Submitted:
20 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection: Clinicopathological Data
2.3. Tissue Sample Preparation and Microarray Analysis
2.4. Immunohistochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Differences in Baseline Characteristics between Patients with Microsatellite Stable (MSS) and Microsatellite Instability-High (MSI-H)
3.2. Differential Expression of Immune Checkpoint Molecules between MSS and MSI-H Groups
3.3. Concomitant Expression of Immune Checkpoint Molecules
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Kunst, N.; Alarid-Escudero, F.; Aas, E.; Coupe, V.M.H.; Schrag, D.; Kuntz, K.M. Estimating population-based recurrence rates of colorectal cancer over time in the united states. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 2710–2718. [Google Scholar] [CrossRef]
- Lee, K.S.; Kwak, Y.; Ahn, S.; Shin, E.; Oh, H.K.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol. Immunother. 2017, 66, 927–939. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.H.; Oh, H.K.; Kim, D.W.; Kang, S.B.; Kim, H.; Shin, E. Programmed cell death ligand-1 protein expression and CD274/PD-L1 gene amplification in colorectal cancer: Implications for prognosis. Cancer Sci. 2018, 109, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jun, S.Y.; Lee, I.H.; Kang, B.W.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S.; Yoon, G.; Kim, J.G. CD274, LAG3, and IDO1 expressions in tumor-infiltrating immune cells as prognostic biomarker for patients with MSI-high colon cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, A.E.; Theochari, M.; Stefanou, D.; Papalambros, A.; Felekouras, E.; Gogas, H.; Ziogas, D.C. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit. Rev. Oncol. Hematol. 2022, 173, 103663. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune checkpoint inhibitors in prostate cancer. Cancers (Basel) 2021, 13, 2187. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. The Lancet Oncology 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. The Lancet Oncology 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Gatalica, Z.; Vranic, S.; Xiu, J.; Swensen, J.; Reddy, S. High microsatellite instability (MSI-H) colorectal carcinoma: A brief review of predictive biomarkers in the era of personalized medicine. Fam. Cancer 2016, 15, 405–412. [Google Scholar] [CrossRef]
