1. Introduction
Colorectal cancer (CRC), one of the most common cancers with a rising global incidence rate [
1,
2,
3,
4]. Recent advancements in immunotherapy, including immune checkpoint inhibitors, offer promising strategies to improve outcomes in CRC [
5,
6,
7,
8,
9,
10]. However, stage IV CRC is associated with a poor prognosis, with a 5-year overall survival of 16.9% [
11], 80% of which are deemed poorly resectable [
2]. The liver is the most common site of distant metastasis in stage IV CRC. Although surgical resection is the cornerstone of treatment for CRC liver metastases (CRCLM), liver metastases recur in 25–30% of patients during their disease course, even after surgery [
2,
5,
6]. This highlights the urgent need for novel therapeutic approaches to improve outcomes in this patient population. However, the role of neoadjuvant or adjuvant chemotherapy in preventing such recurrence remains controversial, with no established strategies to date.
Immune checkpoint inhibitors, CD19 chimeric antigen receptors-T (CAR-T) cells, and mRNA-based COVID-19 vaccines have demonstrated potential to mobilize robust and diverse T cell responses against cancer. Efforts have focused on developing effective cancer vaccines and CAR/T cell receptor-T cell (TCR-T) therapies. We detected the cancer-specific antigen glypican 3 (GPC3) and identified peptides that can induce CTLs in a human leukocyte antigen (HLA) A24- or A2-restricted manner. We then conducted clinical trials of GPC3 peptide vaccines for hepatocellular carcinoma, ovarian clear cell carcinoma, and pediatric cancer [
12,
13,
14,
15,
16,
17,
18,
19], where we demonstrated multiple cases of partial response in advanced cancer and recurrence prevention. Notably, seven children (male: 3, female: 4) with pediatric cancer who received the GPC3 vaccine have remained disease-free for approximately 10 years. Further, we identified T-cell receptors (TCR) from peptide-specific CTL clones derived from a pediatric hepatoblastoma survivor, which enabled the development of TCR-T cell therapy [manuscript submitted]. Moreover, we identified the cancer-specific antigen heat shock protein 105α (HSP105α), which is highly expressed in several cancers, such as CRC and esophageal cancer, and peptides capable of inducing CTLs in an HLA A24- or A2-restricted manner. We also conducted clinical trials for HSP105α peptide vaccines in patients with CRC and esophageal cancer [
20,
21,
22]. From these trials, we verified the safety and immunological effectiveness of TCR-T cell therapy by identifying TCRs from peptide-specific CTL clones derived from patients who received the vaccine.
Cancer heterogeneity presents a challenge because targeting a single antigen is insufficient for complete eradication. Thus, we identified 10 highly immunogenic cancer-specific antigens, including GPC3 and HSP105α, which are widely expressed in solid tumors. These antigens possess peptides that can trigger many CTL responses. We predicted 72 and 73 peptides that bind to HLA-A24 and -A2 in silico from the full-length amino acid sequences of these 10 common cancer antigens. We immunized each HLA transgenic mouse with a cocktail of synthesized peptides together with the poly I:CLC three times weekly to analyze the antigen-specific immune response. As a result, 68 peptide sequences (30 and 38, respectively) were identified that had higher CTL induction ability than GPC3 298-306 and GPC3 144-152, which were used in clinical trials. Furthermore, experiments with cocktail peptide vaccines using mouse models expressing subcutaneous tumors of each antigen showed promising results in terms of safety and efficacy [
23]. For antigens to be promising targets for cancer vaccines and TCR-T cell therapy, they must be expressed on cancer cells, with corresponding HLA class I expressed on the cell membrane. For CAR-T cell therapy, antigens must be expressed on the membranes of cancer cells.
Of the 10 detected antigens, AFP, GPC3, and TGFβI were not specifically expressed in CRC. In contrast, the other seven antigens were confirmed to be expressed in CRC. Immunohistochemical (IHC) analysis of primary CRC and liver metastases, including cancerous and normal areas, also verified the presence of HLA class I on the cancer cell membrane. In this study, we aimed to identify common cancer antigens that are promising targets for cancer vaccines and CAR/TCR-T cell therapies for primary CRC and liver metastases.
2. Results
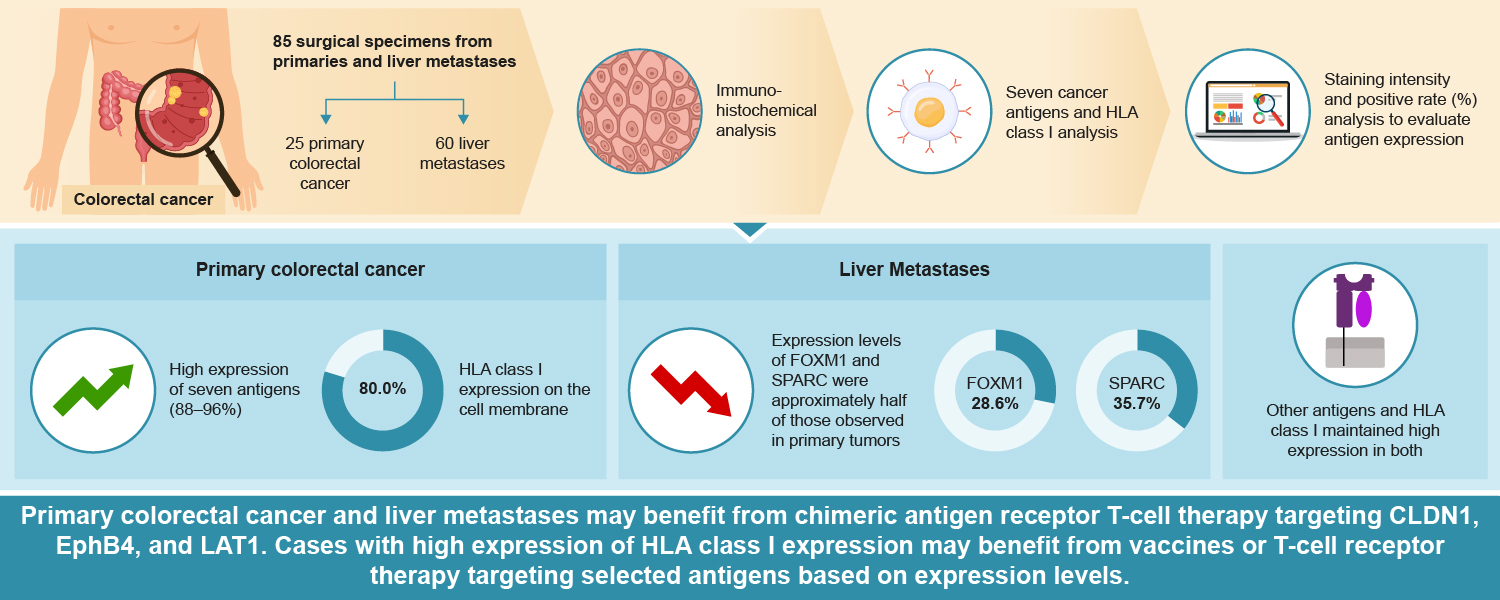
2.1. Expression of Seven Common Cancer Antigens and HLA Class I on the Cell Membrane in 25 Cases of Primary CRC
We included 25 patients with primary CRC in this study. The expression levels of seven common cancer antigens and HLA class I are summarized in
Table 1. For the localization phenotypes of HLA class I, 20 cases (80.0%) were of membrane phenotype, 2 cases (8.00%) were of cytoplasmic phenotype, and 3 cases (12.0%) were of heterogeneity phenotype combined with cytoplasmic and membrane phenotypes. There was no significant difference in the expression of all seven common cancer antigens and HLA class I between sexes (p>0.10).
