Submitted:
31 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background on Cancer and the Immune System
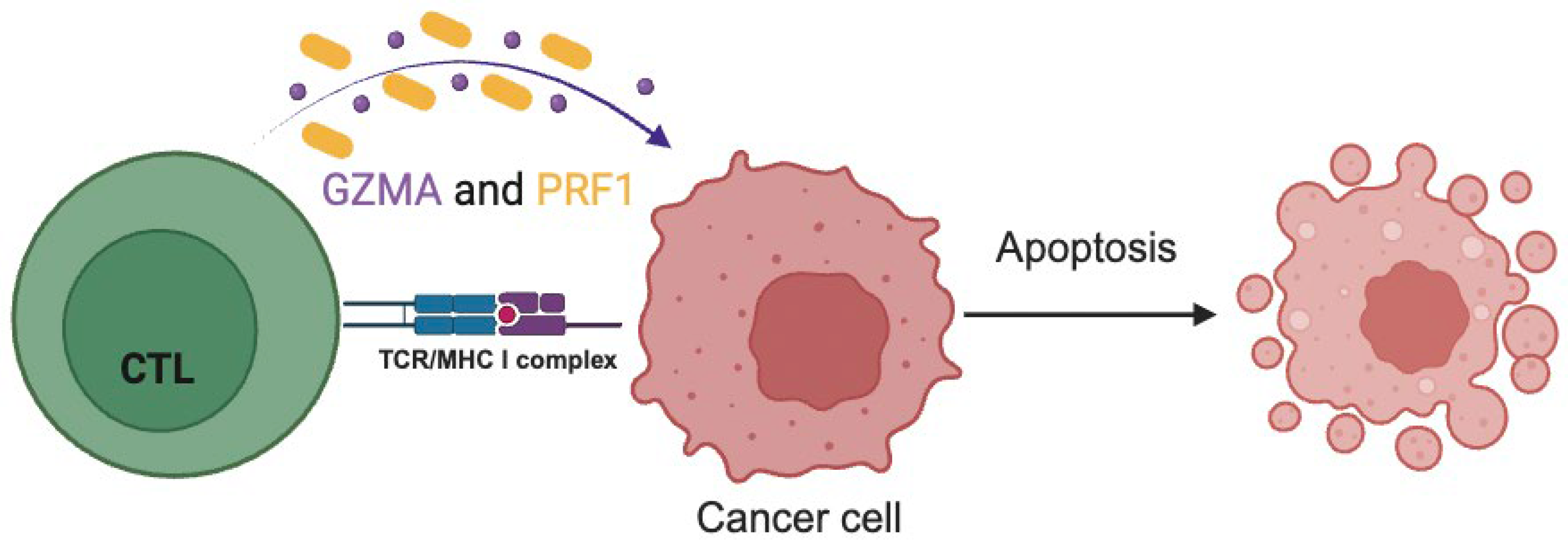
1.2. The Role and Significance of Immune Cytolytic Activity in Tumor Control
2. Results
2.1. Methods for Assesing CYT
2.1.1. Tumor-Infiltrating Lymphocytes
- Isolation and Characterization of TILs
- Analysis of TILs by flow cytometry or immunohistochemistry
2.1.2. Gene Expression Profiling
- Identification of Cytolytic Markers
- Measurement of CYT through RNA expression
2.1.3. Spatial Analysis Techniques
- Multiplex Immunofluorescence (mIF) and Imaging Mass Cytometry (IMC)
- Assessment of CYT within the TME
2.2. Factor Influencing CYT
2.2.1. Tumor Intrinsic Factors
- Tumor Mutational Burden
- Antigen Presentation and MHC Expression
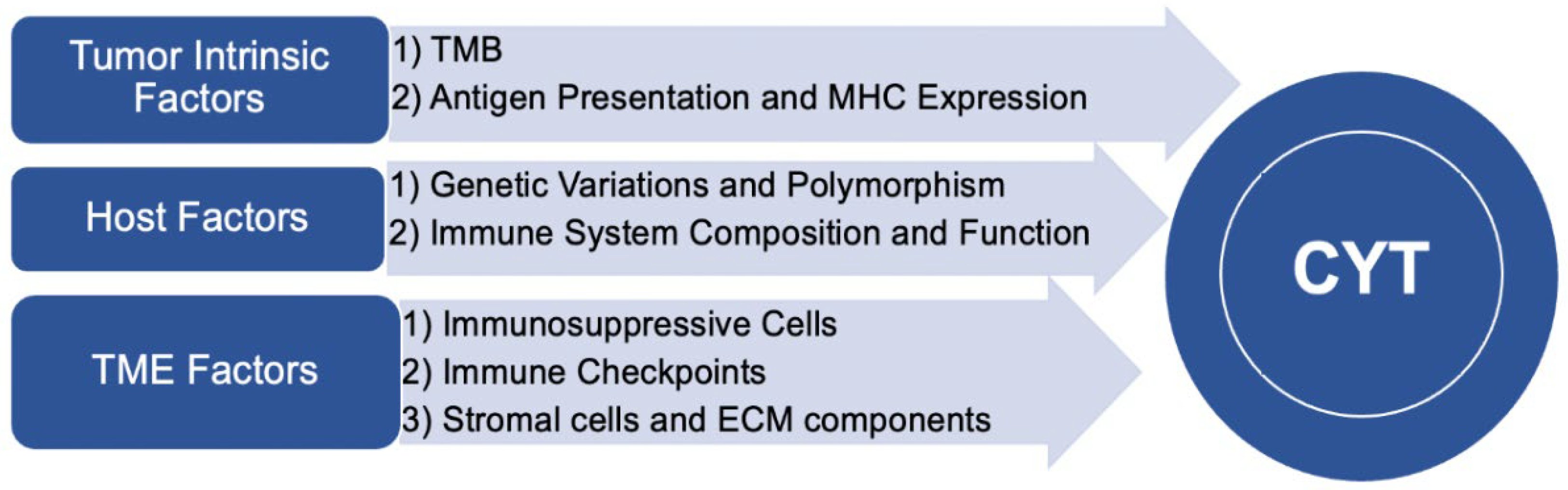
2.2.2. Tumor Microenvironment Factors
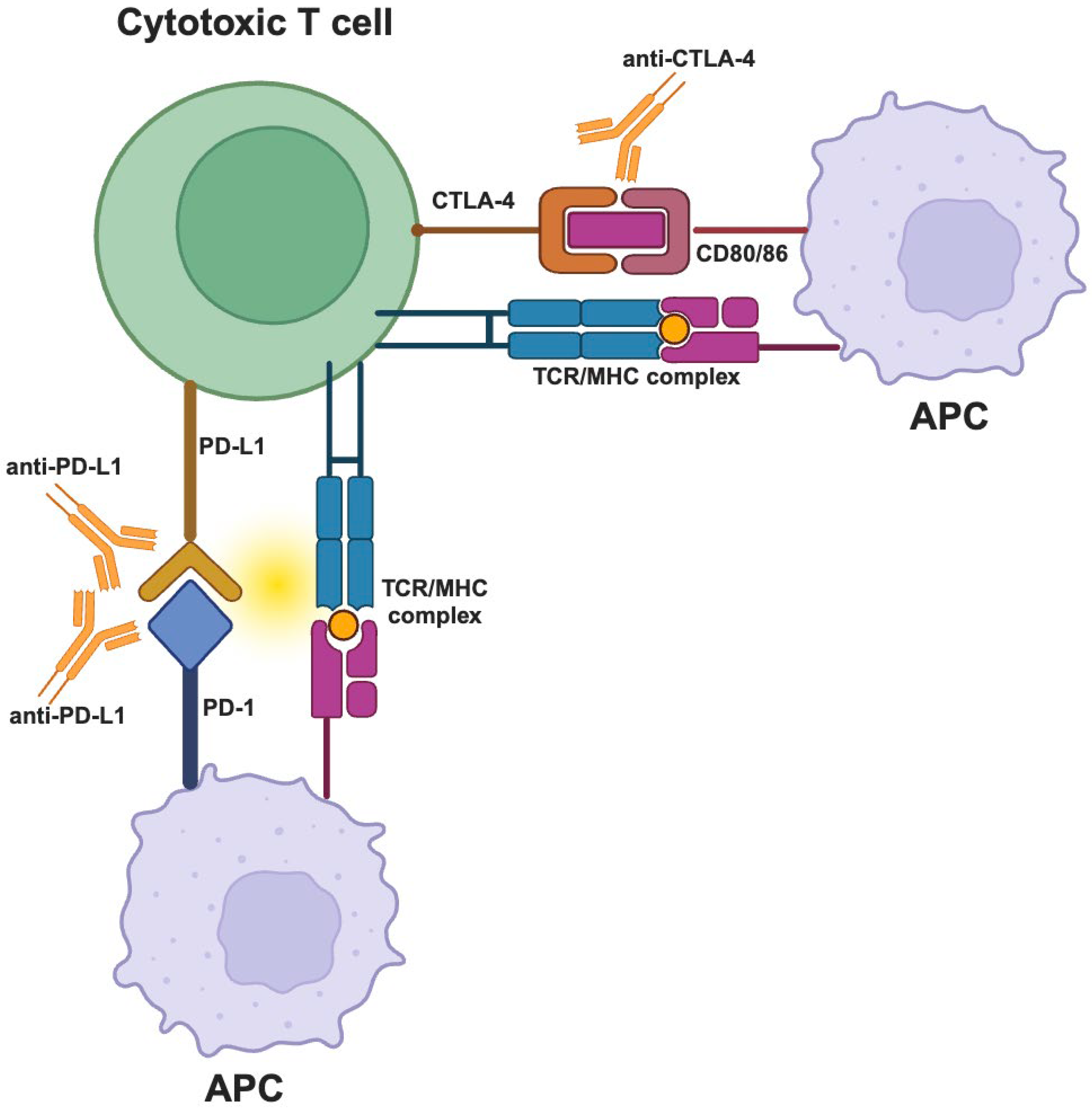
