Submitted:
27 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
SN in sleep and neuropathology
bvFTD as a secondary psychopathy
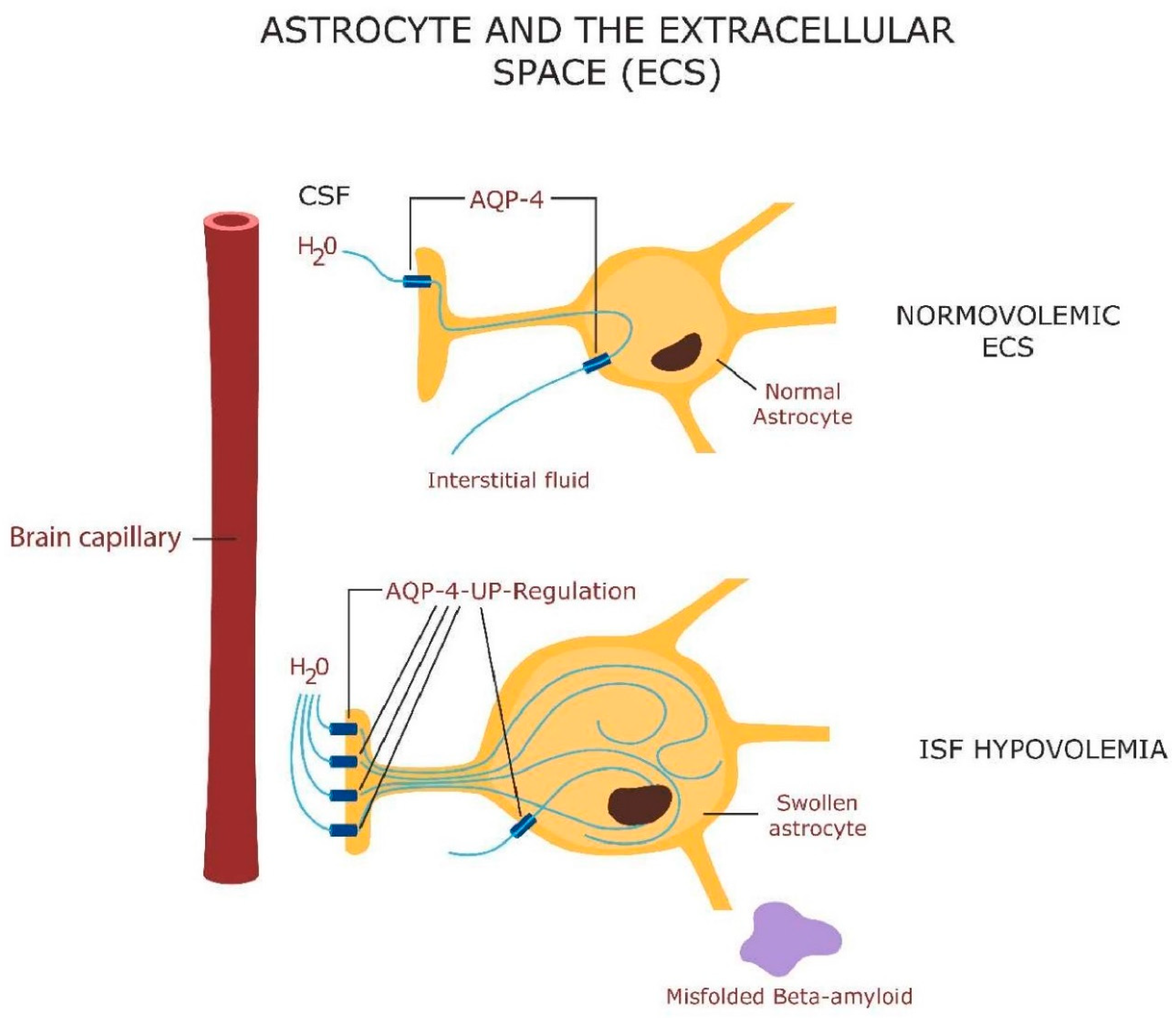
Sleep and glial cells
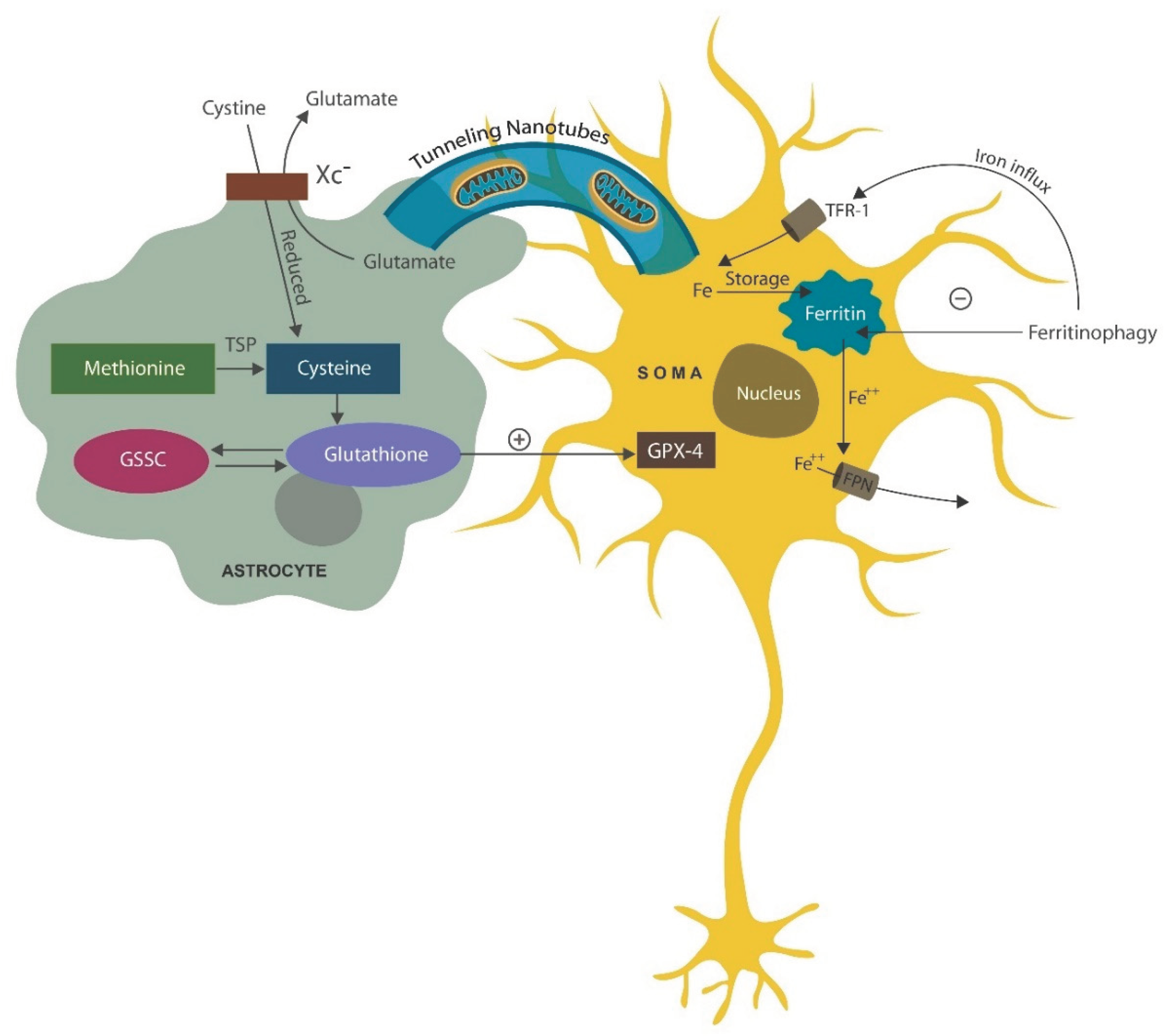
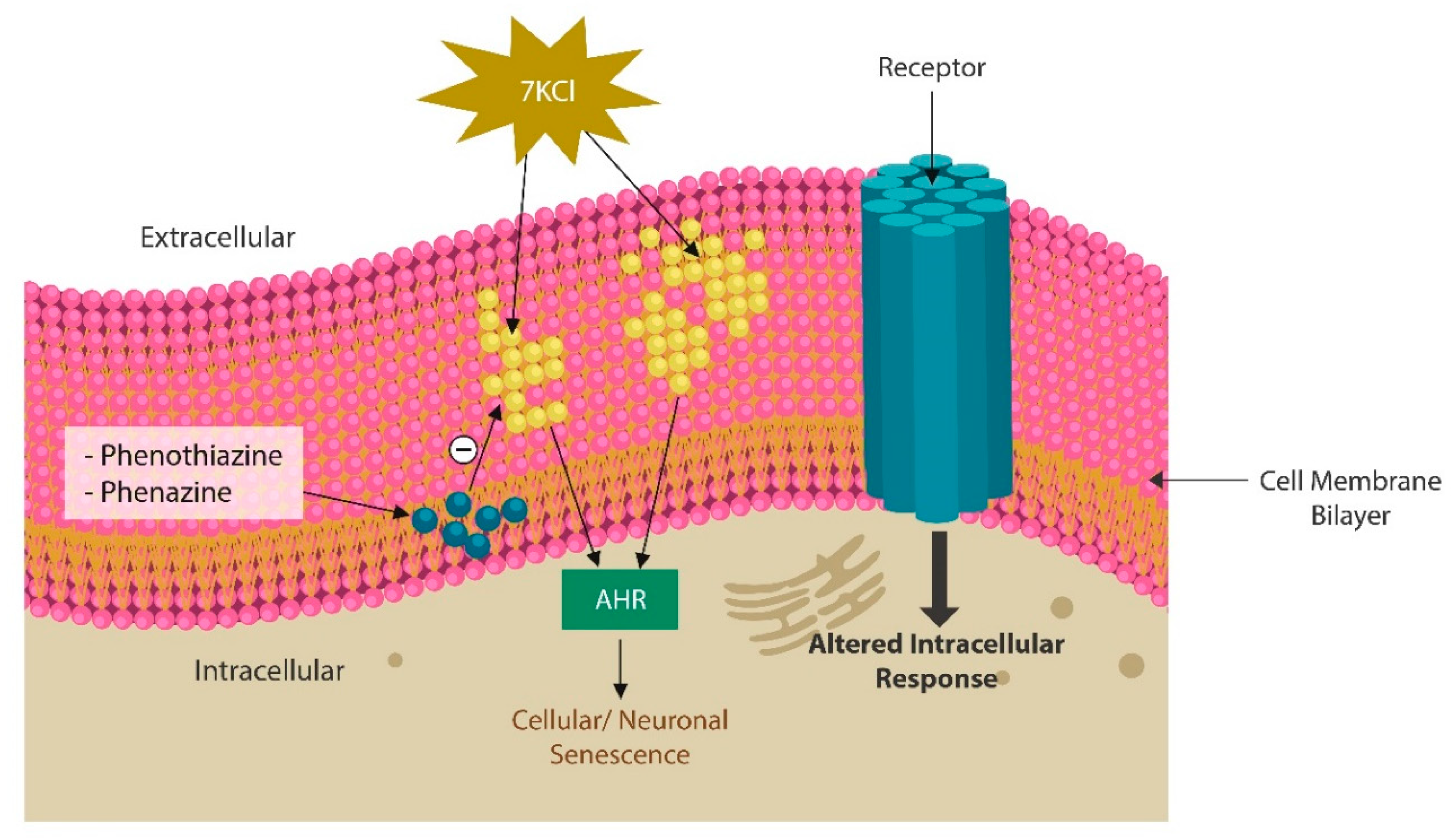
Mitochondria and aryl hydrocarbon receptor
Four cases of bvFTD from our hospital and the community
Discussion
Mitochondria-protective treatments
Membrane Lipid Replacement (MLR)
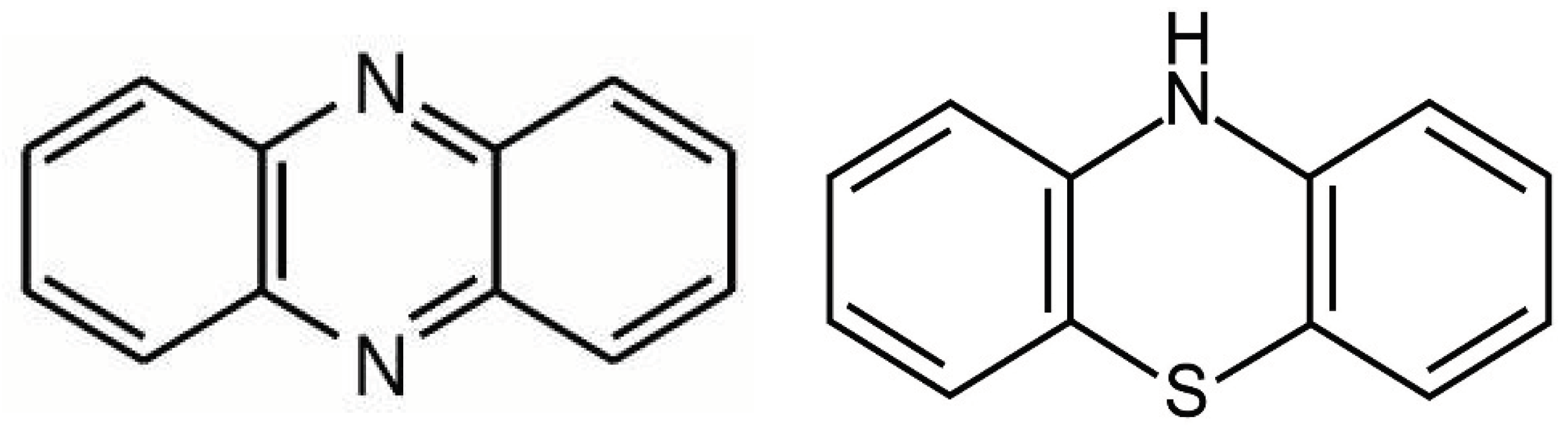
Phenazines and phenothiazine derivatives
Mitochondrial transfer and transplantation
Conclusions
References
- Sateia, M.J.; Doghramji, K.; Hauri, P.J.; Morin, C.M. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 2000, 23, 243–308. [Google Scholar] [CrossRef] [PubMed]
- Dewa, L.H.; Thibaut, B.; Pattison, N.; Campbell, S.J.; Woodcock, T.; Aylin, P.; Archer, S. Treating insomnia in people who are incarcerated: a feasibility study of a multi-component treatment pathway. SLEEP Adv. 2024, 5, zpae003. [Google Scholar] [CrossRef] [PubMed]
- Talih, F.; Ajaltouni, J.; Ghandour, H.; Abu-Mohammad, A.S.; Kobeissy, F. Insomnia in hospitalized psychiatric patients: prevalence and associated factors. Neuropsychiatr. Dis. Treat. 2018, ume 14, 969–975. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Li, W.; Xiao, L.; Huo, X.; Ding, J.; Sun, T. Is the insula linked to sleep? A systematic review and narrative synthesis. Heliyon 2022, 8, e11406. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, M.; Yin, Y.; Hua, K.; Fu, S.; Jiang, G. Aberrant Effective Connectivity of the Right Anterior Insula in Primary Insomnia. Front. Neurol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Wylie, K.P.; Tregellas, J.R. The role of the insula in schizophrenia. Schizophr. Res. 2010, 123, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fathy, Y.Y.; Hoogers, S.E.; Berendse, H.W.; van der Werf, Y.D.; Visser, P.J.; de Jong, F.J.; van de Berg, W.D. Differential insular cortex sub-regional atrophy in neurodegenerative diseases: a systematic review and meta-analysis. Brain Imaging Behav. 2019, 14, 2799–2816. [Google Scholar] [CrossRef]
- Koutsouleris, N.; Pantelis, C.; Velakoulis, D.; McGuire, P.; Dwyer, D.B.; Urquijo-Castro, M.-F.; Paul, R.; Dong, S.; Popovic, D.; Oeztuerk, O.; et al. Exploring Links Between Psychosis and Frontotemporal Dementia Using Multimodal Machine Learning. JAMA Psychiatry 2022, 79, 907–919. [Google Scholar] [CrossRef]
- Triarhou, L.C. The percipient observations of Constantin von Economo on encephalitis lethargica and sleep disruption and their lasting impact on contemporary sleep research. Brain Res. Bull. 2006, 69, 244–258. [Google Scholar] [CrossRef]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Anat. Embryol. 2010, 214, 495–517. [Google Scholar] [CrossRef]
- Berg, M.T.; Rogers, E.M.; Lei, M.-K.; Simons, R.L. Losing Years Doing Time: Incarceration Exposure and Accelerated Biological Aging among African American Adults. J. Heal. Soc. Behav. 2021, 62, 460–476. [Google Scholar] [CrossRef]
- Kaiksow, F.A.; Brown, L.; Merss, K.B. Caring for the Rapidly Aging Incarcerated Population: The Role of Policy. J. Gerontol. Nurs. 2023, 49, 7–11. [Google Scholar] [CrossRef]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Wong, S.L.; Cahaya, H.S.; Atamna, H.; Ames, B.N. Iron Accumulation during Cellular Senescence. Ann. N. Y. Acad. Sci. 2004, 1019, 365–367. [Google Scholar] [CrossRef]
- Urrutia, P.J.; Mena, N.P.; Nãºã±Ez, M.T. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front. Pharmacol. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Carvalhas-Almeida, C.; Cavadas, C.; Álvaro, A.R. The impact of insomnia on frailty and the hallmarks of aging. Aging Clin. Exp. Res. 2022, 35, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Prather, A.A. Sleep and biological aging: A short review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Jamioł-Milc, D.; Borecki, K.; Stachowska, E.; Zabielska, P.; Kamińska, M.; Karakiewicz, B. The Prevalence of Insomnia and the Link between Iron Metabolism Genes Polymorphisms, TF rs1049296 C>T, TF rs3811647 G>A, TFR rs7385804 A>C, HAMP rs10421768 A>G and Sleep Disorders in Polish Individuals with ASD. Int. J. Environ. Res. Public Heal. 2020, 17, 400. [Google Scholar] [CrossRef]
- Nacarino-Palma, A.; Rico-Leo, E.M.; Campisi, J.; Ramanathan, A.; González-Rico, F.J.; Rejano-Gordillo, C.M.; Ordiales-Talavero, A.; Merino, J.M.; Fernández-Salguero, P.M. Aryl hydrocarbon receptor blocks aging-induced senescence in the liver and fibroblast cells. Aging 2022, 14, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Peng, V.; Sudan, R.; Antonova, A.U.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812. [Google Scholar] [CrossRef]
