Submitted:
06 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
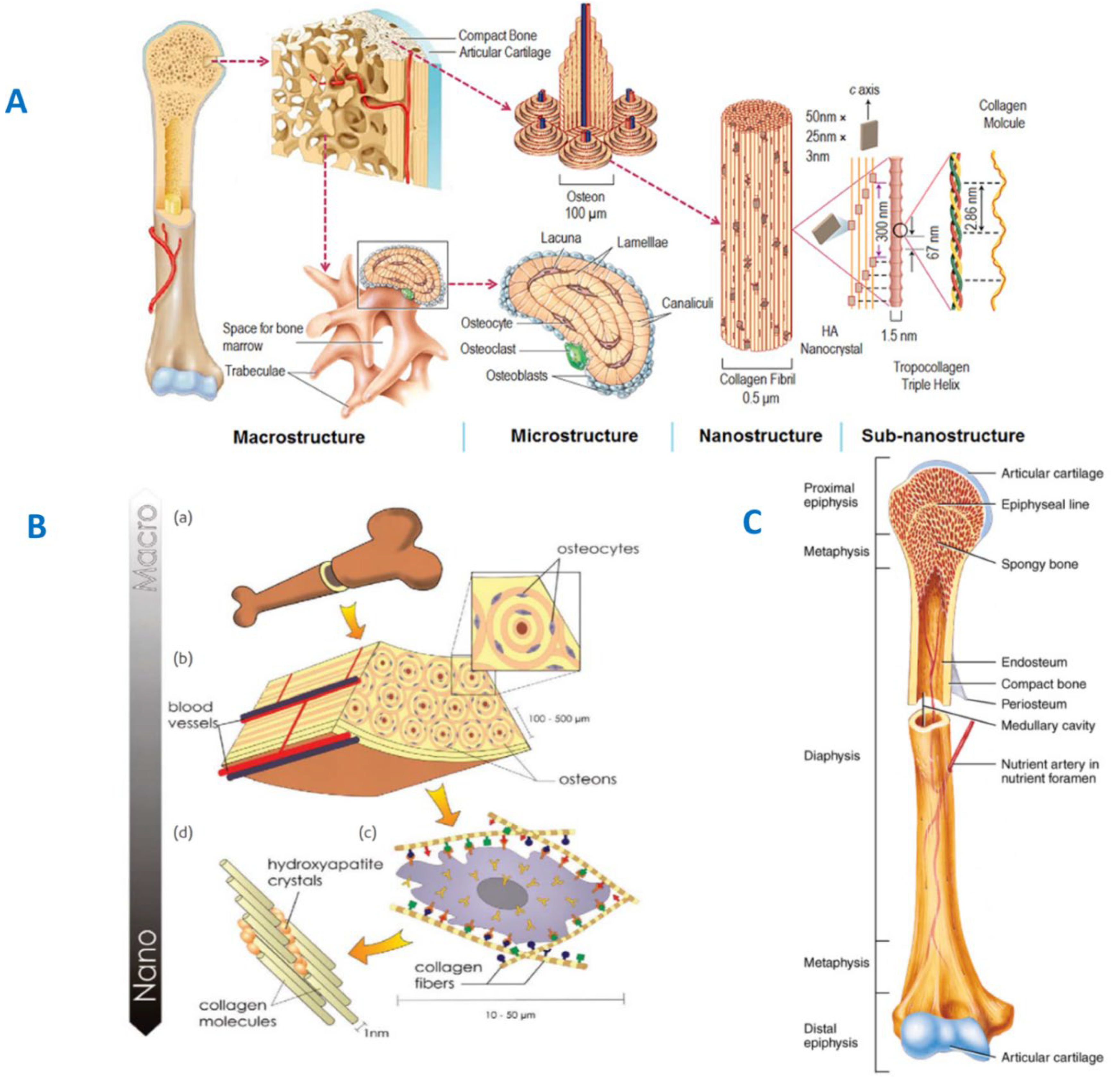
2. Structural and Chemical Properties of Bone
3. GF-CaP: Bioorganisms Interactions
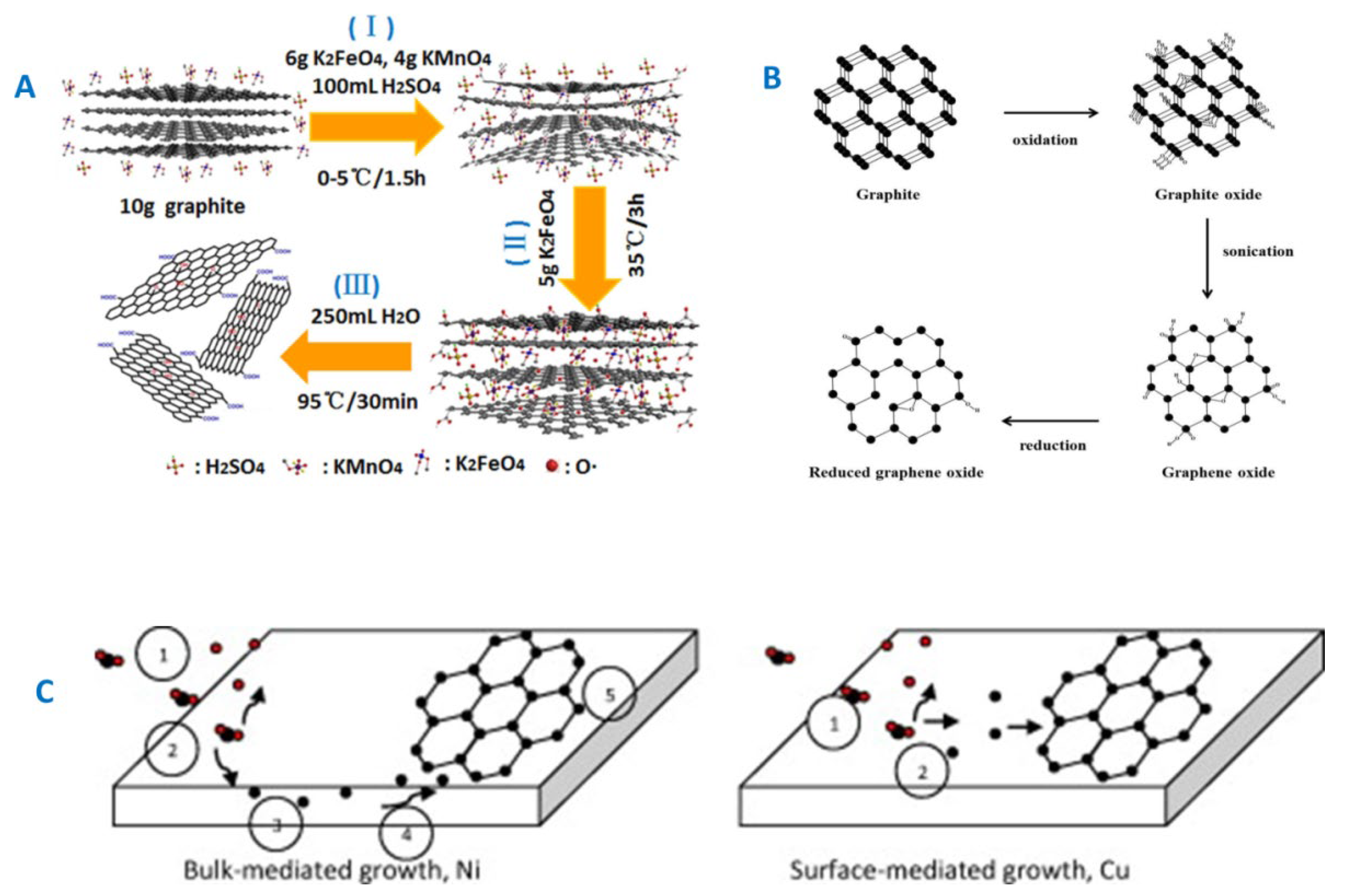
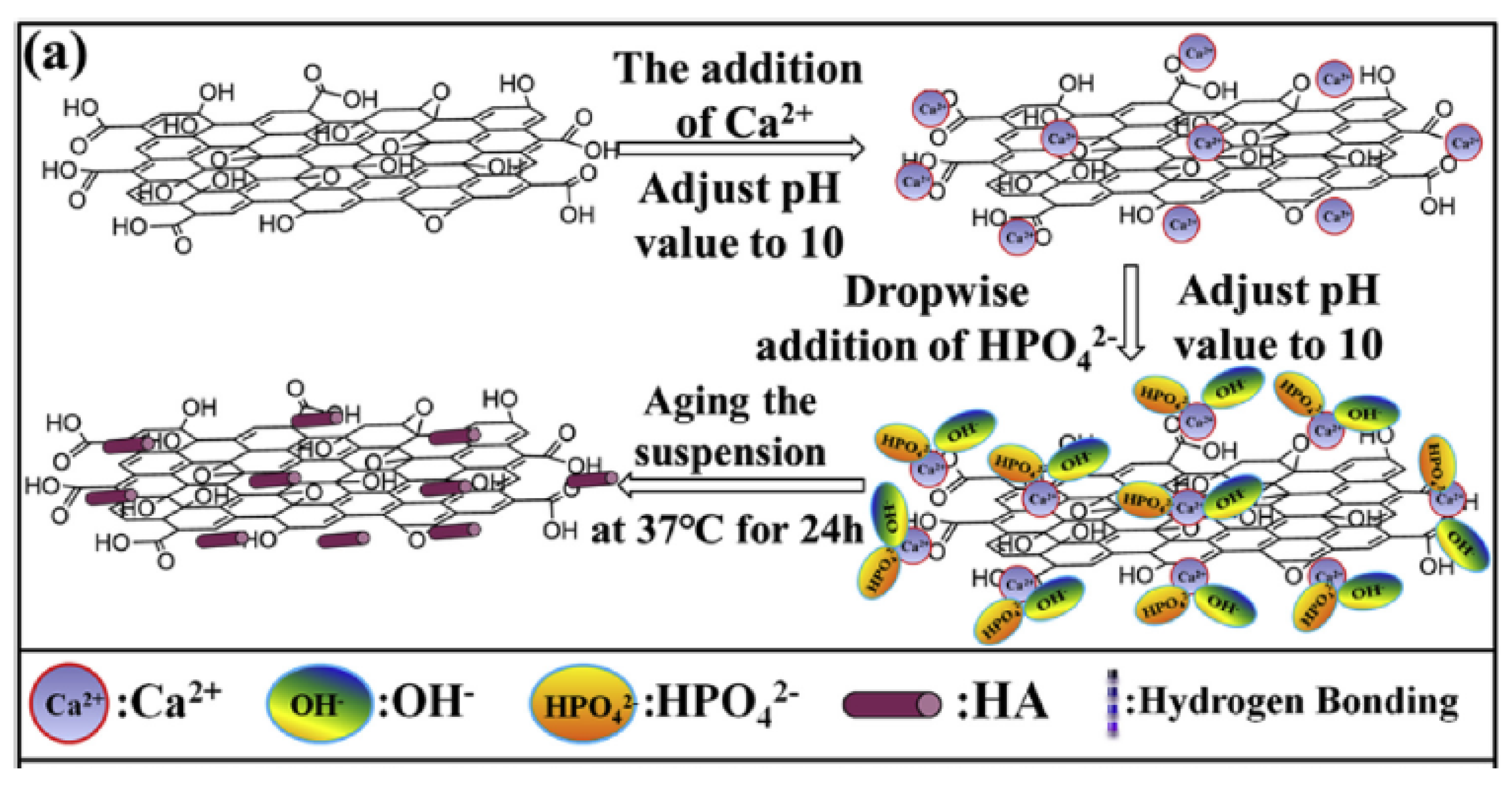
3.1. GF and GF-CaP Coatings: Chemistry
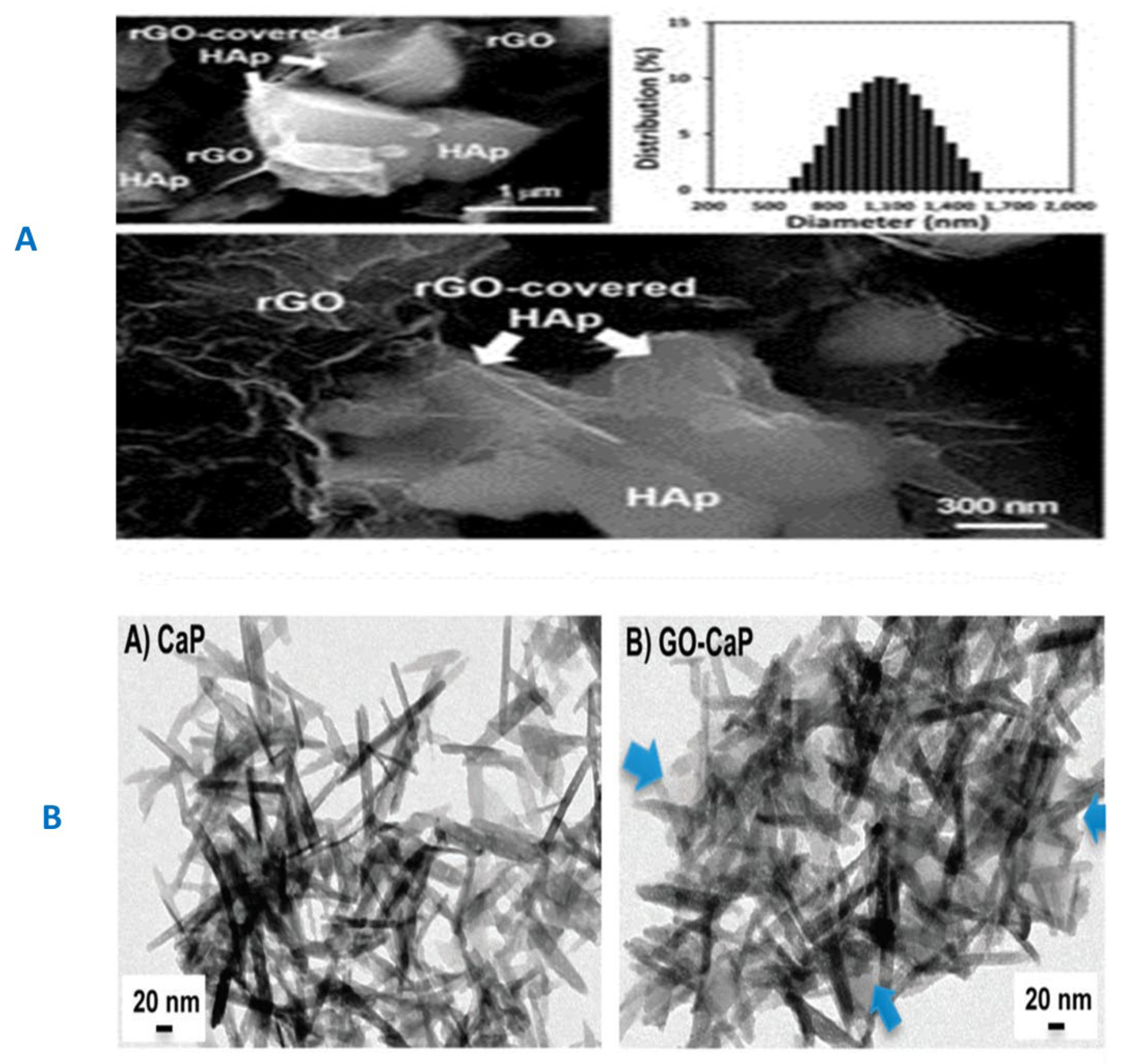
3.2. GF-CaP Biointeractions and Effects
4. GF-CaP Based 2D & 3D Layered Nano Composites and Influences
5. Emerging Biomedical Applications of GF-CaP Based Architectures
6. Standpoint and Future Directions
Acknowledgments
References
- Yi, H.; Rehman, F.U.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, W.; Fan, Y.; Xu, X.; Zhang, K.; Xie, D.; Luo, R.; Yang, X.; Chen, B. Electro-deposited calcium phosphate compounds on graphene sheets: Blossoming flowers. Mater. Lett. 2016, 179, 122–125. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, Y.; He, J.; Shao, W.; Zhou, Y.; Qi, K.; Wang, L.; Cui, S. A graded graphene oxide-hydroxyapatite/silk fibroin biomimetic scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater Biol. Appl. 2017, 80, 232–242. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. ; Synthesis; Properties, M. , and in Vitro Biocompatibility with Osteoblasts of Calcium Silicate–Reduced Graphene Oxide Composites, ACS Appl Mater Interfaces. 2014, 6, 3947–3962. [Google Scholar]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Hydroxyapatite Mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(lactic-co-glycolic acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Talukdar, Y.; Rashkow, J.T.; Lalwani, G.; Kanakia, S.; Sitharaman, B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials 2014, 35, 4863–4877. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shin, Y.C.; Lee, J.-J.; Bae, E.-B.; Jeon, Y.-C.; Jeong, C.-M.; Yun, M.-J.; Lee, S.-H.; Han, D.-W.; Huh, J.-B. The Effect of Reduced Graphene Oxide-Coated Biphasic Calcium Phosphate Bone Graft Material on Osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833–18833. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. In situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013, 48, 175–179. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, Q.; Li, Q.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. , In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosanfunctionalized graphene oxide. J. Mater Chem B. 2013, 1, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Della Bona, A.; Cavalcanti, B.N.; Nör, J.E. Tissue engineering: From research to dental clinics. Dent. Mater. 2012, 28, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Bao, R.-Y.; Shi, X.-J.; Yang, W.; Yang, M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative facile methods for preparing graphene oxide-hydroxyapatite for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2204–2216. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges, Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Rosa, V.; Botero, T.M.; Nör, J.E. Regenerative endodontics in light of the stem cell paradigm. Int. Dent. J. 2011, 61, 23–28. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Lee, J.H.; Lee, E.-J.; Kim, H.-W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Xie, C.; Sun, H.; Wang, K.; Zheng, W.; Lu, X.; Ren, F. Graphene oxide nanolayers as nanoparticle anchors on biomaterial surfaces with nanostructures and charge balance for bone regeneration. J. Biomed. Mater. Res. A 2017, 105, 1311–1323. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Z.; Jiang, F.; Zeng, B.; Huang, M.; Yu, D. Cellular behaviours of bone marrow-derived mesenchymal stem cells towards pristine graphene oxide nanosheets. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Dai, J.; Wang, J.; Tan, Y.; Yang, X.; Yang, S.; Yuan, Q.; Zhang, Y. A 3D graphene coated bioglass scaffold for bone defect therapy based on the molecular targeting approach. J. Mater. Chem. B 2017, 5, 6794–6800. [Google Scholar] [CrossRef]
- Ma, H.; Chang, J.; Wu, C. , in Developments and Applications of Calcium Phosphate Bone Cements, eds. C. Liu and H. 2018. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, J.; Koh, R.H.; Shim, J.; Lee, J.-C.; Kim, T.-I.; Hwang, N.S. Enhanced Osteogenic Commitment of Human Mesenchymal Stem Cells on Polyethylene Glycol-Based Cryogel with Graphene Oxide Substrate. ACS Biomater. Sci. Eng. 2017, 3, 2470–2479. [Google Scholar] [CrossRef]
- Solis-Fernandez, P.; Bissett, M.; Ago, H. Synthesis, structure and applications of graphene-based 2D heterostructures. Chem. Soc. Rev. 2017, 46, 4572–4613. [Google Scholar] [CrossRef]
- Al Faouri, R.; Henry, R.; Biris, A.S.; Sleezer, R.; Salamo, G.J. Adhesive force between graphene nanoscale flakes and living biological cells. J. Appl. Toxicol. 2017, 37, 1346–1353. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of enhanced hydrophilic and hydrophobic graphene oxide nanosheets by a solvothermal method. Carbon 2009, 47, 68–72. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Meng, L.; Sun, G.; Lu, C.; Li, Z. Mechanism of cement paste reinforced by graphene oxide/carbon nanotubes composites with enhanced mechanical properties. RSC Adv. 2015, 5, 100598–100605. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite, Cement and Concrete Comp.2015, 58, 140–147. [CrossRef]
- Lu, Z.; Hou, D.; Ma, H.; Fan, T.; Li, Z. Effects of graphene oxide on the properties and microstructures of the magnesium potassium phosphate cement paste. Constr. Build. Mater. 2016, 119, 107–112. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef]
- Bai, R.G.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.S.; Simões, B.; Mendes, P.M. The increasing dynamic, functional complexity of bio-interface materials. Nat. Rev. Chem. 2018, 2, 0120. [Google Scholar] [CrossRef]
- Hu, X.-B.; Liu, Y.-L.; Wang, W.-J.; Zhang, H.-W.; Qin, Y.; Guo, S.; Zhang, X.-W.; Fu, L.; Huang, W.-H. Biomimetic Graphene-Based 3D Scaffold for Long-Term Cell Culture and Real-Time Electrochemical Monitoring. Anal. Chem. 2018, 90, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Yaragalla, S.; M, A.P.; Kalarikkal, N.; Thomas, S. Chemistry associated with natural rubber–graphene nanocomposites and its effect on physical and structural properties. Ind. Crop. Prod. 2015, 74, 792–802. [Google Scholar] [CrossRef]
