Submitted:
02 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
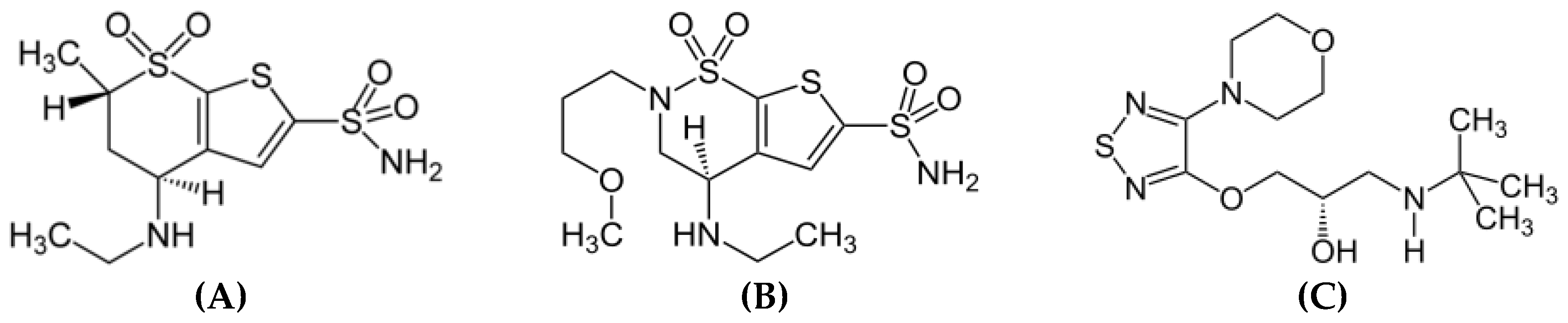
2.1. Chemicals and reagents
2.2. Buffer and Sample Preparation
2.3. Method Validation
2.4. HPLC Analysis
3. Results
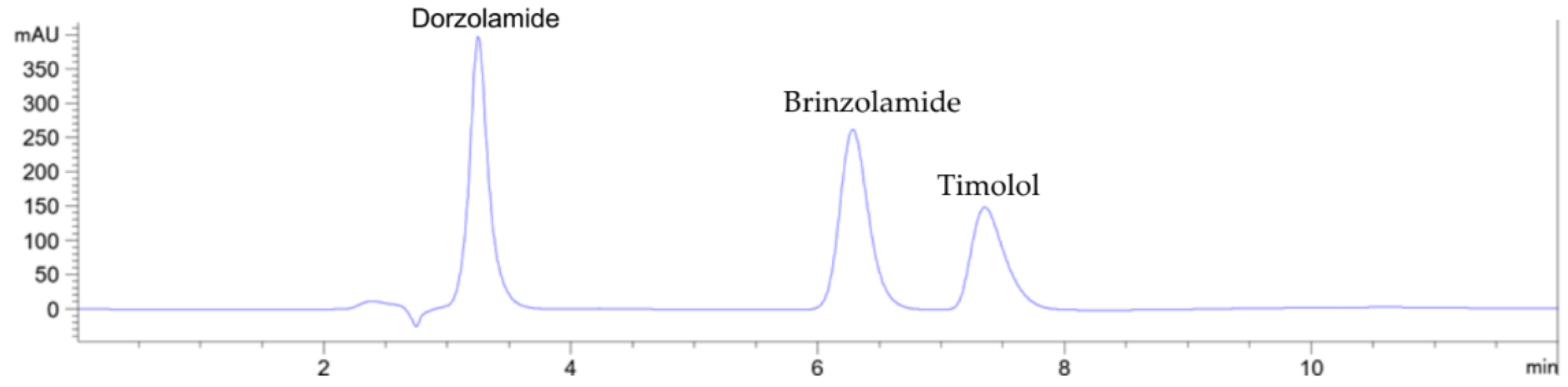
3.1. Method development
3.2. Method Validation
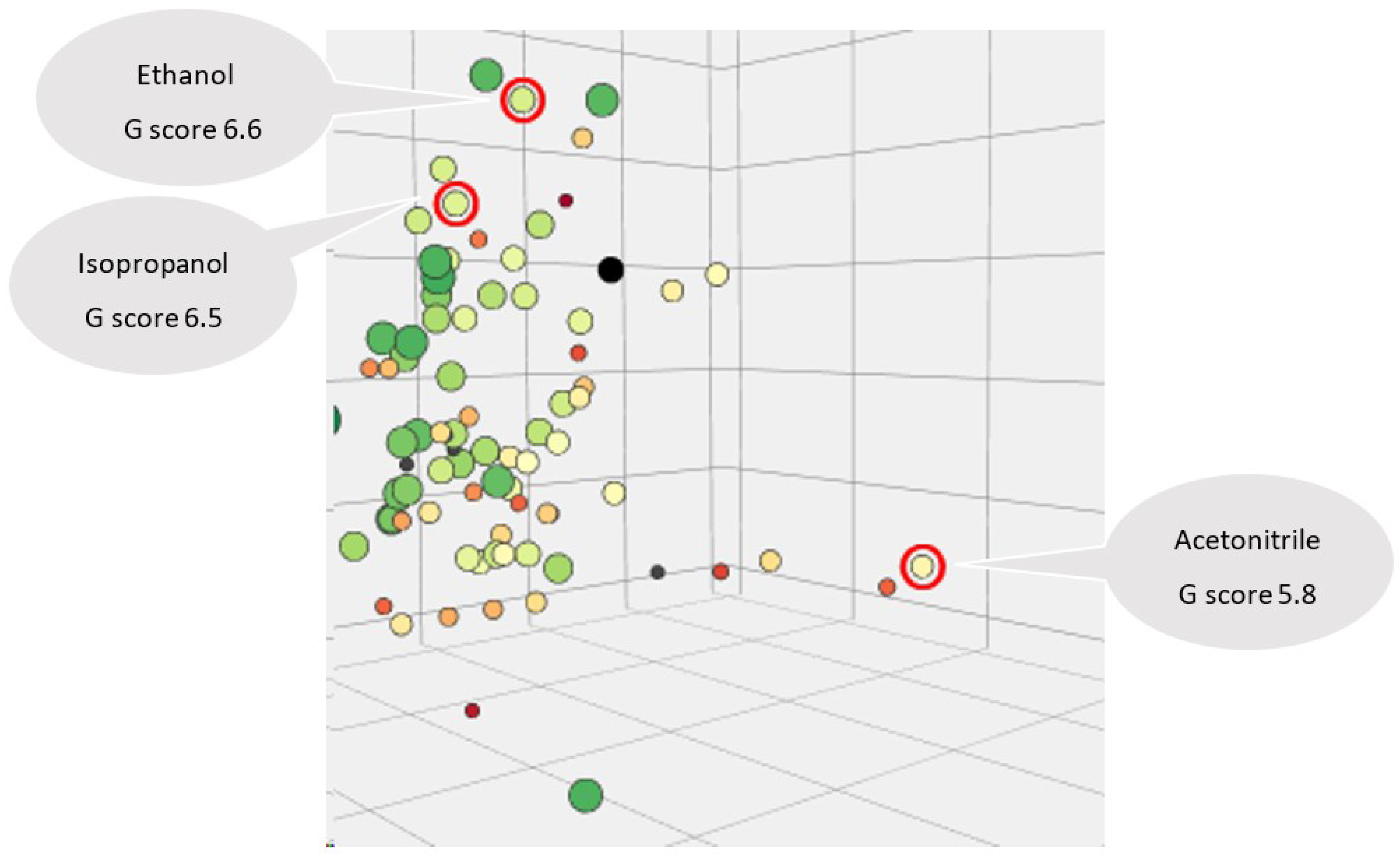
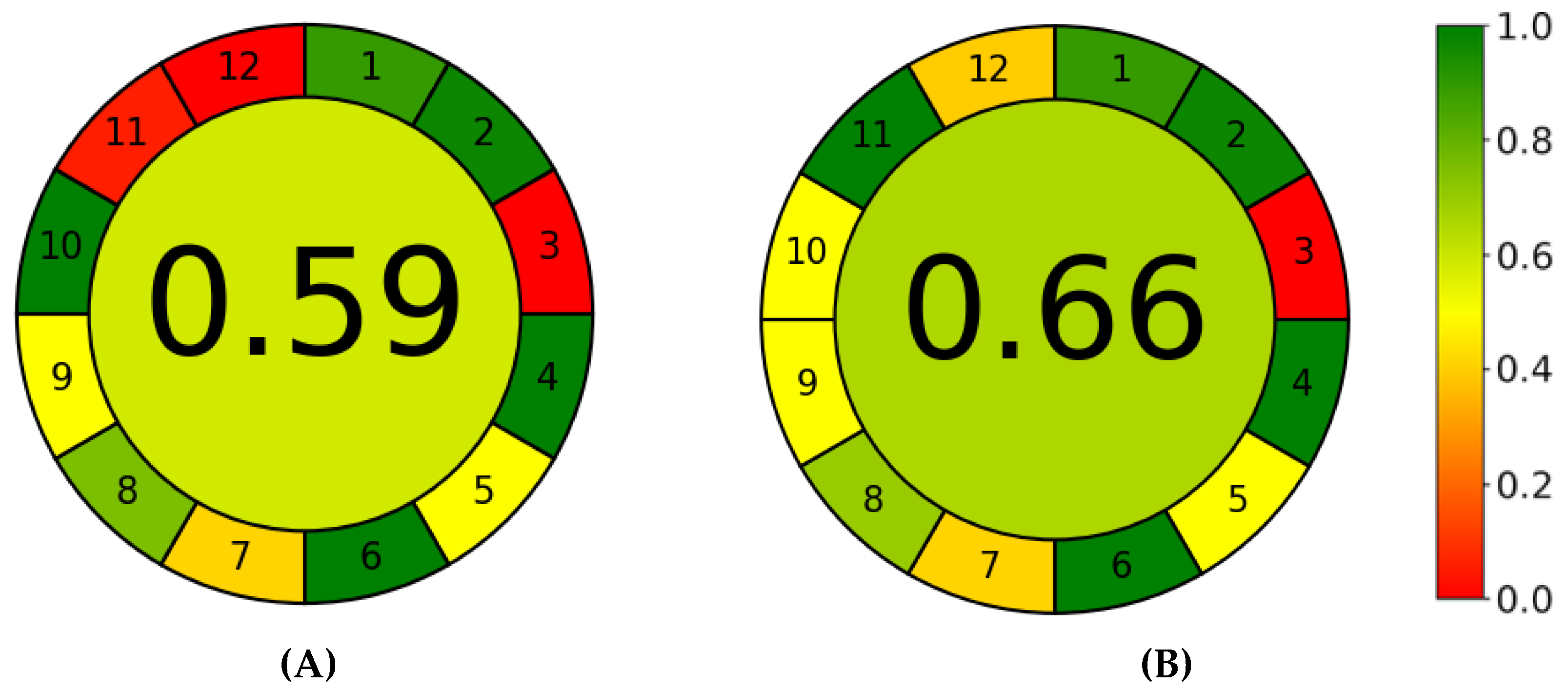
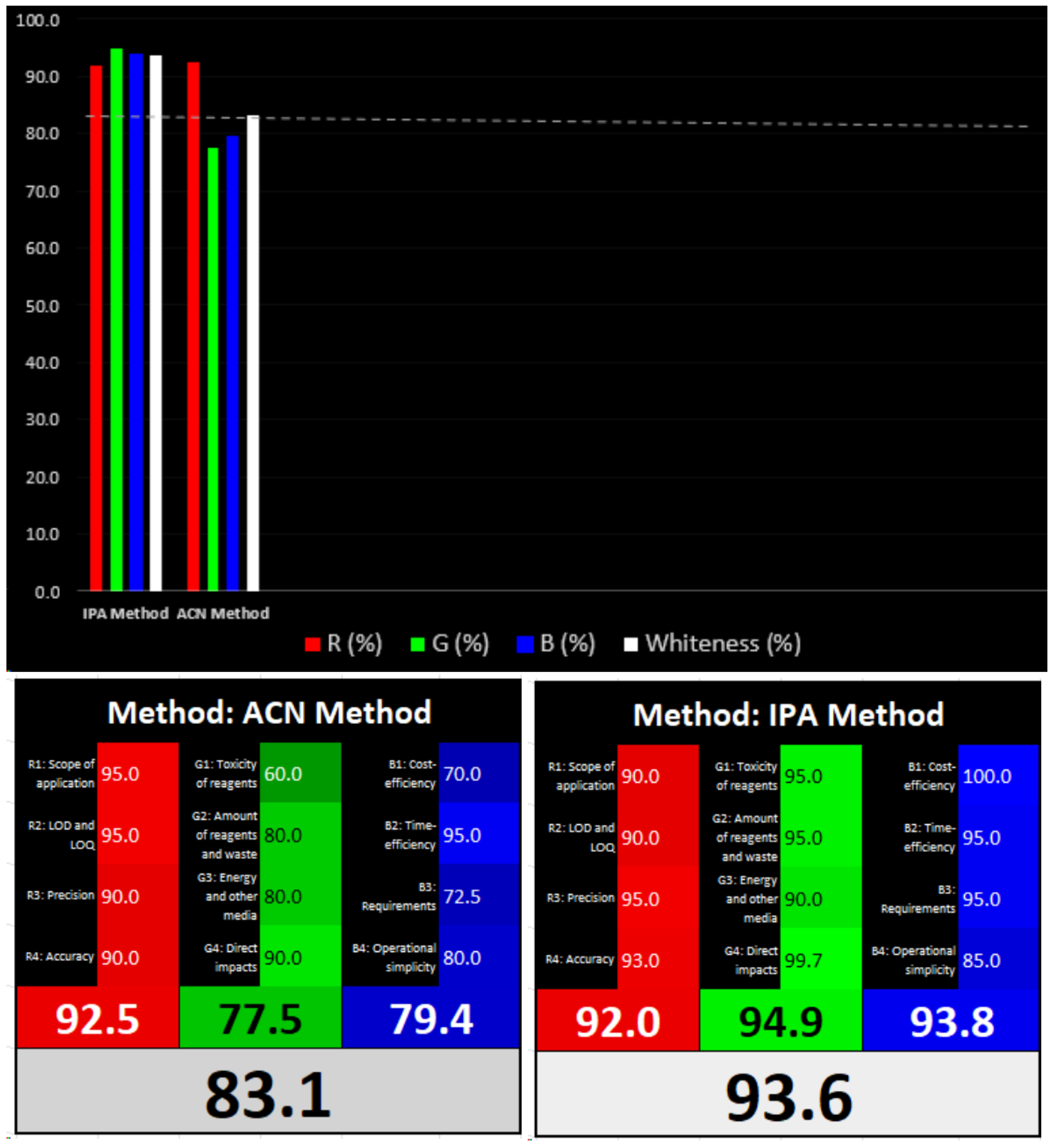
3.3. Greenness and Whiteness Assessments of the Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P. Green Chemistry. Frontiers (Boulder) 1998, 640, 850. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends in Analytical Chemistry 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends in Analytical Chemistry 2012, 37, 61–72. [Google Scholar] [CrossRef]
