Submitted:
02 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
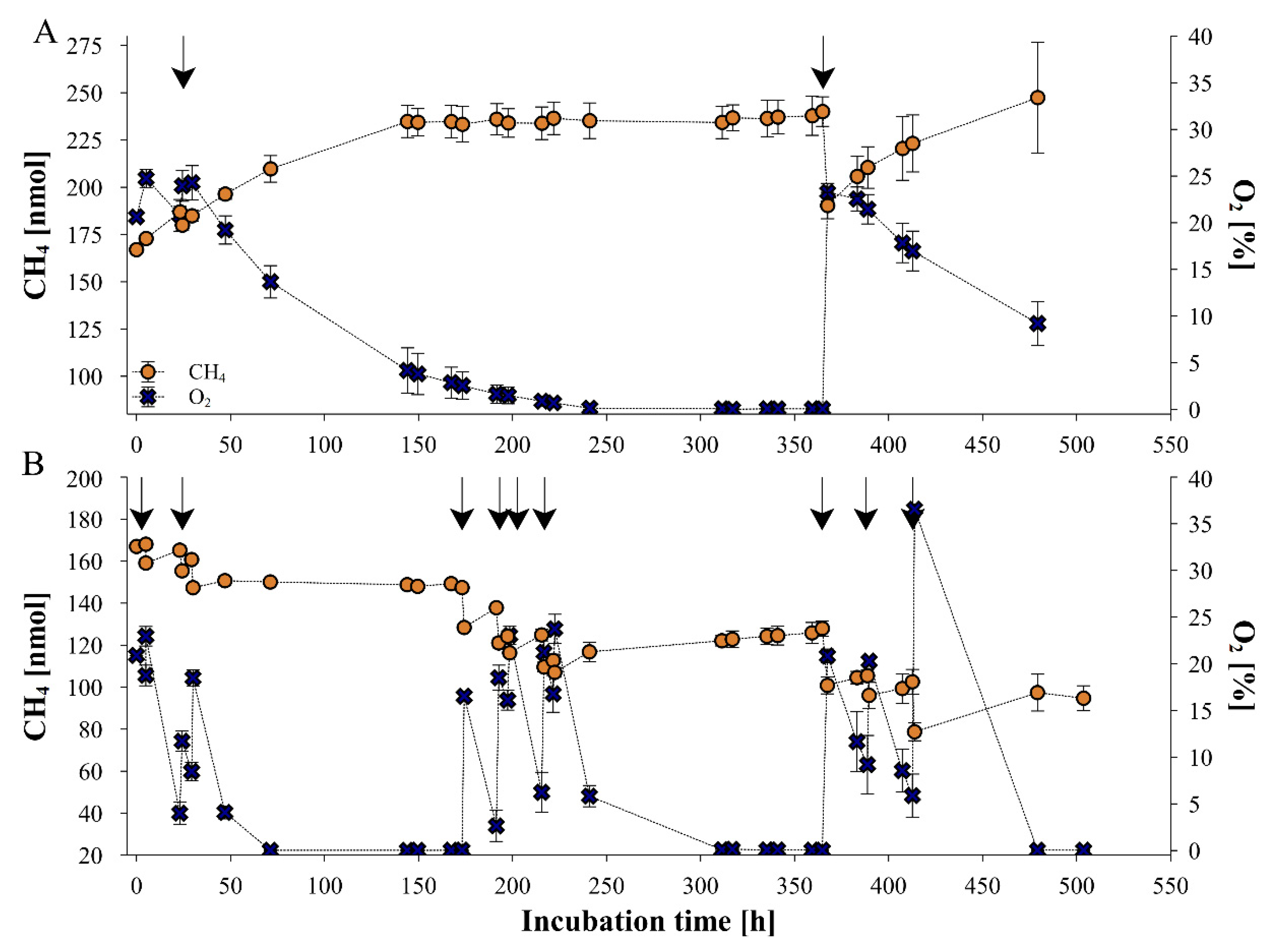
2.1. Dependance of fungal CH4 production on ambient O2 concentrations
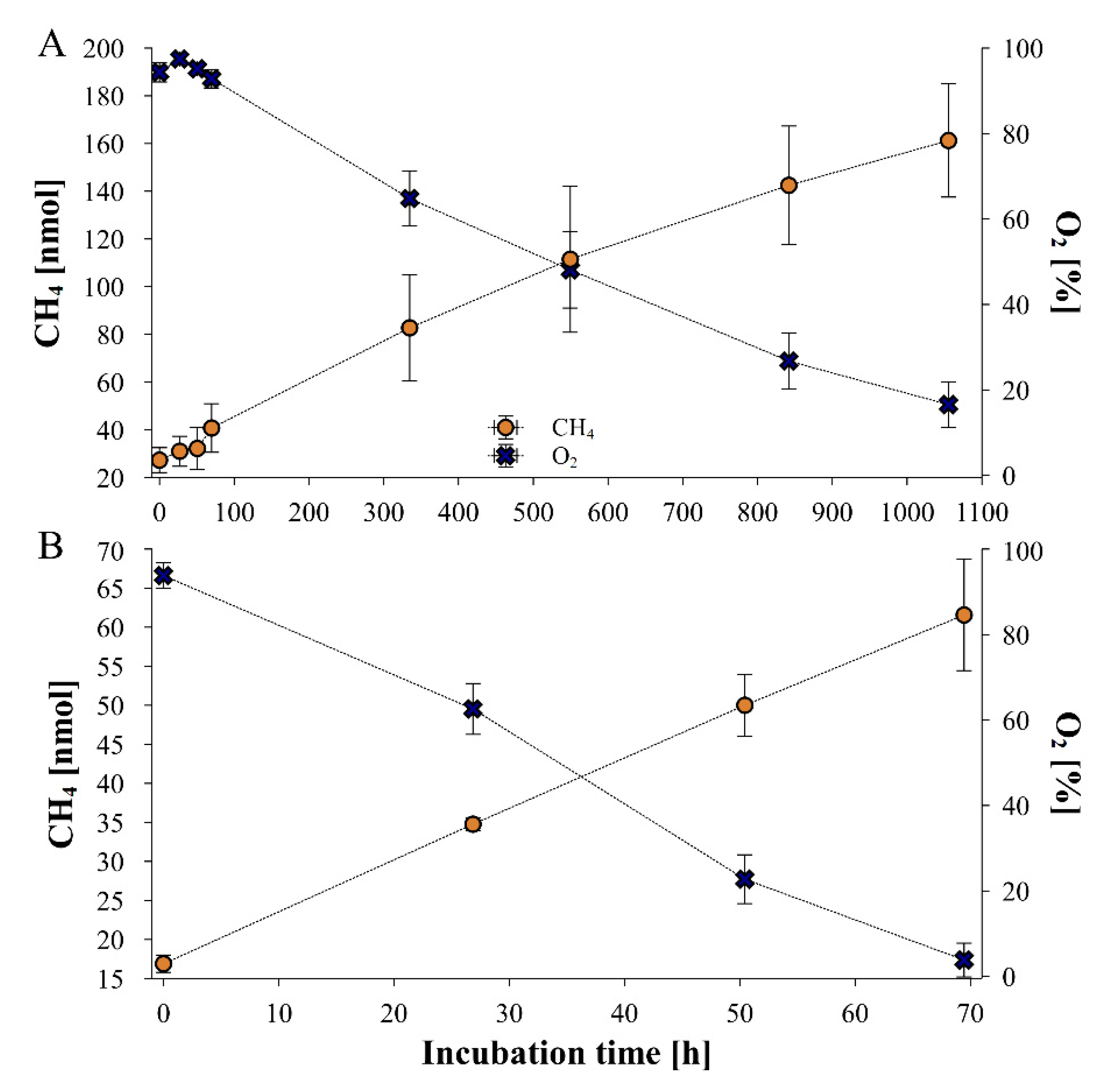
2.2. Fungal CH4 production starting at elevated O2 mixing ratios of near 100%
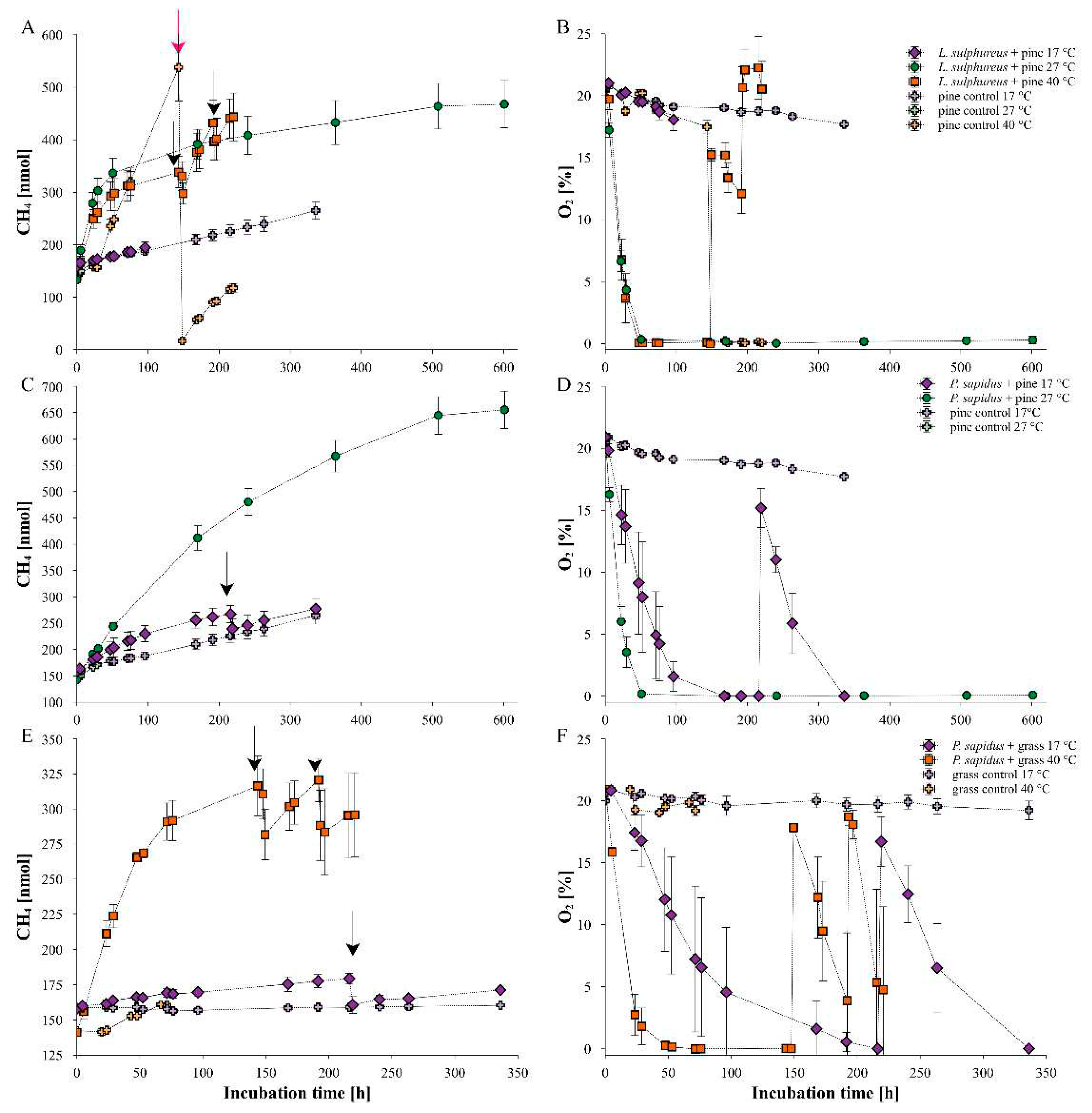
2.3. Temperature dependency of fungal CH4 formation, O2 consumption and CH4_norm rates
3. Discussion
3.1. Dependence of fungal CH4 formation on O2 levels
3.2. Temperature influence on fungal CH4 formation dynamics
4. Materials and Methods
4.1. Selected fungi
4.2. Incubation experiments
4.3. Measurement of CH4, CO2 and O2 concentrations
4.4. Calculations and statistical methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. [CrossRef]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three Decades of Global Methane Sources and Sinks. Nature Geosci 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.G.; Braß, M.; Röckmann, T. Methane Emissions from Terrestrial Plants under Aerobic Conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, K.; Weber, B.; Elbert, W.; Steinkamp, J.; Clough, T.; Crutzen, P.; Pöschl, U.; Keppler, F. Nitrous Oxide and Methane Emissions from Cryptogamic Covers. Glob Change Biol 2015, 21, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, K.; Klintzsch, T.; Langer, G.; Nehrke, G.; Bunge, M.; Schnell, S.; Keppler, F. Evidence for methane production by the marine algae Emiliania huxleyi; Biogeochemistry, 2016, 13, 3163-3174. 13. [CrossRef]
- Klintzsch, T.; Langer, G.; Nehrke, G.; Wieland, A.; Lenhart, K.; Keppler, F. Methane Production by Three Widespread Marine Phytoplankton Species: Release Rates, Precursor Compounds, and Potential Relevance for the Environment. Biogeosciences 2019, 16, 4129–4144. [Google Scholar] [CrossRef]
- Bižić, M.; Klintzsch, T.; Ionescu, D.; Hindiyeh, M.Y.; Günthel, M.; Muro-Pastor, A.M.; Eckert, W.; Urich, T.; Keppler, F.; Grossart, H.-P. Aquatic and Terrestrial Cyanobacteria Produce Methane. Sci. Adv. 2020, 6, eaax5343. [Google Scholar] [CrossRef] [PubMed]