- Peizhen, D.; Juan, S.; Guohong, Z.; Xu, X.; Bo, J.; Jiaxin, Y. , Synthesis spherical porous hydroxyapatite/graphene oxide composites by ultrasonic-assisted method for biomedical applications, Biomed Mater. 2018, 13, 045001.
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; Cheng, Y. , An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Moya, A.; Larochette, N.; Bourguignon, M.; El-Hafci, H.; Potier, E.; Petite, H.; Logeart-Avramoglou, D. Osteogenic potential of adipogenic predifferentiated human bone marrow-derived multipotent stromal cells for bone tissue-engineering. J. Tissue Eng. Regen M. 2018, 2018. 12, e1511–e1524. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Swain, M.V. , Removal of dentin non-collagenous structures results in the unraveling of microfibril bundles in collagen type I, Connect Tissue Res. 2017, 58, 414–423.
- Chakraborty, R.; Seesala, V.S.; Sen, M.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. , MWCNT reinforced bone like calcium phosphate—Hydroxyapatite composite coating developed through pulsed electrodeposition with varying amount of apatite phase and crystallinity to promote superior osteoconduction, cytocompatibility and corrosion protection performance compared to bare metallic implant surface, Surf Coat Tech. 2017, 325, 496–514.
- Chen, C.; Sun, X.; Pan, W.; Hou, Y.; Liu, R.; Jiang, X.; Zhang, L. Graphene Oxide-Templated Synthesis of Hydroxyapatite Nanowhiskers To Improve the Mechanical and Osteoblastic Performance of Poly(lactic acid) for Bone Tissue Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 3862–3869. [Google Scholar] [CrossRef]
- Hernigou, P. , Authorities and foundation of the orthopaedic school in Germany in the 19th century: part II: Richard von Volkmann, Julius Wolff, Albert Hoffa, Friedrich Trendelenburg and other German authors. Int Orthop. 2016, 40, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Hasegawa, T.; Yamamoto, T.; de Freitas, P.H.L.; Oda, K.; Yamauchi, A.; Yokoyama, A. Histochemical examination on the peri-implant bone with early occlusal loading after the immediate placement into extraction sockets. Histochem Cell Bio. 2018, 149, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.A.; Wallace, J.M. Exercise prevents β-aminopropionitrile-induced morphological changes to type I collagen in murine bone. BoneKEy Rep. 2015, 4, 645. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018, 58, 164–207. [Google Scholar] [CrossRef]
- Merk, V.; Chanana, M.; Keplinger, T.; Gaan, S.; Burgert, I. Hybrid wood materials with improved fire retardance by bio-inspired mineralisation on the nano- and submicron level. Green Chem. 2015, 17, 1423–1428. [Google Scholar] [CrossRef]
- Pobloth, A.-M.; Johnson, K.A.; Schell, H.; Kolarczik, N.; Wulsten, D.; Duda, G.N.; Schmidt-Bleek, K. Establishment of a preclinical ovine screening model for the investigation of bone tissue engineering strategies in cancellous and cortical bone defects. BMC Musculoskelet. Disord. 2016, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Boyce, R.W.; Niu, Q.-T.; Ominsky, M.S. Kinetic reconstruction reveals time-dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone 2017, 101, 77–87. [Google Scholar] [CrossRef]
- Omiya, T.; Hirose, J.; Hasegawa, T.; Amizuka, N.; Omata, Y.; Izawa, N.; Yasuda, H.; Kadono, Y.; Matsumoto, M.; Nakamura, M.; Miyamoto, T.; Tanaka, S. The effect of switching from teriparatide to anti-RANKL antibody on cancellous and cortical bone in ovariectomized mice. Bone 2018, 107, 18–26. [Google Scholar] [CrossRef]
- Chirchir, H.; Kivell, T.L.; Ruff, C.B.; Hublin, J.-J.; Carlson, K.J.; Zipfel, B.; Richmond, B.G. , Proc Natl Acad Sci U S A. 2015, 112, 366–371.
- Manske, S.L.; Zhu, Y.; Sandino, C.; Boyd, S.K. , Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT, Bone. 2015, 79, 213–221.
- de Oliveira, B.J.S.; Campanelli, L.C.; Oliveira, D.P. , Bribean.; Guerra, A.P.d.; Bolfarini, C. Surface characterization and fatigue performance of a chemical-etched Ti-6Al-4V femoral stem for cementless hip arthroplasty. Surf Coat Tech. 2017, 309, 1126–1134. [Google Scholar] [CrossRef]
- Ke, P.; Jiao, X.-N.; Ge, X.-H.; Xiao, W.-M.; Yu, B. From macro to micro: structural biomimetic materials by electrospinning. RSC Adv. 2014, 4, 39704–39724. [Google Scholar] [CrossRef]
- Hua, W.; Chen, P.; Xu, M.; Ao, Z.; Liu, Y.; Han, D.; He, F. Quantitative description of collagen fibre network on trabecular bone surfaces based on AFM imaging. J. Microsc. 2016, 262, 112–122. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater Biol Appl. 2016, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhai, H.; Zhang, W.; Wang, L. Monomeric Amelogenin’s C-Terminus Modulates Biomineralization Dynamics of Calcium Phosphate. Cryst. Growth Des. 2015, 15, 4490–4497. [Google Scholar] [CrossRef]
- Xie, Q.; Xue, Z.; Gu, H.; Hu, C.; Yang, M.; Wang, X.; Xu, D. Molecular Dynamics Exploration of Ordered-to-Disordered Surface Structures of Biomimetic Hydroxyapatite Nanoparticles. J. Phys. Chem. C 2018, 122, 6691–6703. [Google Scholar] [CrossRef]
- Bsat, S.; Speirs, A.; Huang, X. Recent Trends in Newly Developed Plasma-Sprayed and Sintered Coatings for Implant Applications. J. Therm. Spray Technol. 2016, 25, 1088–1110. [Google Scholar] [CrossRef]
- Grünewald, T.; Rennhofer, H.; Hesse, B.; Burghammer, M.; Stanzl-Tschegg, S.; Cotte, M.; Löffler, J.; Weinberg, A.; Lichtenegger, H. Magnesium from bioresorbable implants: Distribution and impact on the nano- and mineral structure of bone. Biomaterials 2016, 76, 250–260. [Google Scholar] [CrossRef]
- Tanaka, M.; Hosoya, A.; Mori, H.; Kayasuga, R.; Nakamura, H.; Ozawa, H. , Minodronic acid induces morphological changes in osteoclasts at bone resorption sites and reaches a level required for antagonism of purinergic P2X2/3 receptors. J. Bone Miner Metab. 2018. 36, 54–63. [CrossRef]
- Basirun, W.J.; Nasiri-Tabrizi, B.; Baradaran, S. , Overview of Hydroxyapatite–Graphene Nanoplatelets Composite as Bone Graft Substitute: Mechanical Behavior and In-vitroBiofunctionality, Crit Rev Solid State Mater Sci. 2018, 43, 177–212.