- Hwang, H.J.; Dornbos, P.; Steidemann, M.; Dunivin, T.K.; Rizzo, M.; LaPres, J.J. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol. Appl. Pharmacol. 2016, 304, 121–132. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kontek, B. Lipid peroxidation in patients with schizophrenia. Psychiatry Clin. Neurosci. 2010, 64, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, J.; Qu, C.; Yang, L.; Wu, X.; Wang, S.; Yang, T.; Liu, H.; Fang, Y.; Sun, P. Identification of Ferroptosis-Related Genes in Schizophrenia Based on Bioinformatic Analysis. Genes 2022, 13, 2168. [Google Scholar] [CrossRef] [PubMed]
- Gulec, M.; Ozkol, H.; Selvi, Y.; Tuluce, Y.; Aydin, A.; Besiroglu, L.; Ozdemir, P.G. Oxidative stress in patients with primary insomnia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2012, 37, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Van Remmen, H.; Frohlich, V.; Lechleiter, J.; Richardson, A.; Ran, Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun. 2007, 356, 893–898. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free. Radic. Biol. Med. 2018, 133, 144–152. [Google Scholar] [CrossRef]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free. Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef]
- Fujii, R.; Hasuo, H.; Sakuma, H.; Okada, M.; Uchitani, K. The efficacy and safety of intravenous chlorpromazine treatment for sleep disturbance in patients with incurable cancer, with oral administration difficulty: a 1-week, prospective observational study. Ann. Palliat. Med. 2021, 10, 8547–8556. [Google Scholar] [CrossRef]
- Tischkau, S.A. Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. Eur. J. Neurosci. 2019, 51, 379–395. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef]
- Korade; Liu, W.; Warren, E.B.; Armstrong, K.; Porter, N.A.; Konradi, C. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 2017, 187, 74–81. [Google Scholar] [CrossRef]
- Kiani, A.; Nik, S.H.; Khodadoost, A.; Salimi, A.; Pourahmad, J. Trifluoperazine an Antipsychotic Drug and Inhibitor of Mitochondrial Permeability Transition Protects Cytarabine and Ifosfamide-Induced Neurotoxicity. Drug Res. 2020, 70, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Pathak, D.N.; Singh, R. Effects of Chlorpromazine on the Activities of Antioxidant Enzymes and Lipid Peroxidation in the Various Regions of Aging Rat Brain. J. Neurochem. 1984, 42, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Russo, A.; Barreca, D.; Giunta, E.; Galtieri, A.; Tellone, E. Short-Term Effects of Chlorpromazine on Oxidative Stress in Erythrocyte Functionality: Activation of Metabolism and Membrane Perturbation. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Brunauer, L.S.; Chu, F.C.; Helsel, C.M.; Gedde, M.M.; Huestis, W.H. Selective amphipathic nature of chlorpromazine binding to plasma membrane bilayers. Biochim. et Biophys. Acta (BBA) - Biomembr. 2003, 1616, 95–105. [Google Scholar] [CrossRef]
- Korth, C.; May, B.C.H.; Cohen, F.E.; Prusiner, S.B. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. 2001, 98, 9836–9841. [Google Scholar] [CrossRef]
- Henkel, N.D.; Wu, X.; O’donovan, S.M.; Devine, E.A.; Jiron, J.M.; Rowland, L.M.; Sarnyai, Z.; Ramsey, A.J.; Wen, Z.; Hahn, M.K.; et al. Schizophrenia: a disorder of broken brain bioenergetics. Mol. Psychiatry 2022, 27, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Kempf, A. Mitochondrial control of sleep. Curr. Opin. Neurobiol. 2023, 81, 102733. [Google Scholar] [CrossRef]
- Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. [Google Scholar] [CrossRef]
- Denis, A.A.; Toledo, D.; Hakim, Q.A.; Quintana, A.A.; Escobar, C.R.; Oluwole, S.A.; Costa, A.; Gil Garcia, E.; Hill, A.R.; Agatemor, C. Ligand-Independent Activation of Aryl Hydrocarbon Receptor and Attenuation of Glutamine Levels by Natural Deep Eutectic Solvent. ChemBioChem 2023, 24, e202300540. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Gollihue, J.; Norris, C. Astrocyte mitochondria: Central players and potential therapeutic targets for neurodegenerative diseases and injury. Ageing Res. Rev. 2020, 59, 101039–101039. [Google Scholar] [CrossRef] [PubMed]
- Misgeld, T.; Schwarz, T.L. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci. 2000, 3, 277–283. [Google Scholar] [CrossRef]
- Wolff, M.; Vann, S.D. The Cognitive Thalamus as a Gateway to Mental Representations. J. Neurosci. 2018, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Matsuoka, T.; Kato, Y.; Ayani, N.; Maeda, S.; Takeda, M.; Narumoto, J. Individual Differences in Interoceptive Accuracy Are Correlated With Salience Network Connectivity in Older Adults. Front. Aging Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Blessing, W.W.; Blessing, E.M.; Mohammed, M.; Ootsuka, Y. Clozapine, chlorpromazine and risperidone dose-dependently reduce emotional hyperthermia, a biological marker of salience. Psychopharmacol. 2017, 234, 3259–3269. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Pasquini, L.; Nana, A.L.; Toller, G.; A Brown, J.; Deng, J.; Staffaroni, A.; Kim, E.-J.; Hwang, J.-H.L.; Li, L.; Park, Y.; et al. Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia. Cereb. Cortex 2020, 30, 5387–5399. [Google Scholar] [CrossRef]