- National Environmental Methods Index. Available online: www.nemi.gov.
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends in Analytical Chemistry 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the Move towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chemistry 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal Chem 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends in Analytical Chemistry 2021, 138, 116223. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends in Analytical Chemistry 2023, 159, 116905. [Google Scholar] [CrossRef]
- Nuzzi, R.; Tridico, F. Glaucoma: Biological Trabecular and Neuroretinal Pathology with Perspectives of Therapy Innovation and Preventive Diagnosis. Front Neurosci 2017, 11. [Google Scholar] [CrossRef]
- Nuzzi, R.; Marolo, P.; Nuzzi, A. What Is New in Glaucoma: From Treatment to Biological Perspectives. J Ophthalmol 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of Vision Loss Worldwide, 1990–2010: A Systematic Analysis. Lancet Glob Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. The Number of People with Glaucoma Worldwide in 2010 and 2020. British Journal of Ophthalmology 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Tridico, F. Glaucoma: Biological Trabecular and Neuroretinal Pathology with Perspectives of Therapy Innovation and Preventive Diagnosis. Front Neurosci 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and Its Treatment: A Review. American Journal of Health-System Pharmacy 2005, 62, 691–699. [Google Scholar] [CrossRef]
- Michaud, J.; Friren, B. Comparison of Topical Brinzolamide 1% and Dorzolamide 2% Eye Drops given Twice Daily in Addition to Timolol 0.5% in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. Am J Ophthalmol 2001, 132, 235–243. [Google Scholar] [CrossRef]
- Ingram, C.J.; Brubaker, R.F. Effect of Brinzolamide and Dorzolamide on Aqueous Humor Flow in Human Eyes. Am J Ophthalmol 1999, 128, 292–296. [Google Scholar] [CrossRef]
- Balfour, J.A.; Wilde, M.I. Dorzolamide. Drugs Aging 1997, 10, 384–403. [Google Scholar] [CrossRef]
- SUGRUE, M.F. Review The Preclinical Pharmacology of Dorzolamide Hydrochloride, a Topical Carbonic Anhydrase Inhibitor. Journal of Ocular Pharmacology and Therapeutics 1996, 12, 363–376. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 5284549, Dorzolamide. Retrieved November 2, 2023. Available online: Https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Dorzolamide.