- Wishkerman, A.; Greiner, S.; Ghyczy, M.; Boros, M.; Rausch, T.; Lenhart, K.; Keppler, F. Enhanced Formation of Methane in Plant Cell Cultures by Inhibition of Cytochrome c Oxidase. Plant Cell & Environment 2011, 34, 457–464. [Google Scholar] [CrossRef]
- Ernst, L.; Steinfeld, B.; Barayeu, U.; Klintzsch, T.; Kurth, M.; Grimm, D.; Dick, T.P.; Rebelein, J.G.; Bischofs, I.B.; Keppler, F. Methane Formation Driven by Reactive Oxygen Species across All Living Organisms. Nature 2022, 603, 482–487. [Google Scholar] [CrossRef]
- Boros, M.; Keppler, F. Methane Production and Bioactivity-A Link to Oxido-Reductive Stress. Front. Physiol. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Ghyczy, M.; Torday, C.; Kaszaki, J.; Szabó, A.; Czóbel, M.; Boros, M. Hypoxia-Induced Generation of Methane in Mitochondria and Eukaryotic Cells - An Alternative Approach to Methanogenesis. Cell Physiol Biochem 2008, 21, 251–258. [Google Scholar] [CrossRef]
- Keppler, F.; Boros, M.; Polag, D. Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine. Antioxidants 2023, 12, 1381. [Google Scholar] [CrossRef] [PubMed]
- Polag, D.; Keppler, F. Effect of Immune Responses on Breath Methane Dynamics. J. Breath Res. 2023, 17, 046005. [Google Scholar] [CrossRef] [PubMed]
- Polag, D.; Keppler, F. Global Methane Emissions from the Human Body: Past, Present and Future. Atmospheric Environment 2019, 214, 116823. [Google Scholar] [CrossRef]
- Keppler, F.; Schiller, A.; Ehehalt, R.; Greule, M.; Hartmann, J.; Polag, D. Stable Isotope and High Precision Concentration Measurements Confirm That All Humans Produce and Exhale Methane. J. Breath Res. 2016, 10, 016003. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, K.; Bunge, M.; Ratering, S.; Neu, T.R.; Schüttmann, I.; Greule, M.; Kammann, C.; Schnell, S.; Müller, C.; Zorn, H.; et al. Evidence for Methane Production by Saprotrophic Fungi. Nat Commun 2012, 3, 1046. [Google Scholar] [CrossRef] [PubMed]
- Schroll, M.; Keppler, F.; Greule, M.; Eckhardt, C.; Zorn, H.; Lenhart, K. The Stable Carbon Isotope Signature of Methane Produced by Saprotrophic Fungi. Biogeosciences 2020, 17, 3891–3901. [Google Scholar] [CrossRef]
