Submitted:
31 December 2024
Posted:
03 January 2025
You are already at the latest version
Abstract
This author is not a philosopher nor historian of science, but an engineering thermodynamicist. In that regard and in addition to various philosophical “why & how” treatises and existing historical analyses, the physical and logical “what it is” reflections, as successive Key Points, where a key reasoning infers the next one, along with novel contributions and original generalizations, are presented. We need to keep in mind that in Sadi Carnot’s time (early 1800s) the steam engines were inefficient (below 5%, so the heat in and out were comparable within experimental uncertainty, as if caloric were conserved), the conservation of caloric flourished (might be a fortunate misconception leading to the critical analogy with the waterwheel), and many critical thermal-concepts, including the conservation of energy (The First Law) were not even established. If Clausius and Kelvin were "fathers of thermodynamics" then Sadi Carnot was the "grandfather" [Kostic, 2023 July 24], or better yet, Sadi Carnot was the "Forefather of Thermodynamics-to-become" [Kostic, 2023 October 29].
Keywords:
 “The motive power of heat is independent of the agents employed to realize it; its quantity is fired solely by the temperatures of the bodies between which is effected, finally, the transfer of the caloric.” − by Sadi Carnot, 1824 (English Translation by Robert H. Thurston [1]).
“The motive power of heat is independent of the agents employed to realize it; its quantity is fired solely by the temperatures of the bodies between which is effected, finally, the transfer of the caloric.” − by Sadi Carnot, 1824 (English Translation by Robert H. Thurston [1]).1. Introduction