- Immunosuppressive cells
- Immune checkpoints
- Stromal Cells and Extracellular Matrix Components
2.2.3. Host Factors
- Genetic Variations and Polymorphisms
- Immune System Composition and Function
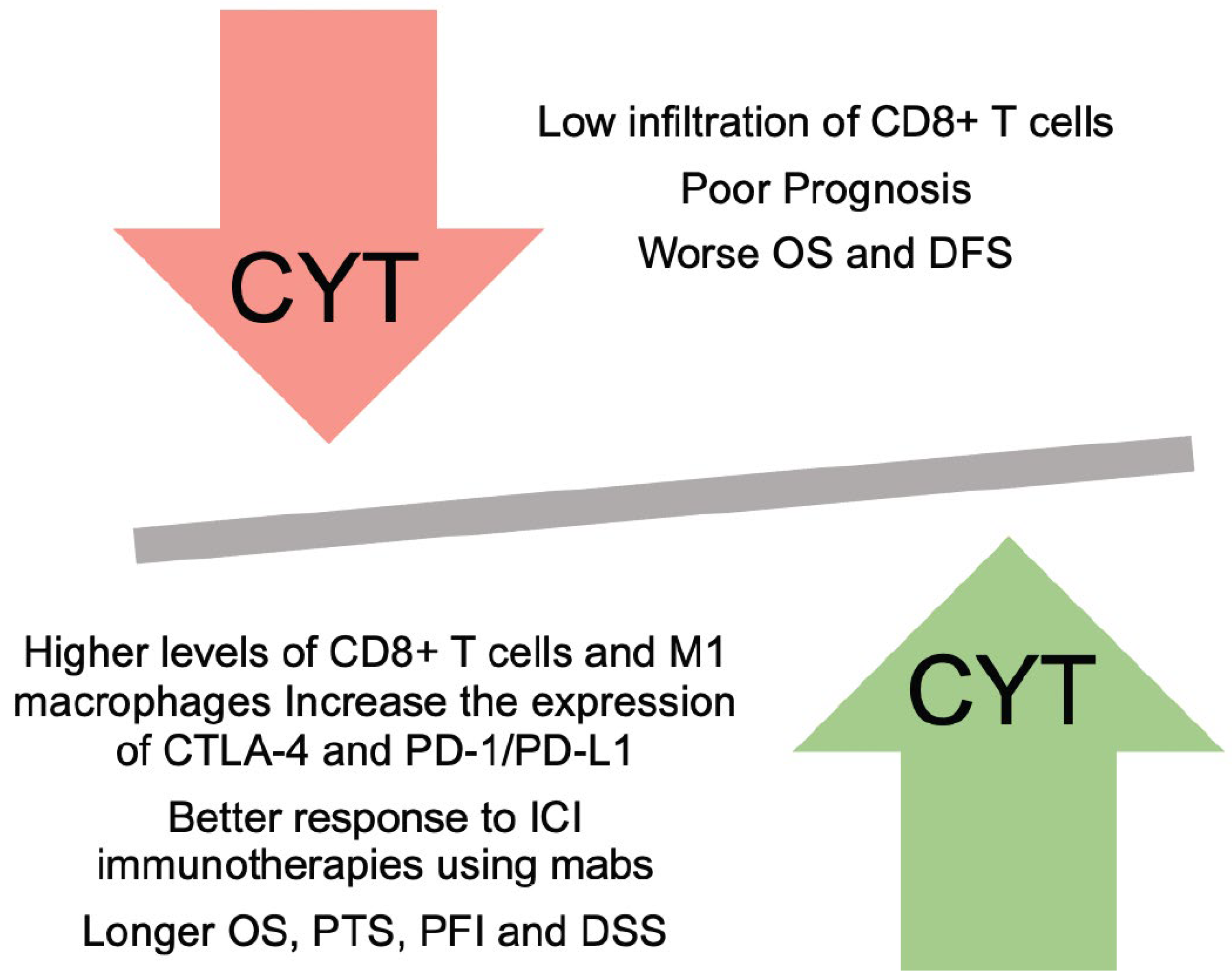
2.3. Clinical Implications and Prognostic Significance
2.3.1. Association between CYT and Patient Outcomes
2.3.2. Predictive Value of CYT for Immunotherapy Response
2.3.3. Integration of CYT Assessment into Clinical Practice
- Potential Biomarkers for CYT Evaluation
- Role of CYT in Patient Stratification and Treatment Selection
2.4. Modulation of CYT
2.4.1. Current and Emerging Immunotherapy Strategies
- Immune Checkpoint Inhibitors
- Adoptive Cell Therapies
- Vaccines and Oncolytic Viruses
2.4.2. Combination Approaches to Enhance CYT
- Dual Immune Checkpoint Blockade
- Modulation of the TME
2.4.3. Challenges and Future Directions in CYT Modulation
3. Conclusions
3.1. Recapitulation of Key Findings
3.2. Potential Clinical Implications and Future Directions
3.3. Overall Significance of Understanding CYT in Cancer
Author Contributions
Funding
Conflicts of Interest
References
- Mattiuzzi C, Lippi G. Current Cancer Epidemiology. J Epidemiol Glob Health 2019;9:217–22. [CrossRef]
- Naidoo J, Page DB, Wolchok JD. Immune checkpoint blockade. Hematol Oncol Clin North Am 2014;28:585–600. [CrossRef]
- M. Candeias S, S. Gaipl U. The Immune System in Cancer Prevention, Development and Therapy. Anticancer Agents Med Chem 2016;16:101–7. [CrossRef]
- Houghton AN, Guevara-Patiño JA. Immune recognition of self in immunity against cancer. J Clin Invest 2004;114:468. [CrossRef]
- Pandya PH, Murray ME, Pollok KE, Renbarger JL. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J Immunol Res 2016;2016. [CrossRef]
- Charles A Janeway J, Travers P, Walport M, Shlomchik MJ. Immunological memory 2001.