- Mendez, M.F.; Anderson, E.; Shapira, J.S. An Investigation of Moral Judgement in Frontotemporal Dementia. Cogn. Behav. Neurol. 2005, 18, 193–197. [Google Scholar] [CrossRef]
- Mendez, M.F. The Neurobiology of Moral Behavior:Review and Neuropsychiatric Implications. CNS Spectrums 2009, 14, 608–620. [Google Scholar] [CrossRef]
- Boeve, B.F. Behavioral Variant Frontotemporal Dementia. Contin. Lifelong Learn. Neurol. 2022, 28, 702–725. [Google Scholar] [CrossRef]
- Arrigoni, E.; Fuller, P.M. The Sleep-Promoting Ventrolateral Preoptic Nucleus: What Have We Learned over the Past 25 Years? Int. J. Mol. Sci. 2022, 23, 2905. [Google Scholar] [CrossRef]
- De Luca, R.; Nardone, S.; Grace, K.P.; Venner, A.; Cristofolini, M.; Bandaru, S.S.; Sohn, L.T.; Kong, D.; Mochizuki, T.; Viberti, B.; et al. Orexin neurons inhibit sleep to promote arousal. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Inutsuka, A.; Yamanaka, A. The regulation of sleep and wakefulness by the hypothalamic neuropeptide orexin/hypocretin. . 2013, 75, 29–36. [Google Scholar]
- Yin, D.; Dong, H.; Wang, T.-X.; Hu, Z.-Z.; Cheng, N.-N.; Qu, W.-M.; Huang, Z.-L. Glutamate Activates the Histaminergic Tuberomammillary Nucleus and Increases Wakefulness in Rats. Neuroscience 2019, 413, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Konadhode, R.R.; Pelluru, D.; Shiromani, P.J. Neurons containing orexin or melanin concentrating hormone reciprocally regulate wake and sleep. Front. Syst. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Bandarabadi, M.; Li, S.; Aeschlimann, L.; Colombo, G.; Tzanoulinou, S.; Tafti, M.; Becchetti, A.; Boutrel, B.; Vassalli, A. Inactivation of hypocretin receptor-2 signaling in dopaminergic neurons induces hyperarousal and enhanced cognition but impaired inhibitory control. Mol. Psychiatry 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, C.; Han, F.; Cheng, H. Histamine H1 receptor in basal forebrain cholinergic circuit: A novel target for the negative symptoms of schizophrenia? Neurosci. Bull. 2022, 38, 558–560. [Google Scholar] [CrossRef]
- Grady, F.S.; Boes, A.D.; Geerling, J.C. A Century Searching for the Neurons Necessary for Wakefulness. Front. Neurosci. 2022, 16, 930514. [Google Scholar] [CrossRef]
- Kerkhofs, M.; Lavie, P. HISTORICAL NOTE: Frédéric Bremer 1892–1982: a pioneer in sleep research. Sleep Med. Rev. 2000, 4, 505–514. [Google Scholar] [CrossRef]
- Fuller, P.; Sherman, D.; Pedersen, N.P.; Saper, C.B.; Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 2010, 519, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P. The sleep theory of Constantin von Economo. J. Sleep Res. 1993, 2, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; De Jesus, O. Von Economo Encephalitis. 2023 Aug 23. In StatPearls (Internet); StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Rosen, D. Asleep: the Forgotten Epidemic That Remains One of Medicine’s Greatest Mysteries. J Clin Sleep Med. 2010, 6, 299. [Google Scholar] [CrossRef]
- Cortelli, P.; Perani, D.; Parchi, P.; Grassi, F.; Montagna, P.; De Martin, M.; Castellani, R.; Tinuper, P.; Gambetti, P.; Lugaresi, E.; et al. Cerebral metabolism in fatal familial insomnia: Relation to duration, neuropathology, and distribution of protease-resistent prion protein. Neurology 1997, 49, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Gallassi, R.; Morreale, A.; Montagna, P.; Cortelli, P.; Avoni, P.; Castellani, R.; Gambetti, R.; Lugaresi, E. Fatal familial insomnia. Neurology 1996, 46, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.E.; Brown, J.A.; Hua, A.Y.; Lwi, S.J.; Zhou, J.; Kurth, F.; Eickhoff, S.B.; Rosen, H.J.; Kramer, J.H.; Miller, B.L.; et al. Network Architecture Underlying Basal Autonomic Outflow: Evidence from Frontotemporal Dementia. J. Neurosci. 2018, 38, 8943–8955. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjun, P.K.; Lalousis, P.A.; Dunne, T.F.; Heinze, K.; Reniers, R.L.; Broome, M.R.; Farmah, B.; Oyebode, F.; Wood, S.J.; Upthegrove, R. Aberrant salience network functional connectivity in auditory verbal hallucinations: a first episode psychosis sample. Transl. Psychiatry 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Cracco, L.; Appleby, B.S.; Gambetti, P. Fatal familial insomnia and sporadic fatal insomnia. Handb Clin Neurol. 2018, 153, 271–299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Li, W.; Xiao, L.; Huo, X.; Ding, J.; Sun, T. Is the insula linked to sleep? A systematic review and narrative synthesis. Heliyon 2022, 8, e11406. [Google Scholar] [CrossRef]
- Levichkina, E.V.; Busygina, I.I.; Pigareva, M.L.; Pigarev, I.N. The Mysterious Island: Insula and Its Dual Function in Sleep and Wakefulness. Front. Syst. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, G.; Shao, Y.; Chen, J.; Li, Y.; Liu, J.; Yao, P.; Zhou, S.; Xu, J.; Hu, S.; et al. Increased connectivity of the anterior cingulate cortex is associated with the tendency to awakening during N2 sleep in patients with insomnia disorder. Sleep 2022, 46. [Google Scholar] [CrossRef]