“The motive power of heat is independent of the agents employed to realize it; its quantity is fired solely by the temperatures of the bodies between which is effected, finally, the transfer of the caloric.” [1]. Namely,
2. Key Points: Most probable sequential developments of Sadi Carnot’s ingenious discoveries
- The source of the heat engine “motive power” is “caloric fall” (“temperature fall” or temperature difference of the caloric)
- The “temperature fall,” as source of engine motive power, should “not be wasted,” but minimized in any “workless heat transfer process”
- All motive frictions and other dissipative processes should be minimized in order to maximize engine power and efficiency
- Reversible Cycles: Isothermal heat transfer and other frictionless processes make an engine process or cycle reversible
- Reversible cycles must all have equal and maximum efficiency
Key Point I: “The source of the heat engine “motive power” is “caloric fall” (“temperature fall” or temperature difference of the caloric)
Key Point II: The “temperature fall,” as source of engine motive power, should “not be wasted,” but minimized in any “workless heat transfer process”
Key Point III: All motive frictions and other dissipative processes should be minimized in order to maximize engine power and efficiency. Mechanical processes should be ideally frictionless to avoid work waste, i.e., dissipation losses.
Key Point IV: Reversible Cycles: Isothermal heat transfer and other frictionless processes make an engine process or cycle reversible
Key Point V: Reversible cycles must all have equal and maximum efficiency.
3. Miss Points: Persistent post-misconceptions and fallacies by others
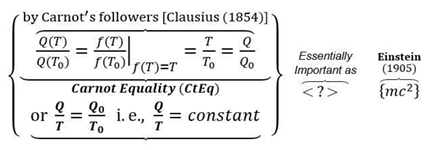
- The well-known Carnot efficiency formula, , was not established by Sadi Carnot, but much later by Kelvin and Clausius
- The cause and source for motive power is the temperature difference, in principle, but not linearly dependent as misstated by some
- The heat transferred out of the Carnot cycle at lower temperature is “not a waste heat” as often stated, but it is “useful quantity”, necessary for completion of the cycle
- Sadi Carnot could not had been thinking of “any other caloric” but heat, to imply “entropy-like quantity” as speculated by some
Miss Point 1: The well-known Carnot efficiency formula, , was not established by Sadi Carnot, but much later by Kelvin and Clausius
Miss Point 2: The cause and source for motive power is the temperature difference, in principle, but not linearly dependent as misattributed by some
Miss Point 3: The heat transferred out of the Carnot cycle at lower temperature is “not a waste heat” as often stated, but it is “useful quantity”, necessary for completion of the cycle
Miss Point 4: Sadi Carnot could not had been thinking of “any ‘other’ caloric” but heat, to imply “entropy-like quantity” as speculated by some
4. Key NOVEL-Points: Novel contributions and original generalizations
- “Reversible and Reverse” Processes and Cycles Dissected
- Maximum Efficiency and “Reversible Equivalency” Scrutinized