- Albéric, M.; Gourrier, A.; Wagermaier, W.; Fratzl, P.; Reiche, I. The three-dimensional arrangement of the mineralized collagen fibers in elephant ivory and its relation to mechanical and optical properties. Acta Biomater. 2018, 72, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, N.; Turunen, M.J.; Guizar-Sicairos, M.; Bech, M.; Schaff, F.; Tägil, M.; Isaksson, H. The compositional and nano-structural basis of fracture healing in healthy and osteoporotic bone. Sci. Rep. 2018, 8, 1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ural, A. Mineralized collagen fibril network spatial arrangement influences cortical bone fracture behavior. J. Biomech. 2018, 66, 70–77. [Google Scholar] [CrossRef]
- Wang, Y.; Ural, A. Effect of modifications in mineralized collagen fibril and extra-fibrillar matrix material properties on submicroscale mechanical behavior of cortical bone. J. Mech. Behav. Biomed. Mater. 2018, 82, 18–26. [Google Scholar] [CrossRef]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Cazalbou, S.; Grossin, D.; Brouillet, F.; Sarda, S. Surface properties of biomimetic nanocrystalline apatites; applications in biomaterials. Prog. Cryst. Growth Charact. Mater. 2014, 60, 63–73. [Google Scholar] [CrossRef]
- Rafeek, A.D.; Choi, G.; Evans, L.A. spectroscopic and crystallographic studies of calcium phosphate bioceramic powders. J. Aust. Ceram. Soc. 2017, 54, 161–168. [Google Scholar] [CrossRef]
- Tadier, S.; Rokidi, S.; Rey, C.; Combes, C.; Koutsoukos, P.G. Crystal growth of aragonite in the presence of phosphate. J. Cryst. Growth 2017, 458, 44–52. [Google Scholar] [CrossRef]
- Yun, J.; Holmes, B.; Fok, A.; Wang, Y. A Kinetic Model for Hydroxyapatite Precipitation in Mineralizing Solutions. Cryst. Growth Des. 2018, 18, 2717–2725. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Boskey, A.; Gundberg, C.; Vashishth, D. Biomolecular regulation, composition and nanoarchitecture of bone mineral. Sci. Rep. 2018, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jacquet, R.; Lowder, E.; Landis, W.J. , Refinement of collagen–mineral interaction: A possible role for osteocalcin in apatite crystal nucleation, growth and development, Bone. 2015, 71, 7–16.
- Wang, J.; Yang, G.; Wang, Y.; Du, Y.; Liu, H.; Zhu, Y.; Mao, C.; Zhang, S. Chimeric Protein Template-Induced Shape Control of Bone Mineral Nanoparticles and Its Impact on Mesenchymal Stem Cell Fate. Biomacromolecules 2015, 16, 1987–1996. [Google Scholar] [CrossRef]
- Hughes, J.M.; Rakovan, J.F. Structurally Robust, Chemically Diverse: Apatite and Apatite Supergroup Minerals. Elements 2015, 11, 165–170. [Google Scholar] [CrossRef]
- Rollo, J.M.D.A.; Boffa, R.S.; Cesar, R.; Schwab, D.C.; Leivas, T.P. , ssessment of Trabecular Bones Microarchitectures and Crystal Structure of Hydroxyapatite in Bone Osteoporosis with Application of the Rietveld Method, Procedia Eng. 2015, 110, 8–14.
- Wu, H.; Xu, D.; Yang, M.; Zhang, X. Surface Structure of Hydroxyapatite from Simulated Annealing Molecular Dynamics Simulations. Langmuir 2016, 32, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, M.B.; Şahin, Ö.; Taskin, H.; Atakol, O.; Inal, A.; Gunes, A. Effect of synthetic nano-hydroxyapatite as an alternative phosphorus source on growth and phosphorus nutrition of lettuce (Lactuca sativa L.) plant. J. Plant Nutr. 2018, 41, 1148–1154. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Bhanja, P.; Chatterjee, S.; Patra, A.K.; Bhaumik, A. A new microporous oxyfluorinated titanium(IV) phosphate as an efficient heterogeneous catalyst for the selective oxidation of cyclohexanone. J. Colloid Interface Sci. 2018, 511, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.B.; Bai, R.; Yan, C.; Science, C.M. 2017, 126, 59-65.
- Ma, G.; Liu, X.Y.; Des, C.G. 2009, 9, 2991–2994.
- Klimek, K.; Przekora, A.; Benko, A.; Niemiec, W.; Blazewicz, M.; Ginalska, G. , The use of calcium ions instead of heat treatment for β-1, 3-glucan gelation improves biocompatibility of the β-1, 3-glucan/HA bone scaffold, Carbohydr Polym. 2017, 164, 170-178.
- Chen, J.-P.; Tsai, M.-J.; Liao, H.-T. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloids Surfaces B: Biointerfaces 2013, 110, 120–129. [Google Scholar] [CrossRef]
- Sammons, R. Woodhead Publishing Sries in Biomaterials; 2015; pp. 53-83.
- Vallet-Regí, M.; Navarrete, D.A.; Arcos, D. , Biomimetic nanoceramics in clinical use: from materials to applications, Royal Society of Chemistry, 2008.
- Nair, A.K.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 4, 1724–9. [Google Scholar] [CrossRef]
- Masic, A.; Bertinetti, L.; Schuetz, R.; Chang, S.-W.; Metzger, T.H.; Buehler, M.J.; Fratzl, P. Osmotic pressure induced tensile forces in tendon collagen. Nat. Commun. 2015, 6, 5942. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C Mater Biol Appl. 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Ion, R.-M.; Turcanu-Caruţiu, D.; Fierăscu, R.-C.; Fierăscu, I.; Bunghez, I.-R.; Ion, M.-L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi–Murfatlar churches ensemble. Appl. Surf. Sci. 2015, 358, 612–618. [Google Scholar] [CrossRef]
- Tsung, L.H. ; J. I. B.; M. K. G.; Peter, R.J., The Effects of Platelet-Rich Plasma on Cell Proliferation and Adipogenic Potential of Adipose-Derived Stem Cells, Tissue Eng A. 2015, 21, 2714–2722. [Google Scholar]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef]
- Niakan, A.; Ramesh, S.; Ganesan, P.; Tan, C.; Purbolaksono, J.; Chandran, H.; Ramesh, S.; Teng, W.D. Sintering behaviour of natural porous hydroxyapatite derived from bovine bone. Ceram. Int. 2015, 41, 3024–3029. [Google Scholar] [CrossRef]
- Gentile, P.; Wilcock, C.J.; Miller, C.A.; Moorehead, R.; Hatton, P.V. Process Optimisation to Control the Physico-Chemical Characteristics of Biomimetic Nanoscale Hydroxyapatites Prepared Using Wet Chemical Precipitation. Materials 2015, 8, 2297–2310. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.-M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.K.; Hoai, T.T.; Viet, P.H. Biomimetic scaffolds based on hydroxyapatite nanorod/poly(d,l) lactic acid with their corresponding apatite-forming capability and biocompatibility for bone-tissue engineering. Colloids Surfaces B: Biointerfaces 2015, 128, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnology 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef]
- Uskoković, V. The role of hydroxyl channel in defining selected physicochemical peculiarities exhibited by hydroxyapatite. RSC Adv. 2015, 5, 36614–36633. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Li, Z.; Hardij, J.; Bagchi, D.P.; Scheller, E.L.; MacDougald, O.A. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone 2018, 110, 134–140. [Google Scholar] [CrossRef]
- Bliley, J.M.; Sivak, W.N.; Minteer, D.M.; Tompkins-Rhoades, C.; Day, J.; Williamson, G.; Liao, H.T.; Marra, K.G. Ethylene Oxide Sterilization Preserves Bioactivity and Attenuates Burst Release of Encapsulated Glial Cell Line Derived Neurotrophic Factor from Tissue Engineered Nerve Guides For Long Gap Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2015, 1, 504–512. [Google Scholar] [CrossRef]
- Krawiec, J.T.; Liao, H.-T.; Kwan, L. (.; D'Amore, A.; Weinbaum, J.S.; Rubin, J.P.; Wagner, W.R.; Vorp, D.A. Evaluation of the stromal vascular fraction of adipose tissue as the basis for a stem cell-based tissue-engineered vascular graft. J. Vasc. Surg. [CrossRef]
- Wu, C.-M.; Chen, Y.-A.; Liao, H.-T.; Chen, C.-H.; Pan, C.; Chen, C.-T. Surgical treatment of isolated zygomatic fracture: Outcome comparison between titanium plate and bioabsorbable plate. Asian J. Surg. 2017, 41, 370–376. [Google Scholar] [CrossRef]
- Lai, G.-J.; Shalumon, K.; Chen, S.-H.; Chen, J.-P. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef]
- Chen, J.-P.; Su, C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O'Brien, F.J. Development of collagen–hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chen, S.-H.; Chen, C.-H.; Chen, S.-H.; Fong, Y.T.; Chen, J.-P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef]
- Jones, M.D.; Martin, P.S.; Williams, J.M.; Kemp, A.M.; Theobald, P. Development of a computational biomechanical infant model for the investigation of infant head injury by shaking. Med. Sci. Law 2014, 55, 291–299. [Google Scholar] [CrossRef]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C.; Engineering, B.T. 2015, 1, 132-140.
- X. Wang; Xu, S.; Zhou, S.; Xu, W.; M.Leary; P.Choong; Qian, M.; Brandt, M., Y.M.Xie.Extracellular matrix-based biomaterial scaffolds and the host response, Biomaterials, 2016, 83, 127–141.
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Seah, C.-M.; Chai, S.-P.; Mohamed, A.R. Mechanisms of graphene growth by chemical vapour deposition on transition metals. Carbon 2014, 70, 1–21. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Park, S.J.; Rhee, K.Y. Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog. Org. Coatings 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Brahmayya, M.; Dai, S.A.; Suen, S.-Y. Sulfonated reduced graphene oxide catalyzed cyclization of hydrazides and carbon dioxide to 1,3,4-oxadiazoles under sonication. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Brahmayya, M.; Suen, S.-Y.; Dai, S.A. Sulfonated graphene oxide-catalyzed N-acetylation of amines with acetonitrile under sonication. J. Taiwan Inst. Chem. Eng. 2018, 83, 174–183. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Brahmayya, M.; Wang, M.-L. , Synthesis and Characterizations of Graphite Bisulphate and Its Selective Catalytic Activity in Presence of Cyclo Hexanone for the Acylation of Aromatic Compounds with the Carboxylic Acids under Ultrasound Irradiation, Int J Chem. 2014, 35, 1522–1530.
- Liu, Z.; Wu, Z.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible In-Plane Micro-Supercapacitors by Direct Printing of Solution-Processable Electrochemically Exfoliated Graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef]
- Yang, S.; Lohe, M.R.; Müllen, K.; Feng, X. New-Generation Graphene from Electrochemical Approaches: Production and Applications. Adv. Mater. 2016, 28, 6213–6221. [Google Scholar] [CrossRef]
- Shinde, D.B.; Brenker, J.; Easton, C.D.; Tabor, R.F.; Neild, A.; Majumder, M. Shear Assisted Electrochemical Exfoliation of Graphite to Graphene. Langmuir 2016, 32, 3552–3559. [Google Scholar] [CrossRef]
- Choi, J.-Y. , Graphene transfer: A stamp for all substrates, Nat nanotechnol.2013, 8, 311.
- Song, J.; Kam, F.-Y.; Png, R.-Q.; Seah, W.-L.; Zhuo, J.-M.; Lim, G.-K.; Ho, P.K.H.; Chua, L.-L. A general method for transferring graphene onto soft surfaces. Nat. Nanotechnol. 2013, 8, 356–362. [Google Scholar] [CrossRef]
- Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D.; et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef]
- Tan, Y.-Z.; Yang, B.; Parvez, K.; Narita, A.; Osella, S.; Beljonne, D.; Feng, X.; Müllen, K. Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, T.-H.; Lee, Y.; Kim, H.; Lee, H.; Yuan, G.; Satija, S.K.; Choi, J.-H.; Ahn, H.; Koo, J. Perpendicular Orientation of Diblock Copolymers Induced by Confinement between Graphene Oxide Sheets. Langmuir 2018, 34, 1681–1690. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene Oxide Sheets at Interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Khandelwal, M.; Li, Y.; Hur, S.H.; Chung, J.S. Surface modification of co-doped reduced graphene oxide through alkanolamine functionalization for enhanced electrochemical performance. New J. Chem. 2017, 42, 1105–1114. [Google Scholar] [CrossRef]
- G. S.; B. A.; S. T.; D. N., Molecular sensitivity of metal nanoparticles decorated graphene-family nanomaterials as surface-enhanced Raman scattering (SERS) platforms. J. Raman Spectrosc. 2018, 49, 438–451.
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017, 46, 7306–7316. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, W.; Zheng, Y.; Liu, A.; Yao, J.; Li, C.; Tang, W.; Chen, B.; Wang, G.; Shi, Z. The structural and biological properties of hydroxyapatite-modified titanate nanowire scaffolds. Biomaterials 2011, 32, 5837–5846. [Google Scholar] [CrossRef]
- Fu, C.; Song, B.; Wan, C.; Savino, K.; Wang, Y.; Zhang, X.; Yates, M.Z. Electrochemical growth of composite hydroxyapatite coatings for controlled release. Surf. Coatings Technol. 2015, 276, 618–625. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coatings Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Gu, Y.; Loh, N.; Khor, K.; Tor, S.; Cheang, P. Spark plasma sintering of hydroxyapatite powders. Biomaterials 2002, 23, 37–43. [Google Scholar] [CrossRef]
- Klébert, S.; Balázsi, C.; Balázsi, K.; Bódis, E.; Fazekas, P.; Keszler, A.M.; Szépvölgyi, J.; Károly, Z. Spark plasma sintering of graphene reinforced hydroxyapatite composites. Ceram. Int. 2015, 41, 3647–3652. [Google Scholar] [CrossRef]
- Bajpai, I.; Kim, D.-Y.; Han, Y.-H.; Jang, B.-K.; Kim, S. Directional property evaluation of spark plasma sintered GNPs-reinforced hydroxyapatite composites. Mater. Lett. 2015, 158, 62–65. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Yue, C.; Zhang, T.; Li, P.; Xing, Z.; Chen, Y. , A tough graphene nanosheet/hydroxyapatite composite with improved in vitrobiocompatibility, Carbon. 2013, 61, 105–115.
