1. Introduction
Autoimmune haemolytic anaemia is an acquired haemolysis caused by a dysfunction of the patient’s immune system, which produces autoantibodies directed against erythrocyte surface antigens [
1]. Disease is, usually defined by positive monospecific direct anti-agglutination [
2]. The WHO 2020 classification of soft tissue tumors recognizes anastomosing hemangioma as a distinct benign vascular neoplasm [
3]. This tumor has been described in all age groups, from 2 to 85 years old; with a median age of 65 years for non-renal tumors [
4]. Anastomosing haemangioma is a tumor with anastomosed vessels the size of sinusoidal capillaries, with scattered nail-like endothelial cells and rare mitoses. Multilayered endothelial cells are absent. Vascular thrombi are typical and the lesions have areas of central sclerosis and focal necrosis [
5]. In addition, there is prominent extra medullary haematopoiesis and immunohistochemically expression of CD31 and CD34 [
6]. The CD31 antigen, also known as PECAM-1 (Plaque Endothelial Cell Adhesion Molecule-1), is a transmembrane glycoprotein that acts as an adhesion molecule for vascular endothelial cells and platelets [
7]. It plays an important role in angiogenesis. CD34 is a transmembrane glycoprotein belonging to the sialomucin family. It is selectively expressed on haematopoietic stem precursor cells, small vessel endothelial cells, embryonic fibroblasts, adipocytes and tumor cells of endothelial origin [
8]. Anastomosing hemangioma develops in more than 90% of cases due to recurrent activating mutations in the GNAQ, GNA 11 and GNA 14 genes, indicating that anastomosing hemangioma is a true clonal vascular neoplasm [
9]. The GNA genes encode the G1 protein subunit a, which interacts with cell membrane receptors and plays an important role in the activation of signaling pathways within cells. These include the PI3K/AKT and MAPK pathways, which are involved in protein synthesis and cell proliferation [
10]. It follows that GNA 11 mutations, as well as the previously known GNAQ and GNA14 mutations, are essential drivers in the pathogenesis of anastomosing haemangiomas [
11].
It is, therefore considered possible that the patient’s autoimmune haemolytic anaemia may be associated with the communicating haemangioma. The aim of this clinical case is to illustrate the difficulties in diagnosing the anastomosing haemangioma, the appropriateness of surgical intervention, and the pre- and post-operative management of the associated autoimmune haemolytic anaemia.
2. Case Reports
2.1. History and Physical Exam
An 80-year-old man, a professional cyclist in his youth, consulted on 22 March 2023 for a retroperitoneal inter-aorto-caval tumor, diagnosed by CT scan in 2022.
