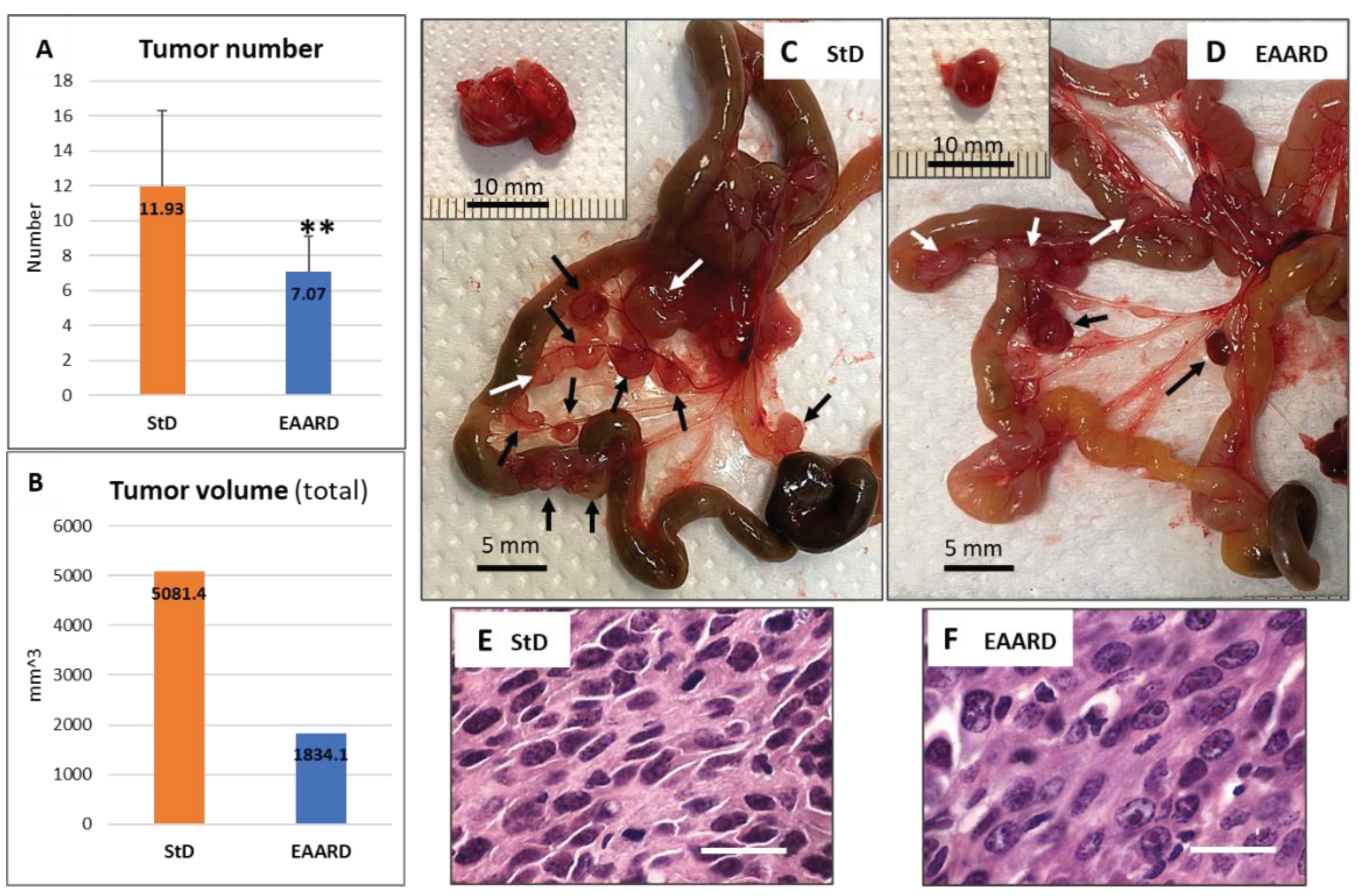
3.6. Mesenteric Tumors
4. Discussion
4.1. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J Clin 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S.; Chen-Scarabelli, C.; Pasini, E.; Corsetti, G.; Scarabelli, T.M. Diet, muscle protein synthesis and autophagy relationships in cancer. An attempt to understand where are we going, and why. Adv in Nutri and Food Sci, 2022; 238. [Google Scholar]
- Saxton, R.A.; Sabatini, D.M. MTOR signaling in growth, metabolism and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; Fornelli, C.; Nisoli, E.; Penna, F. Amino acids in cancer and cachexia: an integrated view. Cancers 2022, 14, 5691. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Pasini, E.; D’Antona, G.; Nisoli, E.; Flati, V.; Assanelli, D.; Dioguardi, F.D.; Bianchi, R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol 2008, 101, 26E–34E. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Stacchiotti, A.; Tedesco, L.; D'Antona, G.; Pasini, E.; Dioguardi, F.S.; Nisoli, E.; Rezzani, R. Essential amino acid supplementation decreases liver damage induced by chronic ethanol consumption in rats. Int J ImmunopatholPharmacol 2011, 24, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Flati, V.; Sanità, P.; Pasini, E.; Dioguardi, F.S. Protect and counter-attack: nutritional supplementation with essential amino acid ratios reduces doxorubicin-induced cardiotoxicity in vivo and promote cancer cell death in vitro. J Cytol Histol 2015, 6, 1. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Pasini, E.; Scarabelli, T.; Chen-Scarabelli, C.; Dioguardi, F.S. Essential amino acids-rich diet increases cardiomyocytes protection in Doxorubicin-treated mice. Nutrients 2023, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- D'Antona, G.; Tedesco, L.; Ruocco, C.; Corsetti, G.; Ragni, M.; Fossati, A.; Saba, E.; Fenaroli, F.; Montinaro, M.; Carruba, M. O. , et al. A peculiar formula of essential amino acids prevents rosuvastatin myopathy in mice. Antioxid Redox Signal 2016, 25, 595–608. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. ClinNutr 2020, 39, 2080–2091. [Google Scholar] [CrossRef]
- Tedesco, L.; Corsetti, G.; Ruocco, C.; Ragni, M.; Rossi, F.; Carruba, M.O.; Valerio, A.; Nisoli, E. A specific amino acid formula preventsalcoholicliverdisease in rodents. Am J PhysiolGastrointest Liver Physiol 2018, 314, G566–G582. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, L.; Rossi, F.; Ragni, M.; Ruocco, C.; Brunetti, D; Carruba, M. O.; Torrente, Y.; Valerio, A.; Nisoli, E. A special amino-acid formula tailored to boosting cell respiration prevents mitochondrial dysfunction and oxidative stress caused by doxorubicin in mouse cardiomyocytes. Nutrients, 2020, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Flati, V.; Corsetti, G.; Pasini, E.; Rufo, A.; Romano, C.; Dioguardi, F.S. Nutrition, nitrogen requirements, exercise and chemotherapy-induced toxicity in cancer patients. A puzzle of contrasting truths? Anticancer Agents Med Chem 2016, 16, 89–100. [Google Scholar] [CrossRef]