2.2. Expression of Seven Common Cancer Antigens and HLA Class I on the Cell Membrane in 60 Cases of CRC Liver Metastases
We analyzed 60 cases of CRCLM for the expression of seven common cancer antigens and HLA class I, with the ratios shown in
Table 1. There was no significant difference in the expression of all seven common cancer antigens and HLA class I between sexes, consistent with the 25 cases of primary CRC (p>0.10). No significant differences in cancer-specific expression levels for each cancer antigen and HLA class I in primary CRC and liver metastases were found between naive and chemotherapy groups (p>0.40) (
Figure S1). The localization phenotype of HLA class I was 37 cases (61.7%) of membrane phenotype, 7 cases (11.7%) of cytoplasmic phenotype, 12 cases (28.3%) of heterogeneity phenotype combined with cytoplasmic and membrane phenotype, and 3 cases (5.00%) of loss of heterozygosity.
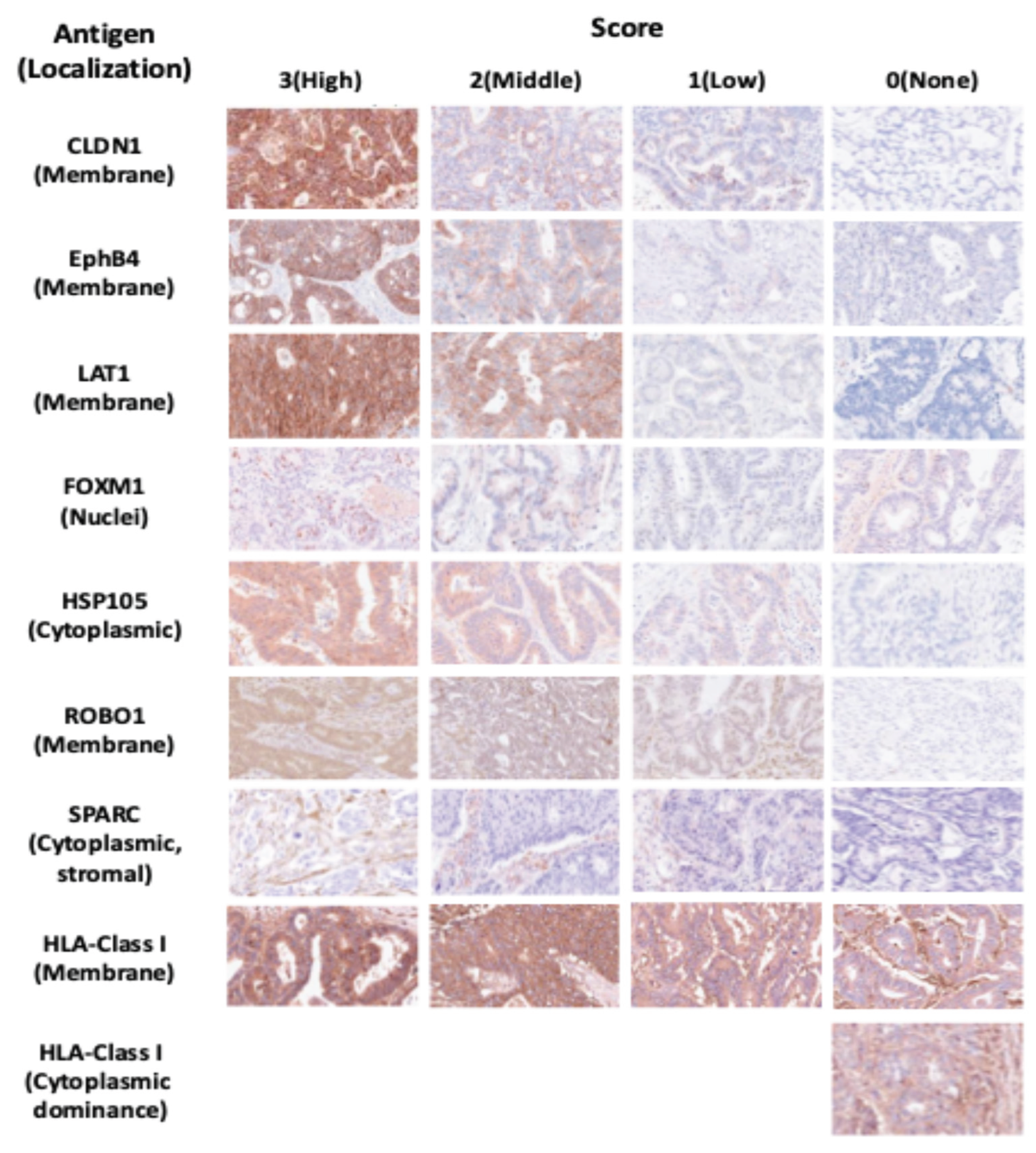
Figure 1.
Scoring of immunohistochemical staining intensity for common cancer antigens and phenotypes, and scoring of staining intensity for HLA class I.
Figure 1.
Scoring of immunohistochemical staining intensity for common cancer antigens and phenotypes, and scoring of staining intensity for HLA class I.
The staining intensity of cancer cells for each of the eight antigens—CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, SPARC, and HLA class I—was scored as follows: negative: 0, weakly positive: 1, weakly to strongly positive: 2, and strongly positive: 3. Intracellular localization of tumor tissue was evaluated using immunohistochemical analysis. For HLA class I expression, the presence and localization (cell membrane or cytoplasm) of staining were evaluated in cancer cells. HLA class I, CLDN1, EphB4, and LAT1 were mostly expressed on the cell membrane of cancer cells, FOXM1 was expressed on the cell nuclei, HSP105α and ROBO1 were expressed in the cytoplasm of cancer cells, and SPARC was expressed in the cytoplasm of cancer-associated fibroblasts (CAF). Loss of heterozygosity was defined as a lack of expression in the cytoplasm or cell membrane.
2.3. Expression of Seven Common Cancer Antigens and HLA Class I on the Cell Membrane in 14 Cases of Primary CRCLM
We analyzed differences in the expression patterns of seven common cancer antigens along with HLA class I among 14 cases of primary CRC with liver metastases (
Table 2). These 14 cases were divided into naïve (11 cases; 78.6%) and chemotherapy (3 cases; 21.4%) groups, while the liver metastases cases were divided into naïve (5 cases; 35.7%) and chemotherapy (9 cases; 64.3%) groups.
CLDN1, EphB4, LAT1, HSP105α, and ROBO1 expression slightly decreased or remained unchanged but remained consistently high across both cancer types. FOXM1 and SPARC exhibited high expression levels in primary CRC. However, their expression levels in liver metastases were lower than those in primary cancer (
Figure S2).
The high expression rate of HLA class I on cancer cell membranes was nearly equivalent in both primary CRC and liver metastases (64.2% and 78.5%, respectively). No significant difference was found in the HLA class expression score level between primary CRC and liver metastases (p=0.18). In the 14 cases of primary CRC with liver metastases, the localization phenotype of HLA class I for primaries in tumor cells was as follows: 9 cases (64.2%) exhibited membrane phenotype, 4 cases (28.5%) showed cytoplasmic phenotype and 1 case (7.14%) showed a heterogenous phenotype (both cytoplasmic and membrane). In contrast, liver metastases showed 13 cases (92.9%) of membrane phenotype and 1 case (7.14%) of cytoplasmic phenotype. Notably, 5 cases (33.3%) showed a phenotypic shift from heterogenous to homogenous membrane phenotype, 3 cases (21.4%) [(b)3 cases] transitioned from cytoplasmic to membrane phenotype, and 1 case (7.13%) [(a) 1 case] changed from heterogenous to homogenous cytoplasmic phenotype.