- Repanas, A.; Andriopoulou, S.; Glasmacher, B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 31, 137–146. [Google Scholar] [CrossRef]
- Ma, H.; Su, W.; Tai, Z.; Sun, D.; Yan, X.; Liu, B.; Xue, Q. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012, 57, 3051–3058. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Berndt, C.C.; Hasan, F.; Tietz, U.; Schmitz, K.-P. , Advances in Calcium Phosphate Biomaterials. 2014, 2, 267-329.
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon 2015, 93, 116–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Biris, A.R.; Mahmood, M.; Lazar, M.D.; Dervishi, E.; Watanabe, F.; Mustafa, T.; Baciut, G.; Baciut, M.; Bran, S.; Ali, S.; Biris, A.S. Novel Multicomponent and Biocompatible Nanocomposite Materials Based on Few-Layer Graphenes Synthesized on a Gold/Hydroxyapatite Catalytic System with Applications in Bone Regeneration. J. Phys. Chem. C 2011, 115, 18967–18976. [Google Scholar] [CrossRef]
- Boilet, L.; Descamps, M.; Rguiti, E.; Tricoteaux, A.; Lu, J.; Petit, F.; Lardot, V.; Cambier, F.; Leriche, A. Processing and properties of transparent hydroxyapatite and β tricalcium phosphate obtained by HIP process. Ceram. Int. 2013, 39, 283–288. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Nasiri-Tabrizi, B.; Basirun, W.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Alias, Y. Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application. Mater. Sci. Eng. C Mater Biol Appl. 2015, 49, 656–668. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, K.-N.; Wang, W.-L.; Wang, Y.-X.; Sun, X.-L.; Liang, Y.-J.; Sun, X.-N.; Chui, P.-F. Microstructure and anisotropic mechanical properties of graphene nanoplatelet toughened biphasic calcium phosphate composite. Ceram. Int. 2013, 39, 7627–7634. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.R.N.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Gao, F.; Xu, C.; Hu, H.; Wang, Q.; Gao, Y.; Chen, H.; Guo, Q.; Chen, D.; Eder, D. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater. Lett. 2015, 138, 25–28. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Bai, D.; Shao, C.; Wang, J.; Xi, P.; Zeng, Z. Gelatin functionalized graphene oxide for mineralization of hydroxyapatite: biomimetic and in vitro evaluation. Nanoscale 2014, 6, 5315–5322. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Meng, L.; Niu, L.; Lu, Q. Simultaneous Reduction and Surface Functionalization of Graphene Oxide by Natural Cellulose with the Assistance of the Ionic Liquid. J. Phys. Chem. C 2012, 116, 16294–16299. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Hou, F.; Bai, D.; Xi, P.; Zeng, Z. Biomimetic and Cell-Mediated Mineralization of Hydroxyapatite by Carrageenan Functionalized Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 3132–3140. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic Mineralized Hierarchical Graphene Oxide/Chitosan Scaffolds with Adsorbability for Immobilization of Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 1707–1717. [Google Scholar] [CrossRef]