- Wang, L.; Ren, F.; Wang, Q.; Baldridge, L.A.; Monn, M.F.; Fisher, K.W.; Sheng, W.; Zhou, X.; Du, X.; Cheng, L. Significance of programmed death ligand 1 (PD-L1) immunohistochemical expression in colorectal cancer. Mol. Diagn. Ther. 2016, 20, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.M.; Di Pinto, F.; Cariola, F.; Guerra, V.; Giannelli, G.; Caruso, M.L.; Pirrelli, M. PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments. Oncotarget 2018, 9, 8584–8596. [Google Scholar] [CrossRef]
- Ho, H.-L.; Chou, T.-Y.; Yang, S.-H.; Jiang, J.-K.; Chen, W.-S.; Chao, Y.; Teng, H.-W. PD-L1 is a double-edged sword in colorectal cancer: The prognostic value of PD-L1 depends on the cell type expressing PD-L1. J. Cancer Res. Clin. Oncol. 2019, 145, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Ko, S.H.; Ko, Y.S.; Kim, N.Y. Clinicopathological significance of PD-L1 expression in colorectal cancer: Impact of PD-L1 expression on pFOXO1 expression. Pathol. Res. Pract. 2020, 216, 152764. [Google Scholar] [CrossRef]
- Jung, D.H.; Park, H.J.; Jang, H.H.; Kim, S.H.; Jung, Y.; Lee, W.S. Clinical impact of PD-L1 expression for survival in curatively resected colon cancer. Cancer Invest. 2020, 38, 406–414. [Google Scholar] [CrossRef]
- Inaguma, S.; Lasota, J.; Wang, Z.; Felisiak-Golabek, A.; Ikeda, H.; Miettinen, M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod. Pathol. 2017, 30, 278–285. [Google Scholar] [CrossRef]
- Ahern, E.; Harjunpää, H.; O'Donnell, J.S.; Allen, S.; Dougall, W.C.; Teng, M.W.L.; Smyth, M.J. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology 2018, 7, e1431088. [Google Scholar] [CrossRef]
- Andre, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Marginean, E.C.; Melosky, B. Is there a role for programmed death ligand-1 testing and immunotherapy in colorectal cancer with microsatellite instability? Part i—Colorectal cancer: Microsatellite instability, testing, and clinical implications. Arch. Pathol. Lab. Med. 2017, 142, 17–25. [Google Scholar] [CrossRef]