In his personal history, he consulted in 2020 with symptoms of asthenia and weight loss and had a diagnosis of autoimmune haemolytic anaemia. Patient not responded to first-line treatment with corticoid 1 mgr./kgr. The second-line treatment with rituximab (4 doses), last dose in 2021. He had Covid-19 in 2022, and then vaccinated. He has had hepatitis B Ag (-), Ac core (+) and has undergone cholecystectomy and prostatectomy. Physical examination reveals epigastric pain.
2.2. Laboratory Tests
A pre-operative hormonal study carried out to determine the levels catecholamine`s, normetanephrine and metanephrine in the blood and urine. Urine tests for adrenaline, dopamine and noradrenaline are also normal, as are serotonin and chromogranin.
An acute flare-up of autoimmune haemolytic anaemia, is diagnosed by the presence of anaemia, total bilirubin 4.58 mg/dl, direct bilirubinemia 0.38 mg/dl and transferrin 183 mg/dl in the preoperative tests. The haptoglobin, during the acute phase, is <15 mg/dl, passing in February 2024 to 33 mg/dl, after surgery.
The total reticulocyte count is 187.3 million /ml, the lactate dehydrogenase (LDH) is 815 UI/mL and the anti-agglutinin test is positive (direct Combs 4+). Indirect Coombs (-). After adding anti-globulin serum to washed red blood cells from the patient, agglutination indicates the presence of immunoglobulins or complement bound to the red cells. In this case, IgG is 778 µmol/L in the blood and cryoglobulins are negative. Among the blood parameters, the progressive increase in alpha-fetoprotein, which reached 17.5 ng/ml in June 2020, was remarkable. In December 2023, in the acute phase of haemolytic anaemia, it is 20.1 ng/ml and after surgical resection, in February 2024, it is 14.56 ng/ml. 6 weeks after surgery, it is 18.2 ng/ml.
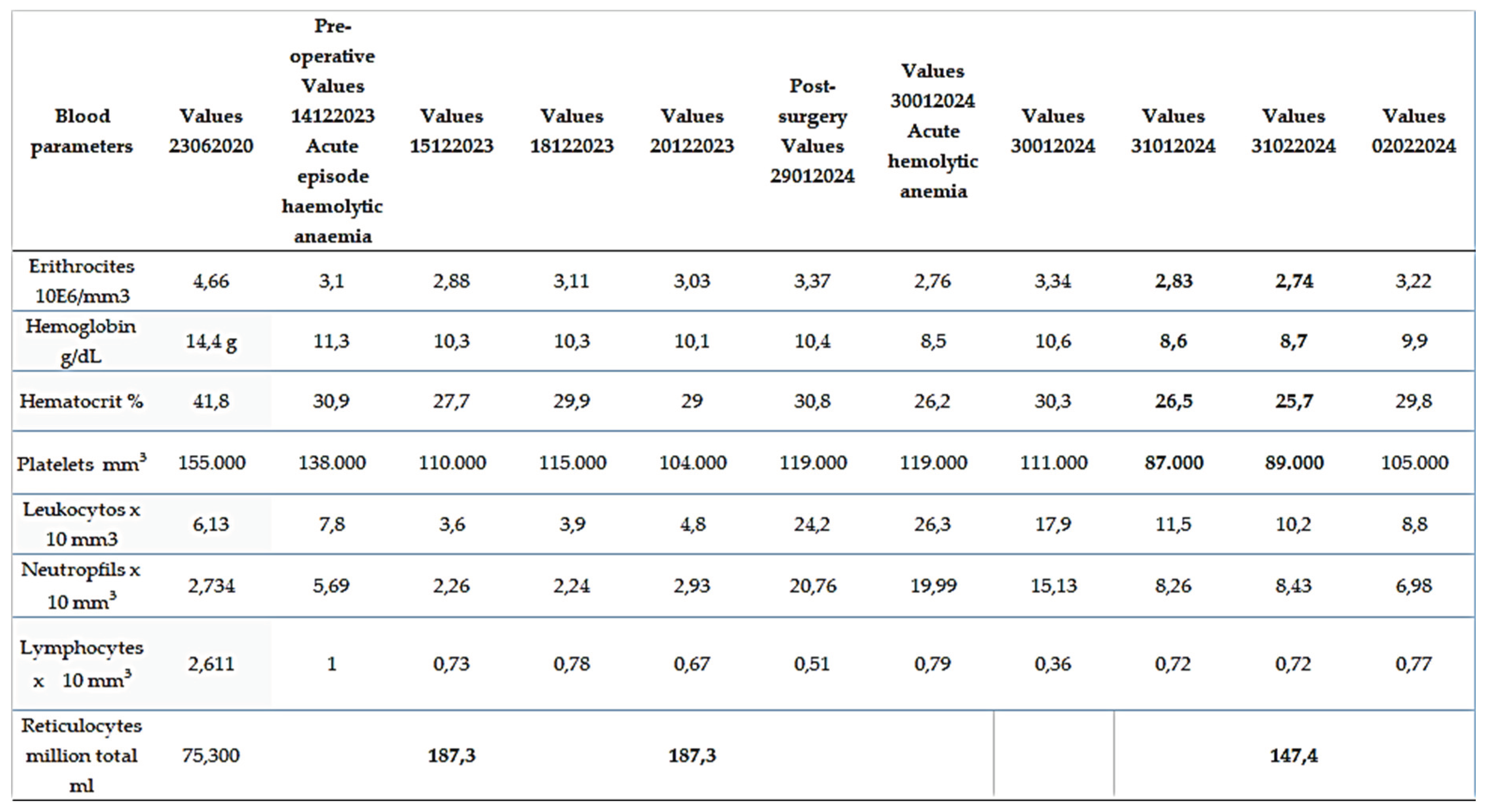
Table 1, shows the evolution over time of the haemacytometry, count and formula, platelets and total reticulocytes.
There was a delay in surgery and he had a month’s treatment with corticosteroids.
2.3. Imaging Tests
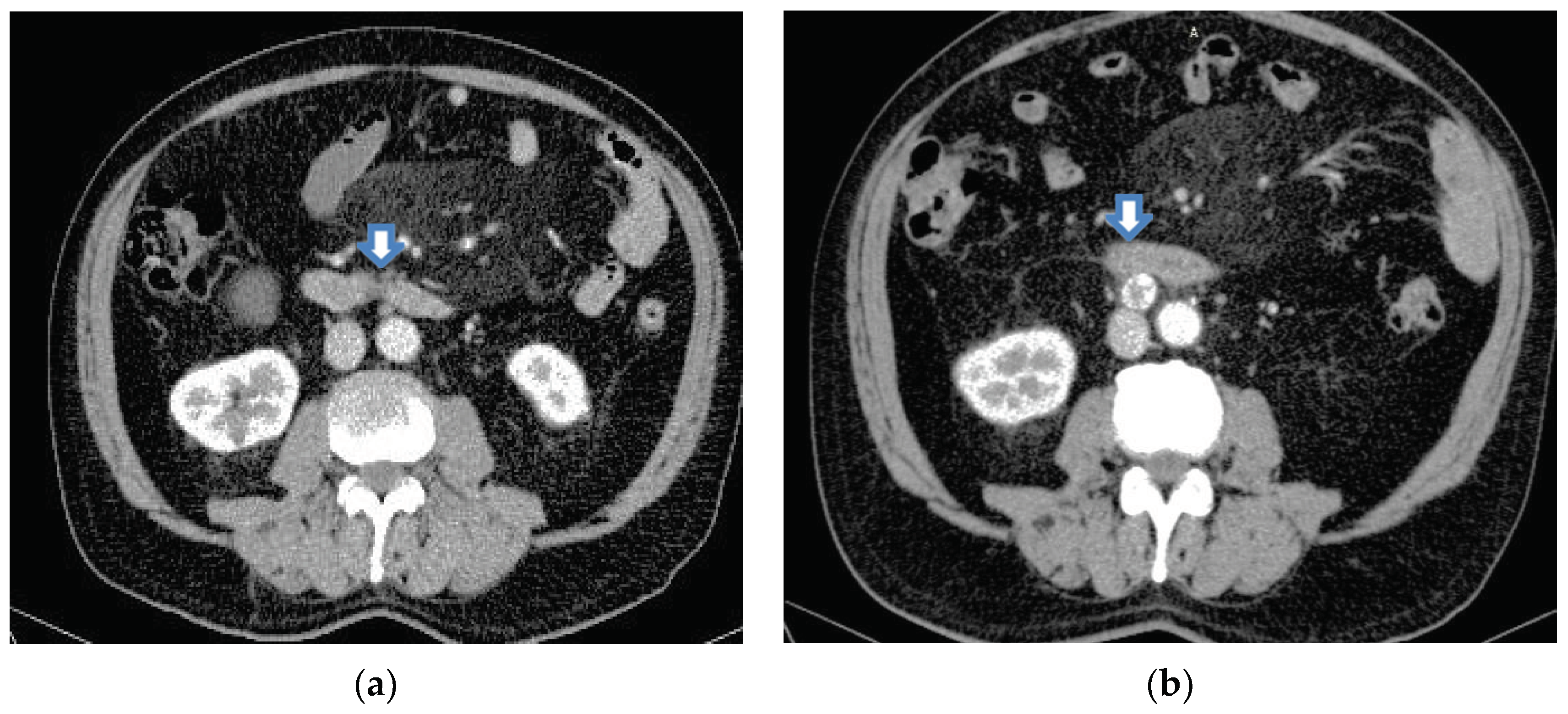
Several imaging studies have been performed since the onset of his illness. These are shown below. Due to a cystic tumor in the right kidney, an abdominal CT scan was performed in 2020. In that year, the inter-aorto-caval space was clear, with only a nodular lesion ventral to the abdominal aorta and caudal to the third duodenal segment, which could be the origin of the tumour,
Figure 1 (a). A CT scan was performed in 2022, which showed a high-attenuation nodular image at the inter-aorto-caval level, with a central area of less intense enhancement, the largest diameter of which was 2 cm. The differential diagnosis was adenopathy or paraganglioma,
Figure 1 (b).