- Guldenmund, P.; Demertzi, A.; Boveroux, P.; Boly, M.; Vanhaudenhuyse, A.; Bruno, M.-A.; Gosseries, O.; Noirhomme, Q.; Brichant, J.-F.; Bonhomme, V.; et al. Thalamus, Brainstem and Salience Network Connectivity Changes During Propofol-Induced Sedation and Unconsciousness. Brain Connect. 2013, 3, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, L.; Zhou, Z.; Xu, K.; Zhang, L.; Liu, X.; Tan, X.; Zhang, J.; Ye, X.; Gao, J.; et al. Functional Connectivity of Anterior Insula Predicts Recovery of Patients With Disorders of Consciousness. Front. Neurol. 2018, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Mashour, G.A. Anesthetizing the Self. Anesthesiology 2016, 124, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.-Z.; Zhang, Y.; Pan, B.; Gao, Y.-H.; Zhan, H.; Liu, Y.; Shao, Y.-C.; Zhang, X. Altered insula-prefrontal functional connectivity correlates to decreased vigilant attention after total sleep deprivation. Sleep Med. 2021, 84, 187–194. [Google Scholar] [CrossRef]
- Li, C.; Dong, M.; Yin, Y.; Hua, K.; Fu, S.; Jiang, G. Aberrant Effective Connectivity of the Right Anterior Insula in Primary Insomnia. Front. Neurol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Chen, M.C.; Chiang, W.-Y.; Yugay, T.; Patxot, M.; Ozcivit, I.B.; Hu, K.; Lu, J. Anterior Insula Regulates Multiscale Temporal Organization of Sleep and Wake Activity. J. Biol. Rhythm. 2016, 31, 182–93. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; White, T.; Liddle, P. The Concept of Salience Network Dysfunction in Schizophrenia: From Neuroimaging Observations to Therapeutic Opportunities. Curr. Top. Med. Chem. 2012, 12, 2324–2338. [Google Scholar] [CrossRef]
- Huang, H.; Chen, C.; Rong, B.; Wan, Q.; Chen, J.; Liu, Z.; Zhou, Y.; Wang, G.; Wang, H. Resting-state functional connectivity of salience network in schizophrenia and depression. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- He, X.; Qin, W.; Liu, Y.; Zhang, X.; Duan, Y.; Song, J.; Li, K.; Jiang, T.; Yu, C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2013, 35, 3446–3464. [Google Scholar] [CrossRef] [PubMed]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Salience and Default Mode Network Coupling Predicts Cognition in Aging and Parkinson’s Disease. J. Int. Neuropsychol. Soc. 2016, 22, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Day, G.S.; Farb, N.A.S.; Tang-Wai, D.F.; Masellis, M.; Black, S.E.; Freedman, M.; Pollock, B.G.; Chow, T.W. Salience Network Resting-State Activity. JAMA Neurol. 2013, 70, 1249–1253. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Rogers, B.P.; Blackford, J.U.; Heckers, S.; Woodward, N.D. Insula functional connectivity in schizophrenia. Schizophr. Res. 2020, 220, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: a selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef]
- Finger, E.C. Frontotemporal Dementias. Contin. Lifelong Learn. Neurol. 2016, 22, 464–489. [Google Scholar] [CrossRef] [PubMed]
- Zago, S.; Scarpazza, C.; Difonzo, T.; Arighi, A.; Hajhajate, D.; Torrente, Y.; Sartori, G. Behavioral Variant of Frontotemporal Dementia and Homicide in a Historical Case. 2021, 49, 219–227. [CrossRef]
- Nilsson, C.; Waldö, M.L.; Nilsson, K.; Santillo, A.; Vestberg, S. Age-Related Incidence and Family History in Frontotemporal Dementia: Data from the Swedish Dementia Registry. PLOS ONE 2014, 9, e94901. [Google Scholar] [CrossRef]
- Hendriks, S.; Peetoom, K.; Bakker, C.; van der Flier, W.M.; Papma, J.M.; Koopmans, R.; Verhey, F.R.J.; de Vugt, M.; Köhler, S.; Withall, A.; et al. Global Prevalence of Young-Onset Dementia. JAMA Neurol. 2021, 78, 1080–1090. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Diehl-Schmid, J.; Perneczky, R.; Koch, J.; Nedopil, N.; Kurz, A. Guilty by Suspicion? Criminal Behavior in Frontotemporal Lobar Degeneration. Cogn. Behav. Neurol. 2013, 26, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Theiss, C.; Brüne, M. Ultrastructural Alterations of Von Economo Neurons in the Anterior Cingulate Cortex in Schizophrenia. Anat. Rec. 2017, 300, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Nana, A.L.; Sidhu, M.; Gaus, S.E.; Hwang, J.-H.L.; Li, L.; Park, Y.; Kim, E.-J.; Pasquini, L.; Allen, I.E.; Rankin, K.P.; et al. Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 2018, 137, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Vohryzek, J.; Cabral, J.; Vuust, P.; Deco, G.; Kringelbach, M.L. Understanding brain states across spacetime informed by whole-brain modelling. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2022, 380, 20210247. [Google Scholar] [CrossRef] [PubMed]