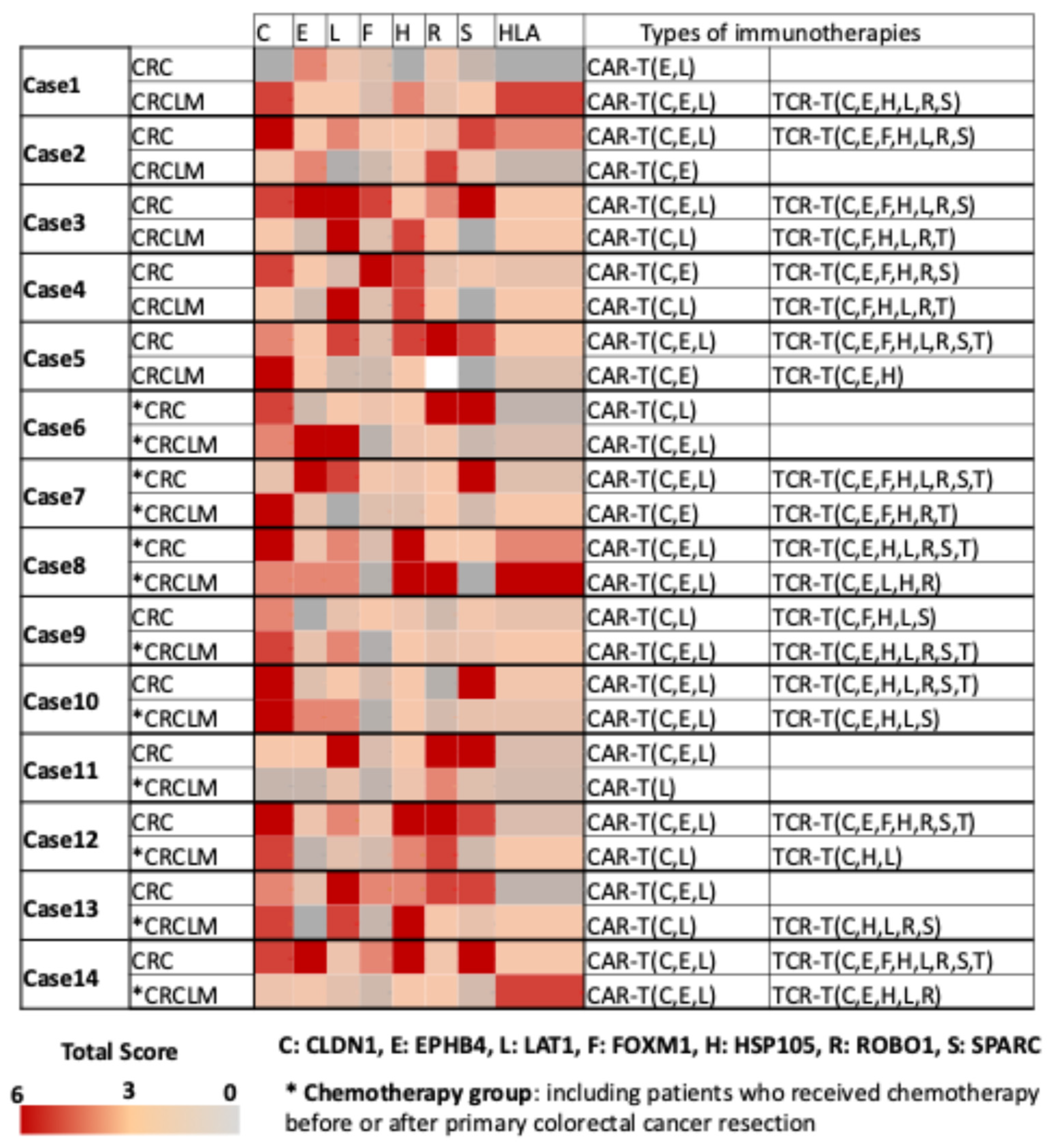
A heatmap was created to visualize the expression levels of seven CRC-specific cancer antigens and HLA class I across each of the 14 cases described in
Figure 2. The data indicated that all cases of primary CRC and CRCLM might be adaptable for CAR-T cell therapy targeting any of the three membrane-type proteins cancer antigens, namely CLDN1, EphB4, and LAT1. Based on the high expression rates of HLA class I on cancer cell membranes, we assumed that cancer vaccine and TCR-T cell therapy could be effective in more than 70% of CRC or CRCLM that are HLA class I positive. Additionally, FOXM1, HSP105α, ROBO1, and SPARC were identified as promising therapeutic targets in CRC, while FOXM1 and SPARC were excluded in liver metastases owing to their significantly reduced expression levels.
Figure 2.
Heatmap and expression score table of seven common cancer antigens and HLA class I in 14 primary colorectal cancer and liver metastases.
Figure 2.
Heatmap and expression score table of seven common cancer antigens and HLA class I in 14 primary colorectal cancer and liver metastases.
The median total score values of staining intensity and positive staining for seven common cancer antigens—CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, and SPARC—and HLA class I on tumor cells from 14 cases each of primary colorectal cancer and liver metastases. Expression levels of each antigen were evaluated, alongside potential T-cell therapies (CAR/TCR-T cell therapy) applicable to each case. A high expression was defined as a score of >3. Cases with high HLA class I expression were suitable for TCR-T, while those with low HLA class I expression may benefit from CAR-T targeting highly expressed CLDN1, EphB4, and LAT1.
The cases were divided into naïve and chemotherapy groups. *: Chemotherapy group, including patients who received chemotherapy before or after primary colorectal cancer resection.
C: CLDN1, E: EphB4, L: LAT1, F: FOXM1, H: HSP105α, R: ROBO1, S: SPARC, HLA: HLA class I. CAR-T: chimeric antigen receptor-T, TCR-T: T Cell receptor-T
2.4. Development of a Multiplex Fluorescence Immunohistochemical Staining System for Common Cancer Antigens and HLA Class I Detection
An MFIH staining system was developed to efficiently determine the expression of five common cancer antigens and HLA class I using fewer tissue sections than necessary for the usual IHC staining. This approach facilitated a comprehensive assessment for personalized immunotherapy.