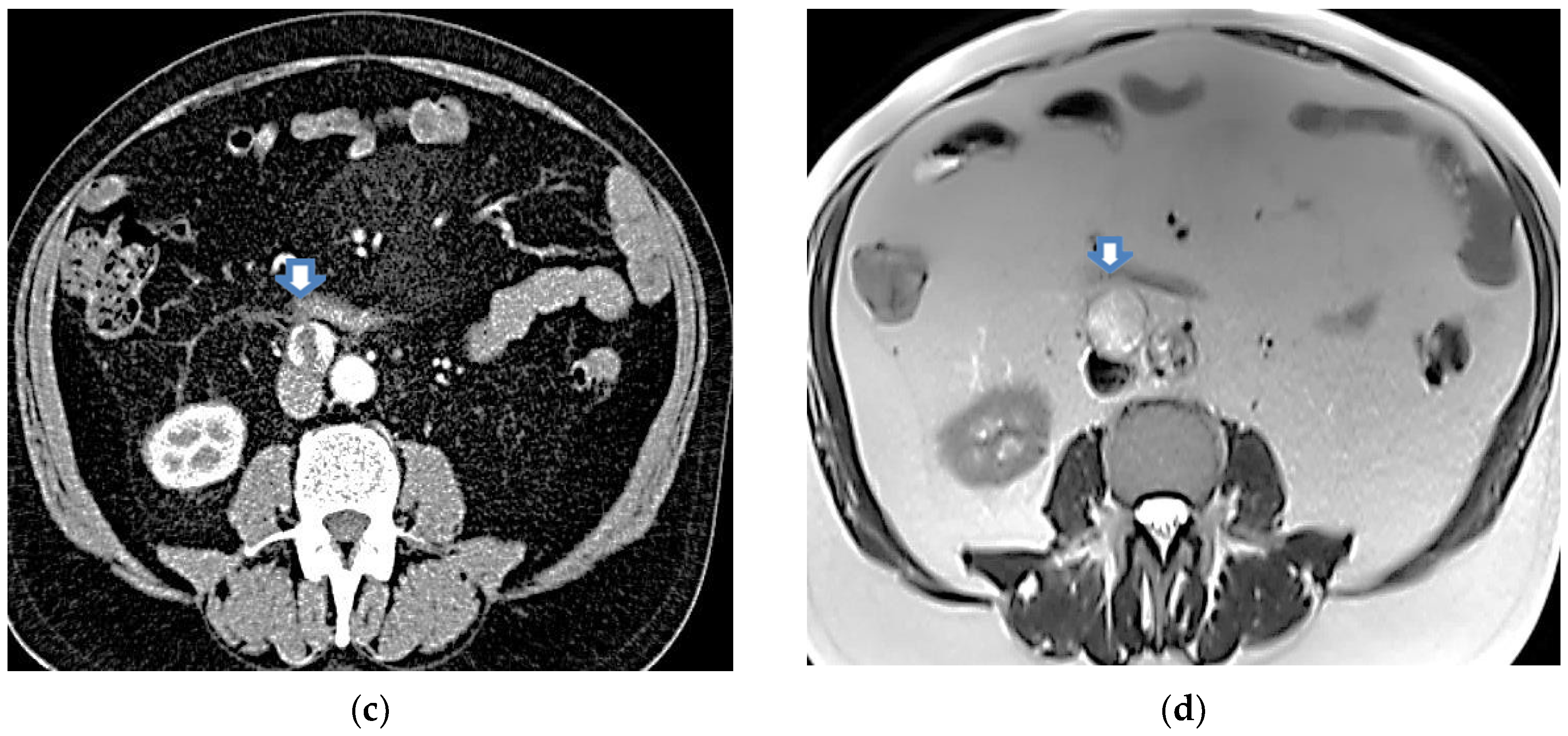
In the same year, a PET scan was performed showing a 2 cm lesion of nodular morphology between the aortocaval cavities with mild metabolic activity (maximum SUV 2.2). In 2023, a control abdominal CT scan was performed, showing a nodular inter- aorto-caval image with a larger diameter of 2.34 cm,
Figure 2 (c). In the same year, sectoral and radial echoendoscopy was performed up to the second duodenal part, distal to the papilla, where a 2.5 cm round lesion was observed, well defined by a pseudo-capsular image, heterogeneous by ultrasound and elastography, in intimate contact with the duodenal wall. FNA was performed in three passes with a 22 G needle and material was obtained for impression and cytoblock. The cytological report described areas of tumour necrosis within the tumour and the pathological anatomy described bands of lympho-polynuclear fibrin. No epithelial cell clusters were identified. Six months later, an MRI was performed in which a retroperitoneal tumour was observed, which imprinted the anterior aspect of the cava, located inter aorto- cava, posterior and inferior to the duodenum in contact with it, measuring 2.7 cm x 2.3 x 3.4 cm, hyper intense in T2-weighted sequences, with heterogeneous enhancement after contrast administration. Conclusion: retroperitoneal tumour, possible paraganglioma,
Figure 2 (c) and (d).
The evolution of tumour size from imaging studies performed between 2020 and 2023 is shown in
Table 2.