- Reversible Contradiction Impossibility (“Reductio ad absurdum”)
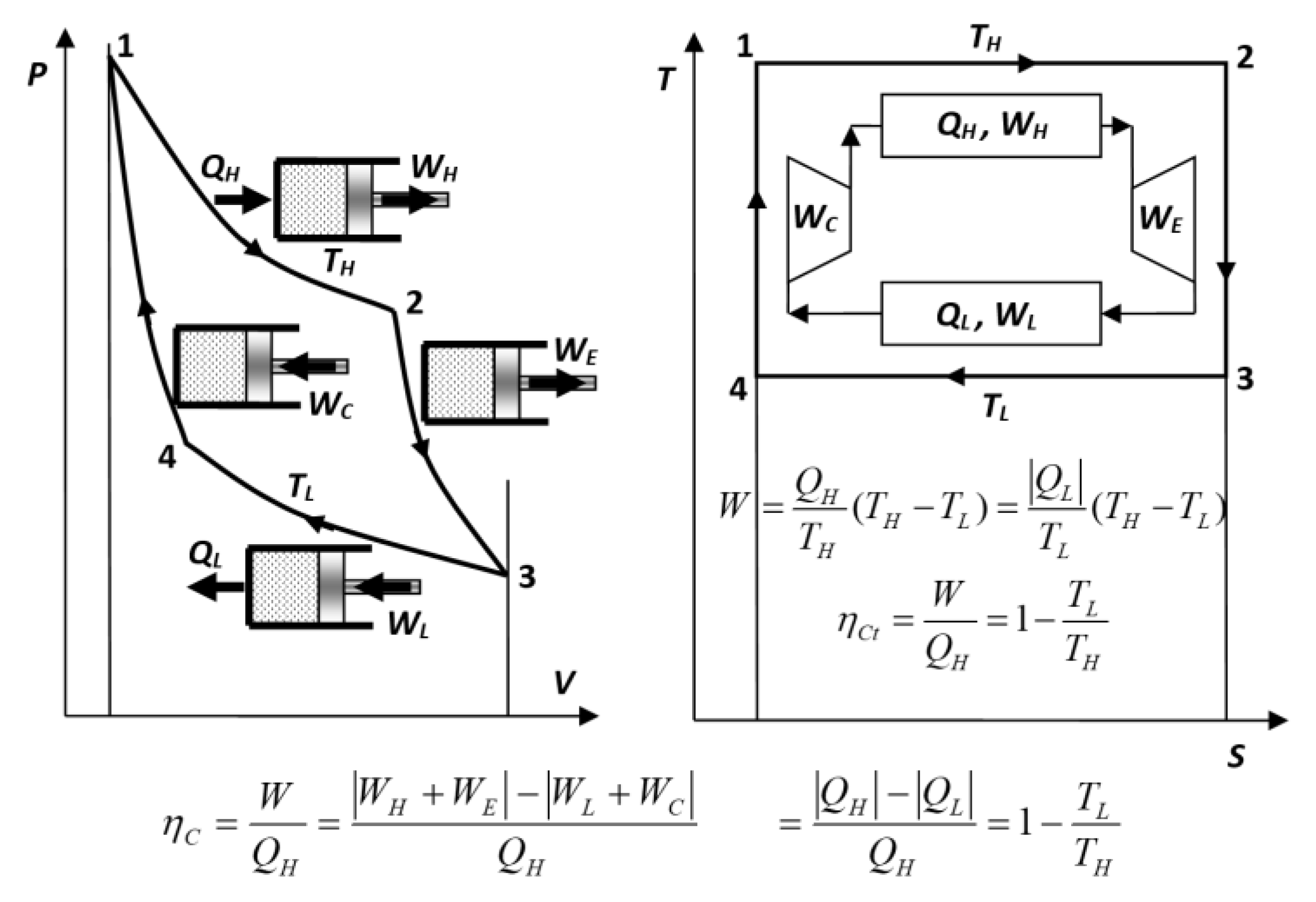
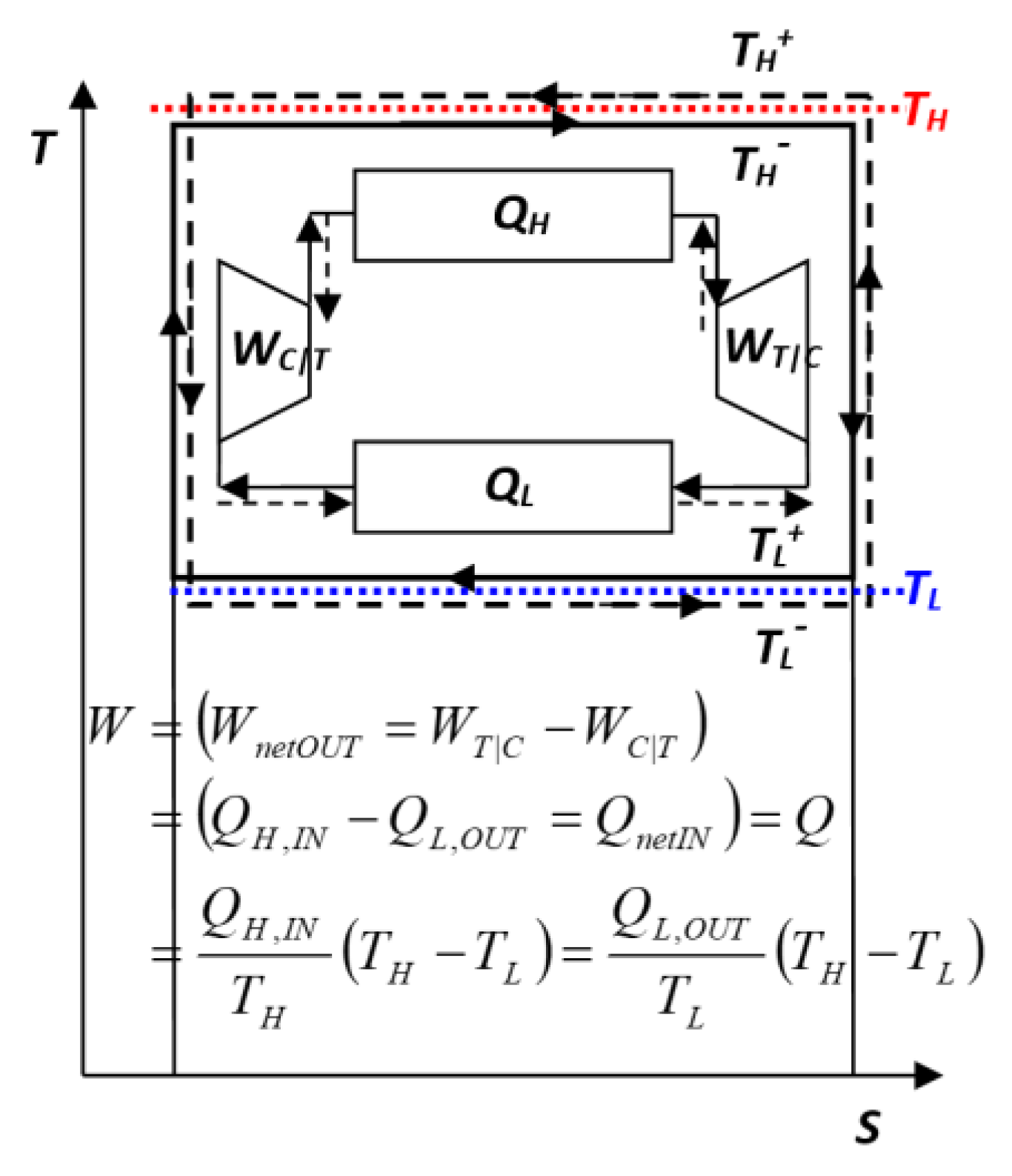
- Reversible Carnot Cycle Efficiency Is Misplaced — It is NOT the “Cycle efficiency” ‘per se’, but a “Thermal energy-source ‘work-potential efficiency’”
- The Carnot-Clausius [Ratio] Equality (CCE) and Clausius Equality (Cyclic integral) are special cases of relevant “Entropy boundary integral” for reversible stationary processes
Key NOVEL-Point 1: “Reversible and Reverse” Processes and Cycles Dissected
Key NOVEL-Point 2: Maximum Efficiency and “Reversible Equivalency” Scrutinized
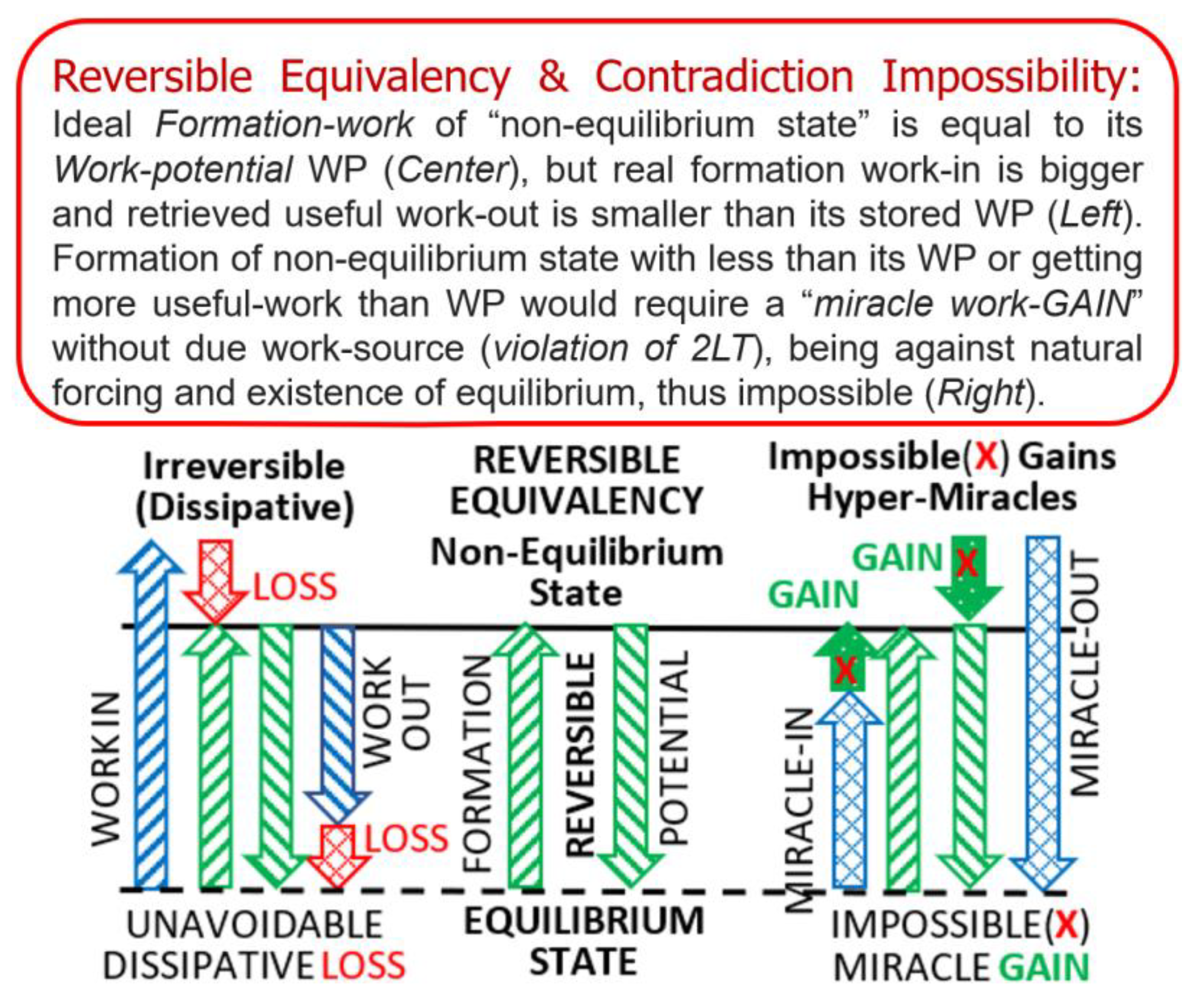
Key NOVEL-Point 3: Reversible Contradiction Impossibility (“Reductio ad absurdum”)
| KEYNOTE 1: It would be logically and otherwise impossible and absurd (“Reductio ad absurdum”) to have a spontaneous process “the one way and/or the opposite way” arbitrarily in opposite directions, as casual by chance (i.e., to have heat transfer “from hot-to-cold or from-cold-to-hot” or “forcing in one direction and accelerating in opposite direction,” by chance). It would be a violation of the Second law of thermodynamics (2LT). |
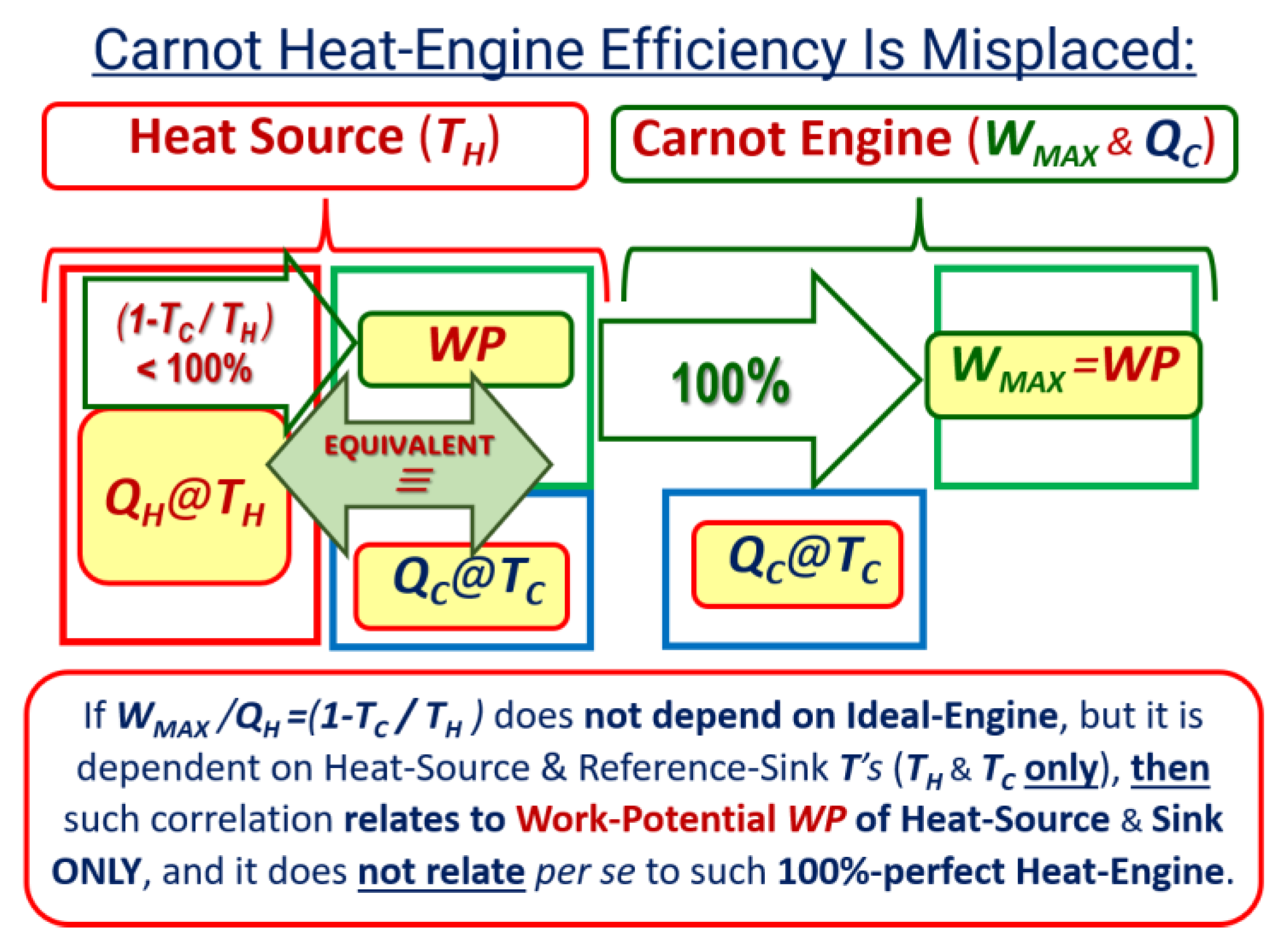
Key NOVEL-Point 4: Reversible Carnot Cycle Efficiency Is Misplaced − It is NOT the “Cycle efficiency” ‘per se’, but a “Thermal energy-source ‘work-potential efficiency’”
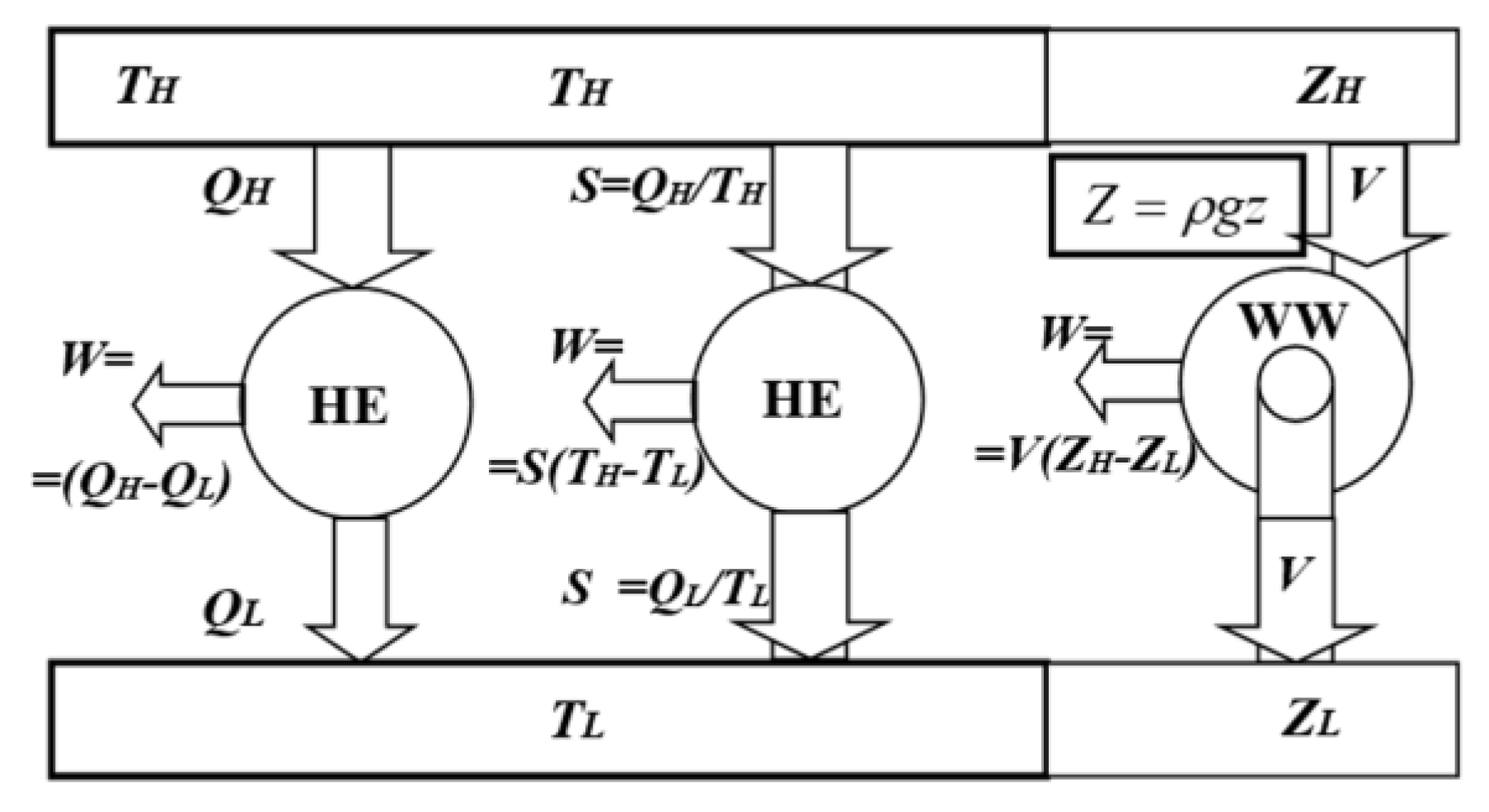