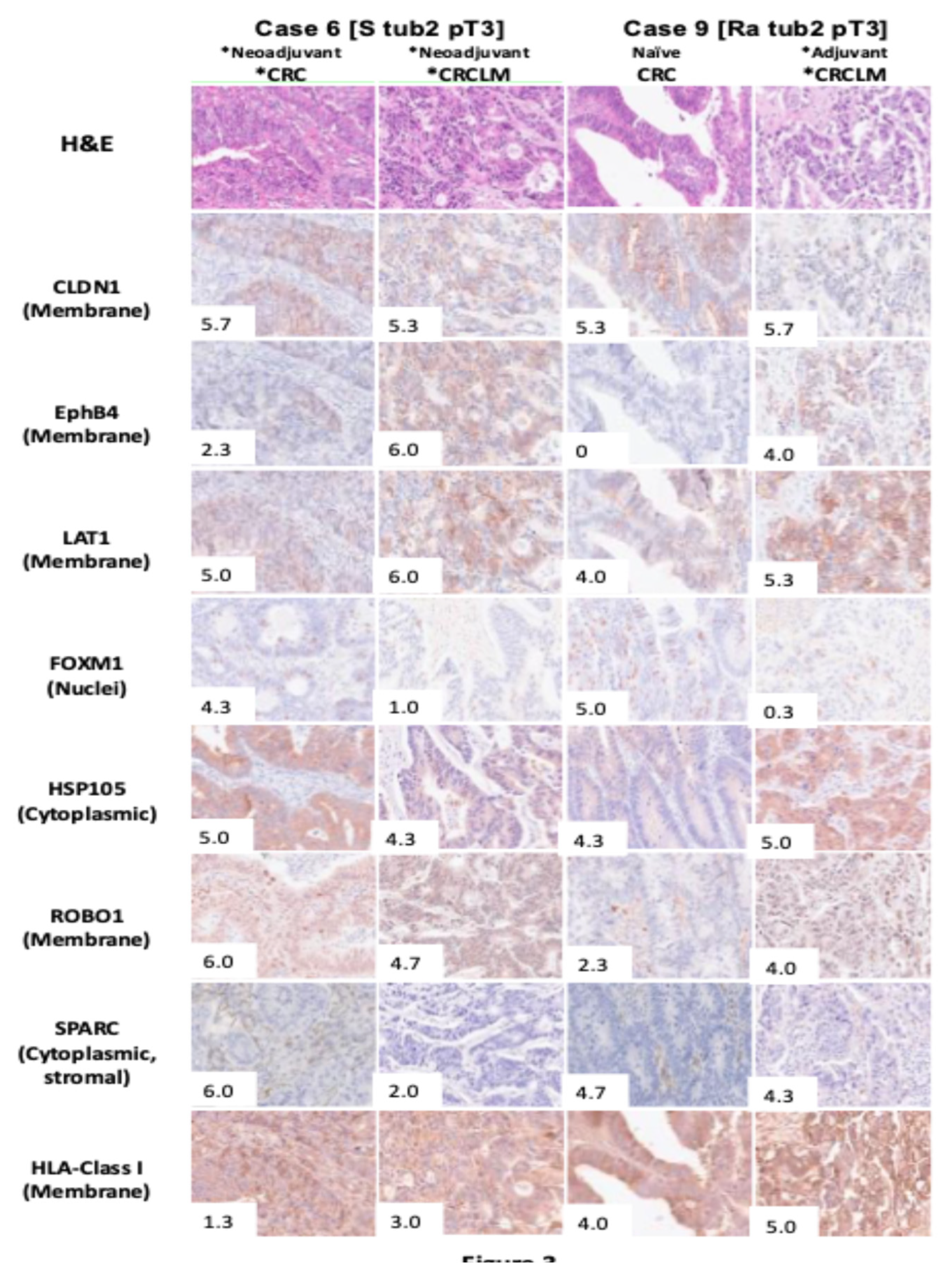
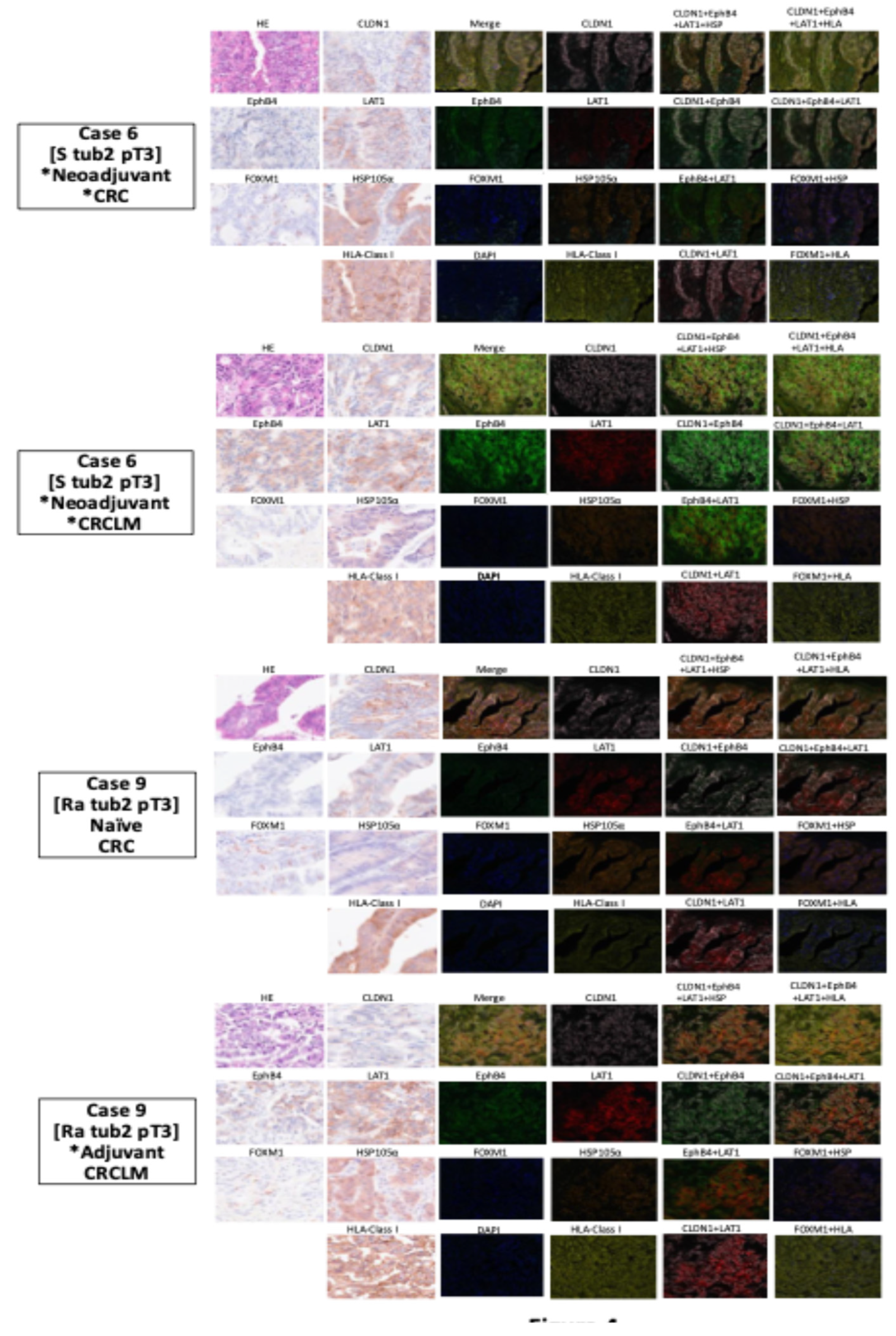
Figure 3 shows the IHC findings of seven common cancer antigens and HLA class I for two representative cases from 14 patients with primary CRC and liver metastases. In one representative case (Case 6), HLA class I exhibited low expression levels in both primary CRC and liver metastases. Conversely, high expression levels of CLDN1 and LAT1 were observed in both primary CRC and liver metastases. MFIH analysis (
Figure 4) visually confirmed the low HLA class I expression and high CLDN1 and LAT1 expression in the primary tumor. Additionally, EphB4 was prominently expressed in liver metastases alongside CLDN1 and LAT1. In another representative case (Case 9), primary CRC showed high expression levels of CLDN1, LAT1, FOXM1, HSP105α, and SPARC, while FOXM1 expression was markedly reduced in liver metastases. MFIH analysis of Case 9 also validated these findings, clearly demonstrating the decreased FOXM1 expression in liver metastases compared to that in primary CRC.
Two representative cases from 14 patients with primary colorectal cancer and liver metastases are presented. Total expression scores for seven cancer antigens and HLA class I in these two representative cases are displayed in the lower-left corner of each image. Case 6, the patient who received chemotherapy prior to primary and liver metastases resection, exhibited high expression of FOXM1, HSP105α, ROBO1, and SPARC in the primary tumor, whereas HLA class I expression was low. CLDN1 and LAT1 were highly expressed, suggesting potential suitability for CAR-T therapy targeting CLDN1 and LAT1. For the liver metastases of Case 6, most antigens showed high expression, except FOXM1 and SPARC, while HLA class I expression remained low.