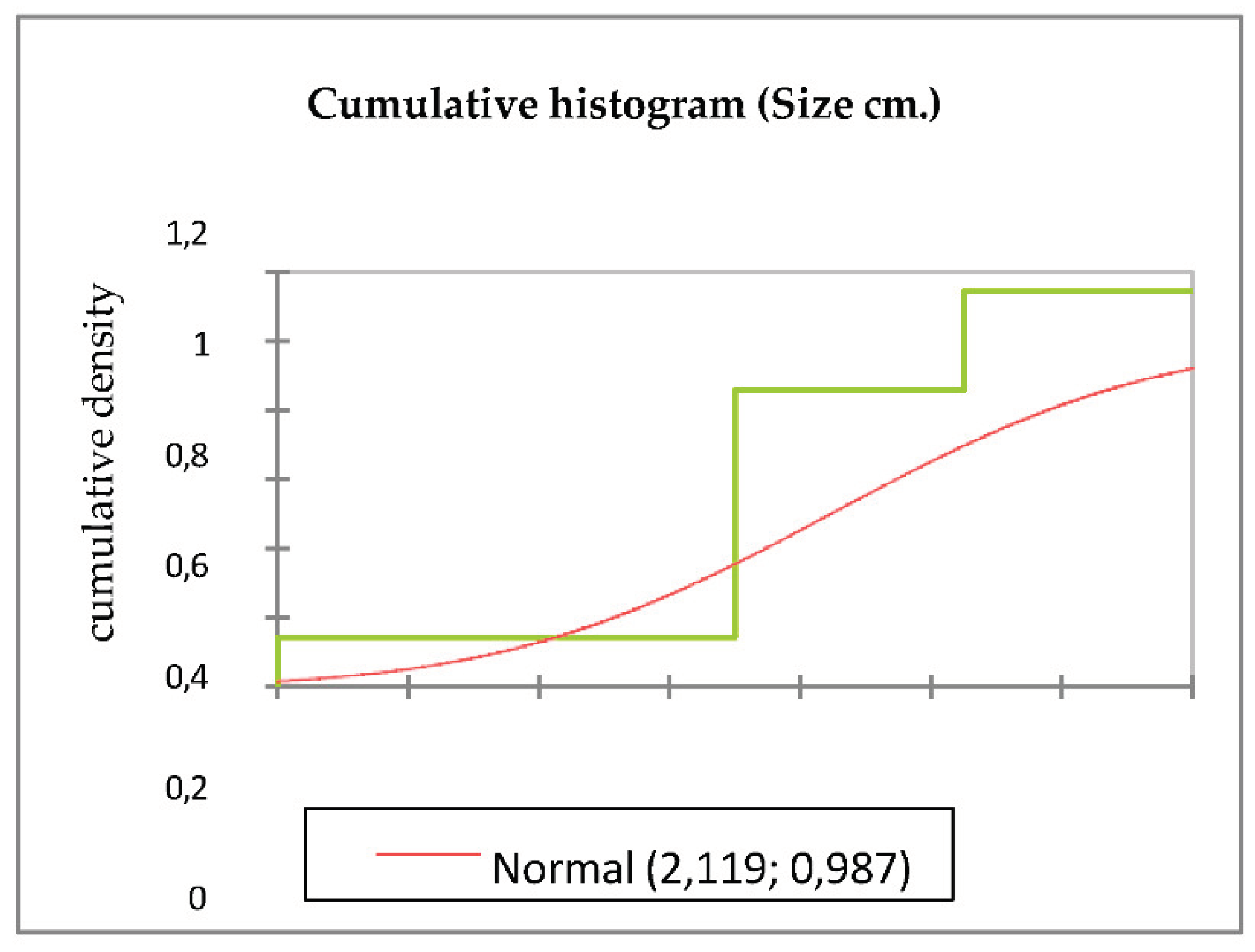
Figure 3(a) shows the cumulative histogram of the data set of tumour size in centimeters and cumulative density. It shows the cumulative proportion of tumour sizes in the different measurements obtained in the imaging studies. The starting point corresponds to the smallest tumor size and the end of the histogram corresponds to the largest tumour size.
As it was a nodular lesion with central apoptosis, increased proliferative activity, inferior cava imprint and likely to increase in size in the coming months, even without a precise diagnosis, it was decided to recommend surgery.
2.4. Surgery
Median supra and infra-umbilical incision. Operative findings: Epiploic adhesions to the bladder bed from previous surgery. Diverticular disease of the sigmoid colon. Retractile mesenteritis. Fibrosis of the fascia of Toldt. Encapsulated cystic inter aorto-caval tumour at the level of the third duodenal portion occupying the entire anterior aspect of the cava.
Procedure: Dissection of the right colon and terminal ileum from lateral to medial. Kocher maneuvre. Identification and dissection of the lateral borders of the cava and the abdominal aorta. Dissection of the third portion of the duodenum. Resection of the tumour.
Perioperative pathological anatomy: vascular tumour without the possibility of determining the degree of malignancy. Haemostasis. Placement of Surgicel hemostatic dressing on the anterior aspect of the cava. Jackson-Pratt drainage in the dissection bed of the tumour. Closure with double BiosynTM suture in two planes. Approximation of subcutaneous cellular tissue with Vicryl suture. Fixation of the skin with staples.
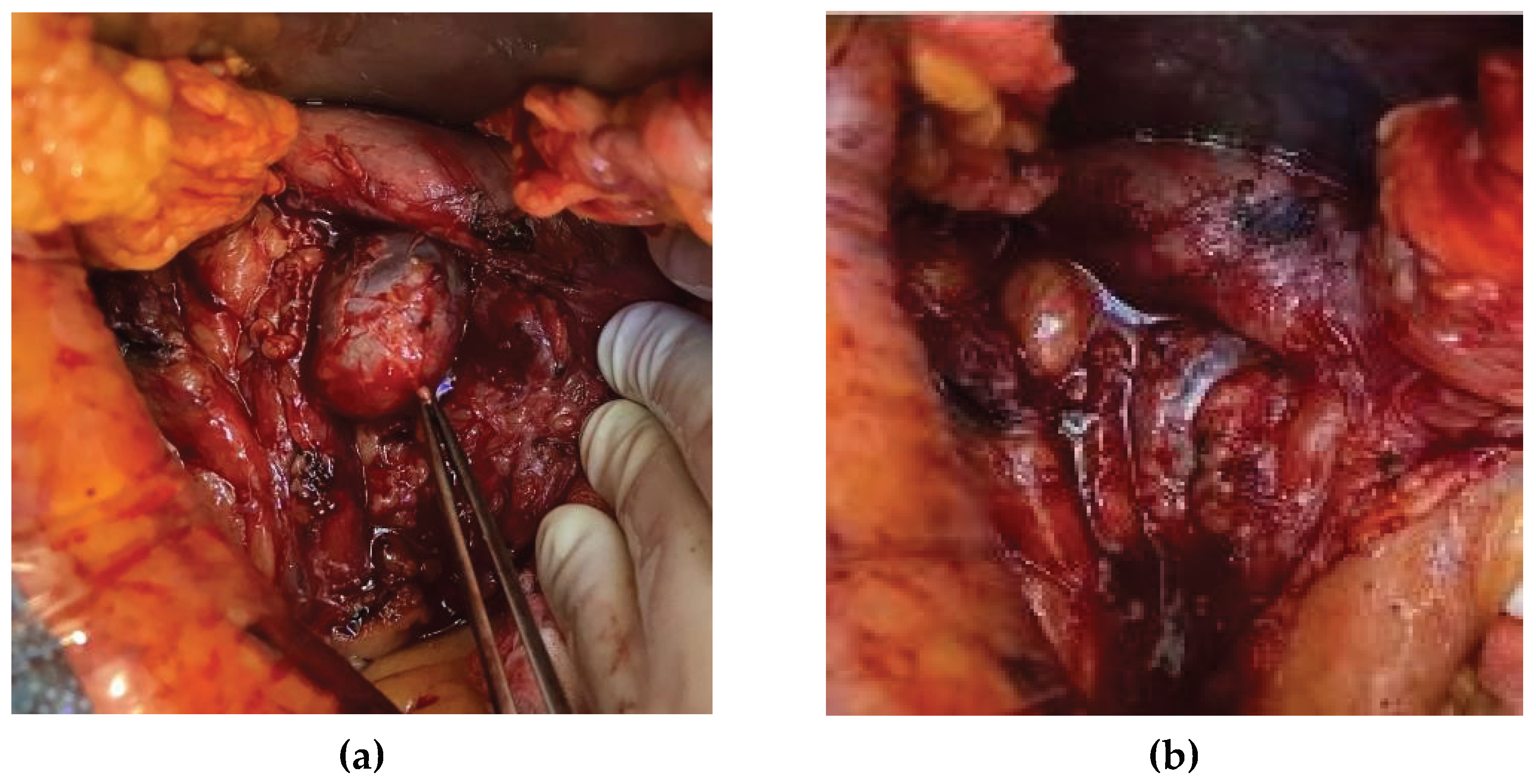
Images of the operative field are shown below,
Figure 4 (a) and (b).