- Swift, M.; Heal, O.; Anderson, J. Decomposition in Terrestrial Ecosystems; University of California Press: Berkley, CA, USA, 1979; Vol. 5. [Google Scholar]
- Mukhin, V.A.; Voronin, P.Yu. Methanogenic Activity of Woody Debris. Russ J Ecol 2009, 40, 149–153. [Google Scholar] [CrossRef]
- Mukhin, V.A.; Voronin, P.Yu. Methane Emission during Wood Fungal Decomposition. Dokl Biol Sci 2007, 413, 159–160. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Ma, X.; Han, M.; Kneeshaw, D.; Sun, H.; Malghani, S.; Chen, H.; Wang, W. Methane Emissions May Be Driven by Hydrogenotrophic Methanogens Inhabiting the Stem Tissues of Poplar. New Phytologist 2022, 233, 182–193. [Google Scholar] [CrossRef]
- Hietala, A.; Dörsch, P.; Kvaalen, H.; Solheim, H. Carbon Dioxide and Methane Formation in Norway Spruce Stems Infected by White-Rot Fungi. Forests 2015, 6, 3304–3325. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Xue, Y.; Pan, B.; Xiao, L.; Wang, S.; Lever, M.A.; Hinrichs, K.-U.; Inagaki, F.; Liu, C. Methane Production by Facultative Anaerobic Wood-Rot Fungi via a New Halomethane-Dependent Pathway. Microbiol Spectr 2022, 10, e01700-22. [Google Scholar] [CrossRef] [PubMed]
- McNally, K.J.; Harper, D.B. Methylation of Phenol by Chloromethane in the Fungus Phellinus Pomaceus. Journal of General Microbiology 1991, 137, 1029–1032. [Google Scholar] [CrossRef]
- Kipping, L.; Gossner, M.M.; Koschorreck, M.; Muszynski, S.; Maurer, F.; Weisser, W.W.; Jehmlich, N.; Noll, M. Emission of CO 2 and CH 4 From 13 Deadwood Tree Species Is Linked to Tree Species Identity and Management Intensity in Forest and Grassland Habitats. Global Biogeochemical Cycles 2022, 36, e2021GB007143. [Google Scholar] [CrossRef]
- Venugopal, P.; Junninen, K.; Linnakoski, R.; Edman, M.; Kouki, J. Climate and Wood Quality Have Decayer-Specific Effects on Fungal Wood Decomposition. Forest Ecology and Management 2016, 360, 341–351. [Google Scholar] [CrossRef]
- Mukhortova, L.; Pashenova, N.; Meteleva, M.; Krivobokov, L.; Guggenberger, G. Temperature Sensitivity of CO2 and CH4 Fluxes from Coarse Woody Debris in Northern Boreal Forests. Forests 2021, 12, 624. [Google Scholar] [CrossRef]