Case 9 represents the patient who underwent primary tumor resection without prior chemotherapy but received chemotherapy before liver metastasis resection. In this case, the primary tumor showed high expression of HLA class I, CLDN1, LAT1, FOXM1, HSP105α, and SPARC. Hence, this case was deemed suitable for TCR-T therapy targeting CLDN1, LAT1, FOXM1, HSP105α, and SPARC and for CAR-T cell therapy targeting CLDN1 and LAT1. The liver metastases of Case 9 displayed consistent HLA class I expression, but FOXM1 expression was notably decreased compared with the primary tumor, suggesting that TCR-T cell therapy targeting antigens excluding FOXM1, along with CAR-T cell therapy targeting CLDN1, EphB4, and LAT1, may be applicable.
MFIH staining using specific antibodies: opal 520 nm for anti-EphB4, opal 540 nm for anti-FOXM1, opal 570 nm for anti-LAT1, opal 620 nm for anti-HSP105α, opal 650 nm for anti-HLA class I, and opal 690 nm for anti-CLDN1.
MFIH results for Case 6 showed low expression of HLA class I in both primary colorectal cancer and liver metastases. CLDN1 and LAT1 were visually confirmed to be highly expressed in primary tumors, with EphB4 also showing high expression in the liver metastases along with CLDN1 and LAT1.
MFIH analysis of Case 9 visually confirmed uniformly high expression of the five cancer antigens and HLA class I in the primary tumor. However, FOXM1 expression was reduced in liver metastases.
3. Discussion
Cancer vaccines, such as peptide and mRNA vaccines, elicit anti-cancer effects by inducing CTLs against tumor cells expressing specific cancer antigens. Our experiments with cocktail peptide vaccines using HLA transgenic mice models expressing subcutaneous tumors of each antigen showed promising results in terms of safety and efficacy. The peptides identified in this study, derived from 10 common cancer antigens covering all solid cancers, are expected to be clinically applicable as cocktail peptide vaccines [
23]. T-cell therapies, including CAR-T and TCR-T cell therapies, have also gained prominence [
24,
25,
26]. The critical role of HLA class I in these therapies lies in their function as mediators of CTL recognition and activation, making them essential for cancer vaccines and TCR-T cell therapy. Conversely, cancer cells frequently evade immune surveillance through partial or total loss of HLA class I expression, thus reducing their susceptibility to CTL-mediated clearance [
27,
28,
29].
The previously reported frequencies of HLA class I between primary CRC and liver metastases (72–74% [
30,
31,
32] and 64%–66.4% [
30,
33]) align closely with those of our study (61.7% and 78.6%). Meanwhile, the observed difference in HLA class I phenotypes between primary CRC and liver metastases in the same patients may result from clonal heterogeneity within the primary tumor. Some metastatic clones may retain the HLA class I phenotype of their primary tumor of origin, while others may acquire new mutations leading to altered phenotypes [
34]. Another possibility is the metastatic spread of HLA class I-positive tumor cells from heterogeneous primary tumors, as observed in liver metastasis derived from mismatch repair-deficient/high-frequency microsatellite instability CRC [
35]. High HLA class I expression in such cases has been associated with poor prognosis [
36]. Furthermore, HLA class I-expressing tumor cells remain, even after HLA class I-negative tumor nests are eradicated by NK cells. These surviving cells can seed distant organs, especially the liver, and form metastases [
34].
Regarding cancer antigens, our findings highlight the expression of FOXM1 and SPARC as being significantly lower in liver metastases than in primary CRC. Both FOXM1 and SPARC, almost undetectable in normal liver tissues [
37,
38], facilitate cancer cell migration and invasion in CRC [
39,
40]. The role of FOXM1 is limited to driving the migration and invasion of cancer cells to distant organs, and its expression may not be significantly expressed or reflected in liver metastases. Similarly, SPARC, although contributing to invasive and metastatic properties of cancer cells [
37], may also exhibit reduced expression in liver metastases due to the minimal stromal content in liver tissue. In addition, the decreased expression of FOXM1 and SPARC in liver metastases compared to primary tumors may mainly reflect the phenotypic changes associated with metastatic progression rather than other cancer antigens. These characteristics likely explain the significant reduction in FOXM1 and SPARC expression and reflection in liver tissue compared to the primaries. Other cancer antigens, while exhibiting downregulated expression in liver tissue compared to primary tumors, may not exhibit as marked a decline as FOXM1 and SPARC, underscoring their distinct biological roles in tumor progression and metastasis.
Our study revealed no significant differences in HLA class I expression between primary CRC and liver metastases. Approximately 70% of patients demonstrated HLA class I expression localized to cancer cell membranes in both primary and liver metastases. CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, and SPARC were identified as potential targets for common cancer antigens for primary CRC. However, FOXM1 and SPARC may have limited applicability as therapeutic targets for liver metastases. Membrane proteins, such as CLDN1, EphB4, and LAT1, were highly expressed in primary CRC and liver metastases, suggesting that CAR-T cell therapy targeting these three cancer antigens could be a viable option, particularly in 30% of cases with reduced HLA class I expression on cancer cell membranes.
Our study has some limitations. Analyses were performed at a single center, which may have limited the generalizability of the findings because of fewer samples. As our immunohistochemical analysis represents a snapshot of antigen expression in tissue sections, it may not fully reflect the heterogeneity of cancer antigen and HLA class I in the entire tumor. The MFIH system requires further modification, including improvements to scoring methods. Of the 60 CRCLM cases analyzed, no significant differences were observed in the expression of cancer antigens or HLA class I between 12 naïve and 48 chemotherapy cases. However, typically, the specimens of liver metastases were not uniformly distributed owing to various background factors, such as liver recurrence after resection of primary CRC or liver metastases of CRC following preoperative treatment. Therefore, this heterogeneity in the background of liver metastasis cases, although not significantly affecting our results, should be considered when interpreting these findings. Although this study compared 25 cases of primary CRC with 60 cases of liver metastases, the lack of identical case-pair comparisons remains a limitation. Despite this, we observed that the median expression levels of all seven cancer antigens and HLA class I were lower in liver metastases than those in primary CRC. Further analysis of 14 identical cases of primary CRC and liver metastases (
Figure S2) showed that while the median expression levels of FOXM1 and SPARC were significantly decreased in liver metastases, the median expression levels of CLDN1, EphB4, LAT1, HSP105α, ROBO1, and HLA class I were only slightly reduced and not statistically significant. Importantly, the median expression levels of CLDN1, EphB4, LAT1, HSP105α, ROBO1, and HLA class I remained above 3.5, and their frequencies exceeded 50% in liver metastases. These findings suggest that CLDN1, EphB4, LAT1, HSP105α, and ROBO1 may serve as promising targets for immunotherapy in liver metastases.
The identification of common cancer antigens adaptable for vaccine development and CAR/TCR-T cell therapy holds great value for advancing immunotherapeutic strategies. These therapeutic strategies hold promise for treating primary CRC and liver metastases. Based on our findings, further in vivo and in vitro studies are planned to validate the potential of these identified antigens as targets for immunotherapy in CRC liver metastases (e.g., EphB4 CAR-T trial for EphB4-positive CRCLM).