- Nathani, M.; Jaleel, V.; Turner, A.; Dirvonas, C.; Suryadevara, U.; Tandon, R. When you hear hoofbeats, think horses and zebras: The importance of a wide differential when it comes to frontotemporal lobar degeneration. Asian J. Psychiatry 2019, 47, 101875. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Herbert, C.; Pollatos, O. On the Relationship Between Interoceptive Awareness and Alexithymia: Is Interoceptive Awareness Related to Emotional Awareness? J. Pers. 2011, 79, 1149–1175. [Google Scholar] [CrossRef] [PubMed]
- Quadt, L.; Critchley, H.D.; Garfinkel, S.N. The neurobiology of interoception in health and disease. Ann. New York Acad. Sci. 2018, 1428, 112–128. [Google Scholar] [CrossRef]
- Cauda, F.; Geminiani, G.C.; Vercelli, A. Evolutionary appearance of von Economo’s neurons in the mammalian cerebral cortex. Front. Hum. Neurosci. 2014, 8, 104. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- López-Ojeda, W.; Hurley, R.A. Von Economo Neuron Involvement in Social Cognitive and Emotional Impairments in Neuropsychiatric Disorders. J. Neuropsychiatry 2022, 34, 302–306. [Google Scholar] [CrossRef]
- Hodge, R.D.; Miller, J.A.; Novotny, M.; Kalmbach, B.E.; Ting, J.T.; Bakken, T.E.; Aevermann, B.D.; Barkan, E.R.; Berkowitz-Cerasano, M.L.; Cobbs, C.; et al. Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- A Dijkstra, A.; Lin, L.-C.; Nana, A.L.; E Gaus, S.; Seeley, W.W. Von Economo Neurons and Fork Cells: A Neurochemical Signature Linked to Monoaminergic Function. Cereb. Cortex 2016, 28, 131–144. [Google Scholar] [CrossRef]
- Azizi, S.A. Monoamines: Dopamine, Norepinephrine, and Serotonin, Beyond Modulation, “Switches” That Alter the State of Target Networks. Neurosci. 2020, 28, 121–143. [Google Scholar] [CrossRef]
- Valli, M.; Cho, S.S.; Uribe, C.; Masellis, M.; Chen, R.; Mihaescu, A.; Strafella, A.P. VMAT2 availability in Parkinson’s disease with probable REM sleep behaviour disorder. Mol. Brain 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Broese, M.; Riemann, D.; Hein, L.; Nissen, C. α-Adrenergic Receptor Function, Arousal and Sleep: Mechanisms and Therapeutic Implications. Pharmacopsychiatry 2012, 45, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Mancama, D.; Kerwin, R.W.; Arranz, M.J. Expression of the α1A-adrenergic receptor in schizophrenia. Neurosci. Lett. 2006, 401, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Minthon, L.; Passant, U.; Blennow, K.; Wallin, A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer’s disease. Neurobiol. Aging 1998, 19, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The Pathophysiology of Insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Kiyoshi, C.M. Astrocyte syncytium: a functional reticular system in the brain. Neural Regen. Res. 2019, 14, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Picard, K.; Limatola, C.; Nadjar, A.; Pascual, O.; Tremblay, M.E. Role of Glia in the Regulation of Sleep in Health and Disease. 10, 101.245. Compr Physiol 2020, 10, 101.245. [Google Scholar] [CrossRef]
- Que, M.; Li, Y.; Wang, X.; Zhan, G.; Luo, X.; Zhou, Z. Role of astrocytes in sleep deprivation: accomplices, resisters, or bystanders? Front. Cell. Neurosci. 2023, 17, 1188306. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Vacca, R.A.; Moro, L.; Atlante, A. Mitochondria Can Cross Cell Boundaries: An Overview of the Biological Relevance, Pathophysiological Implications and Therapeutic Perspectives of Intercellular Mitochondrial Transfer. Int. J. Mol. Sci. 2021, 22, 8312. [Google Scholar] [CrossRef] [PubMed]
- Leow, D.M.-K.; Cheah, I.K.-M.; Fong, Z.W.-J.; Halliwell, B.; Ong, W.-Y. Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 5498. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integr Med (Encinitas). 2014, 13, 35–43. [Google Scholar] [PubMed]
- Hill, V.M.; O’connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLOS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bonora, M.; Ingusci, S.; Previati, M.; Marchi, S.; Zucchini, S.; Perrone, M.; Wieckowski, M.R.; Castellazzi, M.; Pugliatti, M.; et al. Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Heckers, S.; Konradi, C. Hippocampal neurons in schizophrenia. J. Neural Transm. 2002, 109, 891–905. [Google Scholar] [CrossRef]
- Padurariu., M.; Ciobica, A.; Mavroudis, I.; Fotiou, D.; Baloyannis, S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. 2012 Jun;24(2):152-8. Psychiatr Danub. 2012, 24, 152–158. [Google Scholar]
- Zhang, P.; Li, Y.-X.; Zhang, Z.-Z.; Yang, Y.; Rao, J.-X.; Xia, L.; Li, X.-Y.; Chen, G.-H.; Wang, F. Astroglial Mechanisms Underlying Chronic Insomnia Disorder: A Clinical Study. Nat. Sci. Sleep 2020, ume 12, 693–704. [Google Scholar] [CrossRef]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef]
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2017, 285, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, T.; Howes, O. The role of mitochondria in the pathophysiology of schizophrenia: A critical review of the evidence focusing on mitochondrial complex one. Neurosci. Biobehav. Rev. 2021, 132, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, L.M.M.; Brown, G.M.; Braganza, N.A.; Kennedy, J.L.; Gonçalves, V.F. Mitochondria’s role in sleep: Novel insights from sleep deprivation and restriction studies. World J. Biol. Psychiatry 2021, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. [Google Scholar] [CrossRef] [PubMed]