- Lee, L.H.; Cavalcanti, M.S.; Segal, N.H.; Hechtman, J.F.; Weiser, M.R.; Smith, J.J.; Garcia-Aguilar, J.; Sadot, E.; Ntiamoah, P.; Markowitz, A.J.; et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod. Pathol. 2016, 29, 1433–1442. [Google Scholar] [CrossRef]
- Zhou, E.; Huang, Q.; Wang, J.; Fang, C.; Yang, L.; Zhu, M.; Chen, J.; Chen, L.; Dong, M. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8018–8027. [Google Scholar] [PubMed]
- Teng, F.; Meng, X.; Kong, L.; Mu, D.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte–associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl. Res. 2015, 166, 721–732. [Google Scholar] [CrossRef]
- Brandacher, G.; Perathoner, A.; Ladurner, R.; Schneeberger, S.; Obrist, P.; Winkler, C.; Werner, E.R.; Werner-Felmayer, G.; Weiss, H.G.; G√∂bel, G.; et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006, 12, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Young, G.D.; McMiller, T.L.; Chen, S.; Salas, J.T.; Pritchard, T.S.; Xu, H.; Meeker, A.K.; Fan, J.; Cheadle, C.; et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: Implications for PD-1 pathway blockade. Clin. Cancer Res. 2015, 21, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Talaat, I.M.; Elemam, N.M.; Zaher, S.; Saber-Ayad, M. Checkpoint molecules on infiltrating immune cells in colorectal tumor microenvironment. Front. Med. (Lausanne) 2022, 9, 955599. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Smits, E.; Lardon, F.; Pauwels, P.; Deschoolmeester, V. Immune checkpoint modulation in colorectal cancer: What's new and what to expect. J. Immunol. Res. 2015, 2015, 158038. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Dai, W.; Cai, G.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 2016, 15, 55. [Google Scholar] [CrossRef]
- Shan, T.; Chen, S.; Wu, T.; Yang, Y.; Li, S.; Chen, X. PD-L1 expression in colon cancer and its relationship with clinical prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 1764–1769. [Google Scholar]
- Ntomi, V.; Foukas, P.; Papaconstantinou, D.; Antonopoulou, I.; Pikoulis, A.; Panagiotides, I.; Pikoulis, E.; Syrigos, K. The clinical significance of PD-L1 in colorectal cancer (Review). Oncol. Rep. 2021, 45, 92. [Google Scholar] [CrossRef]
