Submitted:
29 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Laboratory Incubation Experiment
2.3. Soil Chemical Analyses
2.4. Microbial Biomass C and N
2.5. Enzyme Analysis
2.6. Phospholipid Fatty Acids (PLFAs) Analysis
2.7. Calculation of Rate, Cumulative Mineralization, and Temperature Sensitivity
2.8. Statistical Analyses
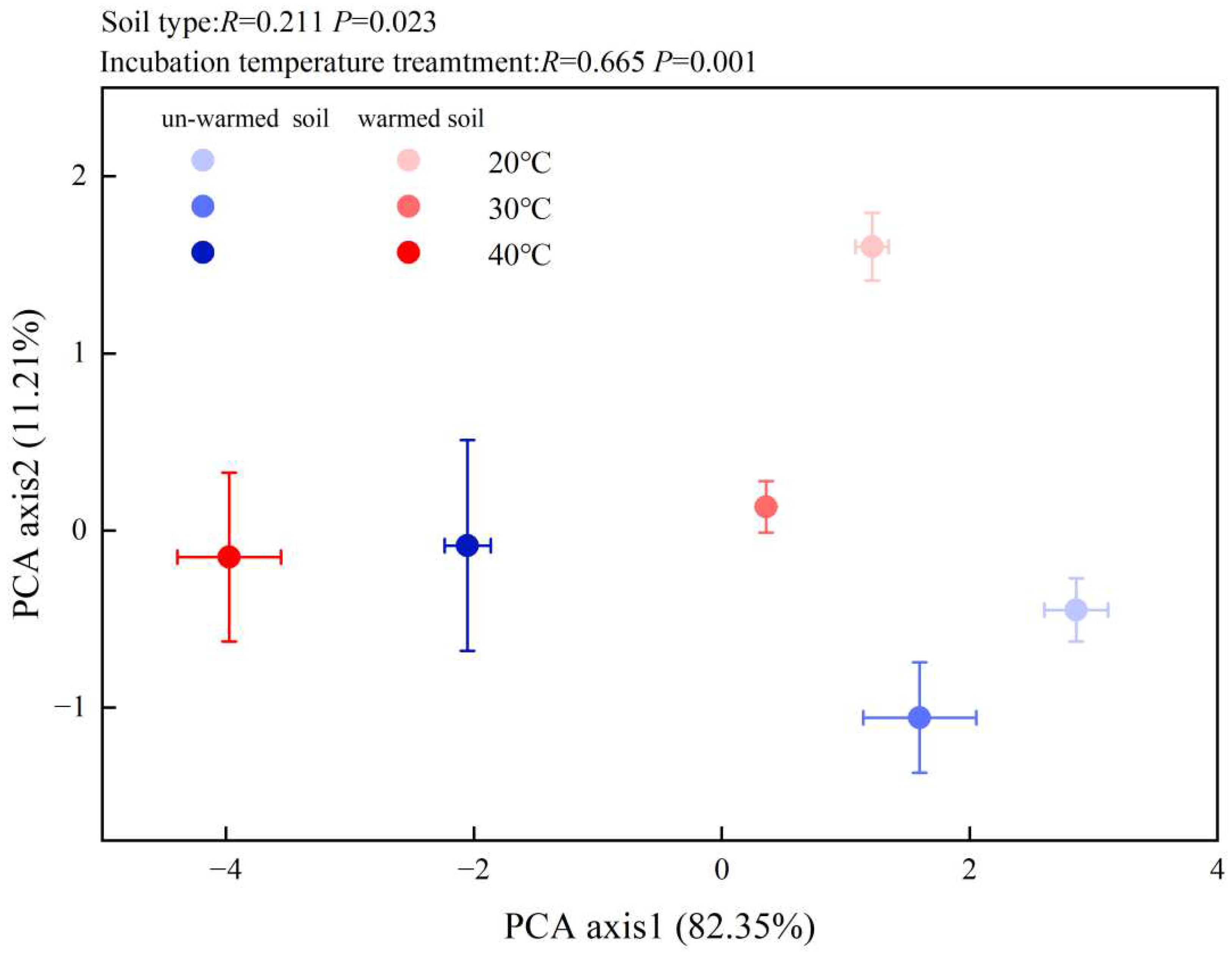
3. Results
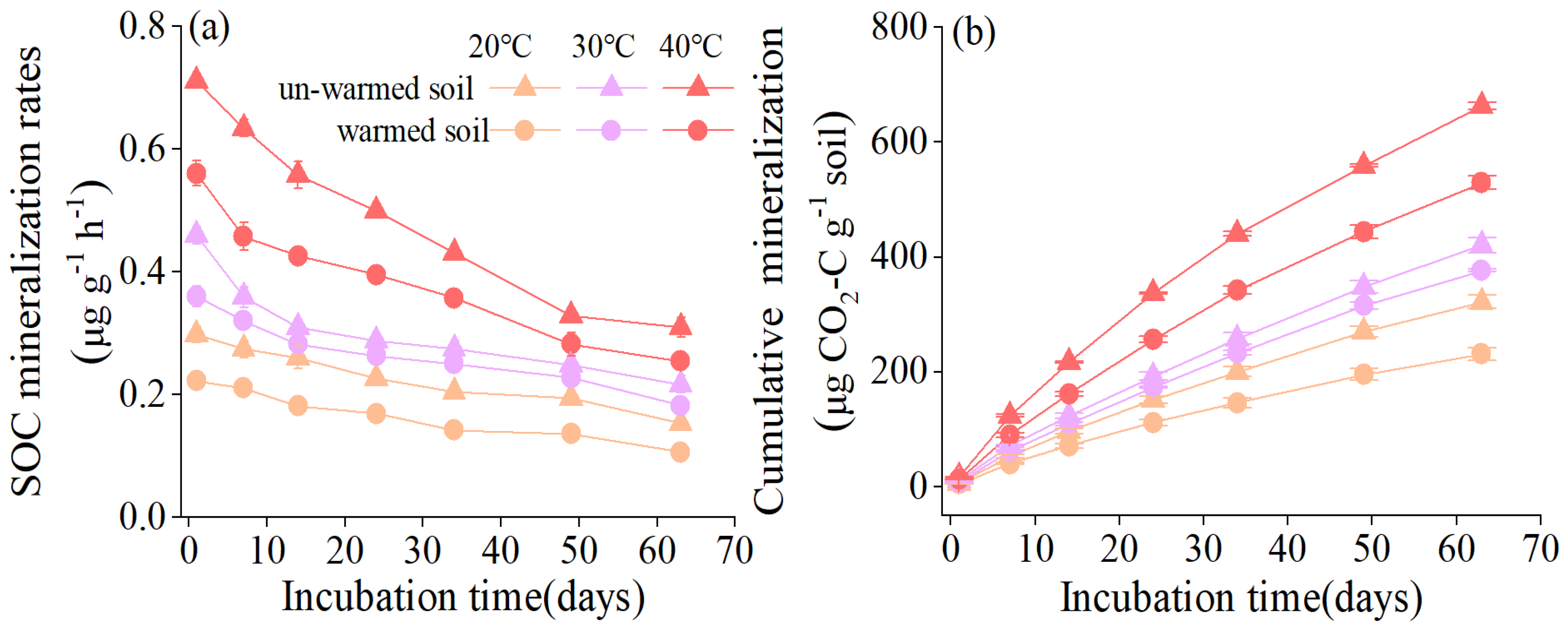
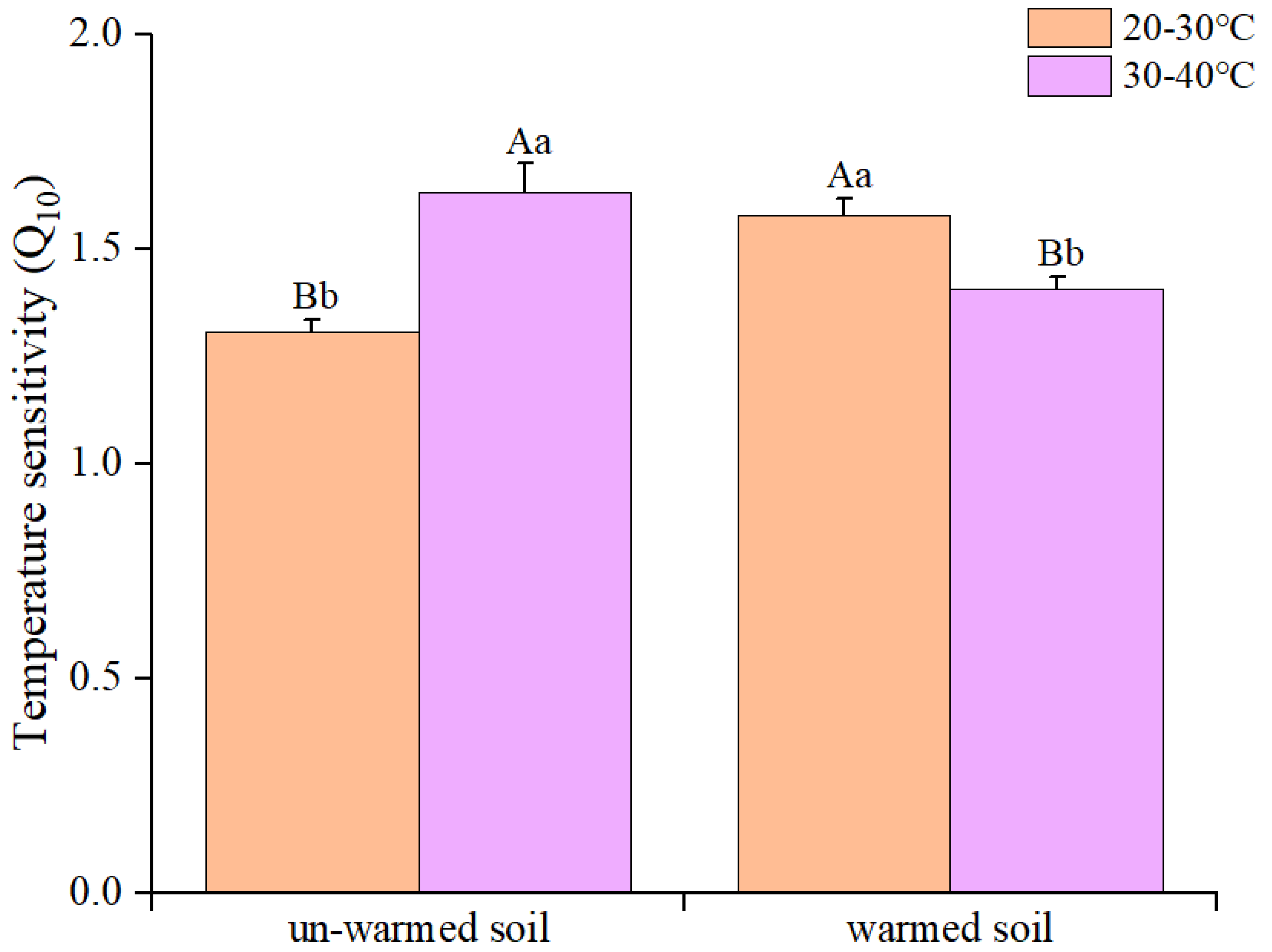
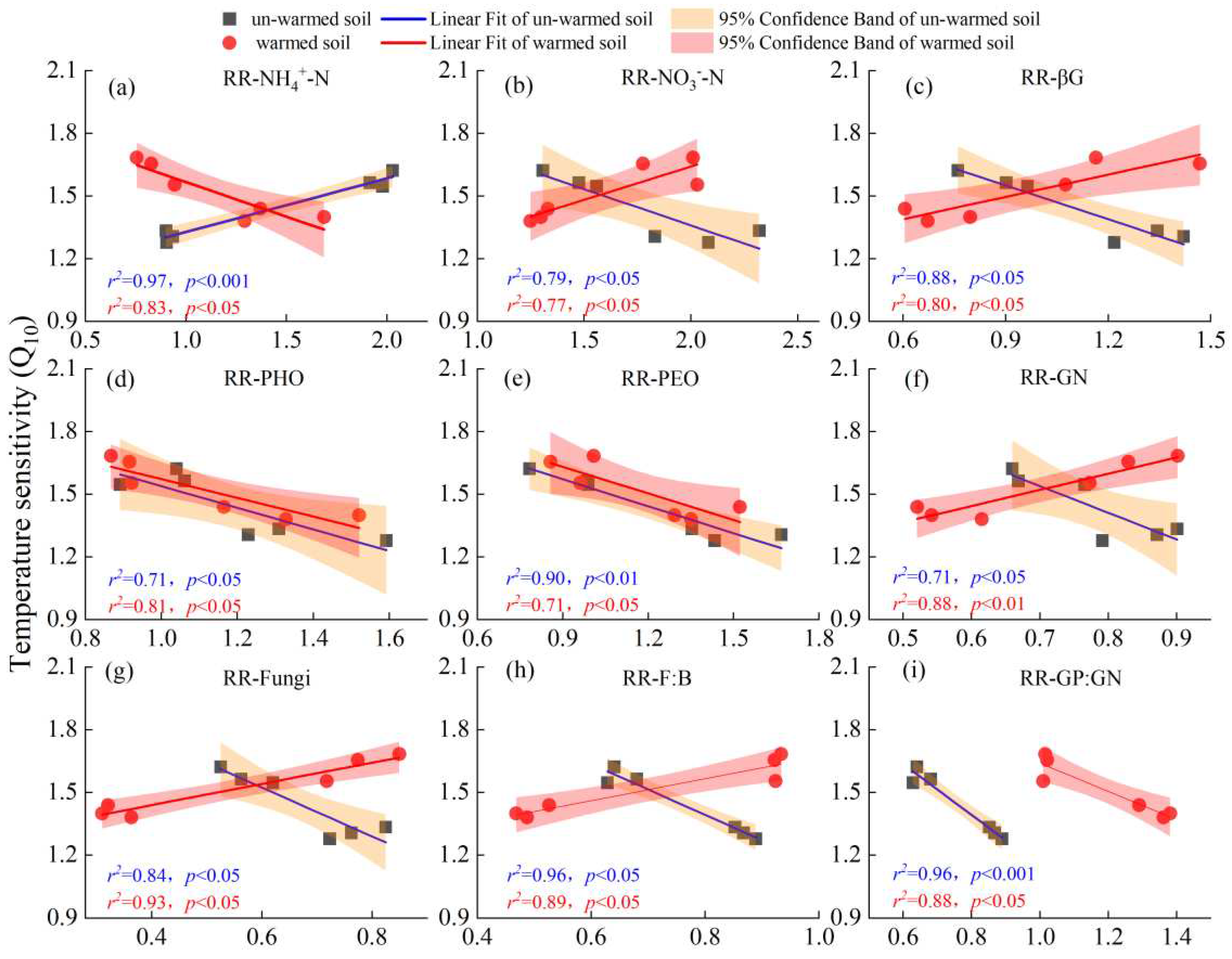
3.1. Soil Organic Carbon Mineralization and Its Temperature Sensitivity
3.2. Soil Nutrients, MBC, Microbial Metabolic Quotients
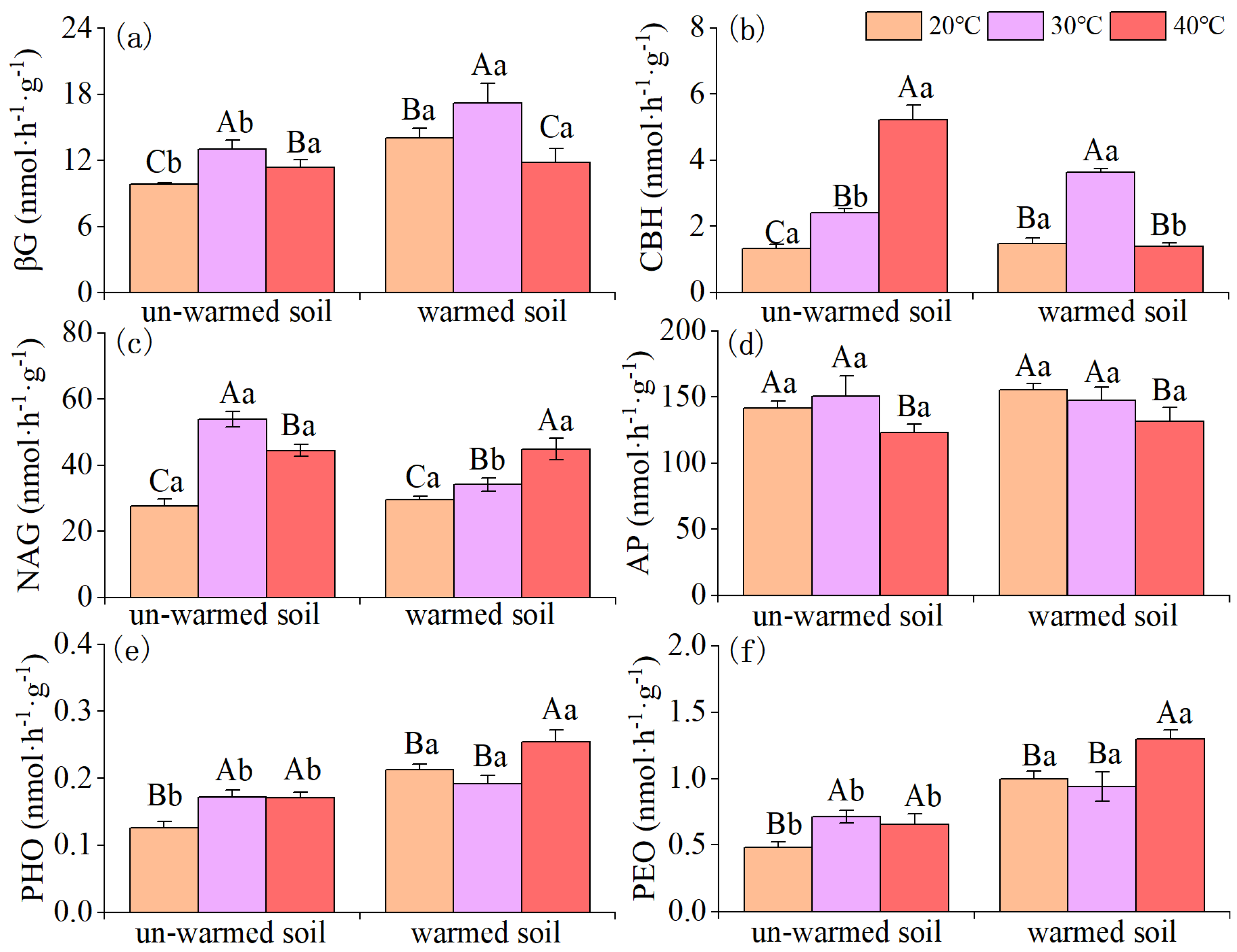
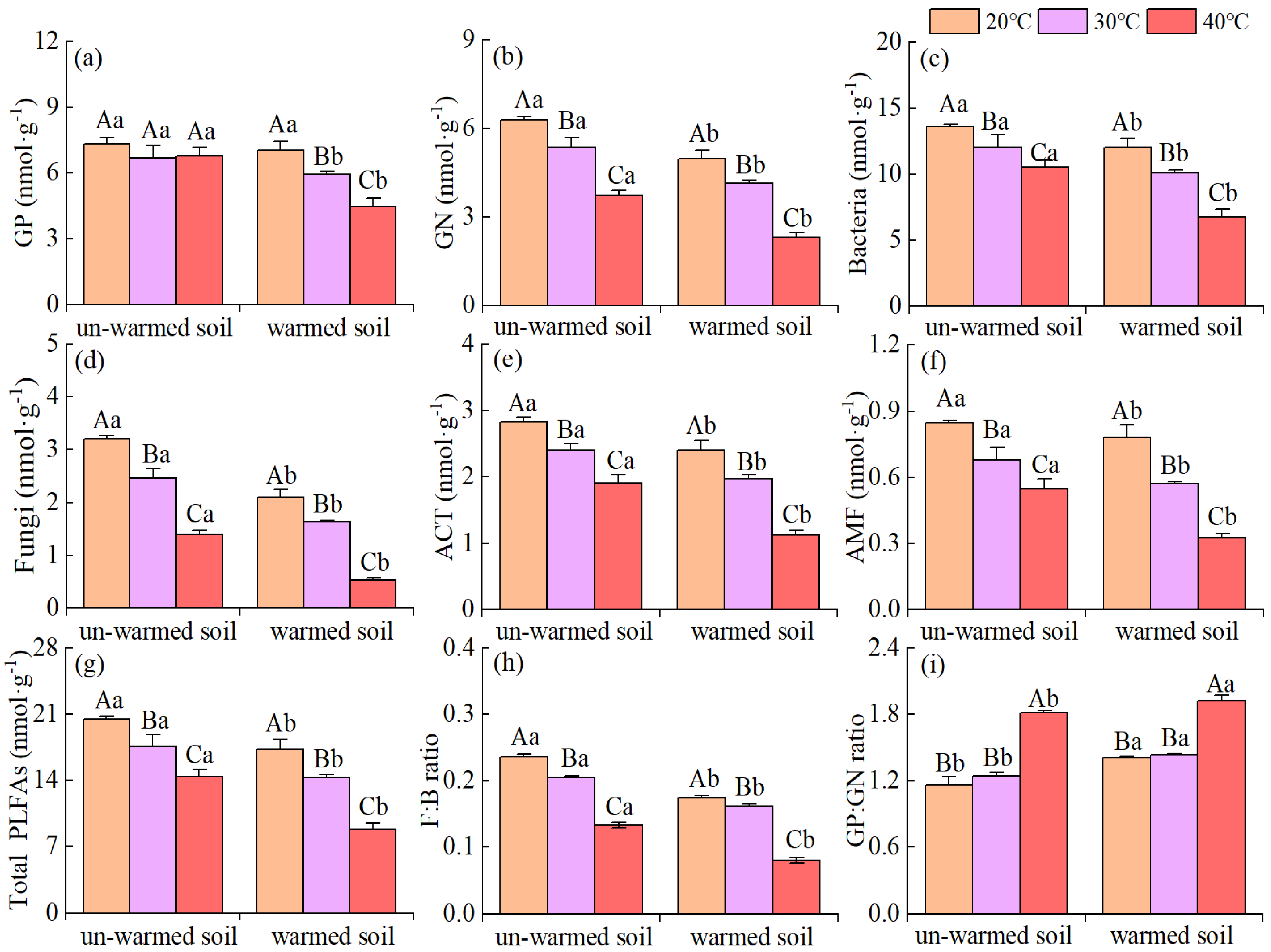
3.3. Soil Enzyme Activities and Microbial PLFAs
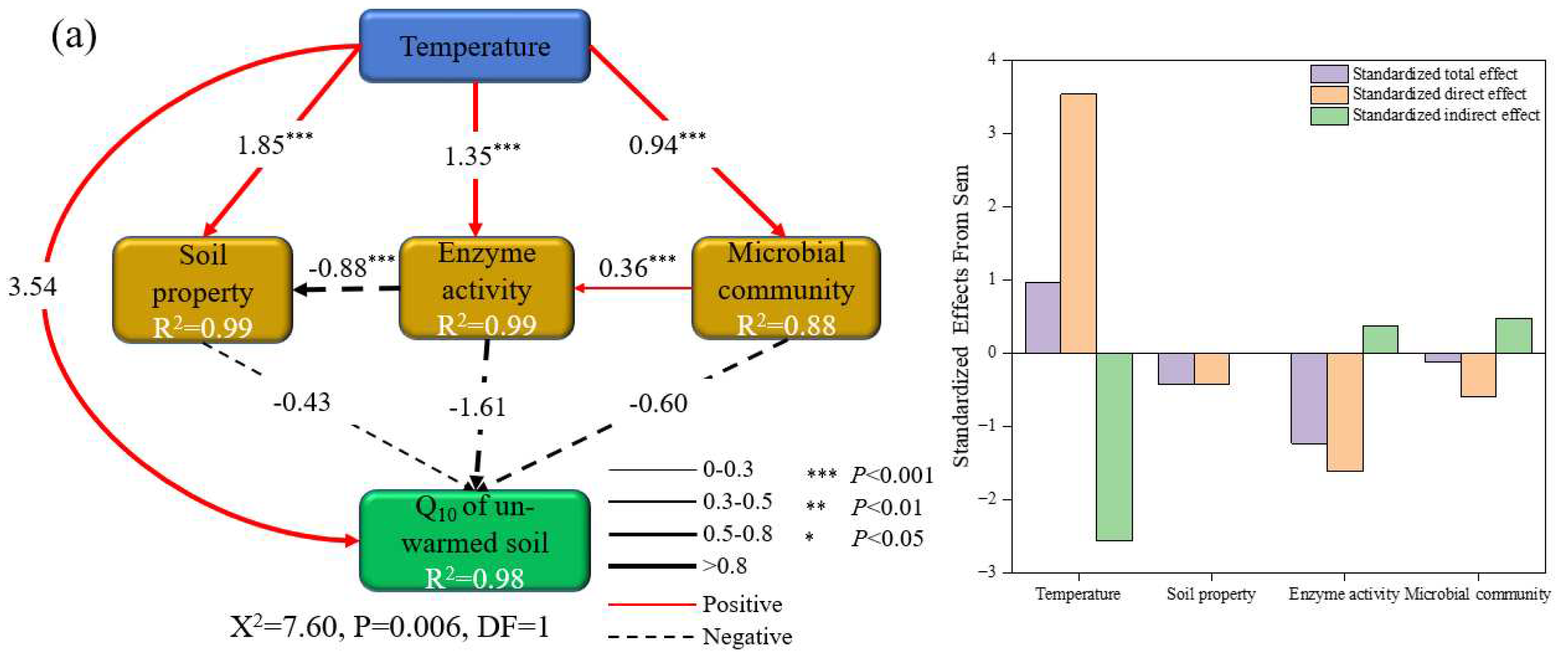
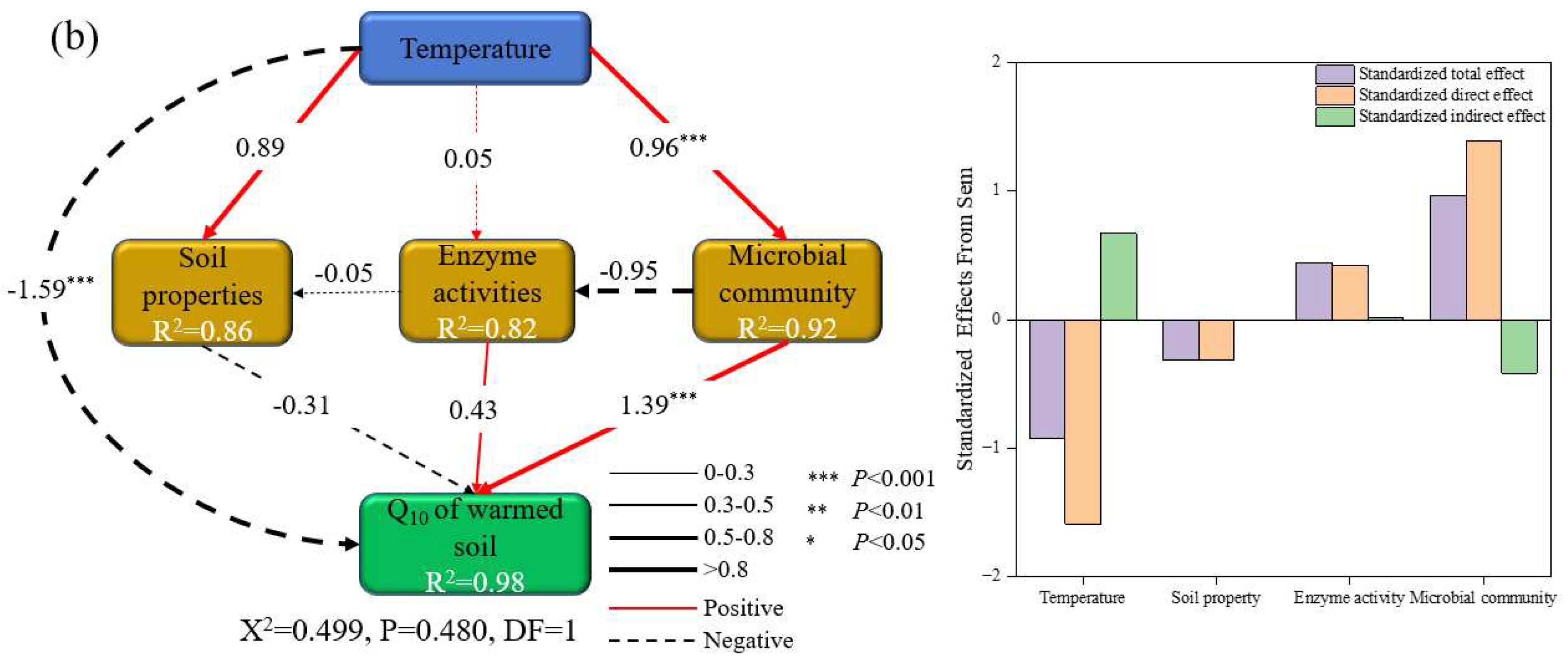
3.4. Factors Affecting the Temperature Sensitivity of SOC Mineralization
4. Discussion
4.1. Response of SOC Mineralization of Un-Warmed and Warmed Soil to Warming
4.2. Response of the Q10 of SOC Mineralization of Un-Warmed and Warmed Soil to Rising Incubation Temperature
5. Conclusions
Supplementary Materials
Funding
Declaration of competing interest
Acknowledgments
References