- Scheffer, T.C. O2 Requirements for Growth and Survival of Wood-Decaying and Sapwood-Staining Fungi. Can. J. Bot. 1986, 64, 1957–1963. [Google Scholar] [CrossRef]
- Tavzes, C.; Pohleven, F.; Koestler, R.J. Effect of Anoxic Conditions on Wood-Decay Fungi Treated with Argon or Nitrogen. International Biodeterioration 2001. [Google Scholar] [CrossRef]
- Mukhin, V.A.; Diyarova, D.K. Eco-Physiological Adaptations of the Xylotrophic Basidiomycetes Fungi to CO2 and O2 Mode in the Woody Habitat. JoF 2022, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Highley, T.L.; Bar-Lev, S.S.; Kirk, T.K.; Larsen, M.J. Influence of O2 and CO2 on Wood Decay by Heartrot and Saprot Fungi. Phytopathology 1982, 630–633. [Google Scholar]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive Effects of Temperature and Soil Moisture on Fungal-Mediated Wood Decomposition and Extracellular Enzyme Activity. Soil Biology and Biochemistry 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Meier, C.L.; Rapp, J.; Bowers, R.M.; Silman, M.; Fierer, N. Fungal Growth on a Common Wood Substrate across a Tropical Elevation Gradient: Temperature Sensitivity, Community Composition, and Potential for above-Ground Decomposition. Soil Biology and Biochemistry 2010, 42, 1083–1090. [Google Scholar] [CrossRef]
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodall, C.W. Release of Coarse Woody Detritus-Related Carbon: A Synthesis across Forest Biomes. Carbon Balance Manage 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-Y.; Xie, G.-J.; Ding, J.; Liu, B.-F.; Xing, D.-F.; Ren, N.-Q.; Wang, Q. Microbial Methane Emissions from the Non-Methanogenesis Processes: A Critical Review. Science of The Total Environment 2022, 806, 151362. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, V.A.; Voronin, P.Yu. Methane Emission during Wood Fungal Decomposition. Dokl Biol Sci 2007, 413, 159–160. [Google Scholar] [CrossRef]
- Epron, D.; Mochidome, T.; Tanabe, T.; Dannoura, M.; Sakabe, A. Variability in Stem Methane Emissions and Wood Methane Production of Different Tree Species in a Cold Temperate Mountain Forest. Ecosystems 2023, 26, 784–799. [Google Scholar] [CrossRef]