- Depan, D.; Pesacreta, T.C.; Misra, R.D.K. The synergistic effect of a hybrid graphene oxide–chitosan system and biomimetic mineralization on osteoblast functions. Biomater. Sci. 2014, 2, 264–274. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Wang, Y.; Li, J.; Su, Z.; Wei, G. Alternate layer-by-layer assembly of graphene oxide nanosheets and fibrinogen nanofibers on a silicon substrate for a biomimetic three-dimensional hydroxyapatite scaffold. J. Mater. Chem. B 2014, 2, 7360–7368. [Google Scholar] [CrossRef]
- Ashkan, A. ; J. A. H.; J. A. C., The effect of graphene substrate on osteoblast cell adhesion and proliferation, J Biomed Mater Res A. 2014, 102, 3282–3290. [Google Scholar]
- Tatavarty, R.; Ding, H.; Lu, G.; Taylor, R.J.; Bi, X. Synergistic acceleration in the osteogenesis of human mesenchymal stem cells by graphene oxide–calcium phosphate nanocomposites. Chem. Commun. 2014, 50, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Wang, Y.; Sun, X.; Sun, K. Reduced graphene oxide/carbon nanotubes reinforced calcium phosphate cement. Ceram. Int. 2017, 43, 13083–13088. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Kim, B.; Park, J.-C.; Han, D.-W. Synergistic effects of reduced graphene oxide and hydroxyapatite on osteogenic differentiation of MC3T3-E1 preosteoblasts. Carbon 2015, 95, 1051–1060. [Google Scholar] [CrossRef]
- Manitha, N.; Nancy, D.; Amit, G.K.; Anjusree, G.S.; Sajini, V.; Shantikumar, V.N. , Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells, Nanotechnol. 2015, 26, 161001.
- Zhang, Q.; Liu, Y.; Zhang, Y.; Li, H.; Tan, Y.; Luo, L.; Duan, J.; Li, K.; Banks, C.E. Facile and controllable synthesis of hydroxyapatite/graphene hybrid materials with enhanced sensing performance towards ammonia. Analyst 2015, 140, 5235–5242. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, K.; Fang, D.; Shang, L.; Tran, S.D.; Cerruti, M. Graphene and hydroxyapatite self-assemble into homogeneous, free standing nanocomposite hydrogels for bone tissue engineering. Nanoscale 2015, 7, 7992–8002. [Google Scholar] [CrossRef]
- Wu, C.; Han, P.; Liu, X.; Xu, M.; Tian, T.; Chang, J.; Xiao, Y. Mussel-inspired bioceramics with self-assembled Ca-P/polydopamine composite nanolayer: Preparation, formation mechanism, improved cellular bioactivity and osteogenic differentiation of bone marrow stromal cells. Acta Biomater. 2014, 10, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is Graphene a Promising Nano-Material for Promoting Surface Modification of Implants or Scaffold Materials in Bone Tissue Engineering? Tissue Eng. Part B: Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.M.; Giugliano, M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014, 5, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Guan, S.; Wang, Y.; Shi, G.; Cao, L.; Gao, Y.; Dong, Z.; Xu, J.; Luo, Q.; Liu, J. Self-assembly of amphiphilic peptides into bio-functionalized nanotubes: a novel hydrolase model. J. Mater. Chem. B 2013, 1, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T., A. H. Castro.; Neto, A.H.C.; Rosa, V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2015, 2015, 12. [Google Scholar] [CrossRef]