- Ene, H.M.; Karry, R.; Farfara, D.; Ben-Shachar, D. Mitochondria play an essential role in the trajectory of adolescent neurodevelopment and behavior in adulthood: evidence from a schizophrenia rat model. Mol. Psychiatry 2022, 28, 1170–1181. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. (BBA)—Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.J.; Suh, J.H.; Lee, S.H.; Poulsen, K.L.; An, Y.A.; Moorthy, B.; Hartig, S.M.; Moore, D.D.; Kim, K.H. Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice. Mol. Metab. 2023, 72, 101717. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Dornbos, P.; Steidemann, M.; Dunivin, T.K.; Rizzo, M.; LaPres, J.J. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol. Appl. Pharmacol. 2016, 304, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah Receptor by Tryptophan and Tryptophan Metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-X.; Wang, C.; Krager, S.L.; Bottum, K.M.; Tischkau, S.A. Aryl Hydrocarbon Receptor Activation Attenuates Per1 Gene Induction and Influences Circadian Clock Resetting. Toxicol. Sci. 2013, 132, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Kempf, A. Mitochondrial control of sleep. Curr. Opin. Neurobiol. 2023, 81, 102733. [Google Scholar] [CrossRef]
- Wei, Y.-D.; Helleberg, H.; Rannug, U.; Rannug, A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem. Interactions 1998, 110, 39–55. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Flaws, J.A.; Mahoney, M.M.; Miller, S.R.; Zacur, H.A.; Gallicchio, L. Genetic polymorphisms in the aryl hydrocarbon receptor-signaling pathway and sleep disturbances in middle-aged women. Sleep Med. 2013, 14, 883–887. [Google Scholar] [CrossRef]
- Frau-Méndez, M.A.; Fernández-Vega, I.; Ansoleaga, B.; Tech, R.B.; Tech, M.C.; del Rio, J.A.; Zerr, I.; Llorens, F.; Zarranz, J.J.; Ferrer, I. Fatal familial insomnia: mitochondrial and protein synthesis machinery decline in the mediodorsal thalamus. Brain Pathol. 2016, 27, 95–106. [Google Scholar] [CrossRef]
- Kishikawa, J.-I.; Inoue, Y.; Fujikawa, M.; Nishimura, K.; Nakanishi, A.; Tanabe, T.; Imamura, H.; Yokoyama, K. General anesthetics cause mitochondrial dysfunction and reduction of intracellular ATP levels. PLOS ONE 2018, 13, e0190213–e0190213. [Google Scholar] [CrossRef]
- Wei, H. The Role of Calcium Dysregulation in Anesthetic-Mediated Neurotoxicity. Anesth. Analg. 2011, 113, 972–974. [Google Scholar] [CrossRef]
- Lee, H.-G.; Choi, S.-I.; Park, S.-K.; Park, S.-J.; Kim, N.-H.; Choi, E.-K. Alteration of glutathione metabolism and abnormal calcium accumulation in the mitochondria of hamster brain infected with scrapie agent. Neurobiol. Aging 2000, 21, 151. [Google Scholar] [CrossRef]
- Glatzel, M.; Sepulveda-Falla, D. Losing sleep over mitochondria: a new player in the pathophysiology of fatal familial insomnia. Brain Pathol. 2016, 27, 107–108. [Google Scholar] [CrossRef]
- Moon, J.-H.; Park, S.-Y. Prion peptide-mediated calcium level alteration governs neuronal cell damage through AMPK-autophagy flux. Cell Commun. Signal. 2020, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.L.; Iraci, N.; Biggi, S.; Cecchetti, V.; Biasini, E. Pharmacological Agents Targeting the Cellular Prion Protein. Pathogens 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Korth, C.; May, B.C.H.; Cohen, F.E.; Prusiner, S.B. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. 2001, 98, 9836–9841. [Google Scholar] [CrossRef] [PubMed]
- Khattar, K.E.; Safi, J.; Rodriguez, A.-M.; Vignais, M.-L. Intercellular Communication in the Brain through Tunneling Nanotubes. Cancers 2022, 14, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, N.E.; Borchert, A.; Bräuer, A.U.; Kuhn, H. Role for glutathione peroxidase-4 in brain development and neuronal apoptosis: Specific induction of enzyme expression in reactive astrocytes following brain injury. Free. Radic. Biol. Med. 2007, 43, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mauri, S.; Favaro, M.; Bernardo, G.; Mazzotta, G.M.; Ziviani, E. Mitochondrial autophagy in the sleeping brain. Front. Cell Dev. Biol. 2022, 10, 956394. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Owens, G.C.; Crossin, K.L.; Edelman, D.B. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol. Cell. Neurosci. 2007, 36, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Nana, A.L.; Hepker, M.; Hwang, J.-H.L.; Gaus, S.E.; Spina, S.; Cosme, C.G.; Gan, L.; Grinberg, L.T.; Geschwind, D.H.; et al. Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol. Commun. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Hogan, D.B.; Jetté, N.; Fiest, K.M.; Roberts, J.I.; Pearson, D.; Smith, E.E.; Roach, P.; Kirk, A.; Pringsheim, T.; Maxwell, C.J. The Prevalence and Incidence of Frontotemporal Dementia: a Systematic Review. Can. J. Neurol. Sci. / J. Can. des Sci. Neurol. 2016, 43, S96–S109. [Google Scholar] [CrossRef]