- Davidson, EA.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; Nannipieri, P.; Rasse, D.P.; Weiner, S.; Trumbore, S.E. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, M.A.; Reed, S.C.; Smith, W.K.; Wood, T.E. Urgent need for warming experiments in tropical forests. Global Change Biology 2015, 21, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liang, N.S.; Sha, Q.; Xu, X.L.; Zhang, Y.P.; Lu, H.Z.; Song, L.; Song, Q.H.; Xie, Y.N. Heterotrophic respiration does not acclimate to continuous warming in a subtropical forest. Scientific Reports 2016, 6, 21561. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Giardina, C.P.; Albano, J.K.; Long, M.S.; Asner, G.P. The magnitude and variability of soil-surface CO2 efflux increase with mean annual temperature in Hawaiian tropical montane wet forests. Biochemistry 2011, 43, 2315–2323. [Google Scholar] [CrossRef]
- Giardina, C.P.; Litton, C.M.; Crow, S.E.; Asner, G.P. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nature Climate Change 2014, 4, 822–827. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 1982, 14, 161–187. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M. V.; Yao, F.; Ye, J.I.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; Deng, Y.E.; Kravchenko, I.; Jiang, Y.; Kuzyakov, Y. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Global Change Biology 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Qin, S.; Kou, D.; Mao, C.; Chen, Y.; Chen, L.; Yang, Y. Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties. Science Advances 2021, 7, eabe3596. [Google Scholar] [CrossRef]
- Liu, X.F.; Yang, Z.J.; Lin, C.F.; Giardina, C.P.; Xiong, D.C.; Lin, W.S.; Chen, S.D.; Xu, C.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; Yang, Y.S. Will nitrogen deposition mitigate warming-increased soil respiration in a young subtropical plantation? Agricultural and Forest Meteorology 2017, 246, 78–85. [Google Scholar] [CrossRef]
- Wang, Q.K.; Liu, S.; Tian, P. Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Global change biology 2018, 24, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Domeignoz-Horta, L.A.; Pold, G.; Erb, H.; Sebag, D.; Verrecchia, E.; Northen, T.; Louie, K.; Eloe-Fadrosh, E.; Pennacchio, C.; Knorr, M.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Substrate availability and not thermal acclimation controls microbial temperature sensitivity response to long-term warming. Global change biology 2023, 29, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; O’Neill, T.A.; Ryburn, J.; Liang, L.L.; Arcus, V.L.; Schipper, L.A. Rapid