During the operation and after the stay in the intensive care unit, in order to avoid changes in body temperature, we have used a device for heating the serum.
2.5. Clinical Course and Follow-Up
Despite treatment with corticosteroids, started preoperatively and postoperatively, 3 days postoperatively he presented with anaemia due to a new onset of autoimmune haemolytic anaemia, during which he maintained the aspiration drainage, showing no significant burden through it. He received with one unit of packed red cell, seven units of platelets and two units of plasma. The patient experienced no new post-operative complications, resumed oral feeding on post-operative day 3 and discharged 7 days later of the operation.
2.6. Histopathological Examination
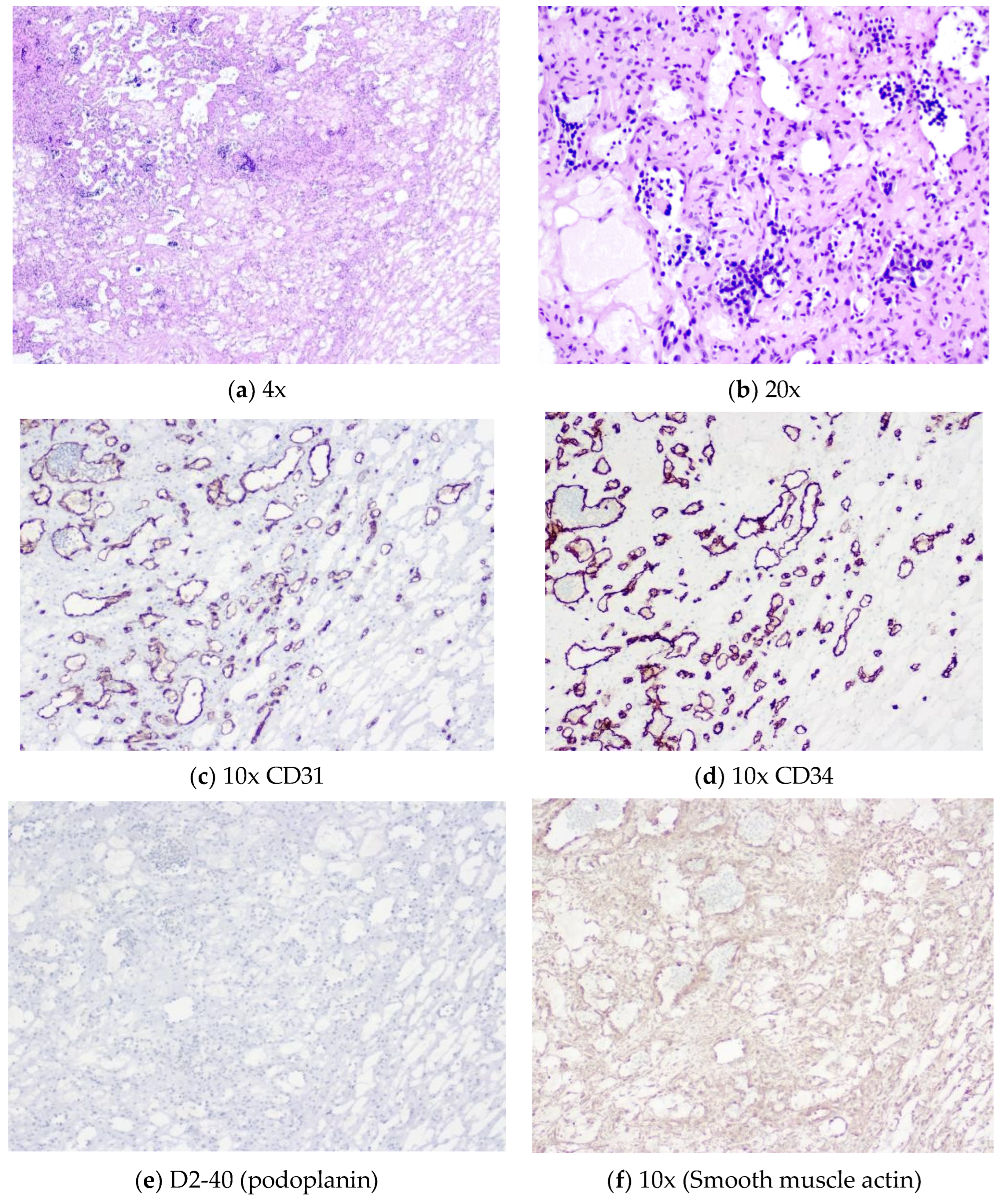
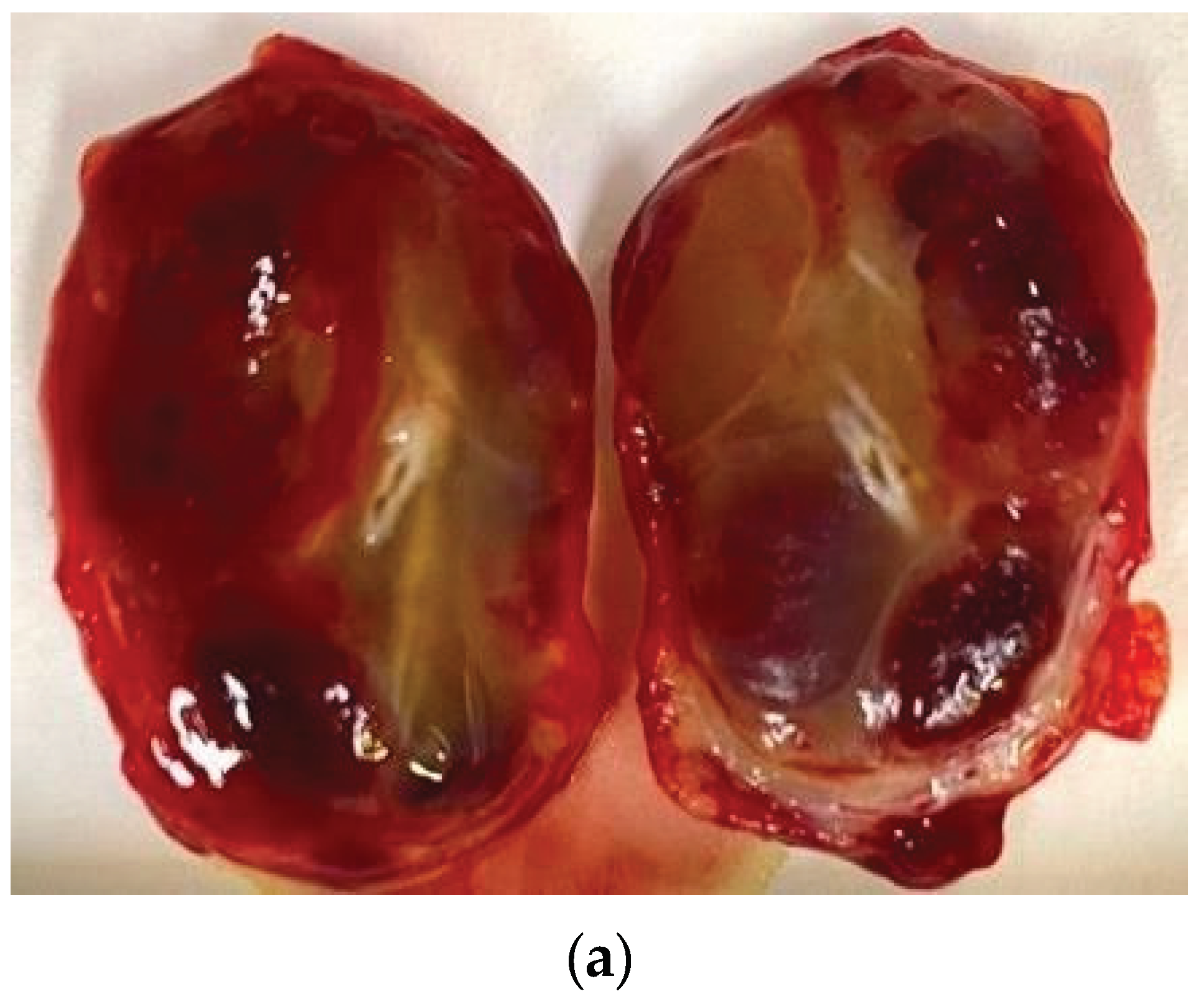
Gross description: well-defined nodule measuring 3.7 x 2.5 x 2.2 cm. Nodular cut surface with haematopoietic and myxoid areas,
Figure 5.
Microscopic description: nodular tumour with a lobulated growth pattern and associated with medium to large vessels, generally in the periphery. There is a marked anastomosing proliferation of capillary channels; the endothelial cells show no atypia, no obvious multilayered pleomorphic and a nail-like appearance. Absence of mitosis. Small fibrin thrombi are common.
Marked extra-medullary haematopoiesis with precursors of erythrocytes and other cell types including megakaryocytes; within the lesion foci of ripe adipose tissue. Stroma shows foci of sclerosis, edema and myxoid changes with central predilection. Isolated intracytoplasmic hyaline globules are visible.
Identification of specific immunohistochemistry techniques: vascular channels of the tumour express high levels of CD31 and CD34, while D2-40 is negative (podoplanin, as a component of extracellular vesicles that reprogrammed the protein content of exosomes). Smooth muscle actin negative in endothelial cells and positive in the vascular wall, with enhancement of the support network of benign pericytes. Ki-67 very low rate of proliferative activity, marked in the haematopoietic component. HHV8 (human herpesvirus 8) negative. Alpha-fetoprotein negative.
Figure 6.