- Course, M.M.; Wang, X. Transporting mitochondria in neurons. F1000Research 2016, 5, 1735. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Youdim, M.B.H. The Neuroprotective Activities of the Novel Multi-Target Iron-Chelators in Models of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis and Aging. Cells 2023, 12, 763. [Google Scholar] [CrossRef]
- Calderon-Montaño, J.M.; Burgos-Morón, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Reviews Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Du, Y.-Y.; Sun, T.; Yang, Q.; Liu, Q.-Q.; Li, J.-M.; Yang, L.; Luo, L.-X. Therapeutic Potential of Kaempferol against Sleep Deprivation-Induced Cognitive Impairment: Modulation of Neuroinflammation and Synaptic Plasticity Disruption in Mice. ACS Pharmacol. Transl. Sci. 2023, 6, 1934–1944. [Google Scholar] [CrossRef]
- Saleem, A.; Ain, Q.U.; Akhtar, M.F. Alternative Therapy of Psychosis: Potential Phytochemicals and Drug Targets in the Management of Schizophrenia. Front. Pharmacol. 2022, 13, 895668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3β. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Roh, M.-S. Glycogen Synthase Kinase-3 (GSK3) in Psychiatric Diseases and Therapeutic Interventions. Curr. Drug Targets 2006, 7, 1421–1434. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Al-Masri, I.M.; Taha, M.O.; Al-Ghussein, M.A.; AlKhatib, H.S.; Najjar, S.; Bustanji, Y. Olanzapine inhibits glycogen synthase kinase-3β: An investigation by docking simulation and experimental validation. Eur. J. Pharmacol. 2008, 584, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Shilovsky, G.A.; Putyatina, T.S.; Morgunova, G.V.; Seliverstov, A.V.; Ashapkin, V.V.; Sorokina, E.V.; Markov, A.V.; Skulachev, V.P. A Crosstalk between the Biorhythms and Gatekeepers of Longevity: Dual Role of Glycogen Synthase Kinase-3. Biochem. (Moscow) 2021, 86, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Dozza, B.; Smith, M.A.; Perry, G.; Tabaton, M.; Strocchi, P. Regulation of glycogen synthase kinase-3β by products of lipid peroxidation in human neuroblastoma cells. J. Neurochem. 2004, 89, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Serafim, B.; Bernardino, A.R.; Freitas, F.; Torres, C.A.V. Recent Developments in the Biological Activities, Bioproduction, and Applications of Pseudomonas spp. Phenazines. Molecules 2023, 28, 1368. [Google Scholar] [CrossRef]
- Jiang, J.; Beltran, D.G.; Schacht, A.; Wright, S.; Zhang, L.; Du, L. Functional and Structural Analysis of Phenazine O-Methyltransferase LaPhzM from Lysobacter antibioticus OH13 and One-Pot Enzymatic Synthesis of the Antibiotic Myxin. ACS Chem. Biol. 2018, 13, 1003–1012. [Google Scholar] [CrossRef]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an Acetylcholinesterase Inhibitor Produced by a Streptomyces Species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef]
- Rusman, Y.; Oppegard, L.M.; Hiasa, H.; Gelbmann, C.; Salomon, C.E. Solphenazines A–F, Glycosylated Phenazines from Streptomyces sp. Strain DL-93. J. Nat. Prod. 2013, 76, 91–96. [Google Scholar] [CrossRef]
- Wang, X.; Abbas, M.; Zhang, Y.; Elshahawi, S.I.; Ponomareva, L.V.; Cui, Z.; Van Lanen, S.G.; Sajid, I.; Voss, S.R.; Shaaban, K.A.; et al. Baraphenazines A–G, Divergent Fused Phenazine-Based Metabolites from a Himalayan Streptomyces. J. Nat. Prod. 2019, 82, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- van Wezel, G. P.; McKenzie, N. L.; Nodwell, J. R. Chapter 5 Applying the genetics of secondary metabolism in model actinomycetes to the discovery of new antibiotics, 1st ed.; Elsevier Inc., 2009; Vol. 458. [Google Scholar]
- Cha, J.W.; Lee, S.I.; Kim, M.C.; Thida, M.; Lee, J.W.; Park, J.-S.; Kwon, H.C. Pontemazines A and B, phenazine derivatives containing a methylamine linkage from Streptomyces sp. UT1123 and their protective effect to HT-22 neuronal cells. Bioorganic Med. Chem. Lett. 2015, 25, 5083–5086. [Google Scholar] [CrossRef]
- Krishnaiah, M.; Rodriguez de Almeida, N.; Udumula, V.; Song, Z.; Chhonker, Y.S.; Abdelmoaty, M.M.; Aragao do Nascimento, V.; Murry, D.J.; Conda-Sheridan, M. Synthesis, biological evaluation, and metabolic stability of phenazine derivatives as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Lavaggi, M.L.; Aguirre, G.; Boiani, L.; Orelli, L.; García, B.; Cerecetto, H.; González, M. Pyrimido[1,2-a]quinoxaline 6-oxide and phenazine 5,10-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1737–1741. [Google Scholar] [CrossRef]
