Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Night – blindness (nyctalopia), that is, difficulties in night vision
- Reduced visual acuity
- A typical hyperpigmentation of the retina in a “bone spicule” pattern in the mid-periphery, visible by fundoscopy
- Attenuation of retinal arteries
- Dysfunction of photoreceptors, distinguishable by electroretinographical abnormalities
- A peripheral ring scotoma and narrowing of the visual field (detectable by visual field testing)
- fundus image photography
- Optical Coherence Tomography (OCT)
- ○
- OCTA Small Field, which is limited to the posterior pole (macula and optic disc)
- ○
- OCTA Wide Field which allows analyzing a larger retinal area.
- Superficial capillary plexus (SCP): Layer of ganglion cells and nerve fibers
- Deep capillary plexus (DCP): Inner nuclear and outer plexiform layer
- Choriocapillary plexus (CC): Between the Bruch membrane (BM) and the Sattler layer, at the choroidal level.
2. Mathematical background
2.1. Fractal Dimension
2.2. Multifractals
3. The algorithms
3.1. Computing the fractal dimension
3.2. Computing the Generalized Renyi Point Centered Dimensions
3.2.1. The case when
3.2.2. The case when
4. Results
4.1. Fractal dimension
| age | FD | Confidence (95%) | p-value | |
| 1 CG | 1.557 | 0.999 | 1.510-1.604 | 2.308E-10 |
| 3a CG | 1.485 | 1.000 | 1.456-1.514 | 1.838E-11 |
| Figure 1A | 1.167 | 0.997 | 1.096-1.237 | 1.350E-07 |