- Yu, M.; Lu, B.; Liu, Y.; Me, Y.; Wang, L.; Zhang, P. Tim-3 is upregulated in human colorectal carcinoma and associated with tumor progression. Mol. Med. Rep. 2017, 15, 689–695. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Rezaei, M.; Sanei, M.H.; Dehghanian, A.; Faghih, Z.; Heidari, Z.; Tavana, S. Tim3 and PD-1 as a therapeutic and prognostic targets in colorectal cancer: Relationship with sidedness, clinicopathological parameters, and survival. Front. Oncol. 2023, 13, 1069696. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast Cancer (Auckl) 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Anderson, Ana C. ; Joller, N.; Kuchroo, Vijay K. Lag-3, tim-3, and tigit: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.; et al. Lag-3 protein expression in non–small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Rhyner Agocs, G.; Assarzadegan, N.; Kirsch, R.; Dawson, H.; Galvan, J.A.; Lugli, A.; Zlobec, I.; Berger, M.D. Lag-3 expression predicts outcome in stage ii colon cancer. J. Pers. Med. 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Mokhtari, Z.; Sanei, M.H.; Heidari, Z.; Dehghanian, A.R.; Faghih, Z.; Rezaei, M. Clinicopathological significance and prognostic role of LAG3 + tumor-infiltrating lymphocytes in colorectal cancer; relationship with sidedness. Cancer Cell Int. 2023, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.J.; Sohn, J.H.; Kim, S.K.; Kim, C.S.; Cho, M.Y.; Kim, B.; An, S.; Kim, K.; Kim, Y. Concurrent upregulation of immune checkpoint molecule genes in colorectal cancer. Mol. Cell. Toxicol. 2022, 19, 521–529. [Google Scholar] [CrossRef]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression analysis and significance of PD-1, lag-3, and tim-3 in human non–small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Han, K.; Tang, J.H.; Liao, L.E.; Jiang, W.; Sui, Q.Q.; Xiao, B.Y.; Li, W.R.; Hong, Z.G.; Li, Y.; Kong, L.H.; et al. Neoadjuvant immune checkpoint inhibition improves organ preservation in t4bm0 colorectal cancer with mismatch repair deficiency: A retrospective observational study. Dis. Colon Rectum 2023, 66, e996–e1005. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Morse, M.A.; Overman, M.J.; Hartman, L.; Khoukaz, T.; Brutcher, E.; Lenz, H.J.; Atasoy, A.; Shangguan, T.; Zhao, H.; El-Rayes, B. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist 2019, 24, 1453–1461. [Google Scholar] [CrossRef]
- Cohen, R.; Bennouna, J.; Meurisse, A.; Tournigand, C.; De La Fouchardiere, C.; Tougeron, D.; Borg, C.; Mazard, T.; Chibaudel, B.; Garcia-Larnicol, M.L.; et al. RECIST and iRECIST criteria for the evaluation of nivolumab plus ipilimumab in patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The GERCOR NIPICOL phase II study. J. Immunother. Cancer 2020, 8, e001499. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
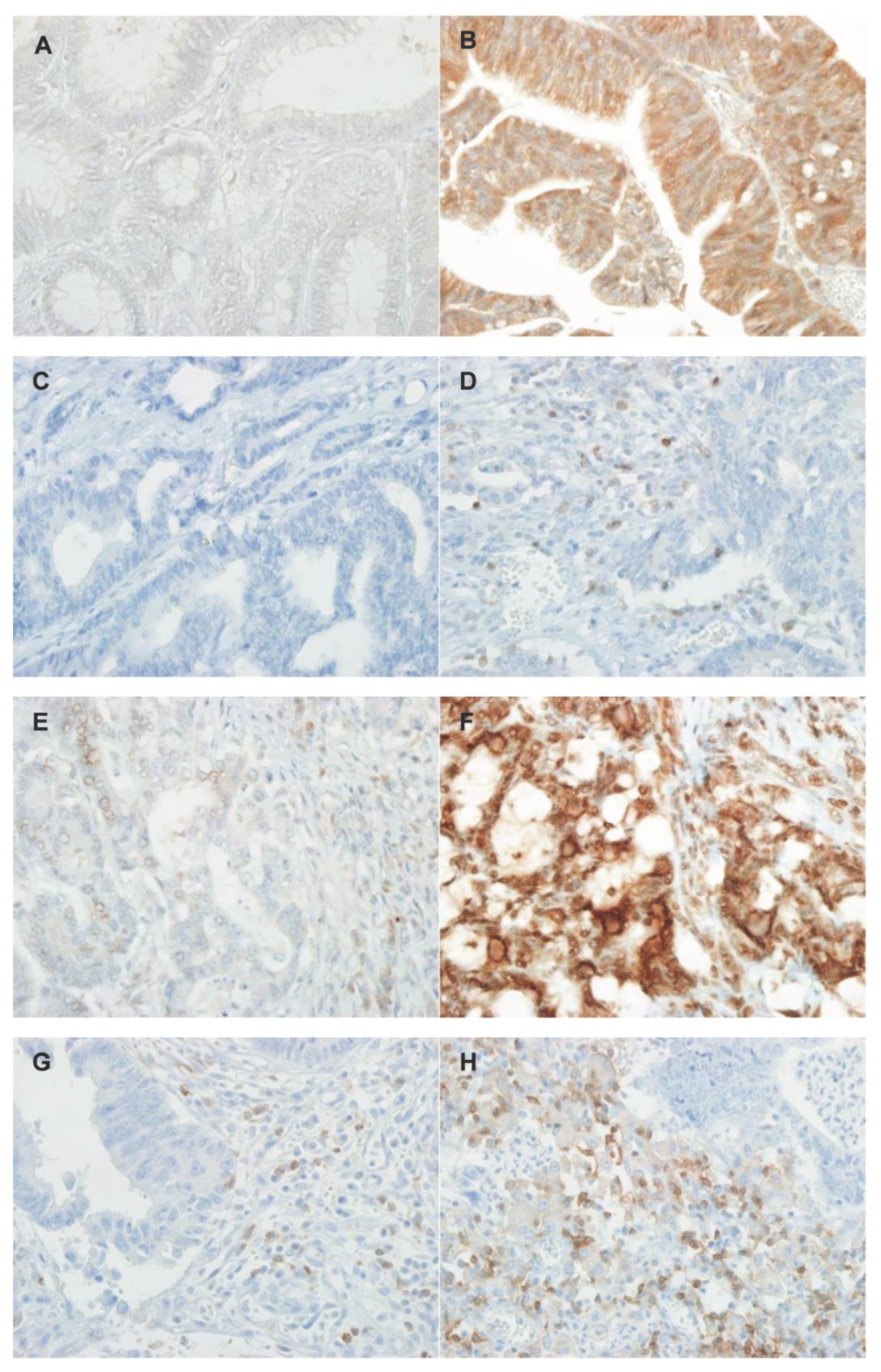
| MSS (%) (n = 40) |
MSI-H (%) (n = 43) |
P value | |
|---|---|---|---|
| Age (year) | |||
| Mean (SD) | 67.4 (13) | 66.3 (14.5) | 0.305 |
| Gender | |||
| Female | 21 (52.5) | 25 (58.1) | 0.662 |
| Male | 19 (47.5) | 18 (41.9) | |
| ASA score | |||
| 1 | 5 (12.5) | 5 (11.6) | 0.784 |
| 2 | 23 (57.5) | 22 (51.2) | |
| 3 | 12 (30.0) | 16 (37.2) | |
| Tumor location | |||
| Proximal | 10 (25.0) | 29 (67.4) | 0.001 |
| Distal | 13 (32.5) | 7 (16.3) | |
| Rectum | 17 (42.5) | 7 (16.3) | |
| CEA | |||
| <5 | 26 (65.0) | 31 (72.1) | 0.636 |
| 5≥ | 14 (35.0) | 12 (27.9) | |
| T stage | |||
| 2-3 | 29 (72.5) | 28 (65.1) | 0.489 |
| 4 | 11 (27.5) | 15 (34.9) | |
| N stage | |||
| 0 | 25 (62.5) | 28 (65.1) | 0.823 |
| 1-2 | 15 (37.5) | 15 (34.9) | |
| TNM stage | |||
| I-II | 24(60.0) | 28(65.1) | 0.656 |
| III | 16(40.0) | 15(34.9) | |
| Differentiation | |||
| High grade | 38 (95.0) | 31 (72.1) | 0.007 |
| Low grade | 2 (5.0) | 12 (27.9) | |
| Lymphatic invasion | |||
| No | 22 (55.0) | 22 (51.2) | 0.827 |
| Yes | 18 (45.0) | 21 (48.8) | |
| Lymph node harvest | |||
| Mean (SD) | 25.48 (11.79) | 33.2 (13.37) | 0.320 |
| Recurrence | |||
| No | 29 (72.5) | 38 (88.4) | 0.067 |
| Yes | 11 (27.5) | 5 (11.6) | |
| Cancer related Mortality | |||
| No | 38 (95.0) | 38 (88.4) | 0.435 |
| Yes | 2 (5.0) | 5 (11.6) |
| MSS (%) (n = 40) |
MSI-H (%) (n = 43) |
P value | |