4. Materials and Methods
4.1. Clinical Samples
We included surgical cases based on two criteria: (1) recent cases with well-preserved surgical specimen blocks, and (2) cases where both tumor and normal tissues were preserved within the same block to allow comparative analyses. We obtained 85 surgical specimens from 25 patients (male: 15, female: 10) with primary CRC (including 14 primary CRC with concurrent liver metastases) and 60 patients (male: 42, female: 18) with CRCLM who underwent surgery at our hospital between December 2016 and July 2021. There was no difference in surgical outcomes and prognoses between the sexes. The CRCLM cases included in our study were categorized into two groups. (a) Naïve: patients who had not received chemotherapy and underwent simultaneous resection of the primary tumor or liver metastases alone, and (b) Chemotherapy: patients who received chemotherapy either before or after primary CRC resection.
4.2. Immunohistochemical Analysis of Common Cancer Antigens and HLA Class I
For IHC analysis, we used specific antibodies against the following seven common cancer antigens and HLA class I: Claudin 1 [CLDN1 (#13255; Cell Signaling Technology®)], Ephrin type-B receptor 4 [EphB4 (#14960; Cell Signaling Technology®)], L-type amino acid transporter 1 [LAT1 (ab208776; Abcam®)], Forkhead box M1 [FOXM1 (ab207298; Abcam®)], heat shock protein 105α [HSP105α (MA5-32408; Novus Bio ®)], roundabout homolog-1 [ROBO1 (25181-1-AP; Proteintech®)], secreted protein acidic and rich in cysteine [SPARC (sc-73472; Santa Cruz Biotechnology®)], and HLA class I [HLA-ABC (IR500; HOKUDO®)]. Formalin-fixed paraffin-embedded tissues (4 μm) were deparaffinized with xylene and rehydrated with ethanol. Endogenous peroxidase activity was blocked using 0.3% H2O2/MeOH. Antigen activation was performed via microwave heat treatment in TRS9 buffer (pH 9.0, Dako/Agilent, Santa Clara, CA, USA) and AR6 buffer (pH 6.0, PerkinElmer) at 95 °C or 121 °C for 20 min. Primary antibodies were incubated at room temperature (20–25°C) for 1 h or overnight at 4 °C. Mouse/rabbit Envision Polymer (Dako/Agilent) was used as the secondary antibody and incubated at room temperature for 30 min, followed by 3,3′-diaminobentidine staining and hematoxylin counterstaining. Slides were dehydrated with xylene and sealed with glass coverslips.
Staining intensity of the tumor tissue was scored for each of the seven antigens using a semi-quantitative method. The percentages of positively stained cells were scored as follows: 0 (0–10%), 1 (10–39%), 2 (40–69%), and 3 (70–100%). These scores were subsequently combined to grade overall expression into three levels: low, middle, and high, with a total score of 0–2, 3–4, and 5–6, respectively. The individual scores were averaged across three random visual fields (40x field of view). Intracellular localization of tumor tissue (membrane, cytoplasm, or nucleus) was also evaluated using IHC analysis [
41]. Virtual slides were created using Nano Zoomer (Hamamatsu Photonics), and three researchers independently analyzed staining using these virtual slides at a 40x field of view. A total score of 3.1–6.0 based on IHC analyses was defined as high expression.
Figure 1 shows the immunostaining patterns and scoring positive staining for each common cancer antigen and HLA class I. SPARC was additionally expressed in the cytoplasm of cancer-associated fibroblasts (CAFs) in addition to cancer cell cytoplasm, suggesting potential therapeutic relevance for targeting SPARC in both cancer cells and CAFs [
37,
39,
42,
43,
44]. Although ROBO1 is a transmembrane protein receptor, its expression is predominantly cytoplasmic in primary CRC and liver metastases [
45,
46]. Therefore, we assessed ROBO1 expression in the cytoplasm. In normal tissues, CLDN1 was expressed in pancreatic acinar cells and skin, LAT1 in the basal layer of pharyngeal and esophageal squamous epithelium, EphB4 in vascular endothelial cells, and HSP105α in hepatocytes.
4.3. Multiplex Fluorescence Immunohistochemical Staining and Analysis of Cancer Antigens and HLA Class I
Multiplex fluorescent immunohistochemical staining (MFIH) was performed on 4-μm-thick tissue sections of primary CRC and liver metastases using the PerkinElmer Opal kit. MFIH images were acquired using the Vectra 3 tissue section quantitative analysis imaging system. Up to 20 regions of interest (ROIs; 699×500 µm) were randomly selected and evaluated in each tumor center and margins using an image analysis program (Inform 2.6.; PerkinElmer, Inc.). The distribution of specific cancer antigens was also analyzed. ROBO1 and SPARC were excluded owing to their complex intracellular localization—ROBO1 being expressed in the nucleus, membrane, and cytoplasm of cancer cells, and SPARC in both the cytoplasm of cancer cells and CAFs. Based on these characteristics, the MFIH panel was finalized with CLDN1, EphB4, LAT1, FOXM1, HSP105α, and HLA class I.
4.4. Statistical Analysis
Patient characteristics were summarized using descriptive statistics, such as medians (range) and proportions. Univariate analyses were conducted to assess differences between primary CRC and liver metastases using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Statistical significance was set at p < 0.05. We also created a heatmap to depict the expression levels of each cancer antigen and HLA class I in the 14 cases of primary CRC with liver metastases. All statistical analyses were performed using EZR software for R (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [
47].
5. Conclusions
Our study highlights that most cases of primary CRC could potentially benefit from CAR-T cell therapy targeting CLDN1, EphB4, and LAT1. Furthermore, CRC with high levels of HLA class I expression on the cell membrane may be suitable for vaccine-based and TCR-T cell therapies targeting CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, and SPARC. For liver metastases, while CAR-T cell therapy targeting CLDN1, EphB4, and LAT1 remains a viable option, vaccines and TCR-T cell therapy targeting CLDN1, EphB4, LAT1, HSP105α, and ROBO1—excluding FOXM1 and SPARC—may be effective in most cases. These findings underscore the potential for personalized immunotherapeutic approaches tailored to the antigenic profiles of primary and metastatic CRCs.
6. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1. Box plot depicting the expression scores and levels of seven common cancer antigens and HLA class I for naïve and chemotherapy groups in 60 cases of colorectal cancer liver metastases; Figure S2. Box plot depicting the expression scores of seven common cancer antigens and HLA class I for 14 cases of primary colorectal cancer and liver metastases.
Author Contributions
Conceptualization, J.K., K.T., and T.N.; methodology, J.K., K.T., and T.N.; software, J.K. and K.T.; validation, K.T., T.S., K.O., and T.N.; formal analysis, J.K., K.T., T.S., K.O., and T.N.; investigation, J.K., K.T., T.S., K.O., and T.N.; resources, J.K., Y.T., N.G., M.I., and T.N.; data curation, J.K. and K.T.; writing—original draft preparation, J.K.; writing—review and editing, K.T., T.S., K.O., N.G., and T.N.; visualization, J.K., K.T., T.S., K.O., Y.T., M.I., and T.N.; supervision, T.S., K.O., N.G., M.I., and T.N.; project administration, T.N.; funding acquisition, T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This Study was partly supported by the National Cancer Center Research and Development Fund (2022-A-9), JST START Project Promotion Type (Commercialization Support), Grant Number JPMJST2211, and AMED under Grant Number JP24bm1123042.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and the use of primary colorectal cancer and liver metastasis specimens was approved by the institutional review board of the National Cancer Center Hospital of East (protocol no. 2020-352).
Informed Consent Statement
The patients provided written informed consent for the publication of this study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We would like to thank Editage (
www.editage.jp) for English language editing.