- Klintzsch, T.; Geisinger, H.; Wieland, A.; Langer, G.; Nehrke, G.; Bizic, M.; Greule, M.; Lenhart, K.; Borsch, C.; Schroll, M.; et al. Stable Carbon Isotope Signature of Methane Released From Phytoplankton. Geophysical Research Letters 2023, 50, e2023GL103317. [Google Scholar] [CrossRef]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and Transformation of Humic Substances by Saprotrophic Fungi: Processes and Mechanisms. Fungal Biology Reviews 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Valášková, V.; Baldrian, P. Degradation of Cellulose and Hemicelluloses by the Brown Rot Fungus Piptoporus Betulinus – Production of Extracellular Enzymes and Characterization of the Major Cellulases. Microbiology 2006, 152, 3613–3622. [Google Scholar] [CrossRef]
- Hammel, K.E.; Kapich, A.N.; Jensen, K.A.; Ryan, Z.C. Reactive Oxygen Species as Agents of Wood Decay by Fungi. Enzyme and Microbial Technology 2002, 30, 445–453. [Google Scholar] [CrossRef]
- Zhu, Y.; Plaza, N.; Kojima, Y.; Yoshida, M.; Zhang, J.; Jellison, J.; Pingali, S.V.; O’Neill, H.; Goodell, B. Nanostructural Analysis of Enzymatic and Non-Enzymatic Brown Rot Fungal Deconstruction of the Lignocellulose Cell Wall†. Front. Microbiol. 2020, 11, 1389. [Google Scholar] [CrossRef]
- Beltrán-Flores, E.; Tayar, S.; Blánquez, P.; Sarrà, M. Effect of Dissolved Oxygen on the Degradation Activity and Consumption Capacity of White-Rot Fungi. Journal of Water Process Engineering 2023, 55, 104105. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0. 05.” The American Statistician 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Van Den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in Aquatic Ecosystems. Nat Rev Microbiol 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B. Ultraviolet Radiation Drives Methane Emissions from Terrestrial Plant Pectins. New Phytologist 2008, 180, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Vigano, I.; Weelden, H.V.; Holzinger, R.; Keppler, F.; Röckmann, T. Effect of UV Radiation and Temperature on the Emission of Methane from Plant Biomass and Structural Components. 2008.