- Kim SK, Cho SW. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front Pharmacol 2022;13. [CrossRef]
- Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 2011;60:319. [CrossRef]
- Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol 2020;11. [CrossRef]
- Salvatore V, Teti G, Focaroli S, Mazzotti MC, Mazzotti A, Falconi M. The tumor microenvironment promotes cancer progression and cell migration. Oncotarget 2017;8:9608. [CrossRef]
- Anderson NM, Simon MC. Tumor Microenvironment. Curr Biol 2020;30:R921. [CrossRef]
- Wei R, Liu S, Zhang S, Min L, Zhu S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal Cell Pathol (Amst) 2020;2020. [CrossRef]
- O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151–67. [CrossRef]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889–94. [CrossRef]
- Lee JB, Kim HR, Ha SJ. Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy. Immune Netw 2022;22. [CrossRef]
- Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G, et al. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front Immunol 2020;11:574271. [CrossRef]
- Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022;29:3044. [CrossRef]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004;21:589–601. [CrossRef]
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2002 210 2002;2:735–47. [CrossRef]
- Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic Cancer. Clin Cancer Res 2017;23:3129–38. [CrossRef]
- Woodsworth DJ, Dreolini L, Abraham L, Holt RA. Targeted Cell-to-Cell Delivery of Protein Payloads via the Granzyme-Perforin Pathway. Mol Ther Methods Clin Dev 2017;7:132. [CrossRef]
- Roufas C, Chasiotis D, Makris A, Efstathiades C, Dimopoulos C, Zaravinos A. The expression and prognostic impact of immune cytolytic activity-related markers in human malignancies: A comprehensive meta-analysis. Front Oncol 2018;8:332192. [CrossRef]
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48–61. [CrossRef]
- Zaravinos A, Roufas C, Nagara M, De Lucas Moreno B, Oblovatskaya M, Efstathiades C, et al. Cytolytic activity correlates with the mutational burden and deregulated expression of immune checkpoints in colorectal cancer. J Exp Clin Cancer Res 2019;38. [CrossRef]
- Hu Q, Nonaka K, Wakiyama H, Miyashita Y, Fujimoto Y, Jogo T, et al. Cytolytic activity score as a biomarker for antitumor immunity and clinical outcome in patients with gastric cancer. Cancer Med 2021;10:3129. [CrossRef]
- Chen Q, Wang C, Lei X, Huang T, Zhou R, Lu Y. Immune Cytolytic Activity for Comprehensive Insights of the Immune Landscape in Endometrial Carcinoma. J Oncol 2022;2022. [CrossRef]
- Narayanan S, Kawaguchi T, Yan L, Peng X, Qi Q, Takabe K. Cytolytic Activity Score to Assess Anticancer Immunity in Colorectal Cancer. Ann Surg Oncol 2018;25:2323–31. [CrossRef]
- Oshi M, Kawaguchi T, Yan L, Peng X, Qi Q, Tian W, et al. Immune cytolytic activity is associated with reduced intra-tumoral genetic heterogeneity and with better clinical outcomes in triple negative breast cancer. Am J Cancer Res 2021;11:3628.
- Takahashi H, Kawaguchi T, Yan L, Peng X, Qi Q, Morris LGT, et al. Immune Cytolytic Activity for Comprehensive Understanding of Immune Landscape in Hepatocellular Carcinoma. Cancers 2020, Vol 12, Page 1221 2020;12:1221. [CrossRef]
- Wakiyama H, Masuda T, Motomura Y, Qingjiang HU, Tobo T, Eguchi H, et al. Cytolytic Activity (CYT) Score Is a Prognostic Biomarker Reflecting Host Immune Status in Hepatocellular Carcinoma (HCC). Anticancer Res 2018;38:6631–8. [CrossRef]
- Zhang H, Liu Y, Hu D, Liu S. Identification of Novel Molecular Therapeutic Targets and Their Potential Prognostic Biomarkers Based on Cytolytic Activity in Skin Cutaneous Melanoma. Front Oncol 2022;12:844666. [CrossRef]
- Melioli G, Meta M, Semino C, Casartelli G, Pasquetti W, Biassoni R, et al. Isolation and In vitro expansion of lymphocytes infiltrating non-small cell lung carcinoma: Functional and molecular characterisation for their use in adoptive immunotherapy. Eur J Cancer 1994;30:97–102. [CrossRef]
- Crossey F, Marx S, Hölters S, Schmitt K, Bohle RM, Schmidt T, et al. Robust method for isolation of tumor infiltrating lymphocytes with a high vital cell yield from small samples of renal cell carcinomas by a new collagenase-free mechanical procedure. Urol Oncol Semin Orig Investig 2018;36:402.e1-402.e10. [CrossRef]
- Finke JH, Rayman P, Alexander J, Edinger M, Tubbs RR, Connelly R, et al. Characterization of the Cytolytic Activity of CD4+ and CD8+ Tumor-infiltrating Lymphocytes in Human Renal Cell Carcinoma1. CANCER Res 1990;50:2363–70.
- Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010;52:370–9. [CrossRef]
- Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H, et al. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol 2022;13:1018962. [CrossRef]
- Fassler DJ, Torre-Healy LA, Gupta R, Hamilton AM, Kobayashi S, Van Alsten SC, et al. Spatial Characterization of Tumor-Infiltrating Lymphocytes and Breast Cancer Progression. Cancers (Basel) 2022;14. [CrossRef]
- Hall ML, Liu H, Malafa M, Centeno B, Hodul PJ, Pimiento J, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer 2016;4:1–12. [CrossRef]
- Kobayashi T, Kumagai S, Doi R, Afonina E, Koyama S, Nishikawa H. Isolation of tumor-infiltrating lymphocytes from preserved human tumor tissue specimens for downstream characterization. STAR Protoc 2022;3:101557. [CrossRef]
- Giraldo NA, Becht E, Vano Y, Petitprez F, Lacroix L, Validire P, et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res 2017;23:4416–28. [CrossRef]
- Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med 1991;173:1099–109. [CrossRef]
- Johnson BJ, Costelloe EO, Fitzpatrick DR, Haanen JBAG, Schumacher TNM, Brown LE, et al. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A 2003;100:2657–62. [CrossRef]
- Haddad AF, Chen JS, Oh T, Pereira MP, Joshi RS, Aghi MK. Higher cytolytic score correlates with an immunosuppressive tumor microenvironment and reduced survival in glioblastoma. Sci Reports 2020 101 2020;10:1–9. [CrossRef]
- Kim EN, Chen PZ, Bressan D, Tripathi M, Miremadi A, Pietro M di, et al. Dual-modality imaging of immunofluorescence and imaging mass cytometry for whole slide imaging with accurate single-cell segmentation. BioRxiv 2023. [CrossRef]
- Viratham Pulsawatdi A, Craig SG, Bingham V, McCombe K, Humphries MP, Senevirathne S, et al. A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment. Mol Oncol 2020;14:2384–402. [CrossRef]
- Lee CW, Ren YJ, Marella M, Wang M, Hartke J, Couto SS. Multiplex immunofluorescence staining and image analysis assay for diffuse large B cell lymphoma. J Immunol Methods 2020;478:112714. [CrossRef]
- Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 2014 114 2014;11:417–22. [CrossRef]
- Schlecht A, Boneva S, Salie H, Killmer S, Wolf J, Hajdu RI, et al. Imaging mass cytometry for high-dimensional tissue profiling in the eye. BMC Ophthalmol 2021;21:1–9. [CrossRef]
- Martinez-Morilla S, Villarroel-Espindola F, Wong PF, Toki MI, Aung TN, Pelekanou V, et al. Biomarker discovery in patients with immunotherapy-treated melanoma with imaging mass cytometry. Clin Cancer Res 2021;27:1987–96. [CrossRef]
- Mori H, Bolen J, Schuetter L, Massion P, Hoyt CC, VandenBerg S, et al. Characterizing the Tumor Immune Microenvironment with Tyramide-Based Multiplex Immunofluorescence. J Mammary Gland Biol Neoplasia 2020;25:417–32. [CrossRef]
- Gu X, Boldrup L, Coates PJ, Fahraeus R, Wang L, Wilms T, et al. High immune cytolytic activity in tumor-free tongue tissue confers better prognosis in patients with squamous cell carcinoma of the oral tongue. J Pathol Clin Res 2019;5:240–7. [CrossRef]
- Roufas C, Georgakopoulos-Soares I, Zaravinos A. Distinct genomic features across cytolytic subgroups in skin melanoma. Cancer Immunol Immunother 2021;70:3137. [CrossRef]
- Lawlor RT, Mattiolo P, Mafficini A, Hong SM, Piredda ML, Taormina S V., et al. Tumor mutational burden as a potential biomarker for immunotherapy in pancreatic cancer: Systematic review and still-open questions. Cancers (Basel) 2021;13:3119. [CrossRef]
- Meléndez B, Van Campenhout C, Rorive S, Remmelink M, Salmon I, D’Haene N. Methods of measurement for tumor mutational burden in tumor tissue. Transl Lung Cancer Res 2018;7:661. [CrossRef]
- Steuer CE, Ramalingam SS. Tumor Mutation Burden: Leading Immunotherapy to the Era of Precision Medicine? J Clin Oncol 2018;36:631–2. [CrossRef]
- Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor Mutational Burden (TMB) as a Predictive Biomarker in Solid Tumors. Cancer Discov 2020;10:1808. [CrossRef]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500. [CrossRef]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [CrossRef]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415–26. [CrossRef]
- Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093–104. [CrossRef]
- Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol 2019;30:1096–103. [CrossRef]
- McWilliam HEG, Villadangos JA. MR1 antigen presentation to MAIT cells and other MR1-restricted T cells. Nat Rev Immunol 2023:1–15. [CrossRef]
- Bruce Sundstrom J, A. Ansari A. Comparative study of the role of professional versus semiprofessional or nonprofessional antigen presenting cells in the rejection of vascularized organ allografts. Transpl Immunol 1995;3:273–89. [CrossRef]
- Wieczorek M, Abualrous ET, Sticht J, Álvaro-Benito M, Stolzenberg S, Noé F, et al. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front Immunol 2017;8:248429. [CrossRef]
- Falk K, Rötzschke O, Stevanovié S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991;351:290–6. [CrossRef]
- Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 2003;110:163. [CrossRef]
- Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol 2000;18:861–926. [CrossRef]
- Holling TM, Schooten E, Van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum Immunol 2004;65:282–90. [CrossRef]
- Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 2021;12:636568. [CrossRef]
- Schrörs B, Lübcke S, Lennerz V, Fatho M, Bicker A, Wölfel C, et al. HLA class I loss in metachronous metastases prevents continuous T cell recognition of mutated neoantigens in a human melanoma model. Oncotarget 2017;8:28312–27. [CrossRef]
- Erdogdu IH. MHC Class 1 and PDL-1 Status of Primary Tumor and Lymph Node Metastatic Tumor Tissue in Gastric Cancers. Gastroenterol Res Pract 2019;2019. [CrossRef]
- Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology 2012;1:908. [CrossRef]
- Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4+ and CD8+ T cells during the effector phase. J Exp Med 2010;207:2469. [CrossRef]
- Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019;79:4557. [CrossRef]
- Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med 2023;12:11149–65. [CrossRef]
- Tie Y, Tang F, Wei Y quan, Wei X wei. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol 2022;15:61. [CrossRef]
- Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med (Berl) 2016;94:509–22. [CrossRef]
- Sceneay J, Griessinger CM, Hoffmann SHL, Wen SW, Wong CSF, Krumeich S, et al. Tracking the fate of adoptively transferred myeloid-derived suppressor cells in the primary breast tumor microenvironment. PLoS One 2018;13. [CrossRef]
- Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol 2018;96:21–33. [CrossRef]
- Kondělková K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kral 2010;53:73–7. [CrossRef]
- Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 2014;20:676–81. [CrossRef]
- Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013;62:909–18. [CrossRef]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80. [CrossRef]
- Herber DL, Nagaraj S, Djeu JY, Gabrilovich DI. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res 2007;67:5067–9. [CrossRef]
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185–98. [CrossRef]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904–12. [CrossRef]
- Sadeghi Rad H, Monkman J, Warkiani ME, Ladwa R, O’Byrne K, Rezaei N, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev 2021;41:1474–98. [CrossRef]
- Gaikwad S, Agrawal MY, Kaushik I, Ramachandran S, Srivastava SK. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol 2022;86:137–50. [CrossRef]
- Mehdizadeh S, Bayatipoor H, Pashangzadeh S, Jafarpour R, Shojaei Z, Motallebnezhad M. Immune checkpoints and cancer development: Therapeutic implications and future directions. Pathol Res Pract 2021;223. [CrossRef]
- Ledford H. Melanoma drug wins US approval. Nature 2011;471:561. [CrossRef]
- Wang Y, Tong Z, Zhang W, Zhang W, Buzdin A, Mu X, et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front Oncol 2021;11. [CrossRef]
- Marin-Acevedo JA, Kimbrough EMO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 2021 141 2021;14:1–29. [CrossRef]
- Otoshi T, Nagano T, Tachihara M, Nishimura Y. Possible Biomarkers for Cancer Immunotherapy. Cancers (Basel) 2019;11. [CrossRef]
- Yu X, Wang X. Tumor immunity landscape in non-small cell lung cancer. PeerJ 2018;2018:e4546. [CrossRef]
- Manetti M. Molecular Morphology and Function of Stromal Cells. Int J Mol Sci 2021;22:13422. [CrossRef]
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol 2001;11:97–104. [CrossRef]
- Zhan H xiang, Zhou B, Cheng Y gang, Xu J wei, Wang L, Zhang G yong, et al. Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett 2017;392:83–93. [CrossRef]
- Lazennec G, Jorgensen C. Concise Review: Adult Multipotent Stromal Cells and Cancer: Risk or Benefit? Stem Cells 2008;26:1387–94. [CrossRef]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401. [CrossRef]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49–61. [CrossRef]
- Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 2012 321 2012;32:303–15. [CrossRef]
- Mun JY, Leem SH, Lee JH, Kim HS. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front Immunol 2022;13:864739. [CrossRef]
- Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol 2003;200:423–8. [CrossRef]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev 2016;97:4–27. [CrossRef]
- Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J 2021;288:6850–912. [CrossRef]
- Trent RJ. DNA Genetic Testing. Mol Med 2012:81–115. [CrossRef]
- Aerts J, Wetzels Y, Cohen N, Aerssens J. Data mining of public SNP databases for the selection of intragenic SNPs. Hum Mutat 2002;20:162–73. [CrossRef]
- Chanock S. Candidate genes and single nucleotide polymorphisms (SNPs) in the study of human disease. Dis Markers 2001;17:89–98. [CrossRef]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 1998;63:861–9. [CrossRef]
- Loktionov A. Common gene polymorphisms, cancer progression and prognosis. Cancer Lett 2004;208:1–33. [CrossRef]
- Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol 2009;39:2059–64. [CrossRef]
- Charles A Janeway J, Travers P, Walport M, Shlomchik MJ. The components of the immune system 2001.
- Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol 2011;7 Suppl 1. [CrossRef]
- Koretzky GA. Multiple roles of CD4 and CD8 in T cell activation. J Immunol 2010;185:2643–4. [CrossRef]
- Loose D, Van De Wiele C. The Immune System and Cancer. Https://HomeLiebertpubCom/Cbr 2009;24:369–76. [CrossRef]
- Yang L, Wang S, Zhang Q, Pan Y, Lv Y, Chen X, et al. Clinical significance of the immune microenvironment in ovarian cancer patients. Mol Omi 2018;14:341–51. [CrossRef]
- Park C, Na KJ, Choi H, Ock CY, Ha S, Kim M, et al. Tumor immune profiles noninvasively estimated by FDG PET with deep learning correlate with immunotherapy response in lung adenocarcinoma. Theranostics 2020;10:10838. [CrossRef]
- Diaz MJ, Fadil A, Kleinberg G, Root KT, Ladehoff L, Batchu S, et al. Omics analysis of uveal melanoma: Leukocyte gene signatures reveal novel survival distinctions and indicate a prognostic role for cytolytic activity scoring. Neurosci Chronicles 2022;3:6.
- McDonald K-A, Kawaguchi T, Qi Q, Peng X, Asaoka M, Young J, et al. Tumor Heterogeneity Correlates with Less Immune Response and Worse Survival in Breast Cancer Patients. Ann Surg Oncol 2019 267 2019;26:2191–9. [CrossRef]
- Huo Q, Ning L, Xie N. Identification of GZMA as a Potential Therapeutic Target Involved in Immune Infiltration in Breast Cancer by Integrated Bioinformatical Analysis. Breast Cancer (London) 2023;15:213. [CrossRef]
- Mauriello A, Zeuli R, Cavalluzzo B, Petrizzo A, Tornesello ML, Buonaguro FM, et al. High Somatic Mutation and Neoantigen Burden Do Not Correlate with Decreased Progression-Free Survival in HCC Patients not Undergoing Immunotherapy. Cancers 2019, Vol 11, Page 1824 2019;11:1824. [CrossRef]
- Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Endo I, et al. Inflammation Is Associated with Worse Outcome in the Whole Cohort but with Better Outcome in Triple-Negative Subtype of Breast Cancer Patients. J Immunol Res 2020;2020. [CrossRef]
- Takeshita T, Yan L, Asaoka M, Rashid O, Takabe K. Late recurrence of breast cancer is associated with pro-cancerous immune microenvironment in the primary tumor. Sci Reports 2019 91 2019;9:1–15. [CrossRef]
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [CrossRef]
- Turnis ME, Andrews LP, Vignali DAA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol 2015;45:1892–905. [CrossRef]
- Vanmeerbeek I, Borras DM, Sprooten J, Bechter O, Tejpar S, Garg AD. Early memory differentiation and cell death resistance in T cells predicts melanoma response to sequential anti-CTLA4 and anti-PD1 immunotherapy. Genes Immun 2021;22:108–19. [CrossRef]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (80- ) 2015;350:207–11. [CrossRef]
- Inoue H, Park JH, Kiyotani K, Zewde M, Miyashita A, Jinnin M, et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology 2016;5. [CrossRef]
- Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, et al. Identification of essential genes for cancer immunotherapy. Nature 2017;548:537–42. [CrossRef]
- Wu X, Wang X, Zhao Y, Li K, Yu B, Zhang J. Granzyme family acts as a predict biomarker in cutaneous melanoma and indicates more benefit from anti-PD-1 immunotherapy. Int J Med Sci 2021;18:1657. [CrossRef]
- Gao Z, Tao Y, Lai Y, Wang Q, Li Z, Peng S, et al. Immune Cytolytic Activity as an Indicator of Immune Checkpoint Inhibitors Treatment for Prostate Cancer. Front Bioeng Biotechnol 2020;8:552034. [CrossRef]
- Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis 2015 66 2015;6:e1792–e1792. [CrossRef]
- Takeshita T, Asaoka M, Katsuta E, Photiadis SJ, Narayanan S, Yan L, et al. High expression of polo-like kinase 1 is associated with TP53 inactivation, DNA repair deficiency, and worse prognosis in ER positive Her2 negative breast cancer. Am J Transl Res 2019;11:6507.