| KEYNOTE 2: Carnot heat-engine efficiency dependance on temperatures of the heat reservoirs would be equally misplaced as attribute the maximum efficiency of an ideal water-wheel (water turbine), based on its motive-power per unit of input water-flow, and then it would also mistakenly depend on the water-reservoirs’ elevations only. All reversible devices are equally and maximally 100% efficient. |
| KEYNOTE 3: For the same fundamental reason, the efficiency of a perfect, ideal Carnot cycle (being below 100%) would also be logically inappropriate (as if there are “some work losses” in the ideal reversible cycles). For the same reason, as for the Heat-pump cycle, it should be called ‘Carnot cycle COP’ but not the ‘Cycle efficiency’. Fundamentally, all ideal, reversible cycles must be “equally and maximally [100%] efficient,” as reasoned by Sadi Carnot [1]. |
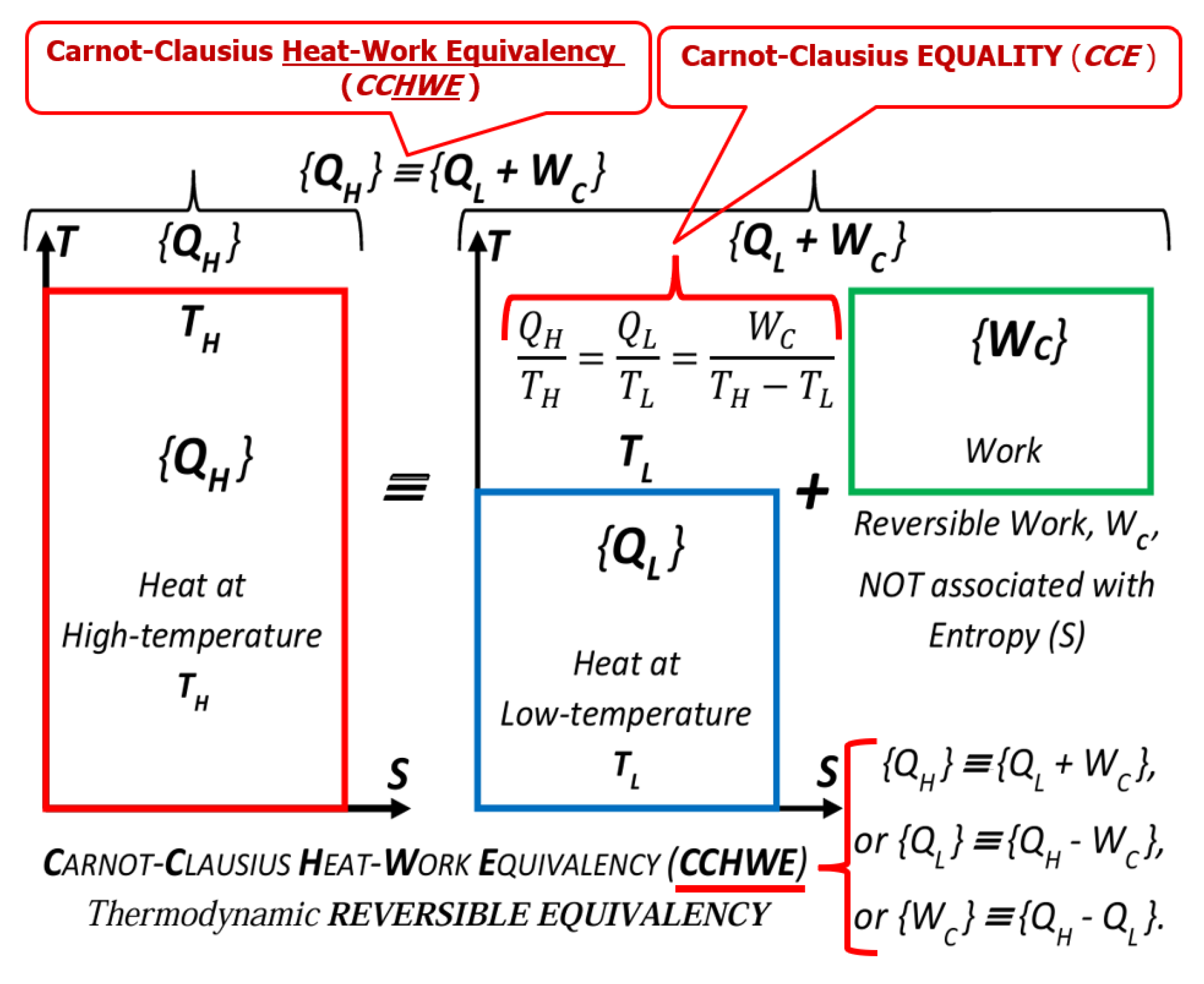
Key NOVEL-Point 5: The Carnot-Clausius [Ratio] Equality (CCE) and Clausius Equality (Cyclic integral) are special cases of related “Entropy boundary integral” for reversible stationary processes
5. Conclusion and “Key Takeaways”
- Conservation of caloric misconception was probably a fortunate catalyst leading to analogy with the waterwheel and Carnot’s hypothesis that the motive power of steam engine was caused and produced by the “fall of caloric” (cooling of hot caloric) since Carnot believed that there was no “consumption” of the caloric (Key Point I).
- Carnot’s reasoning that “wherever there exists a difference of temperature, motive power can be produced” and not be wasted for workless heat transfer, was the most critical and ingenious reasoning (Key Point II) that led to inference of the most critical concept of Carnot’s discovery (Key Point IV & V).