- Ernst, L.; Barayeu, U.; Hädeler, J.; Dick, T.P.; Klatt, J.M.; Keppler, F.; Rebelein, J.G. Methane Formation Driven by Light and Heat Prior to the Origin of Life and Beyond. Nat Commun 2023, 14, 4364. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.G.; McRoberts, W.C.; Vigano, I.; Braß, M.; Röckmann, T. Methoxyl Groups of Plant Pectin as a Precursor of Atmospheric Methane: Evidence from Deuterium Labelling Studies. New Phytologist 2008, 178, 808–814. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B. Ultraviolet Radiation Drives Methane Emissions from Terrestrial Plant Pectins. New Phytologist 2008, 180, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Althoff, F.; Jugold, A.; Keppler, F. Methane Formation by Oxidation of Ascorbic Acid Using Iron Minerals and Hydrogen Peroxide. Chemosphere 2010, 80, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Kerscher, M.; Krause, T.; Schöler, H.F. Iron-Catalysed Oxidation and Halogenation of Organic Matter in Nature. Environ. Chem. 2015, 12, 381. [Google Scholar] [CrossRef]
- Benzing, K.; Comba, P.; Martin, B.; Pokrandt, B.; Keppler, F. Nonheme Iron-Oxo-Catalyzed Methane Formation from Methyl Thioethers: Scope, Mechanism, and Relevance for Natural Systems. Chemistry A European J 2017, 23, 10465–10472. [Google Scholar] [CrossRef]
- Althoff, F.; Benzing, K.; Comba, P.; McRoberts, C.; Boyd, D.R.; Greiner, S.; Keppler, F. Abiotic Methanogenesis from Organosulphur Compounds under Ambient Conditions. Nat Commun 2014, 5, 4205. [Google Scholar] [CrossRef] [PubMed]