(a) (b) (c) (d) (f) (g). 6(a): Thin-walled anastomotic channels lined by a monolayer of bland endothelial cells. Stromal oedema and sclerosis are extensive. Identification of areas of characteristic anastomotic growth with bland, hobnailed endothelial cells is diagnostic. 6(b): Lesional endothelial cells are small and cytologically bland with no multilayering or significant nuclear pleomorphism. Hobnail nuclei are visible. Foci of extramedullary haematopoiesis within vascular channels are common. Erythrocyte and megakaryocyte precursors are seen. 6(c), 6(d) and 6(e): Endothelial marker expression. The lesional channels express high levels of CD31 (c) and CD34 (d) by immunohistochemistry, whereas D2-40 (podoplanin) (e) is typically negative. 6(f): Smooth muscle actin. Although negative in endothelial cells, it is positive in the pericyte support network. Staining for the proliferation index Ki67 is very low in tumour cells and high in the haematopoietic component (not shown).
Figure 6.
(a) (b) (c) (d) (f) (g). 6(a): Thin-walled anastomotic channels lined by a monolayer of bland endothelial cells. Stromal oedema and sclerosis are extensive. Identification of areas of characteristic anastomotic growth with bland, hobnailed endothelial cells is diagnostic. 6(b): Lesional endothelial cells are small and cytologically bland with no multilayering or significant nuclear pleomorphism. Hobnail nuclei are visible. Foci of extramedullary haematopoiesis within vascular channels are common. Erythrocyte and megakaryocyte precursors are seen. 6(c), 6(d) and 6(e): Endothelial marker expression. The lesional channels express high levels of CD31 (c) and CD34 (d) by immunohistochemistry, whereas D2-40 (podoplanin) (e) is typically negative. 6(f): Smooth muscle actin. Although negative in endothelial cells, it is positive in the pericyte support network. Staining for the proliferation index Ki67 is very low in tumour cells and high in the haematopoietic component (not shown).
4. Discussion
Autoimmune haemolytic anaemia is mediated by autoantibodies and/or complement together with activated macrophages, T lymphocytes and cytokines that contribute to the process directed against red blood cells to cause premature destruction of erythrocytes. All of these immune parameters change with age, and immunosenescence is a mechanism associated with autoimmunity [
12].