- Sungjin, K.; Hee, K.S.; Yoon, L.S.; Hong, K.J.; Beum, P.C.; Materials, B.H. ; Mater. A. 2011, 23, 2009–2014. [Google Scholar]
- Liu, Y.; Huang, J.; Li, H. Nanostructural Characteristics of Vacuum Cold-Sprayed Hydroxyapatite/Graphene-Nanosheet Coatings for Biomedical Applications. J. Therm. Spray Technol. 2014, 23, 1149–1156. [Google Scholar] [CrossRef]
- He, C.; Shi, Z.-Q.; Cheng, C.; Lu, H.-Q.; Zhou, M.; Sun, S.-D.; Zhao, C.-S. Graphene oxide and sulfonated polyanion co-doped hydrogel films for dual-layered membranes with superior hemocompatibility and antibacterial activity. Biomater. Sci. 2016, 4, 1431–1440. [Google Scholar] [CrossRef]
- He, C.; Cheng, C.; Nie, S.-Q.; Wang, L.-R.; Nie, C.-X.; Sun, S.-D.; Zhao, C.-S. Graphene oxide linked sulfonate-based polyanionic nanogels as biocompatible, robust and versatile modifiers of ultrafiltration membranes. J. Mater. Chem. B 2016, 4, 6143–6153. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.W.; Haley, L.C. ; Kenry; Chenliang, S. ; Ping, L.K.; Teck, L.C., Cell-assembled graphene biocomposite for enhanced chondrogenic differentiation, Small, 2015, 11, 963–969. [Google Scholar]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Pang, L.; Chen, Y.; Zhang, Y.; Zhang, R.; Lu, B.; Wang, J. Effects of surface charges of graphene oxide on neuronal outgrowth and branching. Analyst 2014, 139, 105–115. [Google Scholar] [CrossRef] [PubMed]
- T. L. A. L.; Cheng, L.W.; Hui, S.; W. E. Y. L.; Anton, S.; Sergey, G.; Jonathan, H.; Teck, L.C.; Ping, L.K., Highly Wrinkled Cross-Linked Graphene Oxide Membranes for Biological and Charge-Storage Applications. Small 2012, 8, 423–431. [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Wang, J.K.; Xiong, G.M.; Zhu, M.; Özyilmaz, B.; Castro Neto, A.H.; Tan, N.S.; Choong, C. Polymer-Enriched 3D Graphene Foams for Biomedical Applications. ACS Appl. Mater. Interfaces 2015, 7, 8275–8283. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Dong, X.; Chen, P.; Wang, X. Electrodeposited Pt on three-dimensional interconnected graphene as a free-standing electrode for fuel cell application. J. Mater. Chem. 2012, 22, 5286–5290. [Google Scholar] [CrossRef]
- Park, J.; Park, H.; Ercius, P.; Pegoraro, A.F.; Xu, C.; Kim, J.W.; Han, S.H.; Weitz, D.A. Direct Observation of Wet Biological Samples by Graphene Liquid Cell Transmission Electron Microscopy. Nano Lett. 2015, 15, 4737–4744. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M.; et al. Nanomaterials for Neural Interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.H.; Seifert, M.; Garrido, J.A. Graphene Transistors for Bioelectronics. Proc. IEEE 2013, 101, 1780–1792. [Google Scholar] [CrossRef]