- Hädeler, J.; Velmurugan, G.; Lauer, R.; Radhamani, R.; Keppler, F.; Comba, P. Natural Abiotic Iron-Oxido-Mediated Formation of C 1 and C 2 Compounds from Environmentally Important Methyl-Substituted Substrates. J. Am. Chem. Soc. 2023; jacs.3c06709. [Google Scholar] [CrossRef]
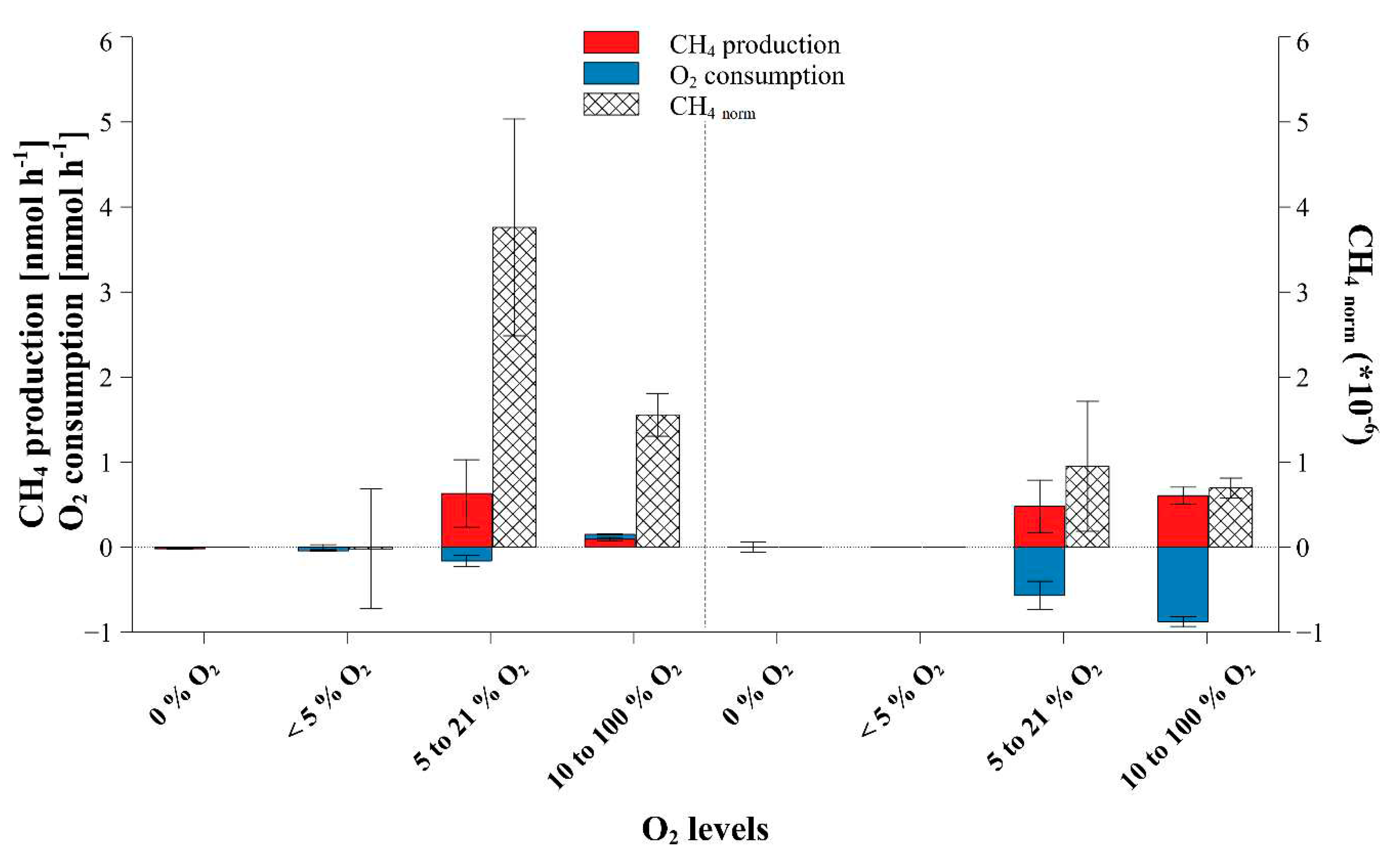
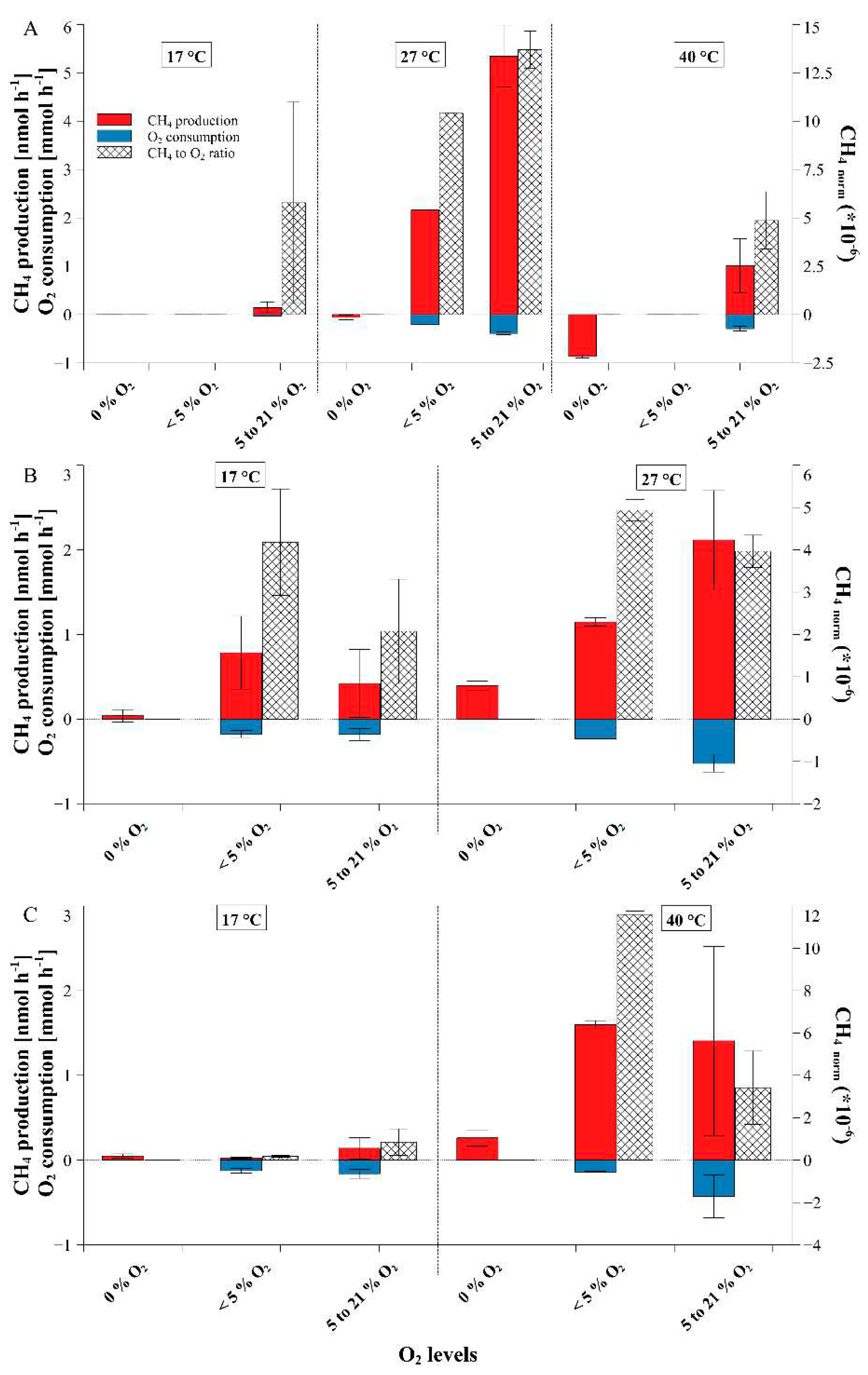
| Fungi | Medium | n | Temperature [°C] |
O2 mixing ratio [%] |
O2 added |
O2 range [%] |
N | CH4 formation rate [nmol h-1] |
O2 consumption rate [mmol h-1] |
CH4_norm [10-6] |
| P. sapidus | beech | 4 | 27 | 0 to 26.0 | 0 | 8 | 0 ± 0.06 | 0 ± 0 | - | |
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 38 | 0.48 ± 0.31 | -0.57 ± 0.17 | 0.95 ± 0.76 | ||||||
| beech | 4 | 27 | 14 to 94.5 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 14 to 94 | 4 | 0.61 ± 0.1 | -0.88 ± 0.06 | 0.7 ± 0.12 | ||||||
| pine | 3 | 17 | 0 to 20.9 | 0 | 3 | 0.04 ± 0.07 | 0 ± 0 | - | ||
| 0 to 5 | 3 | 0.78 ± 0.43 | -0.18 ± 0.04 | 4.18 ± 1.26 | ||||||
| 5 to 20.9 | 6 | 0.42 ± 0.4 | -0.18 ± 0.07 | 2.08 ± 1.23 | ||||||
| pine | 4 | 27 | 0 to 20.9 | 0 | 4 | 0.4 ± 0.05 | 0 ± 0 | - | ||
| 0 to 5 | 3 | 1.15 ± 0.05 | -0.23 ± 0 | 4.94 ± 0.25 | ||||||
| 5 to 20 | 4 | 2.12 ± 0.58 | -0.53 ± 0.1 | 3.97 ± 0.38 | ||||||
| grass | 3 | 17 | 0 to 20.9 | 0 | 2 | 0.05 ± 0.03 | 0 ± 0 | - | ||
| 0 to 5 | 2 | 0.02 ± 0.01 | -0.12 ± 0.03 | 0.19 ± 0.05 | ||||||
| 5 to 20.9 | 6 | 0.14 ± 0.13 | -0.16 ± 0.05 | 0.86 ± 0.62 | ||||||
| grass | 3 | 40 | 0 to 20.9 | 0 | 3 | 0.26 ± 0.09 | 0 ± 0 | - | ||
| 0 to 5 | 2 | 1.6 ± 0.05 | -0.14 ± 0.01 | 11.6 ± 0.17 | ||||||
| 5 to 20.9 | 7 | 1.41 ± 1.11 | -0.43 ± 0.25 | 3.41 ± 1.73 | ||||||