laboratory measurement of the temperature dependence of soil respiration and application to changes in three diverse soils through the year. Biogeochemistry 2017, 133, 101–112. [Google Scholar] [CrossRef]
- Moinet, G.Y.; Dhami, M.K.; Hunt, J.E.; Podolyan, A.; Liáng, L.L.; Schipper, L.A.; Whitehead, D.; Nuñez, J.; Nascente, A.; Millard, P. Soil microbial sensitivity to temperature remains unchanged despite community compositional shifts along geothermal gradients. Global Change Biology 2021, 27, 6217–6231. [Google Scholar] [CrossRef] [PubMed]
- Pold, G.; Billings, A.F.; Blanchard, J.L.; Burkhardt, D.B.; Frey, S.D.; Melillo, J.M.; Schnabel, J.; van Diepen, L.T.A.; DeAngelis, K. Long-term warming alters carbohydrate degradation potential in temperate forest soils. Applied and environmental microbiology 2016, 82, 6518–6530. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biology and Fertility of Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Zhran, M.; Ge, T.; Tong, Y.; Deng, Y.; Wei, X.; Lynn, T.M.; Zhu, Z.; Wu, J.; Gunina, A. Assessment of depth-dependent microbial carbon use efficiency in long-term fertilized paddy soil using an 18O-H2O approach. Land Degradation & Development 2021, 32, 199-207.