- The “Key discovery” ingeniously inferred by Sadi Carnot, that Reversible cycles must all have equal and maximum efficiency, by demonstrating that otherwise would result in creation of conserved caloric and/or perpetual motion: “the maximum of motive power resulting from the employment of steam is also the maximum of motive power realizable by any means whatever [1] (Key Point V).”
- The selected and persistent post-misconceptions and fallacies by others are also presented as Miss Points 1-4, including the misconceptions that the heat transferred out of the ideal Carnot cycle at lower temperature is the “waste heat or loss” as often stated. However, it is the “useful and necessary quantity,” required for the entropy balance and completion of a perfect, reversible cycle.
- The “Reversible and Reverse” processes and cycles are re-examined in Key NOVEL-Point 1. Among others, it is emphasized that the potential qualities of flux quantities (heat and different kinds of works) are not degraded but equipotentially transferred and stored between the system and its boundary surroundings, and thus conserved in every way. The spatial gradients are virtually zero at any instant while time gradients and related fluxes may be arbitrary since the time and energy rates are irrelevant for the reversible analysis of energy balances and properties.
- The reversible cycle “Maximum efficiency” and “Reversible equivalency” are scrutinized in Key NOVEL-Point 2. The reversible efficiency implies maximum work extraction and minimum work expenditure in the reverse process, thereby the two must be equivalent for given conditions, thus