| Figure 2A | 1.130 | 0.999 | 1.083-1.177 | 2.092E-08 |
| Figure 3A | 0.907 | 0.992 | 0.815-0.998 | 1.758E-06 |
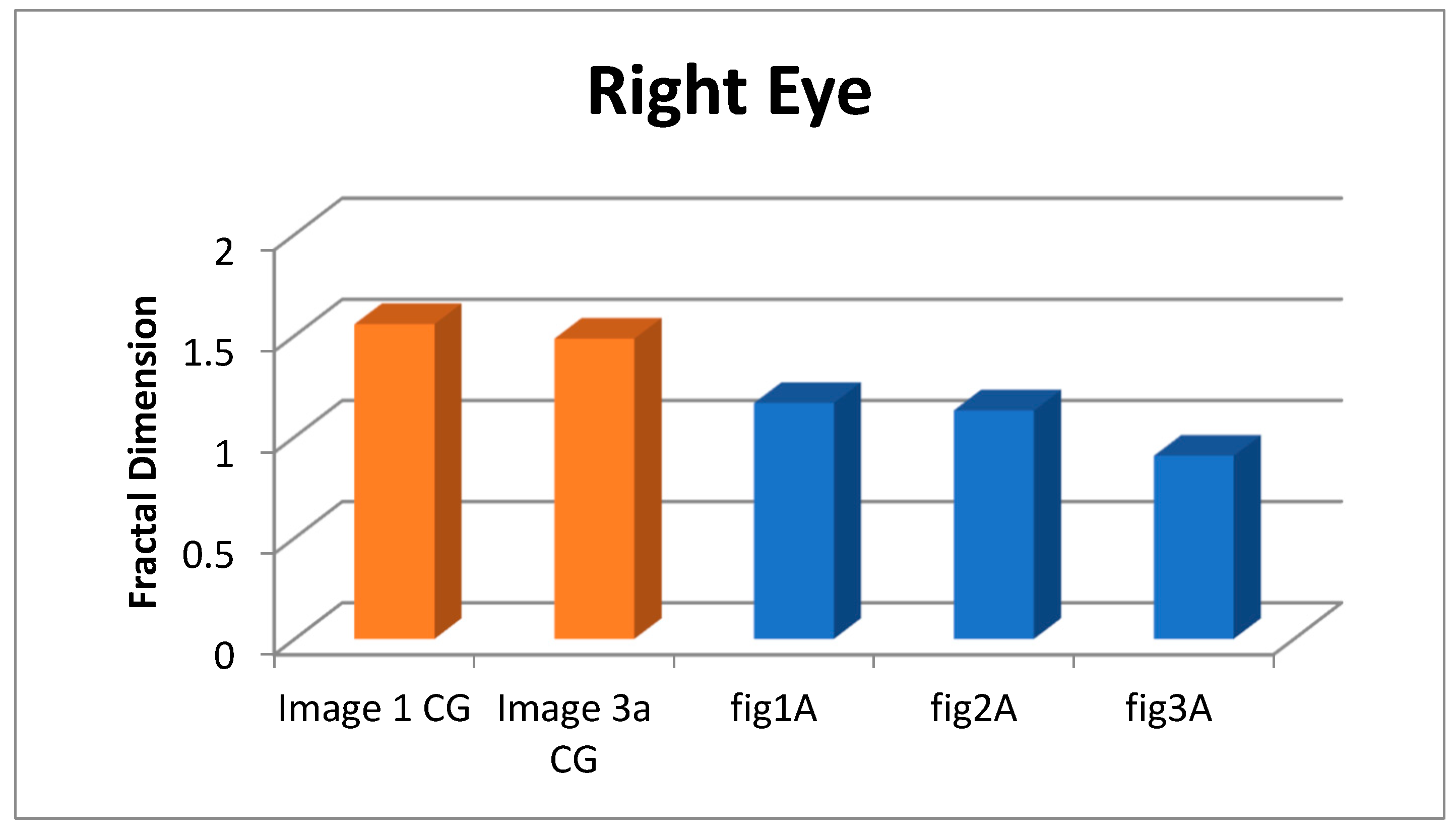
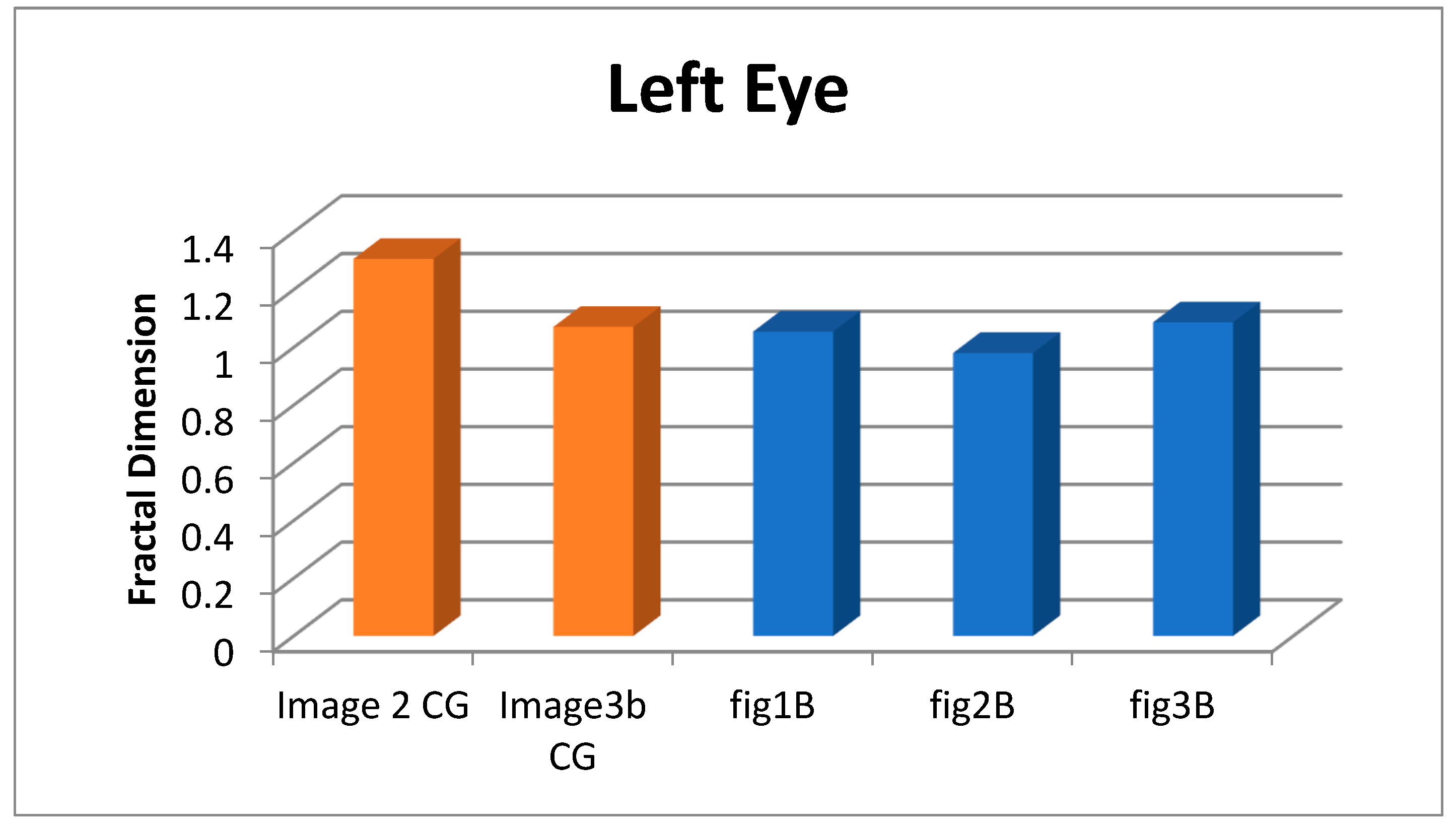
| Image | FD | Confidence (95%) | p-value | |
| 2 CG | 1.307 | 0.989 | 1.172-1.443 | 3.803E-07 |
| 3b CG | 1.071 | 0.999 | 1.034-1.108 | 5.337E-10 |
| Figure 1B | 1.055 | 0.996 | 0.981-1.128 | 2.733E-07 |
| Figure 2B | 0.981 | 0.999 | 0.944-1.018 | 1.328E-08 |
| Figure 3B | 1.087 | 0.990 | 0.964-1.211 | 3.172E-06 |
4.2. Generalized Renyi point centered dimensions
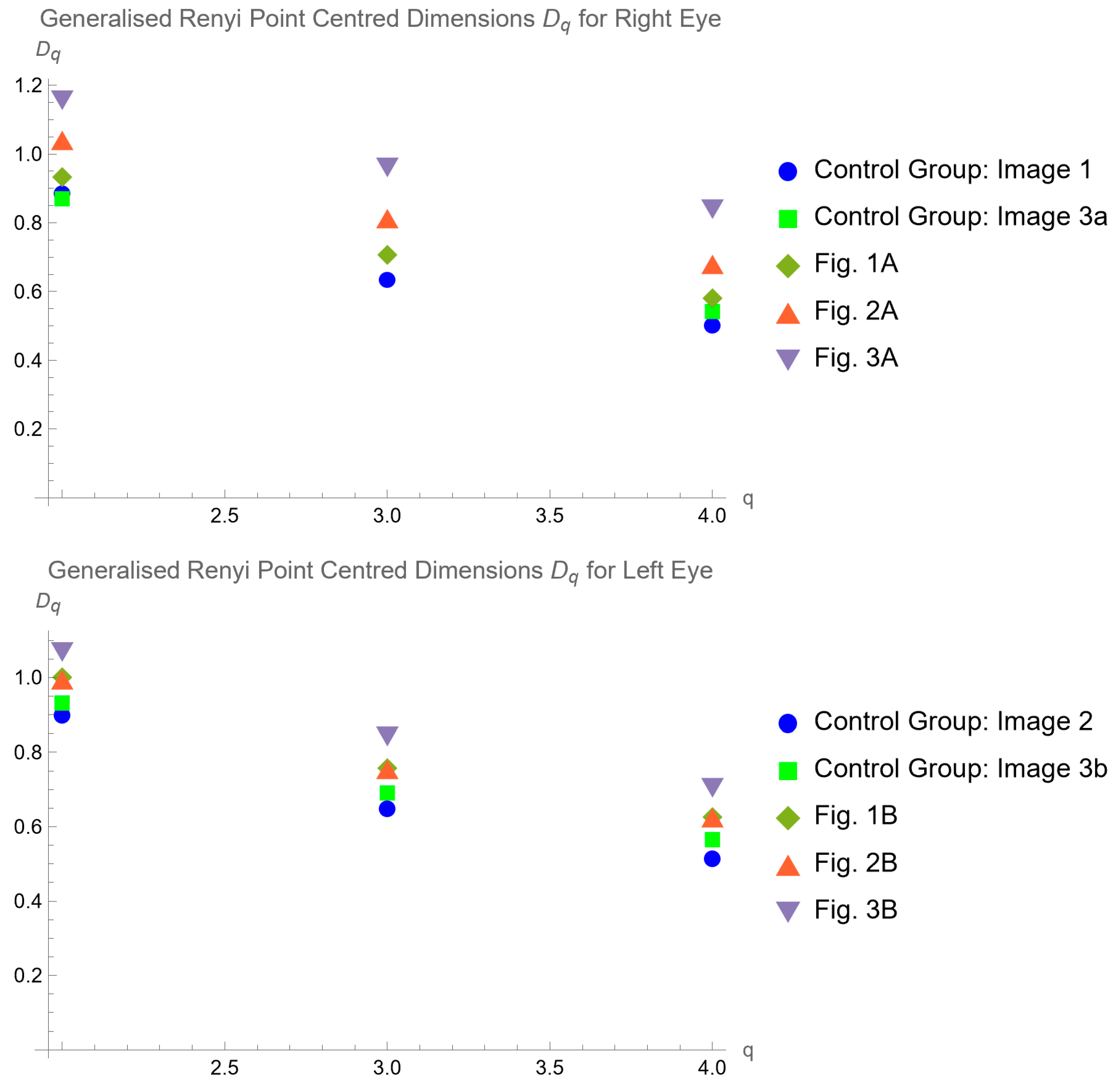
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verbakel, S.K.; Van Huet, R.; Boon, C.; den Hollander, A.I.; Collin, R.; Klaver, C. et al. Nonsyndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–86. [CrossRef]
- Marigo, V. Programmed cell death in retinal degeneration: targeting apoptosis in photoreceptors as potential therapy for retinal degeneration. Cell Cycle, 2007, 6, 652–5. [CrossRef]
- Ferrari, S.; Di Iorio, E.; Barbaro, V.; Ponzin, D.; Sorrentino, F.S.; Parmeggiani, F. Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics. 2011, 12, 238–49. [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. https://doi.org/10.1038/srep42201. [CrossRef]
- Family, F.; Masters, B.R.; Platt, D.E. Fractal Pattern Formation in Human Retinal Vessels. Phys. Nonlinear Phenom. 1989, 38, 98–103;. https://doi.org/10.1016/0167-89(89)90178-4. [CrossRef]