- Iester, M. Brinzolamide. Expert Opin Pharmacother 2008, 9, 653–662. [Google Scholar] [CrossRef]
- Iester, M. Brinzolamide. Expert Opin Pharmacother 2008, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ingram, C.J.; Brubaker, R.F. Effect of Brinzolamide and Dorzolamide on Aqueous Humor Flow in Human Eyes. Am J Ophthalmol 1999, 128, 292–296. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 68844, Brinzolamide. Retrieved November 2, 2023. Available online: Https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Brinzolamide.
- Iester, M. Brinzolamide. Expert Opin Pharmacother 2008, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ingram, C.J.; Brubaker, R.F. Effect of Brinzolamide and Dorzolamide on Aqueous Humor Flow in Human Eyes. Am J Ophthalmol 1999, 128, 292–296. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 68844, Brinzolamide. Retrieved November 2, 2023. Available online: Https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Brinzolamide.
- Narendra, A.; Deepika, D.; Annapurna, M.M. Validated LC Method for the Estimation of Dorzolamide HCl (Carbonic Anhydrase Inhibitor) in Ophthalmic Solutions. E-Journal of Chemistry 2012, 9, 1238–1243. [Google Scholar] [CrossRef]
- Thangabalan, B.; Kahsay, G.; Eticha, T. New RP-HPLC Method Development and Validation for Dorzolamide in Ophthalmic Dosage Form. J Anal Methods Chem 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Tiwari, B.; Shirsat, M.K.; Kulkarni, A. Analytical Method Development and Validation for the Determination of Brinzolamide by RP-HPLC. Journal of Drug Delivery and Therapeutics 2020, 10, 92–96. [Google Scholar] [CrossRef]
- Mitrović, M.; Protić, A.; Malenović, A.; Otašević, B.; Zečević, M. Analytical Quality by Design Development of an Ecologically Acceptable Enantioselective HPLC Method for Timolol Maleate Enantiomeric Purity Testing on Ovomucoid Chiral Stationary Phase. J Pharm Biomed Anal 2020, 180, 113034. [Google Scholar] [CrossRef]
- Mohamed, A.-M.I.; Abdel-Wadood, H.M.; Mousa, H.S. Simultaneous Determination of Dorzolomide and Timolol in Aqueous Humor: A Novel Salting out Liquid–Liquid Microextraction Combined with HPLC. Talanta 2014, 130, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Mathrusri Annapurna, M.; Narendra, A.; Deepika, D. DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF DORZOLAMIDE AND TIMOLOL MALEATE IN PHARMACEUTICAL DOSAGE FORMS. Journal of Drug Delivery and Therapeutics 2012, 2. [Google Scholar] [CrossRef]
- Erk, N. Rapid and Sensitive HPLC Method for the Simultaneous Determination of Dorzolamide Hydrochloride and Timolol Maleate in Eye Drops with Diode-Array and UV Detection. Pharmazie 2003, 58, 491–493. [Google Scholar] [PubMed]
- Tiwari, B.; Shirsat, M.K.; Kulkarni, A. Analytical Method Development and Validation for the Determination of Brinzolamide by RP-HPLC. Journal of Drug Delivery and Therapeutics 2020, 10, 92–96. [Google Scholar] [CrossRef]
- Mandour, A.A.; Nabil, N.; Zaazaa, H.E.; Ibrahim, M.M.; Ibrahim, M.A. Two Stability Indicating Chromatographic Methods: TLC Densitometric versus HPLC Method for the Simultaneous Determination of Brinzolamide and Timolol Maleate in Ophthalmic Formulation in the Presence of Probable Carcinogenic Oxidative Degradation Product of Timolol Maleate. Separations 2023, 10, 37. [Google Scholar] [CrossRef]
- Mandour, A.A. , Nabil, N., Zaazaa, H.E. et al. Review on Analytical Studies of Some Pharmaceutical Compounds Containing Heterocyclic Rings: Brinzolamide, Timolol Maleate, Flumethasone Pivalate, and Clioquinol. Futur J Pharm Sci 2020, 6, 52. [Google Scholar] [CrossRef]