Conflicts of Interest
Tetsuya Nakatsura (TN) received a research grant from BrightPath Biotherapeutics Co., Ltd., Thyas Co., Ltd., ONOPHARMACEUTICAL CO., Ltd., Resonac Corporation, MEDINET Co., Ltd., NapaJen Pharma Inc, Heartseed Inc, Takara Biio Inc, DAICEL CORPORATION, NA Vaccine Institute CO., Ltd., Logomix Inc, Optieum Biotechnologies Inc, and MaxCyte, Inc. TN hold Stock Ownership, Stock Option or Profits from Noile-Immune Biotech Inc., Logomix Inc, and Optieum Biotechnologies Inc, and, TN have royalties from OncoTherapyScience, Inc. Other authors have no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| CAF |
Cancer-associated fibroblast |
| CAR-T |
Chimeric antigen receptors-T |
| CLDN1 |
Claudin 1 |
| CRCLM |
Colorectal cancer liver metastases |
| EphB4 |
Ephrin type-B receptor 4 |
| FOXM1 |
Forkhead box M1 |
| HLA |
Human leukocyte antigen |
| HSP105α |
Heat shock protein 105α |
| IHC |
Immunohistochemical staining |
| LAT1 |
L-type amino acid transporter 1 |
| MFIH |
Multiplex fluorescence immunohistochemistry |
| MMR |
Mismatch repair |
| NCCN |
National Comprehensive Cancer Network |
| ROBO1 |
Roundabout homolog-1 |
| ROI |
Region of interest |
| SPARC |
Secreted protein acidic and rich in cysteine |
| TCR-T |
T cell receptor-T |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [CrossRef]
- Japanese Society for Cancer of the Colon and Rectum: JSCCR Guidelines 2024 for the Treatment of Colorectal Cancer.
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; Teh, C.; Tejpar, S.; Van Cutsem, E.; Vauthey, J.N.; Påhlman, L.; of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015, 41, 729–741. [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; Grem, J.L.; Haste, P.; Hecht, J.R.; Hoffe, S.; Hunt, S.; Hussan, H.; Johung, K.L.; Joseph, N.; Kirilcuk, N.; Krishnamurthi, S.; Malla, M.; Maratt, J.K.; Messersmith, W.A.; Meyerhardt, J.; Miller, E.D.; Mulcahy, M.F.; Nurkin, S.; Overman, M.J.; Parikh, A.; Patel, H.; Pedersen, K.; Saltz, L.; Schneider, C.; Shibata, D.; Shogan, B.; Skibber, J.M.; Sofocleous, C.T.; Tavakkoli, A.; Willett, C.G.; Wu, C.; Gurski, L.A.; Snedeker, J.; Jones, F. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024, 22, e240029. [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Colon Cancer Version 5.2024 [accessed August 22, 2024].
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Rectal Cancer Version 4.2024 [accessed August 22, 2024].
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; Maas, M.; Mertz, M.; Veninga, V.; Bounova, G.; Broeks, A.; Beets-Tan, R.G.; de Wijkerslooth, T.R.; van Lent, A.U.; Marsman, H.A.; Nuijten, E.; Kok, N.F.; Kuiper, M.; Verbeek, W.H.; Kok, M.; Van Leerdam, M.E.; Schumacher, T.N.; Voest, E.E.; Haanen, J.B. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020, 26, 566–576. [CrossRef]
- Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, Dokter S, Büller NV, Grotenhuis BA, Kuhlmann K, Burger JW, Huibregtse IL, Aukema TS, Hendriks ER, Oosterling SJ, Snaebjornsson P, Voest EE, Wessels LF, Beets-Tan RG, Van Leerdam ME, Schumacher TN, van den Berg JG, Beets GL, Haanen JB. LBA7 neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann Oncol 2022, 33, S1389. [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; Hu, J.; Huang, Y.; Huang, L.; Wang, H.; Shi, L.; Cai, Y.; Shen, C.; Ling, J.; Xie, X.; Cai, Y.; He, X.; Dou, R.; Zhou, J.; Ma, T.; Zhang, X.; Luo, S.; Deng, W.; Ling, L.; Liu, H.; Deng, Y. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2022, 7, 38–48. [CrossRef]
- Li, Y.; Liang, F.; Li, Z.; Zhang, X.; Wu, A. Neoadjuvant immunotherapy for patients with microsatellite instability-high or POLE-mutated locally advanced colorectal cancer with bulky tumors: New optimization strategy. Clin Colorectal Cancer 2025 24, 18–31.e2. [CrossRef]
- Cook, A.D.; Single, R.; McCahill, L.E. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: An analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 2005 12, 637–645. [CrossRef]
- Nakatsura, T.; Yoshitake, Y.; Senju, S.; Monji, M.; Komori, H.; Motomura, Y.; Hosaka, S.; Beppu, T.; Ishiko, T.; Kamohara, H.; Ashihara, H.; Katagiri, T.; Furukawa, Y.; Fujiyama, S.; Ogawa, M.; Nakamura, Y.; Nishimura, Y. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003 306, 16–25. [CrossRef]
- Tsuchiya, N.; Yoshikawa, T.; Fujinami, N.; Saito, K.; Mizuno, S.; Sawada, Y.; Endo, I.; Nakatsura, T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology 2017, 6, e1346764. [CrossRef]
- Sayem, M.A.; Tomita, Y.; Yuno, A.; Hirayama, M.; Irie, A.; Tsukamoto, H.; Senju, S.; Yuba, E.; Yoshikawa, T.; Kono, K.; Nakatsura, T.; Nishimura, Y. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology 2016, 5, e1062209. [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; Konishi, M.; Kinoshita, T.; Ikeda, M.; Nakachi, K.; Yamazaki, N.; Mizuno, S.; Takayama, T.; Yamao, K.; Uesaka, K.; Furuse, J.; Endo, I.; Nakatsura, T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016, 5, e1129483. [CrossRef]
- Shimizu, Y.; Mizuno, S.; Fujinami, N.; Suzuki, T.; Saito, K.; Konishi, M.; Takahashi, S.; Gotohda, N.; Tada, T.; Toyoda, H.; Kumada, T.; Miura, M.; Suto, K.; Yamaji, T.; Matsuda, T.; Endo, I.; Nakatsura, T. Plasma and tumoral glypican-3 levels are correlated in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer Sci 2020, 111, 334–342. [CrossRef]
- Taniguchi, M.; Mizuno, S.; Yoshikawa, T.; Fujinami, N.; Sugimoto, M.; Kobayashi, S.; Takahashi, S.; Konishi, M.; Gotohda, N.; Nakatsura, T. Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis. Cancer Sci 2020, 111, 2747–2759. [CrossRef]
- Ukai, M.; Yokoi, A.; Yoshida, K.; Suzuki, S.; Shibata, K.; Kikkawa, F.; Nakatsura, T.; Kajiyama, H. Extracellular miRNAs as predictive biomarkers for glypican-3-derived peptide vaccine therapy response in ovarian clear cell carcinoma. Cancers 2021 13, 550. [CrossRef]
- Suzuki, S.; Sakata, J.; Utsumi, F.; Sekiya, R.; Kajiyama, H.; Shibata, K.; Kikkawa, F.; Nakatsura, T. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology 2016, 5, e1238542. [CrossRef]