|---|---|---|---|
| Center of tumor | |||
| PD-L1 | |||
| negative | 37 (92.5) | 25 (58.1) | < 0.001 |
| positive | 3 (7.5) | 18 (41.9) | |
| PD-1 | |||
| negative | 28(70.0) | 27 (62.8) | 0.643 |
| positive | 12(30.0) | 16 (37.2) | |
| TIM-3 | |||
| negative | 21 (55.2) | 13 (30.2) | 0.047 |
| Positive | 19 (47.5) | 30 (69.8) | |
| LAG-3 | |||
| negative | 10 (30.0) | 10 (23.3) | 1.000 |
| positive | 30 (75.0) | 33 (76.7) | |
| Border of tumor | |||
| PD-L1 | |||
| negative | 32 (80.0) | 31 (72.1) | 0.450 |
| positive | 8 (20.0) | 12 (27.9) | |
| PD-1 | |||
| negative | 24 (60.0) | 19 (44.2) | 0.189 |
| positive | 16 (40.0) | 24 (55.8) | |
| TIM-3 | |||
| negative | 22 (55.0) | 20 (46.5) | 0.512 |
| Positive | 18 (45.0) | 23 (53.5) | |
| LAG-3 | |||
| negative | 11 (27.5) | 6 (14.0) | 0.175 |
| positive | 29 (72.5) | 37 (86.0) |
| Center of tumor | MSS (n = 40) | MSI-H (n = 43) | Total (n = 83) |
|---|---|---|---|
| PD-L1+PD1+TIM3+LAG3 | 1 | 8 | 9 |
| PD-L1+PD1+TIM3 | 0 | 0 | 0 |
| PD-L1+PD1+LAG3 | 1 | 0 | 1 |
| PD-L1+TIM3+LAG3 | 0 | 4 | 4 |
| PD-1+TIM3+LAG3 | 5 | 3 | 8 |
| PD-L1+PD1 | 0 | 2 | 2 |
| PD-L1+TIM3 | 0 | 0 | 0 |
| PD-L1+LAG3 | 1 | 3 | 4 |
| PD1+TIM3 | 0 | 1 | 1 |
| PD1+LAG3 | 3 | 1 | 4 |
| TIM3+LAG3 | 12 | 12 | 24 |
| PD-L1 | 0 | 1 | 1 |
| PD1 | 2 | 1 | 3 |
| TIM3 | 1 | 2 | 3 |
| LAG3 | 7 | 2 | 9 |
| All negative | 7 | 3 | 10 |
| Border of tumor | |||
| PD-L1+PD1+TIM3+LAG3 | 4 | 4 | 8 |
| PD-L1+PD1+TIM3 | 0 | 0 | 0 |
| PD-L1+PD1+LAG3 | 4 | 6 | 10 |
| PD-L1+TIM3+LAG3 | 0 | 0 | 0 |
| PD1+TIM3+LAG3 | 5 | 6 | 11 |
| PD-L1+PD1 | 0 | 1 | 1 |
| PD-L1+TIM3 | 0 | 0 | 0 |
| PD-L1+LAG3 | 0 | 1 | 1 |
| PD1+TIM3 | 1 | 1 | 2 |
| PD1+LAG3 | 1 | 4 | 5 |
| TIM3+LAG3 | 7 | 11 | 18 |
| PD-L1 | 0 | 0 | 0 |
| PD1 | 1 | 2 | 3 |
| TIM3 | 1 | 1 | 2 |
| LAG3 | 8 | 5 | 13 |
| All negative | 8 | 1 | 9 |
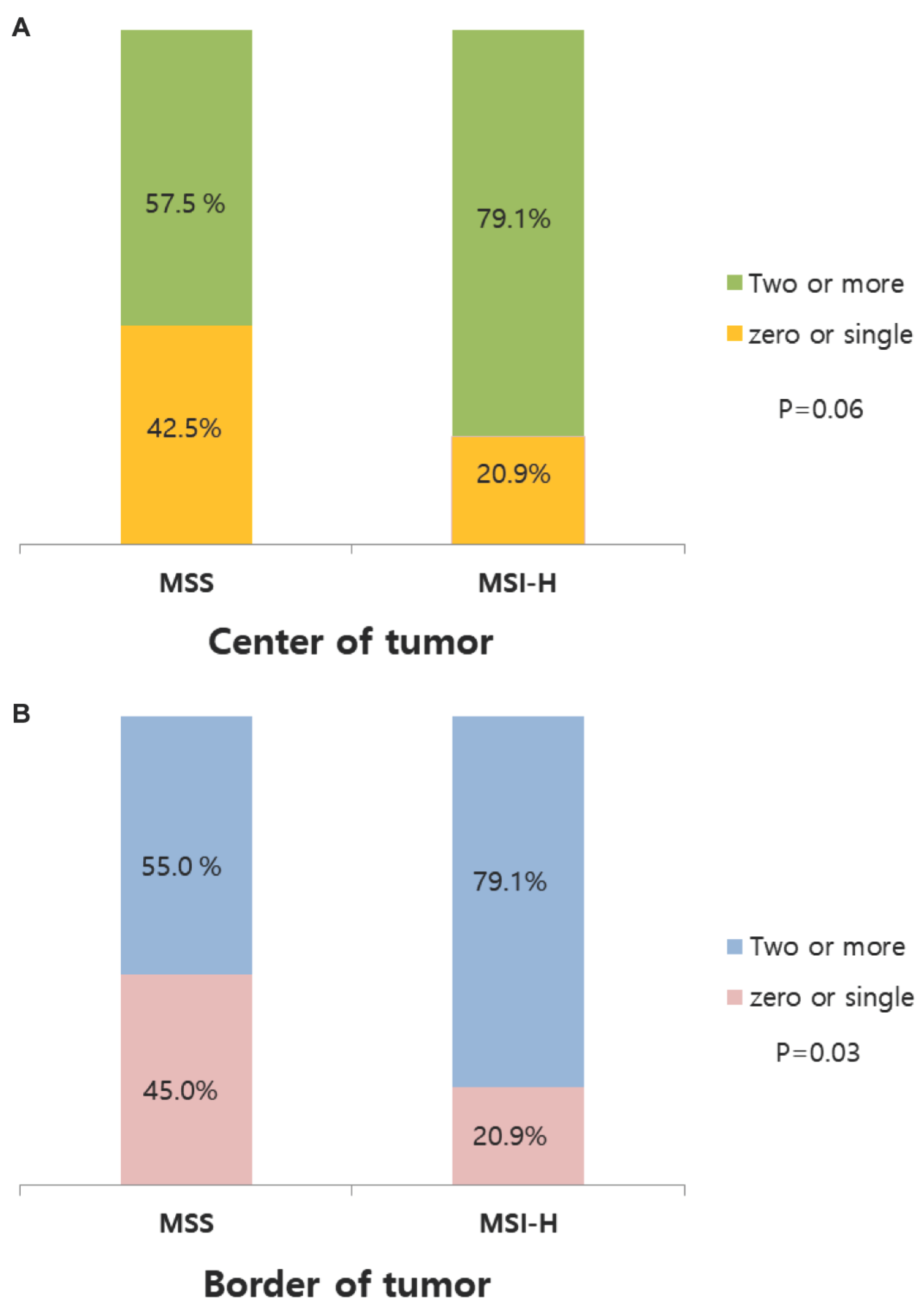
| zero or single | Two or more | P-value | |
|---|---|---|---|
| Center of tumor | |||
| MSS | 17 (42.5) | 23 (57.5) | |
| MSI-H | 9 (20.9) | 34 (79.1) | 0.06 |
| Border of tumor | |||
| MSS | 18 (45.0) | 22 (55.0) | |
| MSI-H | 9 (20.9) | 34 (79.1) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