- Lakshminarayanan, V.; Raghuram, A.; Myerson, J.; Varadharajan, S. The Fractal Dimension in Retinal Pathology. J. Mod. Opt. - J MOD Opt. 2003, 50, 1701–1703;. https://doi.org/10.1080/0950034031000069442. [CrossRef]
- Yu, S.; Lakshminarayanan, V. Fractal Dimension and Retinal Pathology: A Metaanalysis, Appl. Sci. 2021, 11, 2376. Available online: https://doi.org/10.3390/app11052376 (accessed on 9.9.2021). [CrossRef]
- Mitamura, Y.; Mitamura-Aizawa, S.; Nagasawa, T.; Katome, T.; Eguchi, H.; Naito,T. Diagnostic imaging in patients with retinitis pigmentosa. J. Med. Invest. 2012, 59, 1–11. [CrossRef]
- Jauregui, R.; Park, K.S.; Duong, J.K.; Mahajan, V.B.; Tsang, S.H. Quantitative progression of retinitis pigmentosa by optical coherence tomography angiography. Sci. Rep. 2018, 8, 13130. [CrossRef]
- Minicucci F.; Oikonomou F. D. and De Sanctis A. A. Fractal dimensional analysis for retinal vascularization images in retinitis pigmentosa: a pilot study. Chaos, Fractals and Complexity, Springer Proceedings in Complexity, 2023.
- Spaide R.F.; Koizumi H.; Pozzoni M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008, 146, 496–500.
- Margolis R.; Spaide R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009, 147, 811–5. [CrossRef]
- Chhablani J.; Wong I.Y.; Kozak I. Choroidal imaging: a review. Saudi J Ophthalmol.2014, 28, 123–128. [CrossRef]