- Shimizu, Y.; Yoshikawa, T.; Kojima, T.; Shoda, K.; Nosaka, K.; Mizuno, S.; Wada, S.; Fujimoto, Y.; Sasada, T.; Kohashi, K.; Bando, H.; Endo, I.; Nakatsura, T. Heat shock protein 105 peptide vaccine could induce antitumor immune reactions in a phase I clinical trial. Cancer Sci 2019, 110, 3049–3060. [CrossRef]
- Sawada, Y.; Komori, H.; Tsunoda, Y.; Shimomura, M.; Takahashi, M.; Baba, H.; Ito, M.; Saito, N.; Kuwano, H.; Endo, I.; Nishimura, Y.; Nakatsura, T. Identification of HLA-A2 or HLA-A24-restricted CTL epitopes for potential HSP105-targeted immunotherapy in colorectal cancer. Oncol Rep 2014, 31, 1051–1058. [CrossRef]
- Miyazaki, M.; Nakatsura, T.; Yokomine, K.; Senju, S.; Monji, M.; Hosaka, S.; Komori, H.; Yoshitake, Y.; Motomura, Y.; Minohara, M.; Kubo, T.; Ishihara, K.; Hatayama, T.; Ogawa, M.; Nishimura, Y. DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4+ T cells and CD8+ T cells. Cancer Sci 2005, 96, 695–705. [CrossRef]
- Kinoshita, H.; Takenouchi, K.; Tsukamoto, N.; Ohnuki, K.; Suzuki, T.; Nakatsura, T. Identification of 68 HLA-A24 and -A2-restricted cytotoxic T lymphocyte-inducing peptides derived from 10 common cancer-specific antigens frequently expressed in various solid cancers. Neoplasia 2025, 61, 101135. [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; Wasik, M.; Levine, B.L.; Lacey, S.F.; Melenhorst, J.J.; Porter, D.L.; June, C.H. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017, 377, 2545–2554. [CrossRef]
- Ghosh, A.; Smith, M.; James, S.E.; Davila, M.L.; Velardi, E.; Argyropoulos, K.V.; Gunset, G.; Perna, F.; Kreines, F.M.; Levy, E.R.; Lieberman, S.; Jay, H.V.; Tuckett, A.Z.; Zakrzewski, J.L.; Tan, L.; Young, L.F.; Takvorian, K.; Dudakov, J.A.; Jenq, R.R.; Hanash, A.M.; Motta, A.C.; Murphy, G.F.; Liu, C.; Schietinger, A.; Sadelain, M.; van den Brink, M.R. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med 2017, 23, 242–249. [CrossRef]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; Park, S.I.; Gee, A.P.; Eldridge, P.W.; McKay, K.L.; Mehta, B.; Cheng, C.J.; Buchanan, F.B.; Grilley, B.J.; Morrison, K.; Brenner, M.K.; Serody, J.S.; Dotti, G.; Heslop, H.E.; Savoldo, B. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 2020, 38, 3794–3804. [CrossRef]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Pérez-Villar, J.J.; López-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 19971, 8, 89–95. [CrossRef]
- Garrido, F.; Algarra, I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res 2001, 83, 117–158. [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol 2021, 12, 636568. [CrossRef]
- Cabrera, T.; Collado, A.; Fernandez, M.A.; Ferron, A.; Sancho, J.; Ruiz-Cabello, F.; Garrido, F. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens 1998, 52, 114–123. [CrossRef]
- Swets, M.; König, M.H.; Zaalberg, A.; Dekker-Ensink, N.G.; Gelderblom, H.; van de Velde, C.J.; van den Elsen, P.J.; Kuppen, P.J. HLA-G and classical HLA class I expression in primary colorectal cancer and associated liver metastases. Hum Immunol 2016, 77, 773–779. [CrossRef]
- Watson, N.F.; Ramage, J.M.; Madjd, Z.; Spendlove, I.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer 2006, 118, 6–10. [CrossRef]
- Turcotte, S.; Katz, S.C.; Shia, J.; Jarnagin, W.R.; Kingham, T.P.; Allen, P.J.; Fong, Y.; D’Angelica, M.I.; DeMatteo, R.P. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol Res 2014, 2, 530–537. [CrossRef]
- Aptsiauri, N.; Garrido, F. The challenges of HLA class I loss in cancer immunotherapy: facts and hopes. Clin Cancer Res 2022, 28, 5021–5029. [CrossRef]
- Kloor, M.; Michel, S.; von Knebel Doeberitz, M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer 2010, 127, 1001–1010. [CrossRef]
- Ericsson, C.; Seregard, S.; Bartolazzi, A.; Levitskaya, E.; Ferrone, S.; Kiessling, R.; Larsson, O. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Invest Ophtalmol Vis Sci 2001, 42, 2153–2156.
- Inoue, M.; Senju, S.; Hirata, S.; Ikuta, Y.; Hayashida, Y.; Irie, A.; Harao, M.; Imai, K.; Tomita, Y.; Tsunoda, T.; Furukawa, Y.; Ito, T.; Nakamura, Y.; Baba, H.; Nishimura, Y. Identification of SPARC as a candidate target antigen for immunotherapy of various cancers. Int J Cancer 2010, 127, 1393–1403. [CrossRef]
- Wei, T.; Zeng, C.; Li, Q.; Xiao, Z.; Zhang, L.; Zhang, Q.; Ren, L. FOXM1/DEPDC1 feedback loop promotes hepatocarcinogenesis and represents promising targets for cancer therapy. Cancer Sci 2024, 115, 3041–3053. [CrossRef]
- Zhu, J.; Chen, X.; Liao, Z.; He, C.; Hu, X. TGFBI protein high expression predicts poor prognosis in colorectal cancer patients. Int J Clin Exp Pathol 2015, 8, 702–710.
- Zheng, Y.; Guo, J.; Zhou, J.; Lu, J.; Chen, Q.; Zhang, C.; Qing, C.; Koeffler, H.P.; Tong, Y. FoxM1 transactivates PTTG1 and promotes colorectal cancer cell migration and invasion. BMC Med Genomics 2015, 8, 49. [CrossRef]
- Morishita, Y.; Takenouchi, K.; Sakashita, S.; Matsuura, K.; Hayashi, R.; Nakatsura, T. Immunohistochemical analysis of common cancer antigens in head and neck squamous cell carcinoma. Anticancer Res 2022, 42, 5751–5761. [CrossRef]
- Infante, J.R.; Matsubayashi, H.; Sato, N.; Tonascia, J.; Klein, A.P.; Riall, T.A.; Yeo, C.; Iacobuzio-Donahue, C.; Goggins, M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol 2007, 25, 319–325. [CrossRef]
- Porte H, Chastre E, Prevot S, et al. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer 1995, 64, 70–75. [CrossRef]
- Chiavarina, B.; Costanza, B.; Ronca, R.; Blomme, A.; Rezzola, S.; Chiodelli, P.; Giguelay, A.; Belthier, G.; Doumont, G.; Van Simaeys, G.; Lacroix, S.; Yokobori, T.; Erkhem-Ochir, B.; Balaguer, P.; Cavailles, V.; Fabbrizio, E.; Di Valentin, E.; Gofflot, S.; Detry, O.; Jerusalem, G.; Goldman, S.; Delvenne, P.; Bellahcène, A.; Pannequin, J.; Castronovo, V.; Turtoi, A. Metastatic colorectal cancer cells maintain the TGFβ program and use TGFBI to fuel angiogenesis. Theranostics 2021, 11, 1626–1640. [CrossRef]
- Seki, M.; Watanabe, A.; Enomoto, S.; Kawamura, T.; Ito, H.; Kodama, T.; Hamakubo, T.; Aburatani, H. Human ROBO1 is cleaved by metalloproteinases and γ-secretase and migrates to the nucleus in cancer cells. FEBS Lett 2010, 584, 2909–2915. [CrossRef]
- Ballard, M.S.; Hinck, L. A roundabout way to cancer. Adv Cancer Res 2012, 114, 187–235. [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).