- Song, Y.Y.; Liu, C.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Gao, S.Q.; Wang, L.L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, X.; Song, Y.Y.; Gao, S.Q.; Ren, J.S.; Zhang, H.; Wang, X.W. Warming-Induced Labile Carbon Change Soil Organic Carbon Mineralization and Microbial Abundance in a Northern Peatland. Microorganisms 2022, 10, 1329. [Google Scholar] [CrossRef]
- Jagadamma, S.; Mayes, M.; Steinweg, J.M.; Schaeffer, S.M. Substrate quality alters the microbial mineralization of added substrate and soil organic carbon. Biogeosciences 2014, 11, 4665–4678. [Google Scholar] [CrossRef]
- Pold, G.; Grandy, A.S.; Melillo, J.M.; Deangelis, K.M. Changes in substrate availability drive carbon cycle response to chronic warming. Soil Biology and Biochemistry 2017, 110, 68–78. [Google Scholar] [CrossRef]
- Li, X.J.; Xie, J.S.; Zhang, Q.F.; Lyu, M.K.; Xiong, X.L.; Liu, X.F.; Lin, T.C.; Yang, Y.S. Substrate availability and soil microbes drive temperature sensitivity of soil organic carbon mineralization to warming along an elevation gradient in subtropical Asia. Geoderma 2020, 364, 114198. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.-A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.- T.; Gouriveau, F.; Bergkvist, G.; Meir, P.; Nottingham, A.T.; Salinas, N.; Hartley, I. P. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Piñeiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; Dijkstra, P.; Koch, B.J.; Stone, B.W.; Purcell, A.M.; Blazewicz, S.J.; Hofmockel, K.S.; Pett-Ridge, J.; Hungate, B.A. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. The ISME Journal 2021, 15, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biology and Fertility of Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Xu, X.Y.; Liu, X.R.; Li, Y.; Ran, Y.; Liu, Y.P.; Zhang, Q.C.; Li, Z.; He, Y.; Xu, J.H.; Di, H.J. High temperatures inhibited the growth of soil bacteria and archaea but not that of fungi and altered nitrous oxide production mechanisms from different nitrogen sources in an acidic soil. Soil Biology and Biochemistry 2017, 107, 168–179. [Google Scholar] [CrossRef]
- Gommers, P.; Van Schie, B.; Van Dijken, J.; Kuenen, J. Biochemical limits to microbial growth yields: An analysis of mixed substrate utilization. Biotechnology and bioengineering 1988, 32, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gunina, A., & Kuzyakov, Y. From energy to (soil organic) matter. Global change biology 2022, 28, 2169-2182.