- Podila, R.; Moore, T.; Alexis, F.; Rao, A.M. Graphene coatings for enhanced hemo-compatibility of nitinol stents. RSC Adv. 2013, 3, 1660–1665. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Zhao, M.; Deng, J.; Li, X.; Li, D. The enhanced anticoagulation for graphene induced by COOH+ ion implantation. Nanoscale Res. Lett. 2015, 10, 14. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Mao, H.; Huang, Y.; Ding, Q.; Pang, X. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl. Surf. Sci. 2015, 329, 76–82. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan–hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci. Mater. Med. 2016, 27, 48. [Google Scholar] [CrossRef]
- Xie, W.; Song, F.; Wang, R.; Sun, S.; Li, M.; Fan, Z.; Liu, B.; Zhang, Q.; Wang, J. Mechanically Robust 3D Graphene–Hydroxyapatite Hybrid Bioscaffolds with Enhanced Osteoconductive and Biocompatible Performance. Crystals 2018, 8, 105. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Yu, Y.; Zhao, K.; He, P.; Huang, B. Fluoroalkyl-silane-modified 3D graphene foam with improved Joule-heating effects and high hydrophobicity-derived anti-icing properties. J. Mater. Sci. 2018, 53, 528–537. [Google Scholar] [CrossRef]
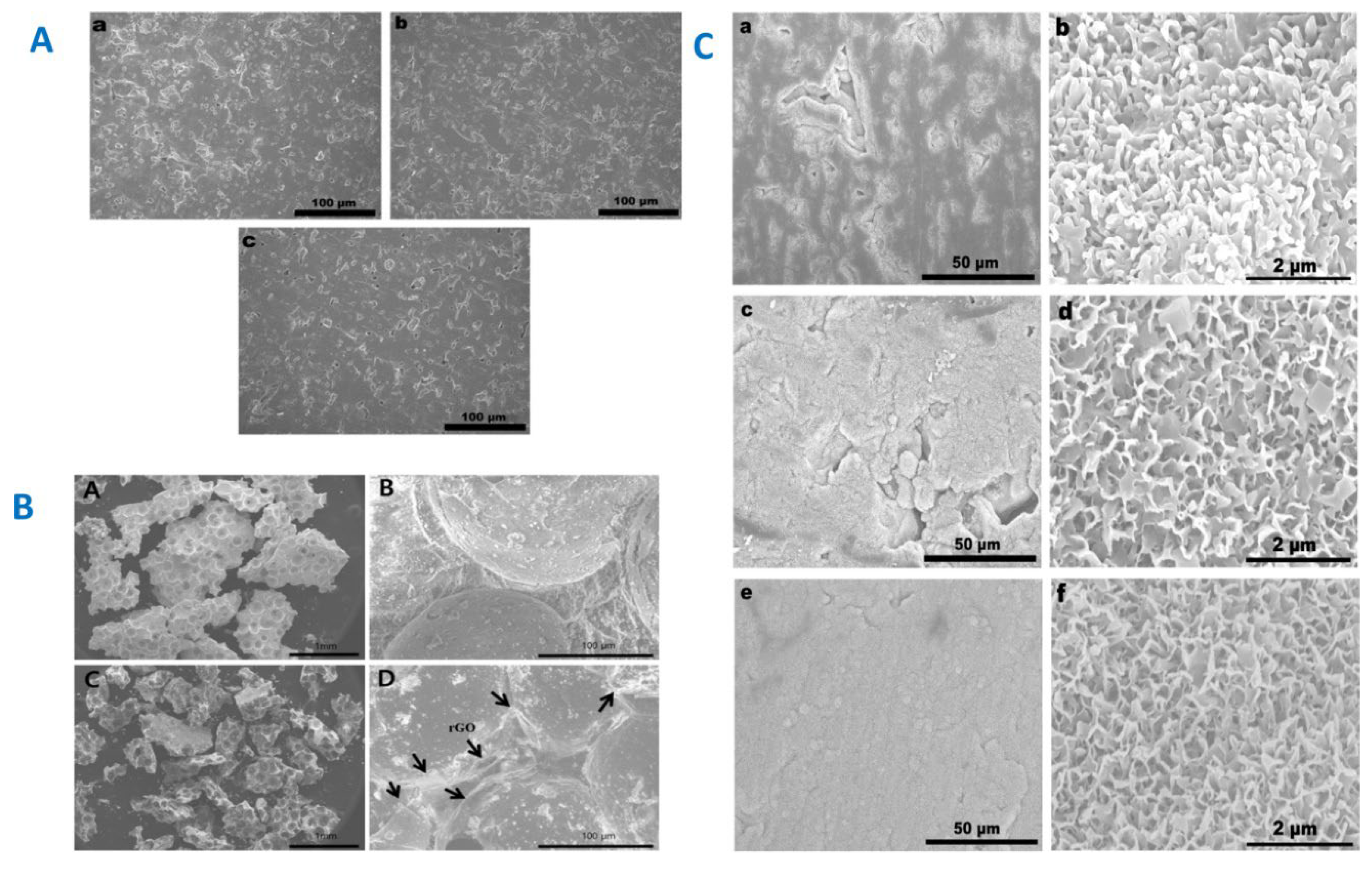
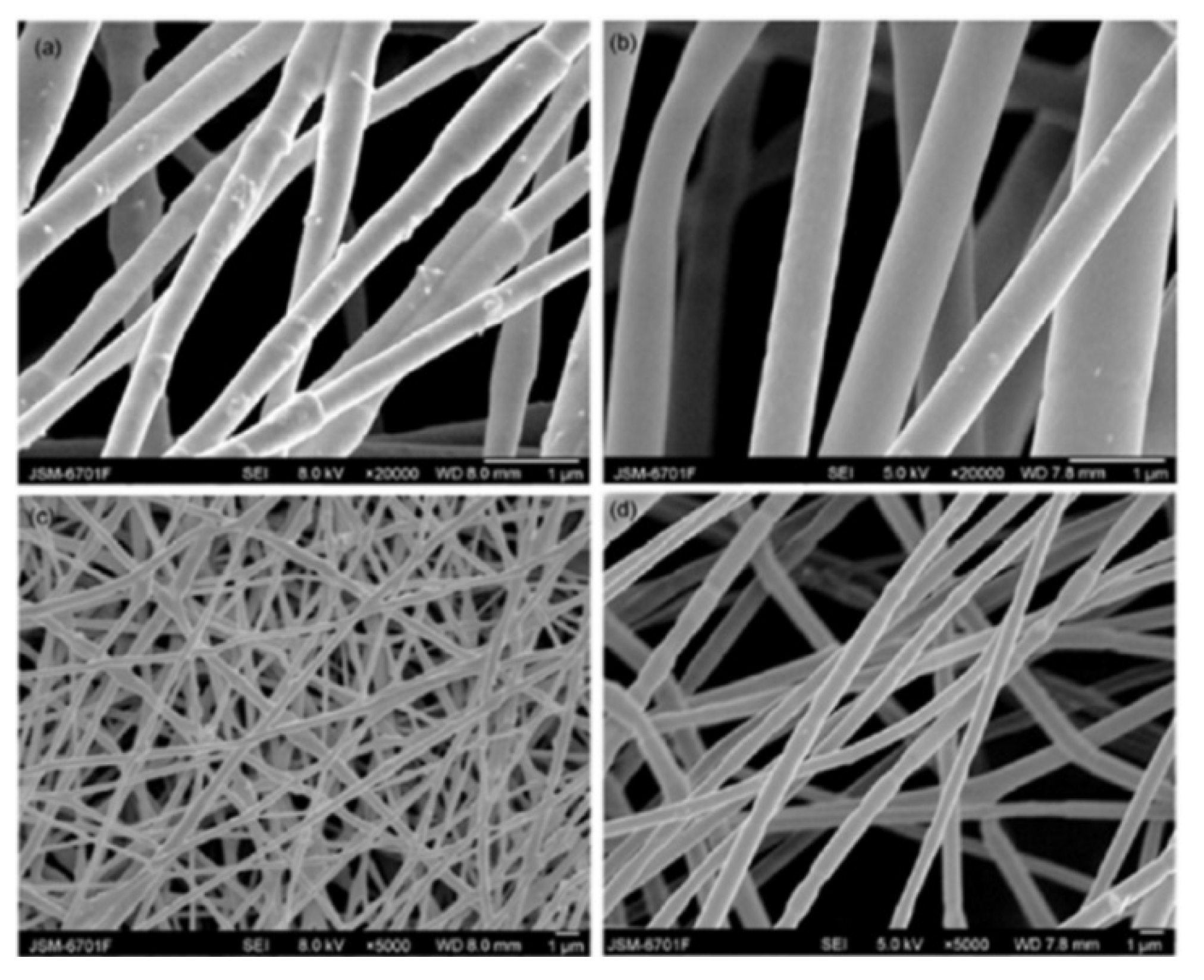
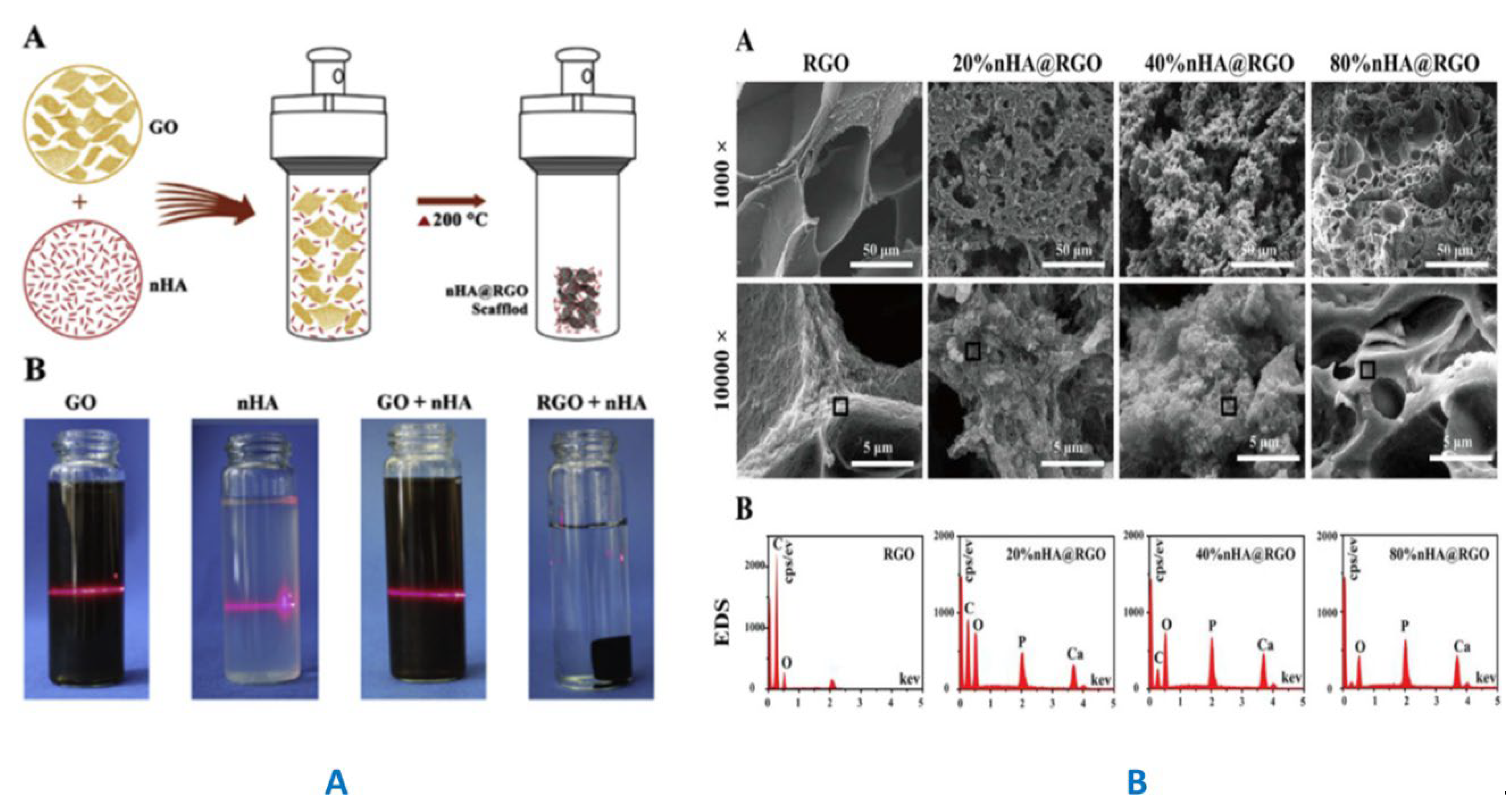
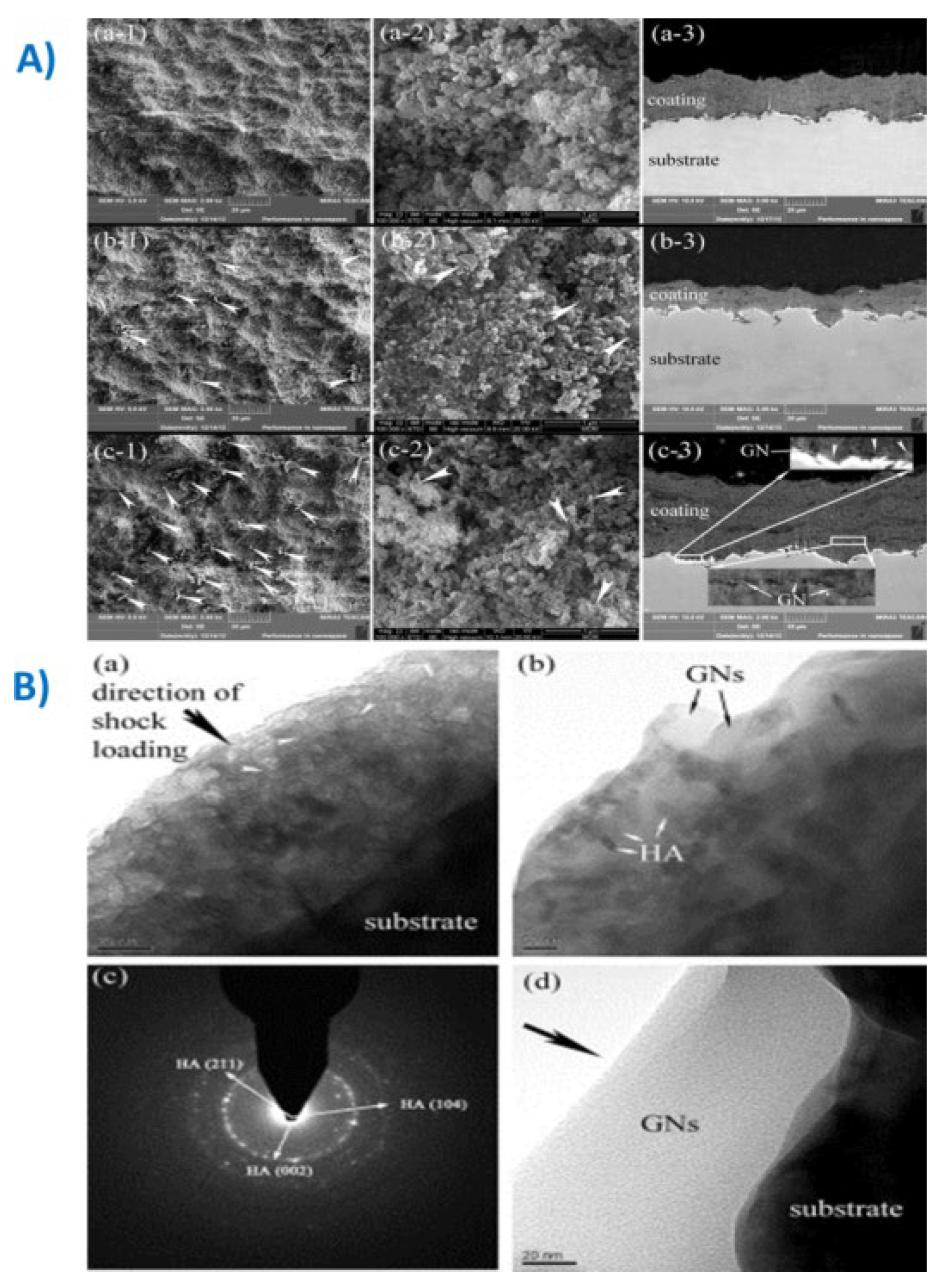
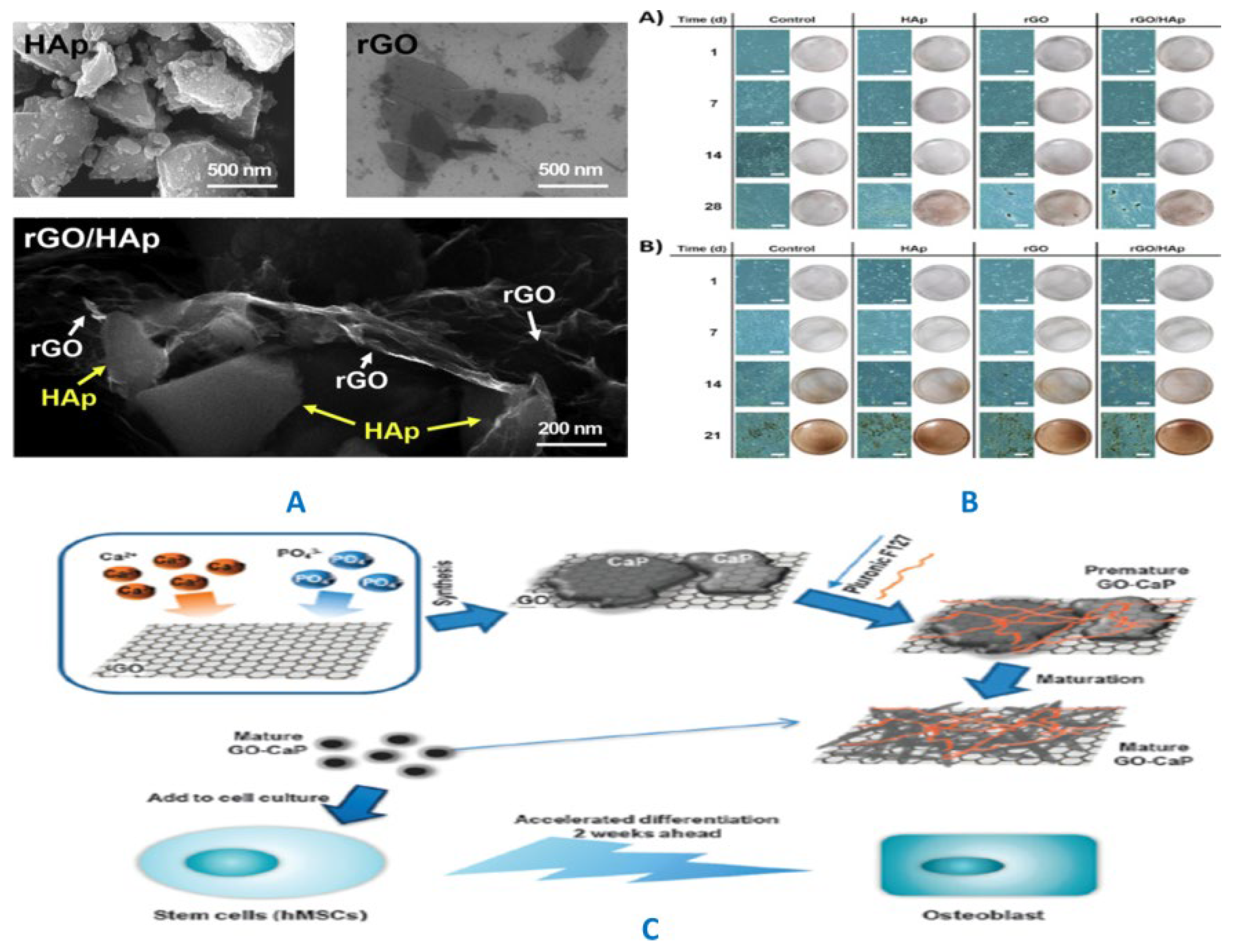
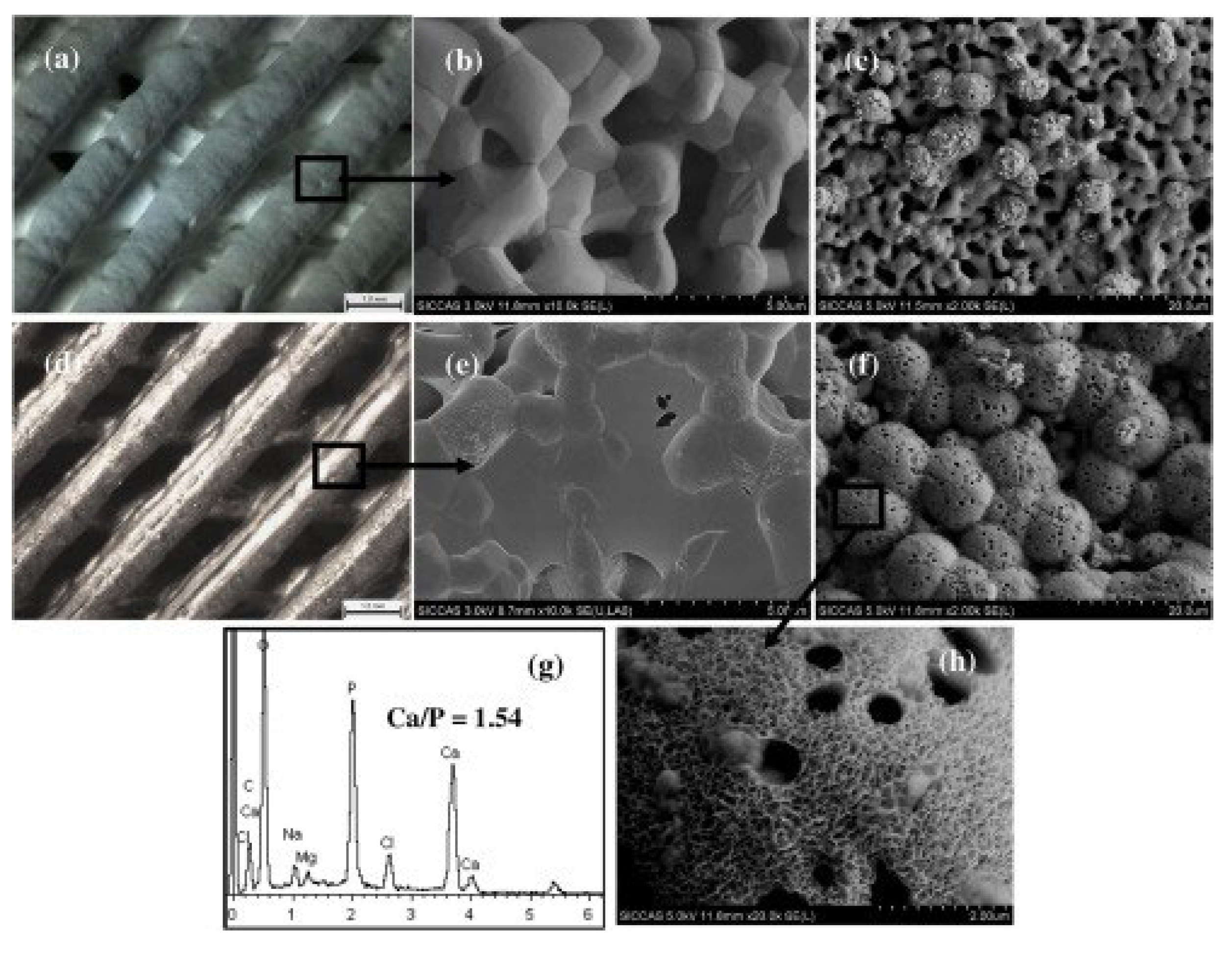
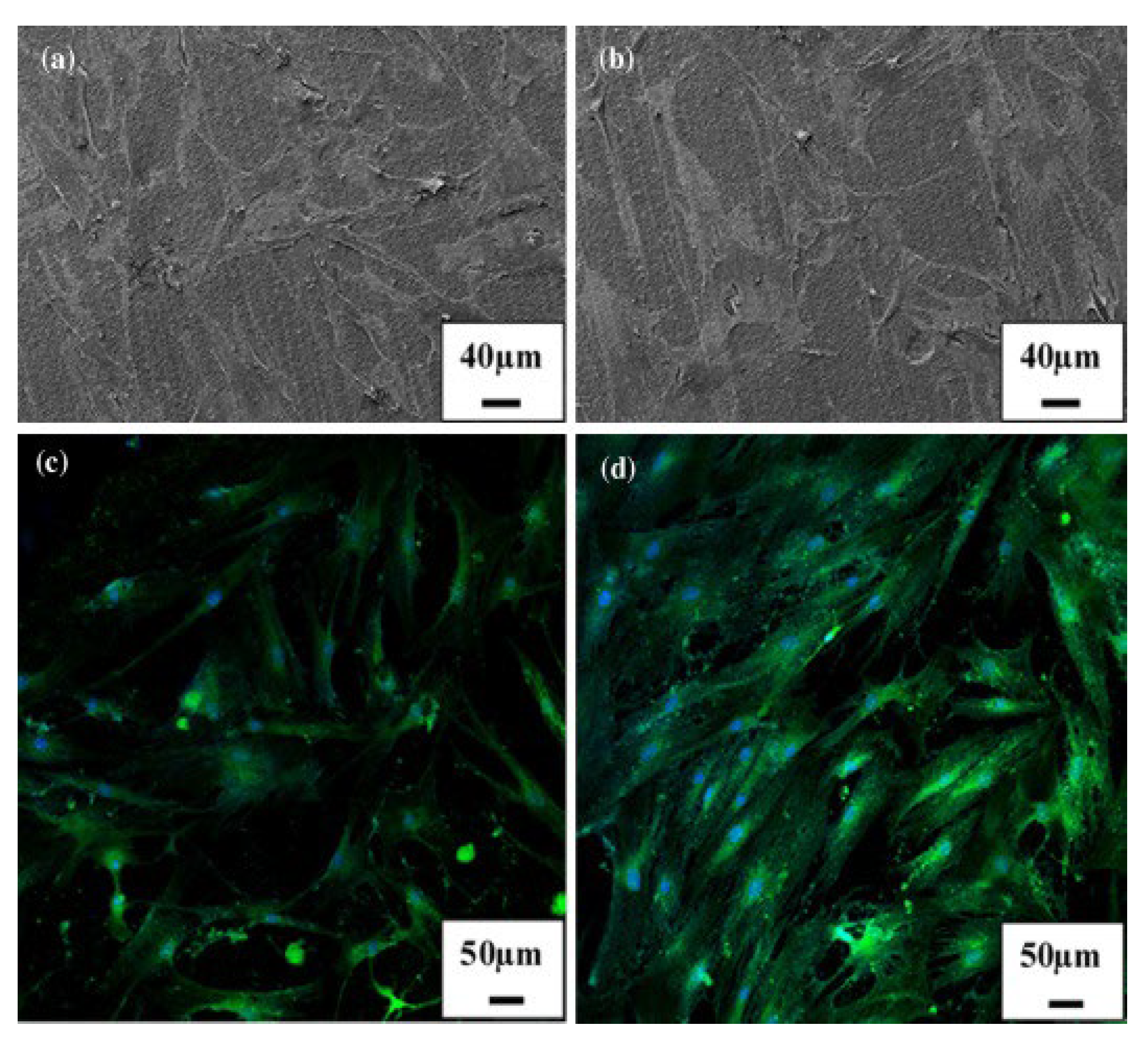
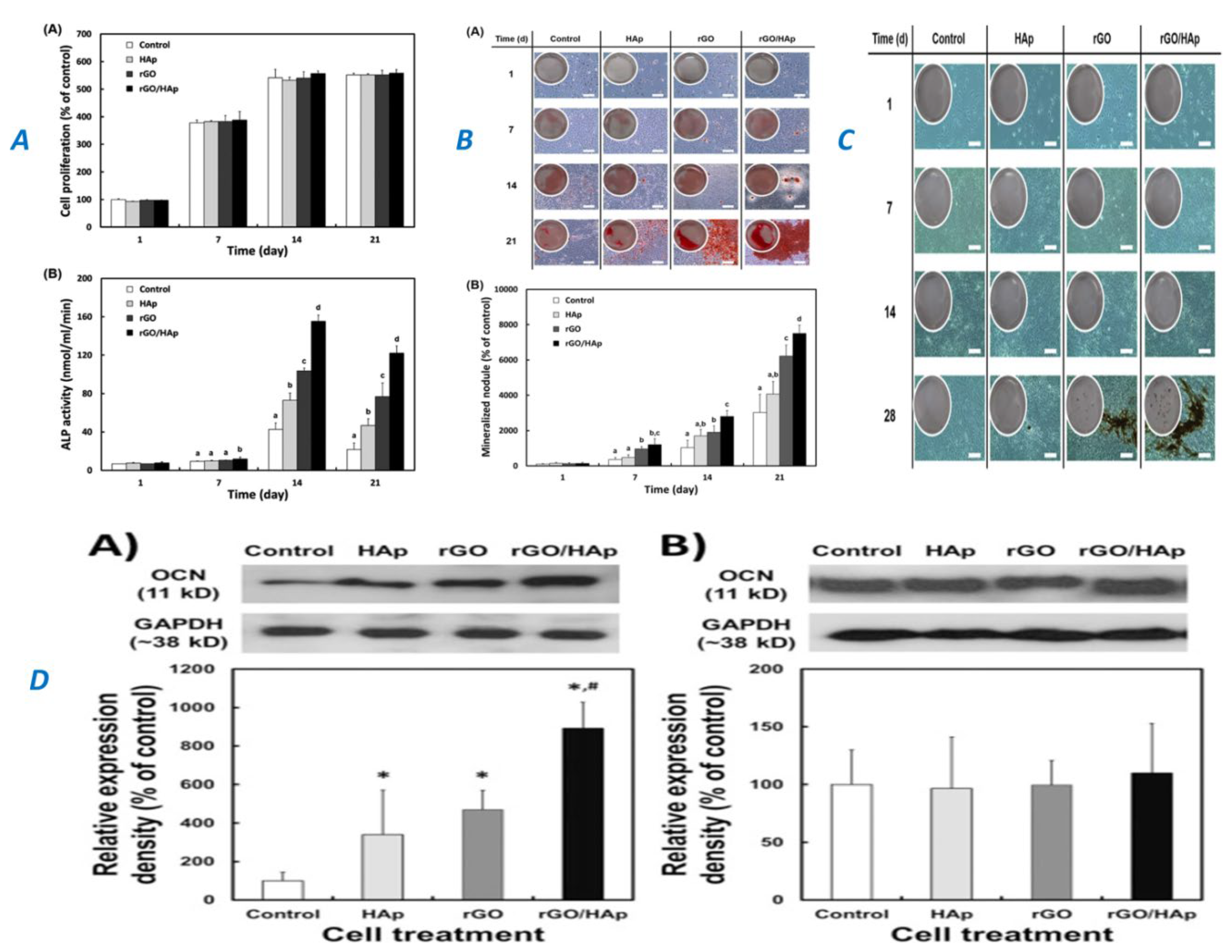
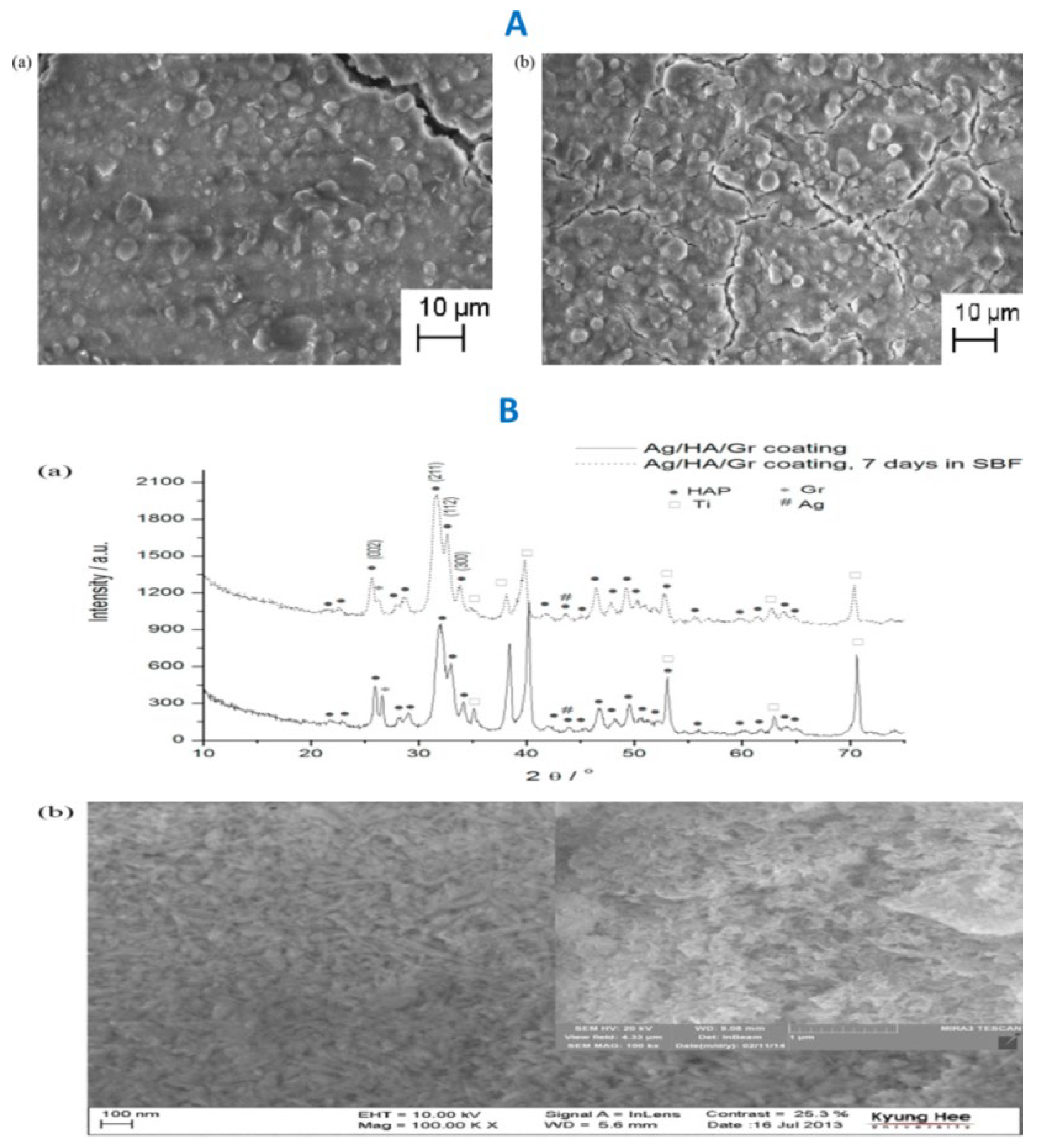
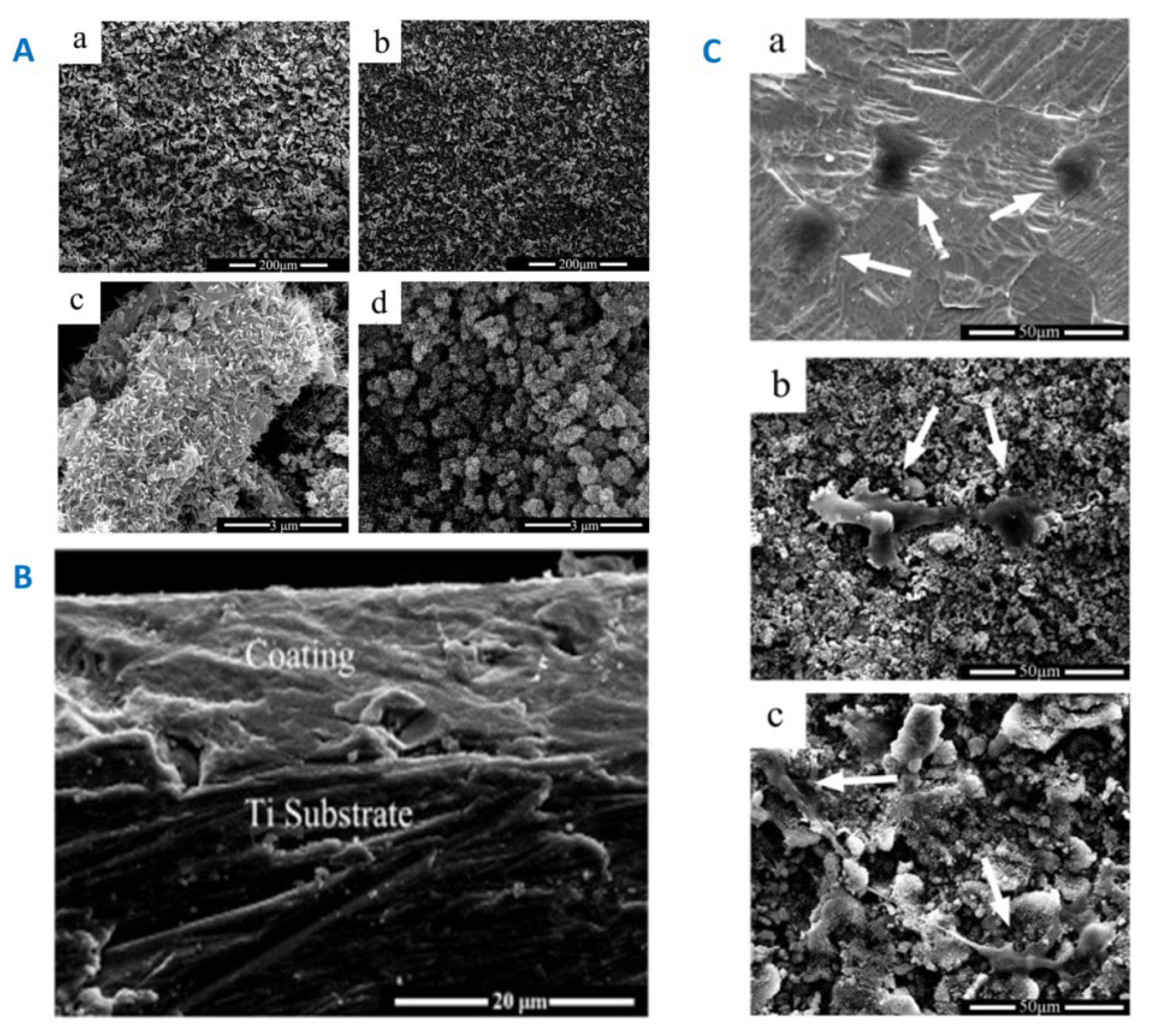
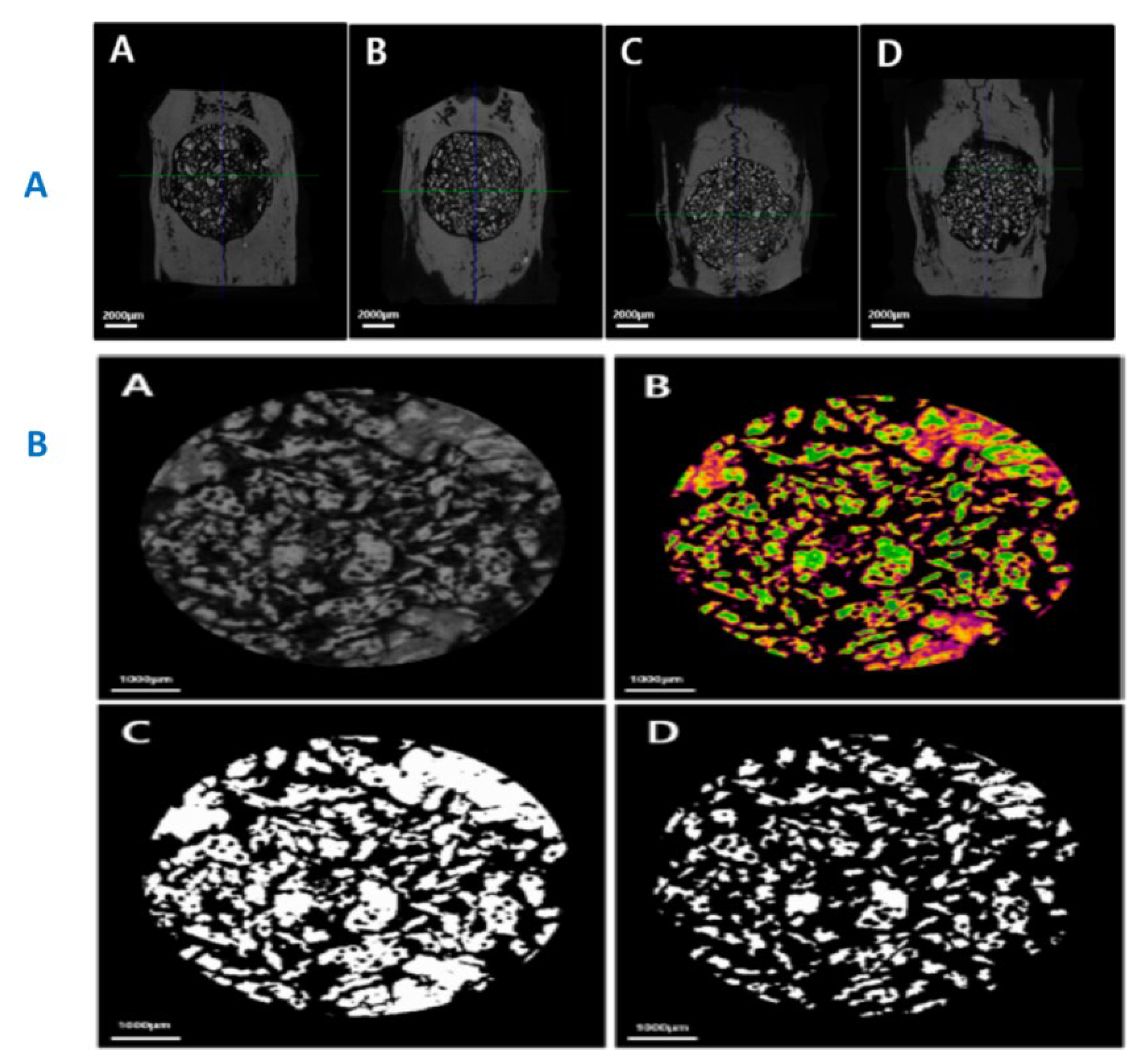
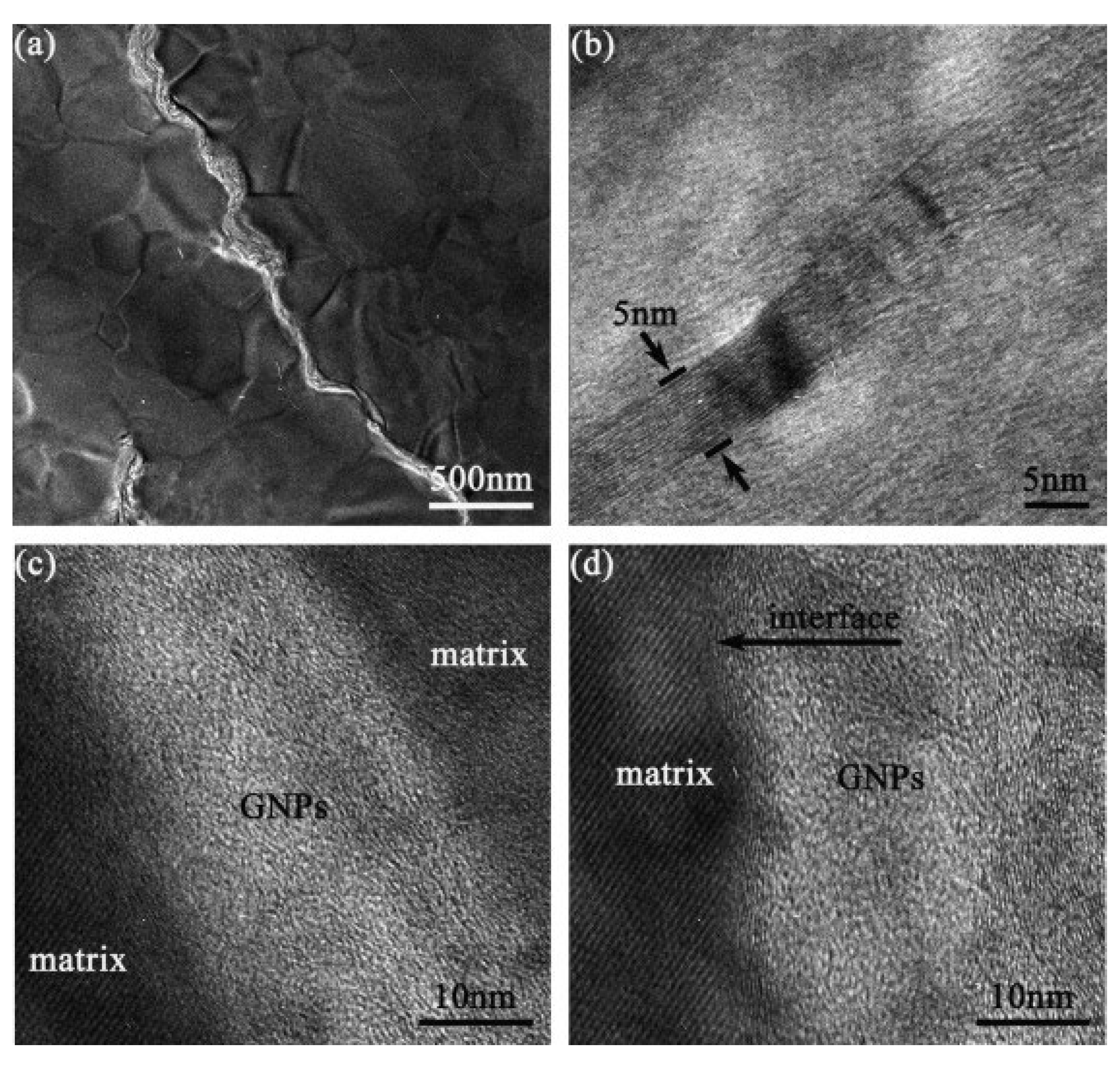
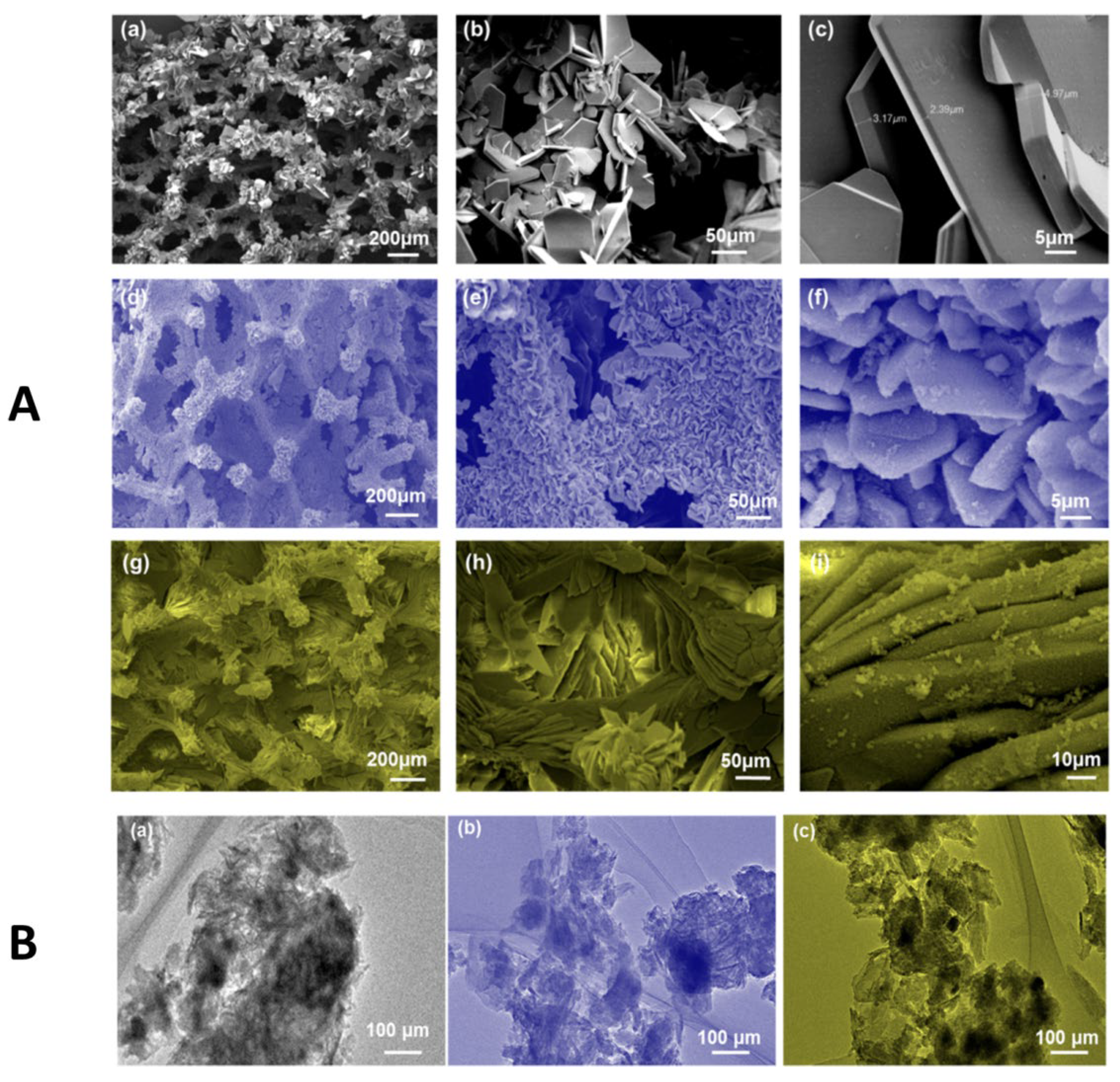
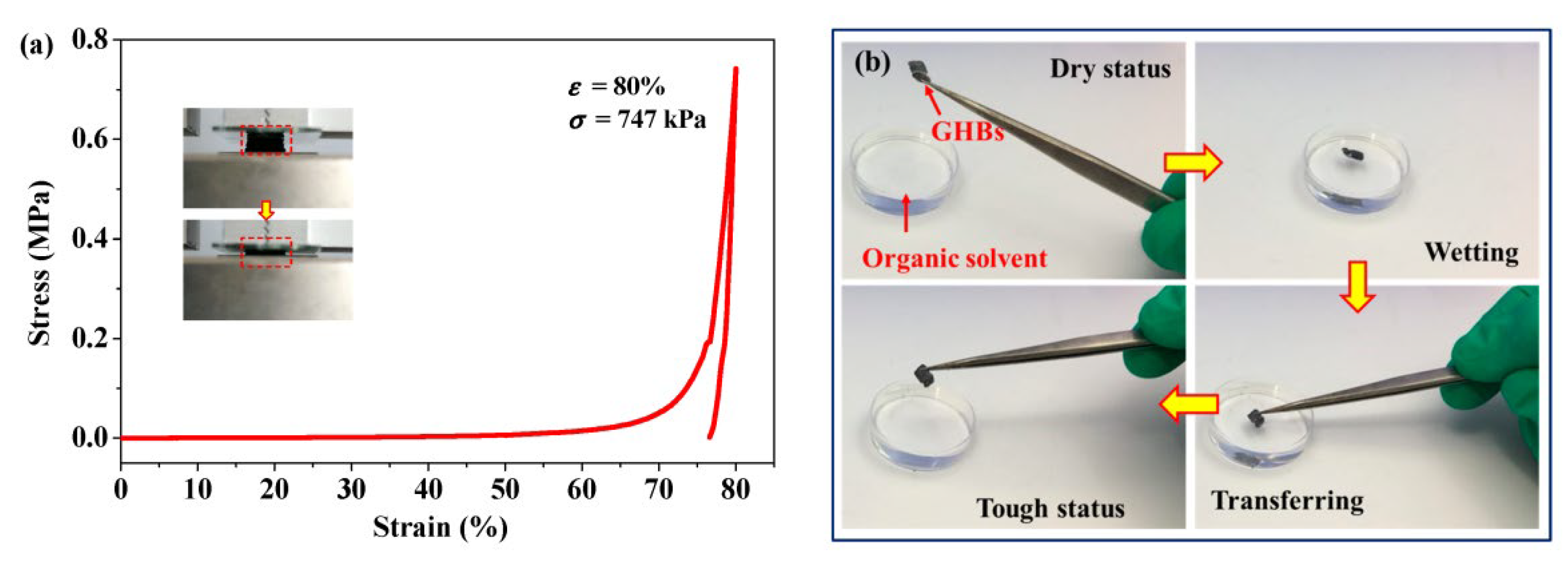
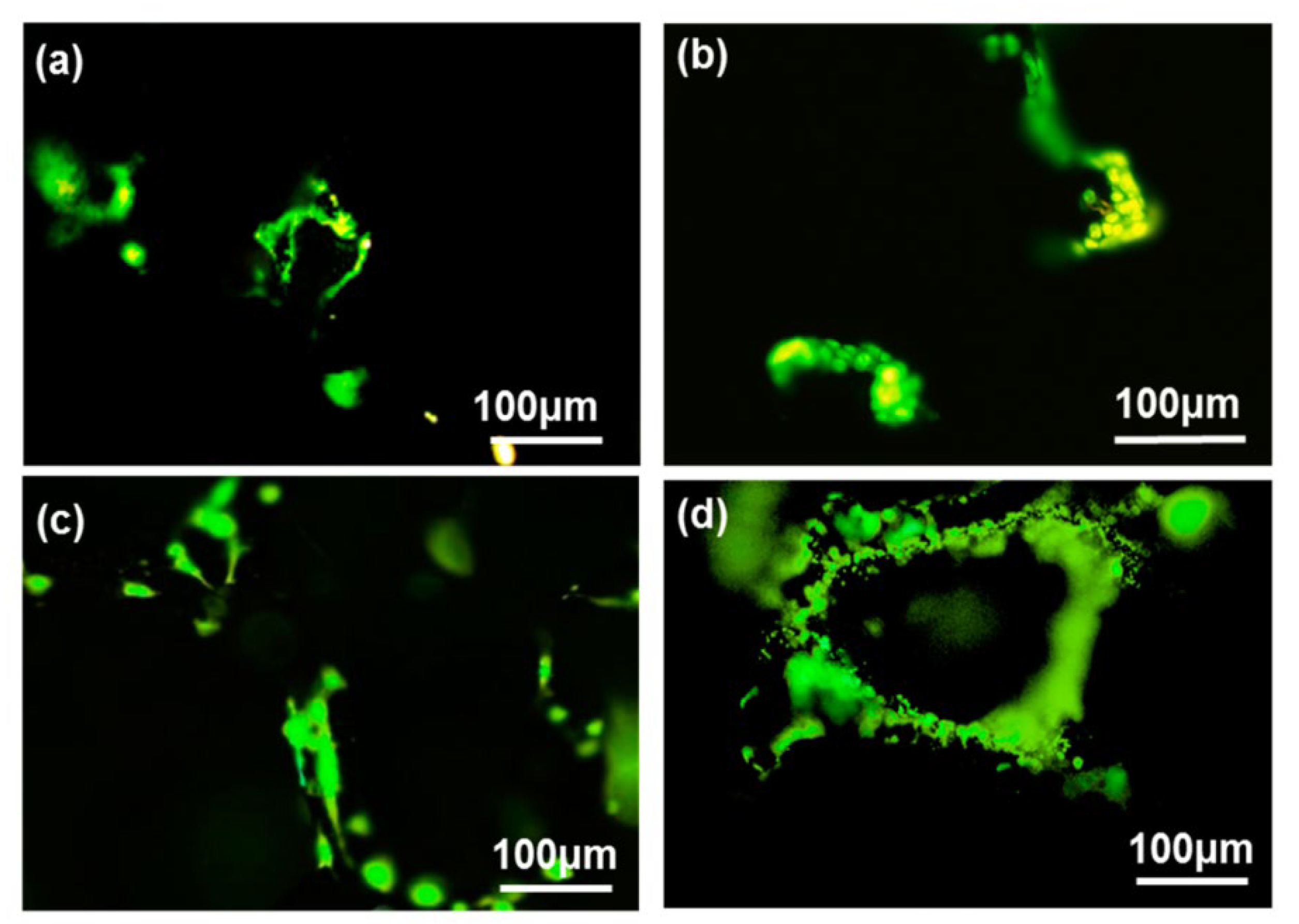
|
Technology |
Categorized procedures |
advantages |
ref |
|---|---|---|---|
|
Synthesis |
To synthesize rGO-coated BCP graft material, as-prepared rGO in water was sonicated for 2h, and then mixed with BCP suspended in deionized water at rGO toBCP with various weight ratios. The rGO-coated BCP graft material got by vigorously mixing colloidal dispersions of rGO nanoplatelets and BCP microparticles for few minutes only (Approximately 10 min) andat room temperature overnight. & Graphene nanoplatelets were used as the toughening agent. For BCP, first, CDA nanopowderswere prepared bychemical precipitationofCa(NO3)2 and (NH4)2HPO4 with an appropriate Ca/Pratio.Then,BCPnano powders,amixtureof70wt% HA and30wt% β-TCP were collectedaftercalcininghe obtained CDAat550 0C. GNPs weredispersed incetyltrimethylammoniumbromide (CTAB) solutionbysonicationfor 1h.Thenthesuspension and BCPnanopowdersweremixedbyballmillingfor8h. After beingdried,suchmixturesweretreatedat500 0C for1h in argontoremovethesurfactant. Eventually,thecomposites were fabricated byhotpressingthescreenedpowdersat 1150 0C inamultipurposehightemperature furnace under a pressureof30MPain an argonatmosphere for1h.ThecontentsofGNPsinthe compositeswere0,0.5,1.0,1.5,2.0and2.5wt%. |