- Kato, S.; Shindo, K.; Yamagishi, Y.; Matsuoka, M.; Kawai, H.; Mochizuki, J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. Taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993, 46, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, M.; Bhat, S.G. Characterization of pyocyanin with radical scavenging and antibiofilm properties isolated from Pseudomonas aeruginosa strain BTRY1. 3 Biotech 2016, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alatawneh, N.; Meijler, M.M. Unraveling the Antibacterial and Iron Chelating Activity ofN-Oxide Hydroxy-Phenazine natural Products and Synthetic Analogs againstStaphylococcus aureus. Isr. J. Chem. 2023, 63. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Armistead, G.; A Rosa, C.; Anderson, A.; Patil, R.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Phenothiazines and their Evolving Roles in Clinical Practice: A Narrative Review. Heal. Psychol. Res. 2022, 10, 38930. [Google Scholar] [CrossRef]
- Heitmann, A.S.B.; Zanjani, A.A.H.; Klenow, M.B.; Mularski, A.; Sønder, S.L.; Lund, F.W.; Boye, T.L.; Dias, C.; Bendix, P.M.; Simonsen, A.C.; et al. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J. Biol. Chem. 2021, 297, 101012. [Google Scholar] [CrossRef]
- Boonnoy, P.; Jarerattanachat, V.; Karttunen, M.; Wong-Ekkabut, J. Bilayer Deformation, Pores, and Micellation Induced by Oxidized Lipids. J. Phys. Chem. Lett. 2015, 6, 4884–4888. [Google Scholar] [CrossRef]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents–A drug repurposing strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef]
- Voronova, O.; Zhuravkov, S.; Korotkova, E.; Artamonov, A.; Plotnikov, E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants 2022, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Keynes, R.G.; Karchevskaya, A.; Riddall, D.; Griffiths, C.H.; Bellamy, T.C.; Chan, A.W.E.; Selwood, D.L.; Garthwaite, J. N10-carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress. Chem. Biol. Drug Des. 2019, 94, 1680–1693. [Google Scholar] [CrossRef] [PubMed]
- Iuga, C.; Campero, A.; Vivier-Bunge, A. Antioxidant vs. prooxidant action of phenothiazine in a biological environment in the presence of hydroxyl and hydroperoxyl radicals: a quantum chemistry study. RSC Adv. 2015, 5, 14678–14689. [Google Scholar] [CrossRef]
- Yue, Y.; Kong, L.; Wang, J.; Li, C.; Tan, L.; Su, H.; Xu, Y. Regional Abnormality of Grey Matter in Schizophrenia: Effect from the Illness or Treatment? PLOS ONE 2016, 11, e0147204–e0147204. [Google Scholar] [CrossRef]
- Martínez, A.; Ibarra, I.A.; Vargas, R. A quantum chemical approach representing a new perspective concerning agonist and antagonist drugs in the context of schizophrenia and Parkinson’s disease. PLOS ONE 2019, 14, e0224691. [Google Scholar] [CrossRef]
- Goode-Romero, G.; Dominguez, L.; Martínez, A. Electron Donor–Acceptor Properties of Different Muscarinic Ligands: On the Road to Control Schizophrenia. J. Chem. Inf. Model. 2021, 61, 5117–5124. [Google Scholar] [CrossRef]
- Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68: 128–137. [CrossRef]
- Veijola, J.; Guo, J.Y.; Moilanen, J.S.; Jääskeläinen, E.; Miettunen, J.; Kyllönen, M.; Haapea, M.; Huhtaniska, S.; Alaräisänen, A.; Mäki, P.; et al. Longitudinal Changes in Total Brain Volume in Schizophrenia: Relation to Symptom Severity, Cognition and Antipsychotic Medication. PLOS ONE 2014, 9, e101689. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ayuk, E.L.; Igbojekwe, B.U.; Unaegbu, M. Potential Antioxidant Activity of New Tetracyclic and Pentacyclic Nonlinear Phenothiazine Derivatives. Biochem. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Clark, M.A.; Shay, J.W. Mitochondrial transformation of mammalian cells. Nature 1982, 295, 605–607. [Google Scholar] [CrossRef]
- Katrangi, E.; D’Souza, G.; Boddapati, S.V.; Kulawiec, M.; Singh, K.K.; Bigger, B.; Weissig, V. Xenogenic Transfer of Isolated Murine Mitochondria into Human ρ0 Cells Can Improve Respiratory Function. Rejuvenation Res. 2007, 10, 561–570. [Google Scholar] [CrossRef]
- Pacak, C.A.; Preble, J.M.; Kondo, H.; Seibel, P.; Levitsky, S.; del Nido, P.J.; Cowan, D.B.; McCully, J.D. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol. Open 2015, 4, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.A.; Hosseinian, S.; Kheradvar, A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am. J. Physiol. Physiol. 2021, 321, C489–C503. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; del Nido, P.J.; McCully, J.D. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-G.; Miao, C.-Y. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm. Sin. B 2023, 13, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Scheiblich, H.; Dansokho, C.; Mercan, D.; Schmidt, S.V.; Bousset, L.; Wischhof, L.; Eikens, F.; Odainic, A.; Spitzer, J.; Griep, A.; et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 2021, 184, 5089–5106. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Ren, D.; Zheng, P.; Zou, S.; Gong, Y.; Wang, Y.; Duan, J.; Deng, J.; Chen, H.; Feng, J.; Zhong, C.; et al. GJA1-20K Enhances Mitochondria Transfer from Astrocytes to Neurons via Cx43-TnTs After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2021, 42, 1887–1895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