- LaRowe, D.E.; Van Cappellen, P. Degradation of natural organic matter: A thermodynamic analysis. Geochimica et Cosmochimica Acta 2011, 75, 2030–2042. [Google Scholar] [CrossRef]
- VandenEnden, L.; Anthony, M.A.; Frey, S.D.; Simpson, M.J. Biogeochemical evolution of soil organic matter composition after a decade of warming and nitrogen addition. Biogeochemistry 2021, 156, 161–175. [Google Scholar] [CrossRef]
- Liu, X.J.A.; Pold, G.; Domeignoz-Horta, L.A.; Geyer, K.M.; Caris, H.; Nicolson, H.; Kemner, K.M.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Soil aggregate-mediated microbial responses to long-term warming. Soil Biology and Biochemistry 2021, 152, 108055. [Google Scholar] [CrossRef]
- Li, Y.Q.; Qing, Y.X.; Lyu, M.K.; Chen, S.D.; Yang, Z.J.; Lin, C.F.; Yang, Y.S. Effects of artificial warming on different soil organic carbon and nitrogen pools in a subtropical plantation. Soil Biology and Biochemistry 2018, 124, 161–167. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, S.D.; Yang, Z.J.; Lin, C.F.; Xiong, D.C.; Lin, W.S.; Xu, C.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; Yang, Y.S. Will heterotrophic soil respiration be more sensitive to warming than autotrophic respiration in subtropical forests?: Warming effect on soil respiration. European Journal of Soil Science 2019, 70, 655–663. [Google Scholar] [CrossRef]
- Hamdi, S.; Chevallier, T.; Aïssa, N.B.; Hammouda, M.B.; Gallali, T.; Chotte, J.; Bernoux, M. Short-term temperature dependence of heterotrophic soil respiration after one-month of pre-incubation at different temperatures. Soil Biology and Biochemistry 2011, 43.9, 1752–1758. [Google Scholar] [CrossRef]
- Huang, J.X.; Lin, T.C.; Xiong, D.C.; Yang, Z.J.; Liu, X.F.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; Yang, Y.S. Organic carbon mineralization in soils of a natural forest and a forest plantation of southeastern China. Geoderma 2019, 344, 119–126. [Google Scholar] [CrossRef]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil sampling and methods of analysis. Soil sampling and methods of analysis 2007, Chapter 19, 207–214. [Google Scholar]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil biology and Biochemistry 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Berninger, F.; Pumpanen, J.; Bai, J.; Yu, L.; Duan, B. Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two Abies faxoniana forest elevations under elevated temperatures. Soil Biology and Biochemistry 2015, 91, 1–13. [Google Scholar] [CrossRef]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: lessons learned about soil quality and indications for improving sustainability. Soil Biology and Biochemistry 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. Effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biology and Biochemistry 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.K.; Linkins, A.E.; McClaugherty, C.A.; Rayburn, L.; Repert, D.A.; Weiland, T. Wood decomposition over a first-order watershed: mass loss as a function of lignocellulase activity. Soil biology and biochemistry 1992, 24, 743–749. [Google Scholar] [CrossRef]
- Wan, X.H.; Huang, Z.Q.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Davis, M.R.; Yang, Y.S. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant and soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biology and Biochemistry 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- McKinley, V.L.; Peacock, A.D.; White, D.C. Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biology and Biochemistry 2005, 37, 1946–1958. [Google Scholar] [CrossRef]
- Hu, R.G.; Hatano, R.; Kusa, K.; Sawamoto, T. Soil respiration and net ecosystem production in an onion field in Central Hokkaido, Japan. Soil science and plant nutrition 2004, 50, 27–33. [Google Scholar] [CrossRef]
- Creamer, C.A.; De Menezes, A.B.; Krull, E.S.; Sanderman, J.; Newton-Walters, R.; Farrell, M. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biology and Biochemistry 2015, 80, 175–188. [Google Scholar] [CrossRef]
- Tian, Q.X.; Wang, X.G.; Wang, D.Y.; Wang, M.; Liao, C.; Yang, X.L.; Liu, F. Decoupled linkage between soil carbon and nitrogen mineralization among soil depths in a subtropical mixed forest. Soil Biology and Biochemistry 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Lefcheck, J.S. Piecewise SEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Wang, X.W.; Li, X.Z.; Hu, Y.M.; Lv, J.J.; Sun, J.; Li, Z.M.; Wu, Z.F. Effect of temperature and moisture on soil organic carbon mineralization of predominantly permafrost peatland in the Great Hing’an Mountains, Northeastern China. Journal of Environmental Sciences 2010, 22, 1057–1066. [Google Scholar] [CrossRef]