It is a nontoxic and stable bone graft material. It can increase the bone regeneration better than BCP alone. GNPs/BCP composite are promising bone substitute materials as well as effective additive for toughening ceramics for bone substituents. |
[9,12] |
|
Electrochemical Deposition |
|
The electro-deposition procedure is a facile, environmental friendly and controllable route towards the synthesis of promising graphene-CaP composite for biomedical applications. |
[2,138,139,140] |
|
Spark plasma sintering processing |
Graphite papers were placed between pure HA, 0.5 wt.% graphene nano sheets (GNS)/HA and 1.0 wt.% and die/punches for easy specimen elimination, using a maximum temperature of 1150 0C and a holding pressure of 40 MPa. A heating rate of 150 0C/min was applied until 1050 0C was reached, with 1150 0C being attained in the next minute; this maximum temperature was kept for 3 min. The samples were then furnace cooled to ambient temperature. |
It is expected that (GNS)/HA could provide more desirable locations for osteoblast adhesion, as well as creates more nucleation sites enabling apatite mineralization. |
[141,142,143,144] |
|
Electro spinning |
The graphene/HA mixture in organic solution and then subjected to high voltage and being subjected out from the spinnerret |
High porosity and connectivity |
[145,146,147] |
|
Self-assembling |
Dispersing the graphene oxide/HA into aqueous solution |
Controllable porosity and connectivity and good mechanical strength |
[148,149] |
| Thermal Spray | Graphene into the aqueous solution, HA preparation using wet chemical synthesis, spray coated onto the substrate material |
Controllable coating thickness and large area deposition and strong adhesion feature | 150 |
| 3D printing | Dispersion of graphene/HA into the specific organic liquid or using 3D printer to synthesize 3d scaffold. | Controllable porosity and connectivity | [151,152] |
|
Chemical vapor deposition |
Au nano particles or clusters are dispersed over HA particles and acetylene and methane are the carbon source using radio frequency chemical vapor deposition |
High graphene purity and large graphene sheets |
[153,154] |
|
Hot isostatic pressing |
Mixing graphene and HA using mechanical milling / ultrasonic dispersion and sintering at high temperatures under high pressures |
Ultrafine microstructures, high HA crystallinity and holding fine grain shapes and sizes |
[155,156,157,158] |
|
Biomimitic mineralization |
Graphene family powdered substances were decorated by bioactive materials |
Increased osteogenic activities with bone like apatite production |
[159,160,161,162,163,164,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