establishing the ‘Reversible equivalency’. The reversible processes are perfect and “equally and maximally efficient,” and they define the concept of “Reversible Equivalency,” — the ‘true equality’ of input and output, where relevant quantities and qualities are conserved in perpetuity.
- Reversible Contradiction Impossibility (“Reductio ad absurdum”) is scrutinized in Key NOVEL-Point 3. Namely, any reversible cycle “under-achievement” (getting less than maximum possible) would become an “over-achievement” when such cycle is reversibly ‘reversed’ (accomplishing with less than minimum required), and such “‘reversed’ over achievement” would be physically impossible (would be the “‘reversible equivalency’ violation” and may violate the conservation laws), thus establishing the “Reversible Contradiction Impossibility” of established fact with numerous consequences as detailed in Key NOVEL-Point 3 and elsewhere.
- Sadi Carnot [1] and his followers, including Kelvin [7] and Clausius [8], ironically referred to the maximum heat-engine cycle efficiency they developed (at the time when most thermal concepts were unknown), with the absurd conclusion, that it does not depend on the cycle design itself nor its operation mode, therefore, the proof that it is not the efficiency of ideal Carnot cycle ‘per se’. Therefore, their attribution is misplaced since the correlation they developed should had referred to the “maximum motive power or ‘work potential’ of the thermal reservoirs” since it depends on their temperatures only, and hence, being the proof of the claim presented here, see Key NOVEL-Point 4 and Figure 6.