- Gudasz, C.; Bastviken, D.; Steger, K.; Premke, K.; Sobek, S.; Tranvik, L.J. Temperature-controlled organic carbon mineralization in lake sediments. Nature 2010, 466, 478–481. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; He, T.X.; Liu, L.; Wu, J.W. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biology and Biochemistry 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Wang, Q.K.; Zeng, Z.Q.; Zhong, M.C. Soil moisture alters the response of soil organic carbon mineralization to litter addition. Ecosystems 2016, 19, 450–460. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.L.; Xu, W.H.; Li, W.; Dai, W.W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytologist 2013, 199, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dawes, M.A.; Schleppi, P.; Hättenschwiler, S.; Rixen, C.; Hagedorn, F. Soil warming opens the nitrogen cycle at the alpine treeline. Global Change Biology 2017, 23, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Butler, S.; Johnson, J.; Mohan, J.; Steudler, P.; Lux, H.; Burrows, E.; Bowles, F.; Smith, R.; Scott, L.; Vario, C.; Hill, T.; Burton, A.; Zhou, Y.M.; Tang, J. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proceedings of the National Academy of Sciences 2011, 108, 9508–9512. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.G.; Valentine, D.W.; Ruess, R.W. Soil and root respiration in mature Alaskan black spruce forests that vary in soil organic matter decomposition rates. Canadian Journal of Forest Research 2005, 35, 161–174. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Ren, X.E.; Xiao, H.A.; Tong, C.L.; Ge, T.; Brookes, P.; Shen, J.L.; Wu, J.S. Organic carbon mineralization responses to temperature increases in subtropical paddy soils. Journal of soils and sediments 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Eberwein, J.R.; Oikawa, P.Y.; Allsman, L.A.; Jenerette, G.D. Carbon availability regulates soil respiration response to nitrogen and temperature. Soil Biology and Biochemistry 2015, 88, 158–164. [Google Scholar] [CrossRef]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecology letters 2008, 11, 1316–1327. [Google Scholar] [CrossRef]
- Hamdi, S.; Moyano, F.; Sall, S.; Bernoux, M.; Chevallier, T. Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biology and Biochemistry 2013, 58, 115–126. [Google Scholar] [CrossRef]
- Gershenson, A.; Bader, N.E.; Cheng, W.C. Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Global Change Biology 2009, 15, 176–183. [Google Scholar] [CrossRef]
- Su, J.; Zhang, H.Y.; Han, X.G.; Penuelas, J.; Filimonenko, E.; Jiang, Y.; Kuzyakov, Y.; Wei, C.Z. Low carbon availability in paleosols nonlinearly attenuates temperature sensitivity of SOM decomposition. Global Change Biology 2022, 28, 4180–4193. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: the eco-physiological approach. Soil Biology and Biochemistry 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annual Review of Ecology, Evolution, and Systematics 2012, 43, 313-343.
- Ding, F.; Sun, W.J.; Huang, Y.; Hu, X.Y. Larger Q10 of carbon decomposition in finer soil particles does not bring long-lasting dependence of Q10 on soil texture. European Journal of Soil Science 2018, 69, 336–347. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Y.Q.; Zhou, J.Z. Carbon quality and the temperature sensitivity of soil organic carbon decomposition in a tallgrass prairie. Soil Biology and Biochemistry 2012, 50, 142–148. [Google Scholar] [CrossRef]
- Bai, Z.; Ma, Q.; Wu, X.; Zhang, Y.L.; Yu, W.T. Temperature sensitivity of a PLFA-distinguishable microbial community differs between varying and constant temperature regimes. Geoderma 2017, 308, 54–59. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
| Treatment | SOC | TN | NH4+-N | NO3--N | DON | DOC | MBC | MBN |
| (mg·g-1) | (mg·g-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | |
| un-warmed soil | 13.02±1.14A | 1.12±0.08A | 4.48±0.60A | 2.04±0.54A | 1.66±0.21A | 13.58±1.46A | 285.50±20.74A | 24.60±2.20A |
| warmed soil | 11.60±1.38A | 0.98±0.09A | 4.08±1.38A | 2.32±0.45A | 1.17±0.25A | 9.86±2.24B | 203.55±28.75B | 19.07±2.43B |
| Treatment | Cumulative SOC mineralization | ||||||
| 1 d | 7 d | 14d | 24d | 34 d | 49d | 63d | |
| Temperature | 1010.31*** | 807.56*** | 1130.97*** | 1718.71*** | 1449.05*** | 1380.53*** | 1515.73*** |
| Soil type | 249.58*** | 215.57*** | 228.79*** | 386.28*** | 307.45*** | 308.32*** | 349.39*** |
| Temperature × Soil type | 10.36*** | 37.93*** | 45.45*** | 58.25*** | 39.60*** | 31.91*** | 29.24*** |
| Treatment | NH4+-N | NO3--N | DON | DOC | MBC | MBN | Microbial quotient | Metabolic quotient | |
| (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (mg·kg-1) | (%) | (mg CO2-C g−1 MBC h−1) | ||
| un-warmed soil | 20 ℃ | 15.50±0.76Ba | 7.73±0.67Ca | 1.42±0.27Ba | 26.03±1.99Aa | 233.18±14.38Aa | 23.31±3.11Aa | 1.80±0.13Aa | 0.66±0.06Ca |
| 30 ℃ | 14.14±0.55Ba | 11.08±0.76Bb | 2.57±0.8Ba | 18.67±1.68Ba | 167.65±9.02Ba | 25.82±4.54Aa | 1.34±0.22Ba | 1.30±0.09Ba | |
| 40 ℃ | 27.88±0.72Aa | 15.96±0.51Ab | 5.68±0.18Aa | 15.88±0.43Ca | 94.26±6.12Ca | 17.54±2.28Ba | 0.78±0.07Ca | 3.30±0.38Aa | |
| warmed soil | 20 ℃ | 15.95±1.47Ba | 6.88±0.28Ca | 1.48±0.37Ba | 15.71±1.05Ab | 178.58±12.37Ab | 18.03±3.31Aa | 1.44±0.15Ab | 0.60±0.08Ca |
| 30 ℃ | 13.46±2.09Ba | 13.32±0.66Ba | 1.71±0.15Ba | 13.45±0.74Bb | 136.89±9.21Bb | 20.69±2.27Aa | 1.15±0.08Ba | 1.34±0.10Ba | |
| 40 ℃ | 19.22±0.63Ab | 17.20±0.30Aa | 4.54±0.41Ab | 10.96±1.08Cb | 79.23±7.18Cb | 13.32±1.72Bb | 0.67±0.06Ca | 3.23±0.25Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