- Abd-AlGhafar, W.N.; Aly, F.A.; Sheribah, Z.A.; Saad, S. Green HPLC Method with Time Programming for the Determination of the Co-Formulated Eye Drops of Tafluprost and Timolol in Their Challengeable Ratio. BMC Chem 2022, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.A.; Nabil, N.; Zaazaa, H.E.; Abdelkawy, M. Review on Analytical Studies of Some Pharmaceutical Compounds Containing Heterocyclic Rings: Brinzolamide, Timolol Maleate, Flumethasone Pivalate, and Clioquinol. Futur J Pharm Sci 2020, 6, 52. [Google Scholar] [CrossRef]
- Ibrahim, F.A.; Elmansi, H.M.; El Abass, S.A. A Versatile HPLC Method with an Isocratic Single Mobile Phase System for Simultaneous Determination of Anti-Glaucoma Formulations Containing Timolol. Ann Pharm Fr 2019, 77, 302–312. [Google Scholar] [CrossRef]
- Hassib, S.T.; Elkady, E.F.; Sayed, R.M. Simultaneous Determination of Timolol Maleate in Combination with Some Other Anti-Glaucoma Drugs in Rabbit Aqueous Humor by High Performance Liquid Chromatography–Tandem Mass Spectroscopy. Journal of Chromatography B 2016, 1022, 109–117. [Google Scholar] [CrossRef]
- Larsen, C.; Lundberg, P.; Tang, S.; Ràfols-Ribé, J.; Sandström, A.; Mattias Lindh, E.; Wang, J.; Edman, L. A Tool for Identifying Green Solvents for Printed Electronics. Nat Commun 2021, 12, 4510. [Google Scholar] [CrossRef]
- Available online: Https://Www.Sigmaaldrich.Com/DE/En/Sds/SIAL/W292912?UserType=undefined (accessed on 1 February 2024).
- Available online: Https://Green-Solvent-Tool.Herokuapp.Com/ (accessed on 2 November 2023).
- International Conference on Harmonization Geneva. ICH Guideline for Validation of Analytical Procedure: Text and Methodology, Q2 (R1); ICH: Geneva, Switzerland, 2005. Available online: Https://Database.Ich.Org/Sites/Default/Files/Q2%28R1%29%20Guideline.Pdf (accessed on 2 November 2023).
| Solvent | UV Cut-off value (nm) | Water solubility | Density (g/cm3) at 20 °C | Polarity Parameter Kamlet-Taft π⁕ | Partition coefficient n-octanol/water (log value) | Boiling Point °C |
Flash Point °C at 1.013 hPa (c.c.) |
G score |
|---|---|---|---|---|---|---|---|---|
| Acetonitrile | 190 | miscible in any proportion | 0.78 | 0,75 | -0.54 | 82 | 2 | 5.8 |
| Methanol | 205 | 1000 g/l at 20 °C - completely miscible | 0.791 | 0,61 | -0.77 | 64.7 | 9.7 | 5.8 |
| Ethanol | 210 | ≥1000 g /l at 20 °C | 0.81 | 0,54 | -0.31 | 78 | 9.7 | 6.6 |
| Isopropanol | 205 | miscible in any proportion | 0.786 | 0,48 | 0.05 | 82.4 | 12 | 6.5 |
| Parameter | Dorzolamide | Brinzolamide | Timolol |
|---|---|---|---|
| Linearity (R2) | 0.9995 | 0.9999 | 0.9979 |
| Equation | y = 3947.2x + 10691 | y = 1624.1x - 1048.1 | y = 117.05x - 213.15 |
| Linearity Range (µg/mL) | 20-70 | 40-140 | 20-70 |
| LOD (µg/mL) | 1.61 | 1.60 | 3.16 |
| LOQ (µg/mL) | 4.87 | 4.86 | 9.59 |
| Accuracy (µg/mL) | 99.1–101.0% | 99.3–100.1% | 95.3–101.8% |
| Precision RSD% | |||
| LQC | 0.08% | 0.04% | 0.03% |
| MQC | 0.02% | 0.01% | 0.02% |
| HQC | 0.02% | 0.07% | 0.04% |
| Method | Elution Conditions | Reference | |
|---|---|---|---|
| A | Reported acetonitrile (ACN)-based nongreen method. | HPLC-DAD using RP-C18 column. Isocratic elution using ACN and phosphate buffer (30:70, v/v) as mobile phase | [41] |
| B | Newly developed green isopropanol-based reference method | HPLC-DAD using the C18 column. Isocratic elution using isopropanol and 0.1 M sodium acetate buffer pH 4.25 (10:90, v/v) as mobile phase | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