- Maddocks, O.D.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br J Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Corrigendum: Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 548, 122, Erratum for: Nature. 2017, 544(7650): 372-376. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S.; Flati, V.; Corsetti, G.; Pasini, E.; Romano, C. Is the response of tumours dependent on the dietary input of some amino acids or ratios among essential and non-essential amino acids? All that glitters is not gold. Int J Mol Sci 2018, 19, 3631. [Google Scholar] [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of diets with varying essential/nonessential amino acid ratios on mouse lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J 2017, 284, 1726–1737. [Google Scholar] [CrossRef]
- Elmore, S.A.; Dixon, D.; Hailey, J.R.; Harada, T.; Herbert, R.A.; Maronpot, R.R.; Nolte, T.; Rehg, J.E.; Rittinghausen, S.; Rosol, T.J.; et al. Recommendations from the INHAND Apoptosis/Necrosis Working Group. Toxicol Pathol 2016, 44, 173–188. [Google Scholar] [CrossRef]
- Chan, J.K. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol 2014, 22, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Dayan, D.; Hiss, Y.; Hirshberg, A.; Bubis, J.J.; Wolman, M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 1989, 93, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.F.; McCullagh, K.G.; Silver, I.A. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res 1984, 12, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Koren, R.; Yaniv, E.; Kristt, D.; Shvero, J.; Veltman, V.; Grushko, I.; Feinmesser, R.; Sulkes, J.; Gal, R. Capsular collagen staining of follicular thyroid neoplasm by picrosirius red: Role in differential diagnosis. Acta Histochem 2001, 103, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; Ruocco, C.; Tedesco, L.; Carruba, M.O.; Valerio, A.; Nisoli, E. An amino acid-defined diet impairs tumour growth in mice by promoting endoplasmic reticulum stress and mTOR inhibition. Mol Metab 2022, 60, 101478. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.M.; Ahmad, H.; Ain, Q.; Anjum, T.; Malik, Z.S.; Hussain, Z.; Naseer, F. Caspase 3 and its role in the pathogenesis of cancer. Clin Oncol 2022, 7, 1941. [Google Scholar]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, CY. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Meyer, M.; Fay, J.; Curry, S.; Bacon, O.; Duessmann, H. , John, K., Boland, K.C.; McNamara, D.A.; Kay, E.; et al. Low levels of Caspase-3 predict favorable response to 5FU-based chemotherapy in advanced colorectal cancer: Caspase-3 inhibition as a therapeutic approach. Cell Death Dis 2016, 7, e2087. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Y.; He, S.; Cheng, J.; Gong, Y.; Zhang, Z. , Yang, X.; Xu, B.; Liu, X.; Li, C.Y.; et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett 2017, 385, 12–20. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Huang, R.; Liu, X.; Yu, Y.; Lu, H.; Xie, J. Apoptosis of colon cancer CT-26 cells induced polysaccharide from Cyclocaryapaliurus and its phosphorylated derivative via intrinsic mitochondrial passway. Food Sci Hum Well 2023, 12, 1545–1556. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. PNAS 2021, 118, e2026507118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Liu, Q.; Ran, D.; Wang, L.; Feng, J.; Deng, W.; Mei, D.; Peng, Y.; Du, C. Association between Ki67 expression and therapeutic outcome in colon cancer. Oncol Lett 2023, 25, 272. [Google Scholar] [CrossRef]