There is evidence that the infantile haemangioma may not be the direct source of alpha-fetoprotein. An interaction between the primitive mesoderm-derived haemangioma and endogenous endodermal tissues, such as the liver, via an intermediary, may explain the elevated serum AFP levels in infants with extrahepatic haemangioma [
13]. Similarly, there has been no evidence of alpha-fetoprotein expression in haemolytic anaemia. A relevant finding was that the patient’s serum level of alpha-fetoprotein was elevated, with a progressive increase in successive analyses up to the preoperative analysis. Four days after removal of the tumour, alpha-fetoprotein levels decreased. However, the blood levels are currently on an upward trend. The active regenerative process within the tumour may explain the increase in alpha-fetoprotein. However, neither the removal of the tumour nor the immunohistochemistry expression of the tumour showed its involvement in the anastomosing haemangioma.
It has, been suggested that the observation of well-defined hyper or iso-intensity in genitourinary tract tumors on T2 WI and progressive vascular enhancement patterns on MRI and CT are diagnostic features of anastomosing hemangioma [
14]. Subsequent radiological investigations considered paraganglioma as a possible differential diagnosis. Several radiological features of anastomosing hemangioma, including morphology, clear borders, density, heterogeneous signal and apparent enhancement, are similar to those of extra adrenal paraganglioma. Both processes are richly vascularized. In our case, the functional hormonal study of the tumour showed no hormonal activity.
Resection of both the aorta and the inferior vena cava (IVC) is a surgical procedure that requires both a favorable tumour biology and a patient suitable for this difficult surgery; however, resection offers the possibility of cure [
15]. The absence of infiltration of the anastomosing haemangioma on the anterior aspect of the cava allowed resection of the tumour without resection of the cava.
The progressive growth of the tumour over time was been used to guide surgery. The average size of communicating haemangiomas is 2.2 cm [
16]. The tumour cell types had low mitotic activity, as shown by the expression of Ki-67 by immunohistochemistry and the SUV of 2.2 observed on PET scan. However, tumour growth may be justified by the increased proliferative activity as evidenced by the immunohistochemistry expression of Ki-67 in haematopoietic tissue observed in the anastomosing haemangioma.
In an anastomosing haemangioma, irregular capillary-sized vascular channels show prominent anastomoses. A monolayer of endothelial cells lines the vascular spaces [
17]. Presence of a high density of co-transplanted fibroblasts has favored anastomoses of endothelial progenitor cell-derived vessels with host vessels [
18]. Reprogramming of adult fibroblasts plays an important role in angiogenesis. Through reprogramming, adult fibroblasts can develop into progenitor cells and even different types of endothelial cells necessary for blood vessel formation.
Several studies have reported small colonies of progenitor cells found in various locations in the arterial wall, particularly in the adventitia. These progenitor cells include endothelial, smooth muscle and haematopoietic stem/progenitor cells [
19,
20]. The nodular image ventral to the abdominal aorta on CT scan 2020 may correspond to these small colonies of progenitor cells, which would be the start of the anastomosing haemangioma. The tumour stroma beside may contain edematous, inflammatory, fibrous components or myxoid infiltrates [
21].
In our case, we also observed mature fatty infiltration, fibrin thrombi, dilated vessels and extra medullary haematopoiesis. Immunohistochemistry demonstrates diffuse staining for endothelial markers, including CD31 and CD34 [
22]. When the tumour expresses endothelial marker it indicates angiogenic activity within the tumour. Angiogenesis is a key component in the biology of haemangiomas, particularly in their growth phase. A feature of the process of erythrocyte differentiation or terminal maturation is that it takes place in anatomical niches, known as erythroblastic islands, which is unique to mammalian erythropoiesis and is preferentially located in the bone marrow [
23].
Erythroblast islands consist of macrophages surrounded by about thirty erythroid cells of varying degrees of maturation. They provide cell-cell interactions, which are necessary for erythrocyte differentiation and proliferation. The main growth factors that regulate erythropoiesis in vivo are stem cell factor, granulocyte colony-stimulating factor, interleukins IL-3, IL-6, IL-11 and erythropoietin. Transforming growth factor β1 (TGF-β1) accelerates terminal erythroid differentiation by delaying cells in the G1 phase [
24].
In most cases of autoimmune haemolytic anaemia there is no clonal disease with erythropoietic proliferation in the bone marrow. This phenomenon only occurs if there are somatic mutations in the erythroid progenitor cells. Mutated genes used to define clonal expansions of haematopoietic cells, termed clonal haematopoiesis, include DNMT3A, TET2, AXL1, JAK2, TP53 and SF3B1. These mutated genes are also present in chronic myeloid leukaemia and myelodysplastic syndromes. Therefore, it would not be surprising if these tumours could develop from erythropoietic clones. However, these mutations are also present in the healthy elderly population [
25]. In conclusion that the clonal erythropoietin proliferation in the anastomosing haemangioma is secondary to autoimmune haemolytic anaemia and compensates for the loss of erythrocytes in a senescence-impaired bone marrow.
Since the prognosis of communicating haemangioma is good, the erythroblast islands observed at the periphery of the tumour and the clonal expansions of viable red blood cells could be beneficial in the patient’s autoimmune haemolytic anaemia. However, since these mutated genes are present in hematologic neoplastic processes, indicated to remove the tumour should be considered.
Future lines of research should go directed towards creating sustainable research infrastructures that facilitate the acquisition of tissue and liquid biopsies, together with clinical and biological data on these tumours, as proposed by the clinical and translational research of the international malignant germ cell consortium [
26]. Therefore, a possible causal relationship between autoimmune haemolytic anaemia and anastomosing haemangioma is proposed. Therefore, a possible causal relationship between autoimmune haemolytic anaemia and anastomosing haemangioma is proposed.
Author Contributions
Conceptualization, J.L. and I.L.; methodology, J.L. and E.I.; software, J.L., E.I.; resources, A.S. and I.S. and B.M.; data curation, J.L and E.I.; writing—original draft preparation, J.L.; writing—review and editing, E.I. and I.L.; visualization, J.L. and A.S. and I.S. and E.I. and B.M.; supervision, I.L. and E.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
While no IRB was required for this study, acquisition, storage, photography, and disposal of all tissues conformed with ethical and biosafety considerations.