| L. sulphureus | beech | 4 | 27 | 0 to 30.6 | 0 | 4 | -0.02 ± 0.01 | 0 ± 0 | - | |
| 0 to 5 | 3 | 0 ± 0.03 | -0.04 ± 0 | -0.02 ± 0.7 | ||||||
| 5 to 20.9 | 12 | 0.63 ± 0.4 | -0.16 ± 0.07 | 3.76 ± 1.28 | ||||||
| beech | 4 | 27 | 16.6 to 97.5 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 16 to 97.5 | 4 | 0.09 ± 0.02 | 0.06 ± 0 | 1.56 ± 0.25 | ||||||
| pine | 3 | 17 | 11.1 to 20.9 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 11.1 to 20.9 | 3 | 0.15 ± 0.11 | -0.03 ± 0.01 | 5.78 ± 5.2 | ||||||
| pine | 4 | 27 | 0 to 20.9 | 0 | 3 | -0.06 ± 0.05 | 0 ± 0 | - | ||
| 0 to 5 | 1 | 2.16 ± 0 | -0.21 ± 0 | 10.4 ± 0 | ||||||
| 5 to 20.9 | 3 | 5.34 ± 0.64 | -0.39 ± 0.03 | 13.69 ± 0.97 | ||||||
| pine | 3 | 40 | 0 to 25.8 | 0 | 3 | -0.86 ± 0.03 | 0 ± 0 | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 5 to 25.8 | 5 | 1.01 ± 0.55 | -0.29 ± 0.05 | 4.87 ± 1.49 | ||||||
| Controls | pine | 3 | 17 | 20.9 | 0 | - | - | - | - | |
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 3 | 0.3 ± 0.05 | -0.01 ± 0 | 35.68 ± 4.8 | ||||||
| pine | 3 | 27 | 20.9 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 3 | 0.91 ± 0.09 | - | - | ||||||
| pine | 3 | 40 | 20.9 | 0 | 3 | 1.37 ± 0.1 | 0 ± 0 | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 3 | 3.26 ± 0.53 | -0.02 ± 0 | - | ||||||
| grass | 3 | 17 | 20.9 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 3 | 0.01 ± 0 | 0 ± 0 | - | ||||||
| grass | 3 | 27 | 20.9 | 0 | - | - | - | - | ||
| 0 to 5 | - | - | - | - | ||||||
| 5 to 20.9 | 3 | 0.31 ± 0.01 | 0 ± 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