- Luo, Z.W.; Zhu, M.G.; Zhang, Z.Q.; Ye, F.J.; Huang, W.H.; Luo, X.Z. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta-analysis. BMC Cancer 2019, 19, 123. [Google Scholar] [CrossRef]
- Kuznetsov, S.S.; Snopova, L.B.; Karabut, М.М.; Sirotkina, М.А.; Buyanova, N.L.; Kalganova, Т.I.; Elagin, V.V.; Senina-Volzhskaya, I.V.; Barbashova, L.N.; Shumilova, A.V.; et al. Features of morphological changes in experimental CT-26 tumors growth. Sovremennyetehnologii v medicine 2015, 7, 32. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W. , Thurston, G., Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. anti-angiogenic therapy: current challenges and future perspectives. Int J Mol Sci 2021, 22, 3765. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumour Biol 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.S.; Dunleavey, J.M.; Szot, C.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Feng, Y.; Lutz, E.M.; Koogle, R.; et al. Cancer cell survival depends on collagen uptake into tumor-associated stroma. Nat Commun 2022, 13, 7078. [Google Scholar] [CrossRef] [PubMed]
- Hotary, K.B; Allen, E.D.; Brooks, P.C.; Datta, N.S. , Long, M.W., Weiss, S.J. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 2003, 114, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest 2021, 131, e146987. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int J Mol Sci 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, J.; Kim, K. Crosstalk between endoplasmic reticulum stress response and autophagy in human diseases. Anim Cells Syst (Seoul) 2023, 27, 29–37. [Google Scholar] [CrossRef]
- Lee, A.S. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Bläss, S.; Union, A.; Raymackers, J.; Schumann, F.; Ungethüm, U.; Müller-Steinbach, S.; De Keyser, F.; Engel, J.M.; Burmester, G.R. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum 2001, 44, 761–771. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Cuchacovich, M.; Llanos, C.; Urzua, C.; Gawdi, G.; Pizzo, S.V. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res 2006, 66, 11424–11431. [Google Scholar] [CrossRef]
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. GRP78 regulates apoptosis, cell survival and proliferation in 5-fluorouracil-resistant snuc5 colon cancer cells. Anticancer Res 2017, 37, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Vig, S.; Buitinga, M.; Rondas, D.; Crèvecoeur, I.; van Zandvoort, M.; Waelkens, E.; Eizirik, D.L.; Gysemans, C.; Baatsen, P.; Mathieu, C.; et al. Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells. Cell Death Dis 2019, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Pizzo, S.V. Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol Ther 2010, 9, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. BiochimBiophys Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol 2019 21, 63–71. [CrossRef]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes. J Biomed Sci 2020, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Hall, M. Multiple amino acid sensing inputs to mTORC1. Cell Res 2016, 26, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Wei, M.; Yunoki, T.; Kakehashi, A.; Yamano, S.; Kato, M.; Wanibuchi, H. Long-term treatment with L-isoleucine or L-leucine in AIN-93G diet has promoting effects on rat bladder carcinogenesis. Food Chem Toxicol 2012, 50, 3934–3940. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci Rep 2019, 9, 15529. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in cancer cachexia: mechanisms and a review of the pre-clinical literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Locasale, J.W.; Cantley, L.C. Genetic selection for enhanced serine metabolism in cancer development. Cell Cycle 2011, 10, 3812–3813. [Google Scholar] [CrossRef]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.K. , Jang, H.G.; Jha, A.K.; et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Snell, K. Enzymes of serine metabolism in normal and neoplastic rat tissues. BiochimBiophys Acta 1985, 843, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; van den Broek, N.J.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Reports 2014, 7, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.P.; Hulea, L.; Toban, N.; Birman, E.; Blouin, M.J.; Zakikhani, M.; Zhao, Y.; Topisirovic, I.; St-Pierre, J.; Pollak, M. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res 2014, 74, 7521–7533. [Google Scholar] [CrossRef]
- Keum, N.N.; Giovannucci, E.L. Folic Acid fortification and colorectal cancer risk. Am J Prev Med 2014, 46, S65–S72. [Google Scholar] [CrossRef]
- Kruman, I.I.; Fowler, A.K. Impaired one carbon metabolism and DNA methylation in alcohol toxicity. J Neurochem 2014, 129, 770–780. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Gupta, V.; Gopinath, P.; Mazurek, S.; Bamezai, R.N. Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett 2014, 588, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Corsetti, G.; Pasini, E.; Flati, V.; Dioguardi, F.S. Dietary modification of nitrogen intake decreases inflammation and promotes rejuvenation of spleen in aged mice. J Food Nutr Res 2018, 6, 419–432. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Pasini, E.; Testa, C.; Dioguardi, F.S. Qualitative nitrogen malnutrition damages gut and alters microbiome in adult mice. A preliminary histopathological study. Nutrients 2021, 13, 1089. [Google Scholar] [CrossRef]
| EAA-mix | NEAA-mix | StD | EAARD | |
|---|---|---|---|---|
| KCal/Kg | -- | -- | 3952 | 3995 |
| Carbohydrates (%) | -- | -- | 54.61 | 61.76 |
| Lipids (%) | -- | -- | 7.5 | 6.12 |
| Nitrogen (%) | -- | -- | 21.8 ° | 20 * |
| Proteins: % of total N content | -- | -- | 95.93 | -- |
| Free AA: % of total N content | -- | -- | 4.07 | 100 |
| EAA/NEAA (% in grams) | -- | -- | < or <<0.9 | 6.14 (86/14) |
| Free AA composition (%) | ||||
| L-Leucine (bcaa) | 13.53 | -- | -- | 13.53 |
| L-Isoleucine (bcaa) | 9.65 | -- | -- | 9.65 |
| L-Valine (bcaa) | 9.65 | -- | -- | 9.65 |
| L-Lysine | 11.6 | -- | 0.97 | 11.6 |
| L-Threonine | 8.7 | -- | -- | 8.7 |
| L-Histidine | 11.6 | -- | -- | 11.6 |
| L-Phenylalanine | 7.73 | -- | -- | 7.73 |
| L-Methionine | 4.35 | -- | 0.45 | 4.35 |
| L-Tyrosine | 5.80 | 1.0 | -- | 5.80 |
| L-Triptophan | 3.38 | -- | 0.28 | 3.38 |
| L-Cystine/Cysteine | 8.20 | -- | 0.39 | 8.20 |
| L-Alanine | -- | 35.0 | -- | -- |
| L-Glycine | -- | 15.0 | 0.88 | -- |
| L- Arginine | -- | 14.0 | 1.1 | -- |
| L-Proline | -- | 12.0 | -- | -- |
| L-Glutamine | -- | 12.0 | -- | -- |
| L-Serine | 2.42 | 6.0 | -- | 2.42 |
| L-Glutamic Acid | -- | 2.0 | -- | -- |
| L-Asparagine | -- | 2.0 | -- | -- |
| L-Aspartic Acid | -- | 1.0 | -- | -- |
| Ornithine-αKG | 2.42 | -- | -- | 2.42 |
| N-acetylcysteine | 0.97 | -- | -- | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