- Mellor-Heineke S, Villanueva J, Jordan MB, Marsh R, Zhang K, Bleesing JJ, et al. Elevated Granzyme B in Cytotoxic Lymphocytes is a Signature of Immune Activation in Hemophagocytic Lymphohistiocytosis. Front Immunol 2013;4. [CrossRef]
- Verschoor CP, Picard E, Andrew MK, Haynes L, Loeb M, Pawelec G, et al. NK- and T-cell granzyme B and K expression correlates with age, CMV infection and influenza vaccine-induced antibody titres in older adults. Front Aging 2022;3:1098200. [CrossRef]
- Wang QX, Qu CH, Gao YH, Ding PR, Yun JP, Xie D, et al. The degree of microsatellite instability predicts response to PD-1 blockade immunotherapy in mismatch repair-deficient/microsatellite instability-high colorectal cancers. Exp Hematol Oncol 2021;10:1–4. [CrossRef]
- Saini A, Kumar M, Bhatt S, Saini V, Malik A. CANCER CAUSES AND TREATMENTS. Int J Pharm Sci Res 2020;11:3121. [CrossRef]
- Rao S, Horwitz SB, Ringel I. Direct photoaffinity labeling of tubulin with taxol. J Natl Cancer Inst 1992;84:785–8. [CrossRef]
- Sartiano G, Darrell Bullington W, Lynch W. Mechanism of action of the anthracycline anti-tumor antibiotics, doxorubicin, daunomycin and rubidazone: preferential inhibition of DNA polymerase alpha. J Antibiot (Tokyo) 1979;32:1038–45. [CrossRef]
- Reedijk J, Lohman PHM. Cisplatin: synthesis, antitumour activity and mechanism of action. Pharm Weekbl Sci 1985;7:173–80. [CrossRef]
- Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother 2001;50:445–55. [CrossRef]
- Okano M, Oshi M, Butash AL, Asaoka M, Katsuta E, Peng X, et al. Estrogen Receptor Positive Breast Cancer with High Expression of Androgen Receptor has Less Cytolytic Activity and Worse Response to Neoadjuvant Chemotherapy but Better Survival. Int J Mol Sci 2019, Vol 20, Page 2655 2019;20:2655. [CrossRef]
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:330851. [CrossRef]
- Wu L, Yun Z, Tagawa T, Rey-McIntyre K, De Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther 2012;11:1809–19. [CrossRef]
- Li B, Chan HL, Chen P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr Med Chem 2017;26:3009–25. [CrossRef]
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018 1181 2018;118:9–16. [CrossRef]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [CrossRef]
- Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1–13. [CrossRef]
- Atkins MB, Kudchadkar RR, Sznol M, McDermott DF, Lotem M, Schachter J, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. Https://DoiOrg/101200/Jco20143215_suppl9001 2014;32:9001–9001. [CrossRef]
- Jiang X, Xu J, Liu M, Xing H, Wang Z, Huang L, et al. Adoptive CD8+ T cell therapy against cancer:Challenges and opportunities. Cancer Lett 2019;462:23–32. [CrossRef]
- Cohen JE, Merims S, Frank S, Engelstein R, Peretz T, Lotem M. Adoptive cell therapy: past, present and future. Http://DxDoiOrg/102217/Imt-2016-0112 2017;9:183–96. [CrossRef]
- Hawkins RE, Gilham DE, Debets R, Eshhar Z, Taylor N, Abken H, et al. Development of Adoptive Cell Therapy for Cancer: A Clinical Perspective. Https://HomeLiebertpubCom/Hum 2010;21:665–72. [CrossRef]
- Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch 2019;474:449–61. [CrossRef]
- Ylösmäki E, Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr Opin Biotechnol 2020;65:25–36. [CrossRef]
- de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. Armed oncolytic viruses: A kick-start for anti-tumor immunity. Cytokine Growth Factor Rev 2018;41:28–39. [CrossRef]
- Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer 2013 121 2013;12:1–16. [CrossRef]
- Elsedawy NB, Russell SJ. Oncolytic vaccines. Expert Rev Vaccines 2013;12:1155–72. [CrossRef]
- Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther 2014;22:1949–59. [CrossRef]
- Carloni R, Sabbioni S, Rizzo A, Ricci AD, Palloni A, Petrarota C, et al. Immune-Based Combination Therapies for Advanced Hepatocellular Carcinoma. J Hepatocell Carcinoma 2023;10:1445. [CrossRef]
- Selby M, Engelhardt J, Lu L-S, Quigley M, Wang C, Chen B, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. Https://DoiOrg/101200/Jco20133115_suppl3061 2013;31:3061–3061. [CrossRef]
- Mariniello A, Novello S, Scagliotti G V., Ramalingam SS. Double immune checkpoint blockade in advanced NSCLC. Crit Rev Oncol Hematol 2020;152:102980. [CrossRef]
- van der Leun AM, Traets JJH, Vos JL, Elbers JBW, Patiwael S, Qiao X, et al. Dual Immune Checkpoint Blockade Induces Analogous Alterations in the Dysfunctional CD8+ T-cell and Activated Treg Compartment. Cancer Discov 2023;13:2212–27. [CrossRef]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23–34. [CrossRef]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med 2015;372:2006–17. [CrossRef]
- Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol 2015;35:S199–223. [CrossRef]
- Chen L xun, Zeng S jie, Liu X dong, Tang H bin, Wang J wu, Jiang Q. Cell-cell communications shape tumor microenvironment and predict clinical outcomes in clear cell renal carcinoma. J Transl Med 2023;21. [CrossRef]
- Kolb D, Kolishetti N, Surnar B, Sarkar S, Guin S, Shah AS, et al. Metabolic Modulation of the Tumor Microenvironment Leads to Multiple Checkpoint Inhibition and Immune Cell Infiltration. ACS Nano 2020;14:11055–66. [CrossRef]
- Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013;2. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