Informed Consent Statement
The patient gave informed consent prior to surgery.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
To the patient and family for their comprehension and help, during the diagnosis and treatment of the disease.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hill QA, Hill A, Berentsen S. Defining autoimmune hemolytic anemia: a systematic review of the terminology used for diagnosis and treatment. Blood Adv. 2019 Jun 25;3(12):1897-1906. PMID: 31235526; PMCID: PMC6595261. [CrossRef]
- Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, Kuter DJ, Michel M, Montillo M, Röth A, Zeerleder SS, Berentsen S. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020 May; 41:100648. Epub 2019 Dec 5. [CrossRef] [PubMed]
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021 Apr;113(2):70-84. Epub 2020 Nov 3. PMID: 33179614; PMCID: PMC8167394. [CrossRef]
- Johnstone KJ, Strutton GM, Perry-Keene JL, Hazratwala K, Delahunt B. Multifocal anastomosing haemangioma of the kidney with intravascular growth and sinus fat invasion: a rare benign mimic of angiosarcoma. Pathology. 2020 Apr;52(3):394-396. Epub 2020 Feb 26. [CrossRef] [PubMed]
- Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009 Sep;33(9):1364-9. [CrossRef] [PubMed]
- Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol. 2011 Sep;136(3):450-7. [CrossRef] [PubMed]
- Newman PJ, Berndt MC, Gorski J, White GC 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219-22. [CrossRef] [PubMed]
- Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents. 2001 Jan-Mar;15(1):1-13. 15. [PubMed]
- Bean GR, Joseph NM, Folpe AL, Horvai AE, Umetsu SE. Recurrent GNA14 mutations in anastomosing haemangiomas. Histopathology. 2018 Aug;73(2):354-357. Epub 2018. 21 May. [CrossRef] [PubMed]
- Lim YH, Bacchiocchi A, Qiu J, Straub R, Bruckner A, Bercovitch L, et al. Somatic GNA14 mutation causes congenital and sporadic vascular tumors through MAPK activation. J Hum Genet. 2016;99(2):443-50.
- Liau JY, Tsai JH, Lan J, Chen CC, Wang YH, Lee JC, Huang HY. GNA11 joins GNAQ and GNA14 as a recurrently mutated gene in anastomosing hemangioma. Virchows Arch. 2020 Mar;476(3):475-481. Epub 2019 Nov 9. [CrossRef] [PubMed]
- Goronzy JJ, Li G, Yang Z, Weyand CM. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol. 2013 Jun 4;4:131. PMID: 23761790; PMCID: PMC3671290. [CrossRef]
- Itinteang T, Chibnall AM, Marsh R, Dunne JC, de Jong S, Davis PF, Leadbitter P, Tan ST. Elevated Serum Levels of Alpha-Fetoprotein in Patients with Infantile Hemangioma Are Not Derived from within the Tumor. Front Surg. 2016 Feb 9;3:5. PMID: 26904545; PMCID: PMC4746268. [CrossRef]
- Shanbhogue K, Khandelwal A, Hajdu C, Cao W, Surabhi VR, Prasad SR. Anastomosing hemangioma: a current update on clinical, pathological and imaging features. Abdom Radiol (NY). 2022 Jul;47(7):2335-2346. Epub 2022 Jun 9. [CrossRef] [PubMed]
- Newton DH, Chen SL, Wu K, Fraker D, Guzzo TJ, Roses RE, Foley PJ 3rd, Fairman RM, Jackson BM. Technique and Outcomes of Concomitant Aortic and Caval Resection and Reconstruction for Retroperitoneal Tumors. Ann Vasc Surg. 2024 Jan; 98:251-257. Epub 2023 Oct 5. [CrossRef] [PubMed]
- Perdiki M, Datseri G, Liapis G, Chondros N, Anastasiou I, Tzardi M, Delladetsima JK, Drakos E. Anastomosing hemangioma: report of two renal cases and analysis of the literature. Diagn Pathol. 2017 Jan 24;12(1):14. PMID: 28118845; PMCID: PMC5260082. [CrossRef]
- Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009 Sep;33(9):1364-9. [CrossRef] [PubMed]
- Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010 Feb;16(2):585-94. PMID: 19737050; PMCID: PMC2813071. [CrossRef]
- Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015 Apr 10;116(8):1392-412. 16. [CrossRef] [PubMed]
- Sellahewa SG, Li JY, Xiao Q. Updated Perspectives on Direct Vascular Cellular Reprogramming and Their Potential Applications in Tissue Engineered Vascular Grafts. J Funct Biomater. 2022 Dec 30;14(1):21. PMID: 36662068; PMCID: PMC9866165. [CrossRef]
- Jin LU, Liu J, Li Y, Sun S, Mao X, Yang S, Lai Y. Anastomosing hemangioma: The first case report in the bladder. Mol Clin Oncol. 2016 Feb;4(2):310-312. Epub 2015 Dec 8. PMID: 26893881; PMCID: PMC4734195. [CrossRef]
- Johnstone KJ, Strutton GM, Perry-Keene JL, Hazratwala K, Delahunt B. Multifocal anastomosing haemangioma of the kidney with intravascular growth and sinus fat invasion: a rare benign mimic of angiosarcoma. Pathology. 2020 Apr;52(3):394-396. Epub 2020 Feb 26. [CrossRef] [PubMed]
- Seu KG, Papoin J, Fessler R, Hom J, Huang G, Mohandas N, Blanc L, Kalfa TA. Unraveling Macrophage Heterogeneity in Erythroblastic Islands. Front Immunol. 2017 Sep 20;8:1140. PMID: 28979259; PMCID: PMC5611421. [CrossRef]
- Zermati Y, Fichelson S, Valensi F, Freyssinier JM, Rouyer-Fessard P, Cramer E, et al. El factor de crecimiento transformante inhibe la eritropoyesis bloqueando la proliferación y acelerando la diferenciación de los progenitores eritroides. Exp Hematol. 2000; 28 (8): 885–894. [CrossRef]
- Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019 Nov 1;366(6465):eaan4673. PMID: 31672865; PMCID: PMC8050831. [CrossRef]
- Fonseca A, Lobo J, Hazard FK, Gell J, Nicholls PK, Weiss RS, Klosterkemper L, Volchenboum SL, Nicholson JC, Frazier AL, Amatruda JF, Bagrodia A, Lockley M, Murray MJ. Advancing clinical and translational research in germ cell tumors (GCT): recommendations from the Malignant Germ Cell International Consortium. Br J Cancer. 2022 Nov; 127(9):1577-1583. Epub 2022 Oct 13. PMID: 36229581; PMCID: PMC9596690. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).