- Zhang Y.; Harrison J. M.; Nateras O. S.; Chalfin S.; Duong T. Q. Decreased retinal-choroidal blood flow in retinitis pigmentosa as measured by MRI. Doc Ophthalmol. 2013, 126, 187–97. [CrossRef]
- Finzi A.; Cellini M.; Strobbe E.; Campos EC. ET-1 plasma levels, choroidal thickness and multifocal electroretinogram in retinitis pigmentosa. Life Sci. 2014, 118, 386–90. [CrossRef]
- Strobbe E.; Cellini M.; Fresina M.; Campos EC. ET-1 plasma levels, aqueous flare, and choroidal thickness in patients with retinitis pigmentosa. J Ophthalmol. 2015, 292615. [CrossRef]
- Mandelbrot, B.B.; Wheeler, J.A. The Fractal Geometry of Nature. Am. J. Phys. 1983, 51, 286–287;. https://doi.org/10.1119/1.13295. [CrossRef]
- Mandelbrot, B.B. Les Objets Fractals; Flammarion: Paris, France, 1999.
- Falconer, K. Fractal Geometry Mathematical Foundations and Applications, John Wiley & Sons: England, 1990. [CrossRef]
- Barnsley, M.F. Fractals Everywhere, 3d ed.; Academic Press: San Diego, USA, 1993.
- Peitgen, Heinz-Otto; Jürgens, H.; Saupe, D. Chaos and Fractals, Springer: New York, USA, 2004.
- Harte, D. Multifractals theory and applications; CHAPMAN & HALL/CRC Boca Raton, London, New York, Washington, D.C. © 2001.
- Abdolrahimzadeh S.; Di Pippo M.; Ciancimino C.; Di Staso F. and Lotery A. J. Choroidal vascularity index and choroidal thickness: potential biomarkers in retinitis pigmentosa. Eye, Springer Nature, 2022. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