- The Clausius-Carnot [Ratio] Equality and Clausius Equality [Cyclic integral] are elucidated to be the special cases of related “Entropy [balance] boundary integral” for reversible stationary or cyclic processes, see Key NOVEL-Point 5.
References
- Carnot, S. Reflections on the Motive Power of Heat (Translation by Thurston, R.H. Chapman & Hall, Ltd. London, UK, 1897. Available online: https://books.google.com/books?id=tgdJAAAAIAAJ. Also: Reflections on the Motive Power of Heat - Wikisource, the free online library (Accessed on December 30, 2024).
- Carnot Lille 2024 - Sciencesconf.org; Celebration of 200 years since Sadi Carnot's Réflexions sur la puissance motrice du Feu, 1824–2024, Lille, France 11-13 September 2024. Available online: https://carnotlille2024.sciencesconf.org/ (Accessed on December 30, 2024).
- Sadi Carnot's Legacy - Celebrating 200 years of thermodynamics - Sciencesconf.org. École Polytechnique, 16-18 September 2024. Available online: https://carnot-legacy.sciencesconf.org/?lang=en. Also: Colloquium: Sadi Carnot's Legacy - École Polytechnique, école d'ingénieur (Accessed on December 30, 2024).
- Entropy | Special Issue : 200 Years Anniversary of “Sadi Carnot, Réflexions Sur La Puissance Motrice Du Feu”; Bachelier: Paris, France, 1824. Available online: https://www.mdpi.com/journal/entropy/special_issues/4BAM6VCY2L (Accessed on December 30, 2024).
- Professor Kostic - Sadi Carnot's Réflexions. Available online: http://Carnot.MKostic.com (Accessed on December 30, 2024).
- Kostic, M. Engineering Thermodynamics: Fundamentals and Challenges. École Polytechnique, Palaiseau, France, 16-18 September 2024. Available online: PPT(Updated) →Vidéo. See also Bases documentaires. (Accessed on December 30, 2024).
- Thomson, W. (Lord Kelvin). On the Dynamical Theory of Heat. Transactions of the Royal Society of Edinburgh, March 1851, and Philosophical Magazine IV. 1852. Available online: https://zapatopi.net/kelvin/papers/ (Accessed on December 30, 2024).
- Clausius, R. The Mechanical Theory of Heat; Macmillan: London, UK, 1879; pp. 21–38, 69–109, 212–215. [Google Scholar] (Accessed on December 30, 2024).
- Kostic, M. Sadi Carnot’s Ingenious Reasoning of Ideal Heat Engine Reversible Cycles. In Proceedings of the 4th IASME/WSEAS International Conference on Energy, Environment, Ecosystems and Sustainable Development (EEESD’08), Algarve, Portugal, 11–13 June 2008; Available online: https://www.researchgate.net/publication/228561954 (Accessed on December 30, 2024).
- Kostic, M. Revisiting the Second Law of Energy Degradation and Entropy Generation: From Sadi Carnot’s Ingenious Reasoning to Holistic Generalization. AIP Conf. Proc. 2011, 1411, 327. Available online: http://kostic.niu.edu/kostic/_pdfs/Second-Law-Holistic-Generalization-API.pdf [CrossRef][Green Version] (Accessed on December 30, 2024).
- Kostic, M. Reasoning and Logical Proofs of the Fundamental Laws: “No Hope” for the Challengers of the Second Law of Thermodynamics. Entropy 2023, 25, 1106. [CrossRef]
- Pisano, R. On Principles In Sadi Carnot’s Thermodynamics (1824). Epistemological Reflections, Almagest 2(2), January 2010. Online ISSN: 2507-0371, Print ISSN: 1792-2593. Available online: https://www.researchgate.net/publication/265680441 Accessed on Nov. 15, 2023). [CrossRef]
- Fox, R. The Savant and the State: Science and Cultural Politics in Nineteenth-Century France. The John Hopkins University Press, ISBN 1-4214-0522-9, 2012. Available online: https://books.google.com/books?id=EGn3fJfMRxQC and https://a.co/d/diV1suX (Accessed on December 30, 2024).
- Yamamoto, Y, Yoshida, H. Historical Development in the Thought of Thermal Science—Heat and Entropy. IHTC-16 Fourier Lecture, 2008. Available online: https://ihtcdigitallibrary.com/conferences/ihtc16,677693cb75c8b2ed.html. Also (PDF): http://www.aihtc.org/pdfs/IHTC-16-Yoshida-paper.pdf (Accessed on December 30, 2024).
- Norton, J.D. How Analogy Helped Create the New Science of Thermodynamics. Synthese. 200 (2022), article 269, pp. 1-42. Available online: https://sites.pitt.edu/~jdnorton/papers/Analogy_Carnot.pdf (Accessed on December 30, 2024). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




